Abstract
Proton therapy treatments are prescribed using a biological effectiveness relative to photon therapy of 1.1, that is, proton beams are considered to be 10% more biologically effective. Debate is ongoing as to whether this practice needs to be revised. This short review summarizes current knowledge on relative biological effectiveness variations and uncertainties in vitro and in vivo. Clinical relevance is discussed and strategies toward biologically guided treatment planning are presented.
Keywords: proton therapy, relative biological effectiveness, proton dose response
Introduction
Energy deposition events caused by radiation interactions with tissues can damage biological structures. While apoptosis or programmed cell death may also be initiated by damage to the cell membrane, for most cell types damage to the DNA molecules is decisive for mutation induction and killing. The type and spatial distribution of DNA damage and the properties of cell repair mechanisms determine the resulting biological effect.
The energy deposition pattern of a given radiation is determined by the particle's track structure. It is defined as a track core causing interactions of the primary particle and a surrounding halo generated by δ-electrons. Radiation of different linear energy transfer (LET) differs in track structure, which affects the type of damage and the capacity of the cell to repair it [1–3]. Clusters of strand breaks that are more concentrated in space and the associated damage is less likely to be repaired correctly [4–6]. For instance, even if photons and protons would cause the same number of DNA double-strand breaks per unit dose, their distribution may differ. In addition to differences in track structure for single tracks, the energy deposited per incident proton is significantly higher than the energy deposited per incident photon. For the same dose, the number of protons crossing a region of interest is typically lower than the corresponding number of photons. This further causes energy depositions in a cellular structure to be more heterogeneous for proton radiation compared with photon radiation [7].
Radiation therapy treatments are prescribed based on absorbed dose. Therefore, in order to account for differences in energy deposition patterns, a scaling parameter is needed when comparing different modalities such as photons and protons for the same physical absorbed dose. The proton relative biological effectiveness (RBE) is the ratio of the absorbed doses that produce the same biological effect between a reference radiation and a proton irradiation equation 1).
 |
In addition to LET, the proton RBE varies with dose and the biological endpoint (as well as the intrinsic radiosensitivity of the tissue). Doses in proton therapy are prescribed as Gy(RBE) to reflect that the dose was multiplied with an RBE value [8]. The RBE is typically defined for a region of uniform absorbed dose. In a patient, variable RBE values would have to be assigned on a computer tomography voxel by voxel basis. Organ effect modeling would then be based on the RBE-weighted dose distribution. In the clinical use of RBE, fractionation effects are typically not considered in the RBE concept, that is, the RBE is considered for the dose per fraction [9, 10].
Cell survival curves measured in vitro are typically described using the linear quadratic dose-response curve with parameters α and β. Accordingly, one can deduce a relationship of RBE with the αx and βx values for the reference photon radiation, the αp and βp of the proton radiation, and the proton dose per fraction (Dp). Assuming αp and βp, depending on the dose-averaged LET (LETd), this leads to equation 2.
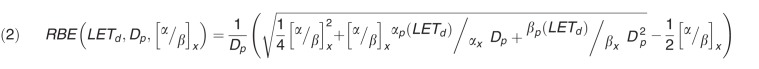 |
Because the RBE depends on the photon reference radiation, the reference has to be stated when reporting RBE values. Clinically, one is interested in the RBE relative to 6-MV photons. However, cell experiments are often based on lower energy photons requiring RBE adjustment to account for differences among photon radiations.
Use of 1.1 as a Generic RBE in Proton Therapy
Because there is no simple relationship between dose and clinical endpoint, clinical prescription doses and dose constraints are assigned mostly empirically. For consistency and to benefit from the large pool of clinical results obtained with photon beams, prescription doses are defined as photon doses. Proton therapy tumor prescriptions and organ constraints are based on physical dose times a generic constant RBE of 1.1. This value is based on in vivo measurements relative to 60Co reference radiation that were performed in the early days of proton therapy (eg [11–14]). It was initially reported for the center of the target volume, a 2 Gy(RBE) fraction size, and averaged over various endpoints, such as skin reaction or median lethal dose. Based on a more recent analysis of all published cell survival data in vitro, the estimated average RBE is about 1.15 in the center of a typical spread-out Bragg peak (SOBP) at 2 Gy(RBE) per fraction [15, 16]. Because one aims at a conservative RBE definition for tumor control, this is in line with the clinical use of 1.1.
RBE Variations in Laboratory Experiments
RBE Dependency on LET
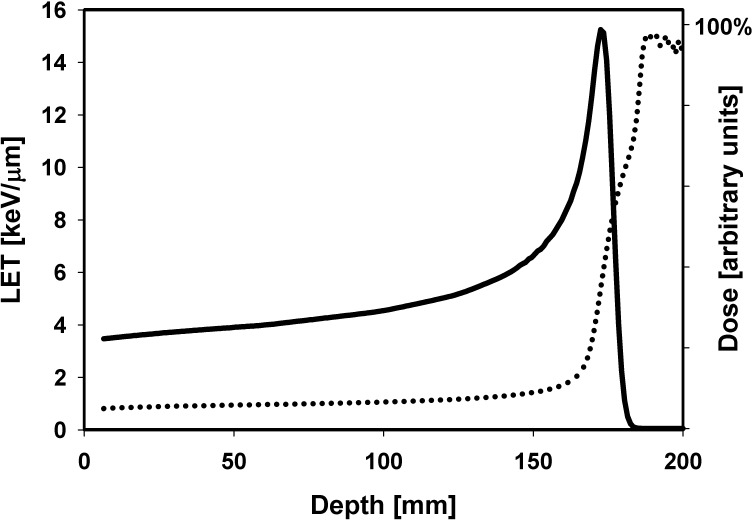
The slowing down of protons causes an LET increase resulting in an increasing RBE with depth in a pristine Bragg curve or SOBP (see, eg, [17–19]). The region of maximum LET is not at the Bragg peak position but downstream (Figure 1). The LET is a macroscopic dosimetric parameter and does not describe energy deposition in a biological target but, rather, the energy deposition per path length of a particle. Nevertheless, the use of LET to describe local energy depositions is a valid approximation for proton beams where the number of particle tracks crossing a subcellular structure is quite large [20].
Figure 1.
Dose (solid line; right axis scale) and dose-averaged LET (dotted line; left axis scale) as a function of depth in a water phantom for a 160 MeV beam. Abbreviation: LET, linear energy transfer.
For protons and heavier ions, the RBE increases with increasing LET up to a maximum and then decreases [21]. For protons, the maximum RBE occurs at very low proton energies that are dosimetrically negligible. Thus, one can safely assume that RBE increases with LET, with the slope depending on the biological endpoint.
When specifying RBE, one needs to consider the LET relative to the LET of the photon reference radiation. For instance, the LET caused by electrons of a 60Co photon beam is ∼ 0.3 keV/μm [22]. Relative to this photon value, the LETd values in the entrance region of an SOBP up to the center of the SOBP are typically between 0 and 3 keV/μm; in the downstream half of an SOBP they are between 3 and 6 keV/μm, in distal edge region between 6 and 9 keV/μm, and in the dose fall-off region between 9 and 15 keV/μm. The LETd in the central region of an SOBP is thus typically between ∼2 and ∼3 keV/μm.
Based on these typical LETd values in an SOBP and based on the average of cell survival results obtained in vitro, the RBE for 2 Gy does increase with depth from ∼1.1 in the entrance region to ∼1.15 in the center, ∼1.35 at the distal edge, and ∼1.7 in the distal fall-off [16]. The rise in LET and RBE also results in an extension of the biologically effective range by 1 to 2 mm (see, eg, [19, 23–26]).
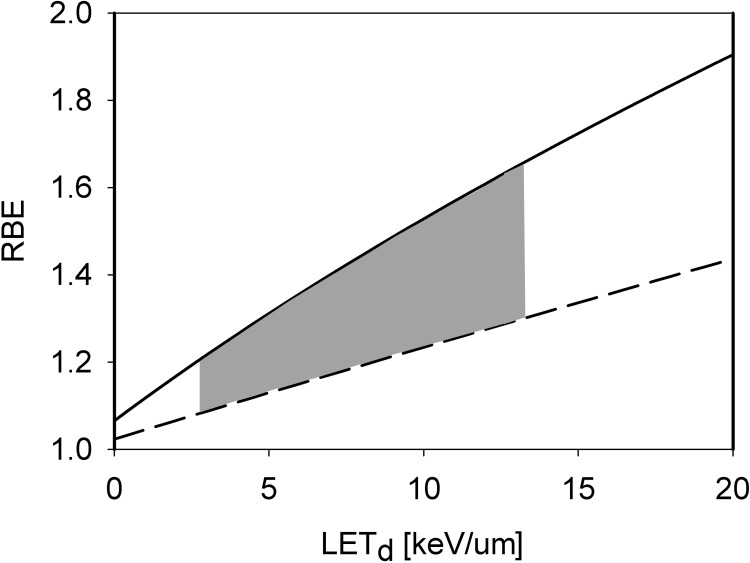
Phenomenologic models based on measured in vitro cell survival data predict a more or less linear relationship between RBE and LETd (see Figure 2) [27]. The RBE also depends on the tissue (eg, the (α/β)x) and models predict a steeper slope as (α/β)x decreases. There is some evidence that the relationship becomes nonlinear at high LET values, with an increasing slope in RBE [28, 29].
Figure 2.
Proton RBE for clonogenic cell survival as a function of LETd at 2 Gy photon dose for tissue (α/β)x of 2 Gy (solid) and 10 Gy (dashed) as predicted by an empirical model [27]. The grey area shows the clinically and dosimetrically most relevant region as LET values are typically between 2.5 and 13 keV/μm [16]. Abbreviations: LET, linear energy transfer; LETd, dose-averaged LET; RBE, relative biological effectiveness.
RBE Dependency on Dose
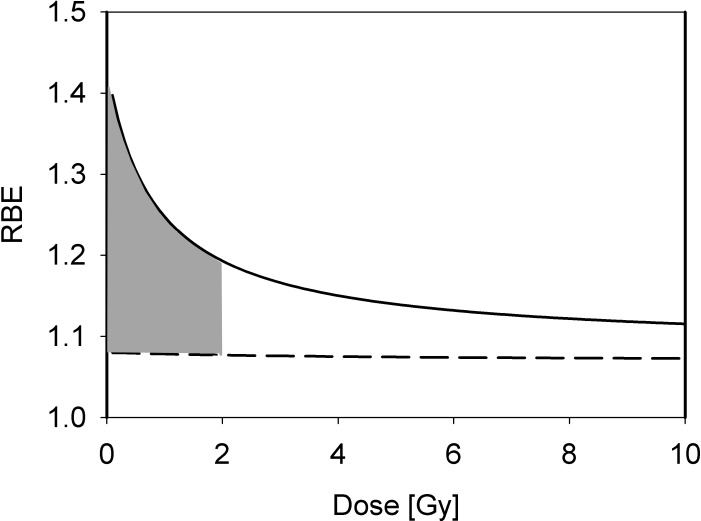
Due to the more pronounced shoulder in the photon dose response curve for cell survival compared with the proton dose response curve, the RBE increases with decreasing dose for cell survival [15, 16]. This trend is more pronounced for late-responding tissues (low α/β) compared with early responding tissues (high α/β) [19, 30–33]. Figure 3 shows the RBE as a function of dose for clonogenic cell survival in vitro as predicted by a phenomenologic model.
Figure 3.
Proton RBE for clonogenic cell survival as a function of dose for an LETd of 2.5 keV/μm for tissue (α/β)x of 2 Gy (solid) and 10 Gy (dashed) as predicted by an empirical model [27]. The grey area shows the clinically most relevant region for standard fractionation with doses at 2 Gy and doses to organs at risk <2Gy. Abbreviations: LETd, dose-averaged linear energy transfer; RBE, relative biological effectiveness.
Experimental data on dose dependency of the RBE for clinically relevant doses are limited. Most in vitro studies do not report detailed cell survival data below 2 Gy. Furthermore, most experimental RBE studies in vivo have used large doses for which an RBE effect may be expected to be small.
RBE Dependency on Endpoint
Most in vitro experiments study cell survival, that is, colony formation. Furthermore, they have predominantly used CHO and V79 hamster cells, which exhibit large shoulders on their photon dose response curve, that is, low α/β. In studies with low energy beams, the data on human cells [34, 35] are significantly lower than the RBE values determined for hamster cells [36–41]. In contrast, most in vivo studies have used early reacting tissues having a high α/β. Also, the in vivo response reflects the more complex expression of radiation damage to 3-dimensional tissue systems and biological processes.
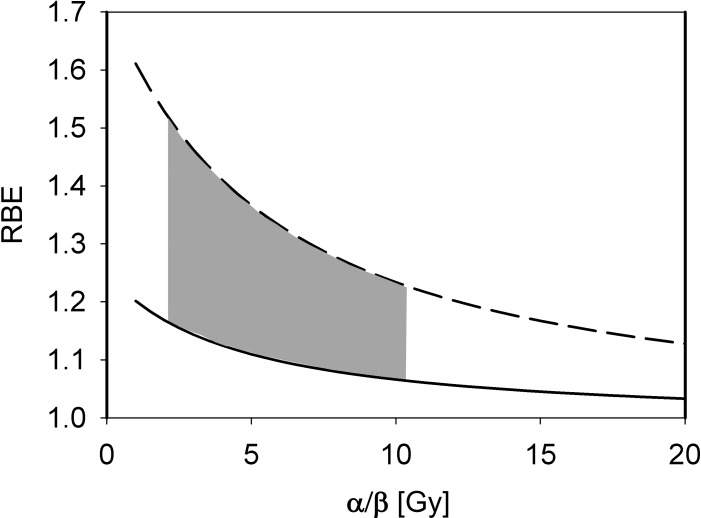
From the linear-quadratic model one expects a higher RBE for low (α/β)x [26]. Studies have indicated that the increase of RBE with decreasing (α/β)x is significant only at low (α/β)x values (<∼5 Gy) [30, 42]. One would also expect the slope to be bigger for high LETd values, which was not shown in a compilation of experimental data due to uncertainties in the experiments [16]. Figure 4 shows RBE values as a function of the tissues' (α/β)x based on a phenomenologic model.
Figure 4.
Proton RBE for clonogenic cell survival as a function of (α/β)x for a photon dose of 2 Gy and LETd values of 2 keV/μm (solid) and 10 keV/μm (dashed) as predicted by an empirical model [27]. The grey area shows the clinically most relevant region as treatment planning considerations are typically based on (α/β)x between 3 and 10 Gy. Abbreviations: LETd, dose-averaged linear energy transfer; RBE, relative biological effectiveness.
Various RBE values for endpoints other than cell survival have been measured in vitro, for example, induction of reactive oxygen species leading to oxidative stress, which regulates a variety of response pathways, DNA single or double-strand breaks, foci formation, repair proteins, gene expression, chromosome aberrations, mutations, micronuclei formation, apoptosis, and cell cycle effects (see eg, [43–46]). The majority of data support an average RBE of ∼1.1. In vivo, the magnitude of RBE variation with physical or biological parameters is usually small relative to our abilities to determine RBE values. The required number of animals to measure a 5% RBE difference can be several hundred [15].
RBE Variations in Patients
RBE Dependency on LET
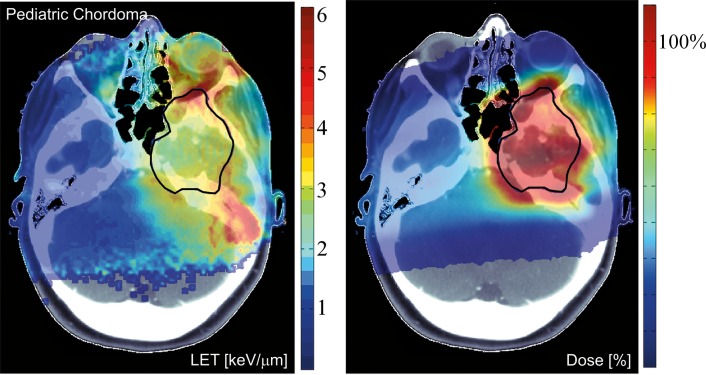
In patients, the increasing LETd, (and RBE) with increasing depth is of concern for critical structures immediately downstream of the target area, particularly if single-field uniform dose (SOBP) fields are being delivered. It potentially causes an underestimation of the RBE-weighted dose. For multiple fields, and particularly in intensity-modulated proton therapy (IMPT) delivering inhomogeneous dose distributions per field, the LET distribution can be more inhomogeneous, although maximum values would still be mostly in the periphery of the target [47, 48]. Figure 5 illustrates the distributions of dose and LETd in a patient. LETd values in patients can typically be >10 keV/um in the distal fall-off, but only between 1.5 and 4 keV/um in the target for typical beam arrangements [48]. Due to margins added in treatment planning, high LET regions may extend well into normal tissues.
Figure 5.
Dose distribution and distribution of LETd for an intensity-modulated proton therapy treatment plan. The contour for the clinical target volume is shown in black. Left: LETd distribution in keV/μm. The LET distribution is a potential measure of biological effectiveness. Right: dose in percent of the prescribed dose. The figure uses dose and LET cut-offs at <0.1% of the maximum, respectively. See reference [48] for more details. Abbreviations: LET, linear energy transfer; LETd, dose-averaged LET.
The clinical evidence that the use of 1.1 causes unexpected rates of toxicities in high LET regions is weak, perhaps due to interpatient variability and other cofounding factors. Even so, it seems clear from in vitro data that if regions of high LETd are within organs at risk with a low (α/β)x we most likely underestimate the RBE. Treatment planners have to keep potential RBE effects in mind. One example is the brainstem, which sometimes is located at the end of range of an SOBP field in patients with an ependymoma [49–52].
The clinical significance of RBE variability may become more apparent as other sources of uncertainty are reduced, leading to a reduction in margins, which in turn might expose RBE variations [26, 53]. Furthermore, the increasing use of IMPT could lead to variations in RBE as a function of delivery and/or planning parameters [48, 54–56].
RBE Dependency on Dose
An increase of RBE as dose decreases suggests higher RBE values in organs at risk compared with the target. Furthermore, it implies that hypofractionated regimens will result in lower RBE values [57]. Several theoretical studies have addressed the issue of RBE spatial variations in patients [57–59] and have analyzed the impact of RBE on fractionation [10, 60, 61].
RBE Dependency on Endpoint
In vitro data on cell survival suggest a tendency toward an increased RBE in cells with smaller (α/β)x. Thus, in vivo, one might expect the biggest variation in RBE for late-responding normal tissues [62]. While most tumors types might on average show higher RBE values, there are also tumors with high (α/β)x which would imply lower RBE values. It has been speculated that medulloblastoma, a tumor with a high (α/β)x, could be underdosed when using protons because of an RBE below 1.1 [63–65]. However, when analyzing patients treated with protons no indication was found that the RBE was overestimated [65]. The trend with (α/β)x does suggest an advantage of proton therapy when treating, for example, prostate carcinoma, which are expected to show low (α/β)x [66]. For normal tissue toxicities, this trend might suggest an increased risk for low (α/β)x tissues like the spinal cord that often have to be partially irradiated in order to achieve sufficient tumor coverage. For tumors and toxicities, the impact of (α/β)x could affect the interpretation of clinical trials comparing photon and proton treatments.
We have not clearly identified toxicities or recurrences that were definitively a result of RBE effects [49, 65, 67]. However, it is difficult to assess RBE effects in patients because of patient variability in tissue radiosensitivity [68, 69].
A subset of human cancers may have defects in DNA repair pathways that influence the RBE. For example, homologous recombination is required for the repair of DSBs in late S- and G2-phases of the cell cycle. It has been shown that defective homologous recombination increases the RBE for cell survival [44, 68, 70–72]. There are research efforts toward identifying biomarkers to identify patients with RBE values either low or high compared with the overall patient population [44, 68, 70].
Tumor Control Probability
While cell survival might be a valid surrogate for tumor control, there are various pathways leading to cell death or to tumor regression. In terms of tumor control probability (TCP), clinically more relevant than cell survival studies might be measurements of tumor control dose 50% (the dose for 50% local control) using human tumor cells that have been implanted in immune-deficient animals. Studies on tumor growth delay in mice as well as recurrence of mouse mammary carcinoma found RBE values between 1.0 and 1.2 at doses >20 Gy [73–75].
In some TCP models radiosensitivity is parameterized with cell kill parameters. When analyzing the impact of (α/β)x on TCP, one needs to consider that not only the RBE but also the TCP depends on (α/β)x [69]. Assuming interpatient variability in linear-quadratic radiosensitivity parameters for a given tumor type, one might expect a lower TCP for patients with low average (α/β)x. In proton therapy compared with photon therapy, the magnitude of such TCP variations in patients is potentially reduced because it is, in part, compensated by an increase in RBE as (α/β)x decreases.
Normal Tissue Complication Probability
Considering typically lower (α/β)x of healthy tissue for cell survival, as well as lower doses than in the target, one might expect larger RBE values for normal tissue. However, endpoints other than cell survival are presumably more relevant for most normal tissue complication probabilities (NTCP). Organ-specific effects of interest are early effects, such as erythema, and late effects, such as lung fibrosis, brain necrosis, or spinal cord injury. The relationship between cell kill and normal tissue effects is complex [46]. For instance, no relation has been found between fibroblast radiosensitivity and the development of late normal tissue effects such as fibrosis [76, 77].
Many endpoints have been studied in animal models, such as skin reactions or organ weight loss. The variety of endpoints does not allow a comprehensive analysis toward a clinical RBE for NTCP considerations. Organ effects are dependent on the dose distribution, and the mean dose is not necessarily a valid approximation. Thus, deducing RBE values for organs at risk based on clinical data is only meaningful if data analysis is done on a voxel-by-voxel basis because proton dose distributions in critical structures are typically more heterogeneous compared with photon therapy.
When considering NTCP models that parameterize radiosensitivity with (α/β)x, patients with a lower (α/β)x are predicted to have a lower complication probability, which is counteracted by an increase in RBE as (α/β)x decreases. Consequently, toxicities in proton therapy would be more affected by variations in (α/β)x compared with photon therapy [69]. While this does not imply an overall higher risk for side effects from proton therapy, it does suggest wider distribution of the severity of toxicities.
Modeling Cellular Radiation Effects
Modeling radiation action mechanistically is challenging because the radiation field can be complex—with primary and secondary particles—and because the biological target is highly structured. Most models are mechanistic when considering the underlying physics but take a phenomenologic approach toward biology.
For example, track structure models [78] are based on the physical details of a particle track, including its secondary particles. They ignore the actual mechanisms of damage initiation and repair. The key assumption is that the difference in biological efficacy, when comparing photons with protons, is caused by different microscopic dose deposition patterns. Other models consider radiation damage and repair more explicitly and are thus capable of describing a range of endpoints (eg, DNA repair, genetic aberration, and cellular survival) by incorporating the kinetics of different DNA repair processes, the spatial distribution of double-strand breaks and the resulting probability and severity of misrepair [79, 80].
In contrast to these more complex approaches, simple LET-based models, while entirely phenomenologic, can be a valuable approximation because they can be based on few parameters. LET-based models follow the linear-quadratic dose-response curve and parameterize the change in α and β with dose-averaged LETd relative to the reference radiation according to Table 1.
Table 1.
Functional form and parameters of the most common linear energy transfer (LET)–based models to estimate relative biological effectiveness. Each model was fitted to a subset of available experimental data from the literature. The largest data set was used by McNamara et al [27].
|
Parameters | ||||||
|
ϕ |
ψ |
A1 |
A2 |
B1 |
B2 |
Reference |
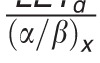 |
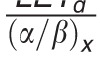 |
0.843 | 0.413644 Gy / (keV/μm) | 1.09 | 0.01612 Gy / (keV/μm) | [26] |
| LETd | 1 | 0.01 / αx, Gy−1 | 0.02 / αx Gy−1, (keV/μm)−1 | 1 | 0 | [81] |
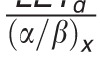 |
1 | 1 | 0.434 Gy / (keV/μm) | 1 | 0 | [82] |
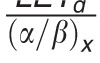 |
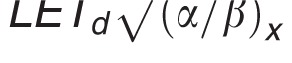 |
0.99064 | 0.35605 Gy / (keV/μm) | 1.1012 | –0.00387 Gy-1/2 / (keV/μm) | [27] |
Equation for αp: 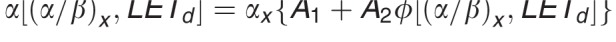 .
.
Equation for βp: 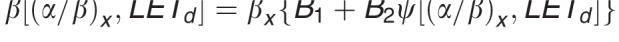 .
.
Biological Optimization
Due to the uncertainties in RBE, treatment plan optimization based on RBE models is currently not feasible with clinically acceptable accuracy [57, 83]. RBE variations are thus only taken into account intuitively (for instance by avoiding certain beam angles), but not quantitatively, during the planning process. The use of robust planning techniques has been suggested to consider RBE uncertainties [84, 85].
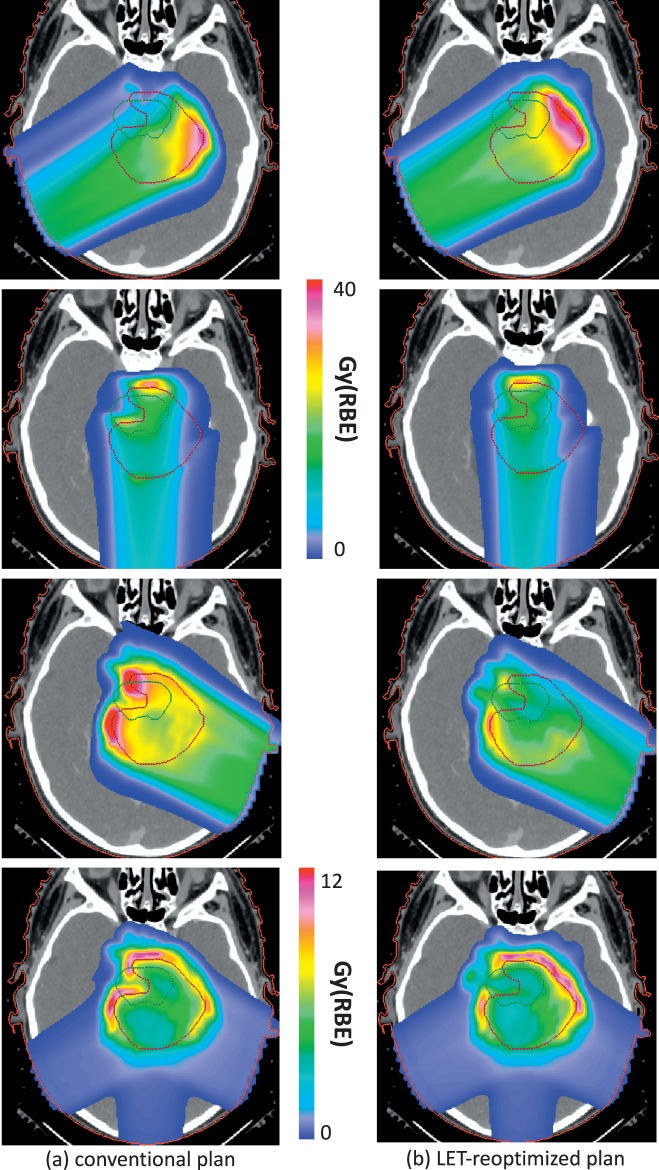
Even though RBE values are currently associated with large uncertainties, biological treatment optimization can be achieved. Algorithms have already been developed in the research setting or implemented in research versions of treatment planning systems. Because for a given dose and (α/β)x, the RBE increases steadily with LETd, the changes of the latter can be used as a surrogate for RBE changes. Interestingly, LET distributions can be influenced in IMPT without significantly altering the dose constraints, that is, dosimetrically equivalent plans can show differences in LET distributions (Figure 6) [48, 84, 86]. This can be utilized to increase the efficacy of proton therapy, thus turning the disadvantage of variable RBE values into a clinical opportunity. It allows biological dose optimization despite uncertainties in RBE values.
Figure 6.
Two intensity-modulated proton therapy plans for a patient with an ependymoma in whom the target volume involves parts of the brainstem. The patient was treated with 3 posterior oblique beams. The left panel shows the 3 fields as prescribed by the planning system (created based on a 2 mm clinical target volume to planning target volume expansion). The right panel shows the 3 fields as prescribed based on LET optimization obtained after minimizing (LETd × dose × empirical constant (0.04 μm/keV)) in the brainstem while constraining the dose distribution to remain close to the conventional plan. The fourth row shows LETd × dose × empirical constant (0.04 μm/keV) for all 3 fields clearly illustrating the reduction in biological effect in the brainstem. See reference [84] for more details. Abbreviation: LETd, dose-averaged linear energy transfer.
The LET-based planning concept was demonstrated in a multicriteria optimization framework [47]. Significant differences in LETd distributions were observed in different base plans, in particular for organs at risk, while preserving target coverage. Subsequently, optimization using a parameter proportional to (LETd × dose) was proposed [87]. This parameter can, to first approximation, be interpreted as a measure of the biological extra dose that is caused by an elevated LET. From a mathematical perspective, (LETd × dose) has the advantage that it is a linear function of pencil beam fluence. Therefore, the same optimization algorithms that are well established for physical dose optimization can be applied.
Summary and Conclusion
Experimental data in vivo and in vitro as well as biophysical models show clear trends in RBE as a function of physical and biological parameters. Nevertheless, other than assuming a 10% difference in required prescription doses and dose constraints, the biological difference between proton and photon therapy is not considered quantitatively in treatment planning. Treatment planning based on variable RBE values is not done clinically because of significant uncertainties, particularly for normal tissues. While the value of 1.1 is appropriate if a generic RBE is being applied, the proton therapy community will for sure move toward variable RBE values in the future after more research has been done. Ideally, this research would include in vivo experiments on normal tissue toxicities.
While RBE uncertainties might impact the efficacy of proton therapy and the interpretation of trials, RBE variations also offer an opportunity. It is important to identify biomarkers recognizing patients with RBE values either low or high compared with the general patient population for either tumor or normal tissue. Furthermore, biological optimization based on LET can lead to a decrease in patient-specific RBE values for organs at risk despite patient specific RBE uncertainties.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The author has no conflicts to disclose.
Acknowledgment: The author wishes to acknowledge funding by the National Institutes of Health (NCI U19 CA21239).
References
- 1.Goodhead DT, Nikjoo H. Track structure analysis of ultrasoft x-rays compared to high- and low-LET radiations. Int J Radiat Biol. 1989;55:513–529. doi: 10.1080/09553008914550571. [DOI] [PubMed] [Google Scholar]
- 2.Prise KM, Ahnstroem G, Belli M, Carlsson J, Frankenberg D, Kiefer J, Loebrich M, Michael BD, Nygren J, Simone G, Stenerloew B. A review of dsb induction data for varying quality radiations. Int J Radiat Biol. 1998;74:173–184. doi: 10.1080/095530098141564. [DOI] [PubMed] [Google Scholar]
- 3.Pastwa E, Neumann RD, Mezhevaya K, Winters TA. Repair of radiation-induced DNA double-strand breaks is dependent upon radiation quality and the structural complexity of double-strand breaks. Radiat Res. 2003;159:251–261. doi: 10.1667/0033-7587(2003)159[0251:roridd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Rydberg B. Clusters of DNA damage induced by ionizing radiation: formation of short DNA fragments. II. Experimental detection. Radiat Res. 1996;145:200–209. [PubMed] [Google Scholar]
- 5.Holley WR, Chatterjee A. Clusters of DNA damage induced by ionizing radiation: formation of short DNA fragments. I. Theoretical hodeling. Radiat Res. 1996;145:188–99. [PubMed] [Google Scholar]
- 6.Goodhead DT. Initial events in the cellular effects of ionizing radiations: clustered damage in DNA. Int J Radiat Biol. 1994;65:7–17. doi: 10.1080/09553009414550021. [DOI] [PubMed] [Google Scholar]
- 7.Loebrich M, Cooper PK, Rydberg B. Non-random distribution of DNA double-strand breaks induced by particle irradiation. Int J Radiat Biol. 1996;70:493–503. doi: 10.1080/095530096144680. [DOI] [PubMed] [Google Scholar]
- 8.International Commission on Radiation Units and Measurements. Report No. 78. Bethesda, MD: International Commission on Radiation Units and Measurements.; 2007. Prescribing, Recording, and Reporting Proton-Beam Therapy. [Google Scholar]
- 9.Denekamp J, Waites T, Fowler JF. Predicting realistic RBE values for clinically relevant radiotherapy schedules. Int J Radiat Biol. 1997;71:681–694. doi: 10.1080/095530097143699. [DOI] [PubMed] [Google Scholar]
- 10.Carabe-Fernandez A, Dale RG, Hopewell JW, Jones B, Paganetti H. Fractionation effects in particle radiotherapy: implications for hypo-fractionation regimes. Phys Med Biol. 2010;55:5685–5700. doi: 10.1088/0031-9155/55/19/005. [DOI] [PubMed] [Google Scholar]
- 11.Dalrymple GV, Lindsay IR, Ghidoni JJ, Hall JD, Mitchell JC, Kundel HL, Morgan IL. Some effects of 138-Mev protons on primates. Radiat Res. 1966;28:471–488. [PubMed] [Google Scholar]
- 12.Dalrymple GV, Lindsay IR, Hall JD, Mitchell JC, Ghidoni JJ, Kundel HL, Morgan IL. The relative biological effectiveness of 138-MeV protons as compared to cobalt-60 gamma radiation. Radiat Res. 1966;28:489–506. [PubMed] [Google Scholar]
- 13.Tepper J, Verhey L, Goitein M, Suit HD. In vivo determinations of RBE in a high energy modulated proton beam using normal tissue reactions and fractionated dose schedules. Int J Radiat Oncol Biol Phys. 1977;2:1115–1122. doi: 10.1016/0360-3016(77)90118-3. [DOI] [PubMed] [Google Scholar]
- 14.Urano M, Goitein M, Verhey L, Mendiondo O, Suit HD, Koehler A. Relative biological effectiveness of a high energy modulated proton beam using a spontaneous murine tumor in vivo. Int J Radiat Oncol Biol Phys. 1980;6:1187–1193. doi: 10.1016/0360-3016(80)90172-8. [DOI] [PubMed] [Google Scholar]
- 15.Paganetti H, Niemierko A, Ancukiewicz M, Gerweck LE, Loeffler JS, Goitein M, Suit HD. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407–421. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 16.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419–R472. doi: 10.1088/0031-9155/59/22/R419. [DOI] [PubMed] [Google Scholar]
- 17.Courdi A, Brassart N, Herault J, Chauvel P. The depth-dependent radiation response of human melanoma cells exposed to 65 MeV protons. Br J Radiol. 1994;67:800–804. doi: 10.1259/0007-1285-67-800-800. [DOI] [PubMed] [Google Scholar]
- 18.Coutrakon G, Cortese J, Ghebremedhin A, Hubbard J, Johanning J, Koss P, Maudsley G, Slater CR, Zuccarelli C, Robertson J. Microdosimetry spectra of the Loma Linda proton beam and relative biological effectiveness comparisons. Med Phys. 1997;24:1499–1506. doi: 10.1118/1.598038. [DOI] [PubMed] [Google Scholar]
- 19.Wouters BG, Lam GKY, Oelfke U, Gardey K, Durand RE, Skarsgard LD. RBE measurement on the 70 MeV proton beam at TRIUMF using V79 cells and the high precision cell sorter assay. Radiat Res. 1996;146:159–170. [PubMed] [Google Scholar]
- 20.Paganetti H. Interpretation of proton relative biological effectiveness using lesion induction, lesion repair and cellular dose distribution. Med Phys. 2005;32:2548–2556. doi: 10.1118/1.1949807. [DOI] [PubMed] [Google Scholar]
- 21.Goodhead DT. Radiation effects in living cells. Can J Phys. 1990;68:872–886. [Google Scholar]
- 22.Linear Energy Transfer., Report No. 16. Bethesda, MD: International Commision on Radiation Units and Measurements; 1970. International Commision on Radiation Units and Measurements. [Google Scholar]
- 23.Robertson JB, Williams JR, Schmidt RA, Little JB, Flynn DF, Suit HD. Radiobiological studies of a high-energy modulated proton beam utilizing cultured mammalian cells. Cancer. 1975;35:1664–1677. doi: 10.1002/1097-0142(197506)35:6<1664::aid-cncr2820350628>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 24.Paganetti H, Goitein M. Radiobiological significance of beam line dependent proton energy distributions in a spread-out Bragg peak. Med Phys. 2000;27:1119–1126. doi: 10.1118/1.598977. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto Y, Matsuura T, Wada M, Egashira Y, Nishio T, Furusawa Y. Enhanced radiobiological effects at the distal end of a clinical proton beam: in vitro study. J Radiat Res. 2014;55:816–822. doi: 10.1093/jrr/rrt230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carabe A, Moteabbed M, Depauw N, Schuemann J, Paganetti H. Range uncertainty in proton therapy due to variable biological effectiveness. Phys Med Biol. 2012;57:1159–1172. doi: 10.1088/0031-9155/57/5/1159. [DOI] [PubMed] [Google Scholar]
- 27.McNamara AL, Schuemann J, Paganetti H. A phenomenological relative biological effectiveness (RBE) model for proton therapy based on all published in vitro cell survival data. Phys Med Biol. 2015;60:8399–8416. doi: 10.1088/0031-9155/60/21/8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abolfath R, Peeler CR, Newpower M, Bronk L, Grosshans D, Mohan R. A model for relative biological effectiveness of therapeutic proton beams based on a global fit of cell survival data. Sci Rep. 2017;7:8340. doi: 10.1038/s41598-017-08622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan F, Bronk L, Titt U, Lin SH, Mirkovic D, Kerr MD, Zhu XR, Dinh J, Sobieski M, Stephan C, Peeler CR, Taleei R, Mohan R, Grosshans DR. Spatial mapping of the biologic effectiveness of scanned particle beams: towards biologically optimized particle therapy. Sci Rep. 2015;5:9850. doi: 10.1038/srep09850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerweck L, Kozin SV. Relative biological effectiveness of proton beams in clinical therapy. Radiother Oncol. 1999;50:135–142. doi: 10.1016/s0167-8140(98)00092-9. [DOI] [PubMed] [Google Scholar]
- 31.Bettega D, Calzolari P, Chauvel P, Courdi A, Herault J, Iborra N, Marchesini R, Massariello P, Poli GL, Tallone L. Radiobiological studies on the 65 MeV therapeutic proton beam at Nice using human tumour cells. Int J Radiat Biol. 2000;76:1297–303. doi: 10.1080/09553000050151565. [DOI] [PubMed] [Google Scholar]
- 32.Blomquist E, Russell KR, Stenerloew B, Montelius A, Grusell E, Carlsson J. Relative biological effectiveness of intermediate energy protons. Comparisons with 60Co gamma-radiation using two cell lines. Radiother Oncol. 1993;28:44–51. doi: 10.1016/0167-8140(93)90184-a. [DOI] [PubMed] [Google Scholar]
- 33.Tang JT, Inoue T, Inoue T, Yamazaki H, Fukushima S, Fournier-Bidoz N, Kolzumi M, Ozeki S, Hatanaka K. Comparison of radiobiological effective depths in 65-MeV modulated proton beams. Br J Cancer. 1997;76:220–225. doi: 10.1038/bjc.1997.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belli M, Bettega D, Calzolari P, Cera F, Cherubini R, Dalla Vecchia M, Durante M, Favaretto S, Gialanella G, Grossi G, Marchesini R, Moschini G, Piazzola A, Poli G, Pugliese M, Sapora O, Scampoli P, Simone G, Sorrentino E, Tabocchini MA, Tallone L, Tiveron P. Inactivation of human normal and tumour cells irradiated with low energy protons. Int J Radiat Biol. 2000;76:831–839. doi: 10.1080/09553000050028995. [DOI] [PubMed] [Google Scholar]
- 35.Bettega D, Birattari C, Bombana M, Fuhrmann Conti AM, Gallini E, Pelucchi T, Tallone Lombardi L. Relative biological effectiveness for protons of 3nergies up to 31 MeV. Radiat Res. 1979;77:85–97. [PubMed] [Google Scholar]
- 36.Bettega D, Calzolari P, Marchesini R, Noris Chiorda GL, Piazzolla A, Tallone L, Cera F, Cherubini R, Dalla Vecchia M, Favaretto S, Tiveron P. Inactivation of C3H10T1/2 cells by low energy protons and deuterons. Int J Radiat Biol. 1998;73:303–309. doi: 10.1080/095530098142400. [DOI] [PubMed] [Google Scholar]
- 37.Sgura A, Antoccia A, Cherubini R, Dalla Vecchia M, Tiveron P, Degrassi F, Tanzarella C. Micronuclei, CREST-positive micronuclei and cell inactivation induced in Chinese hamster cells by radiation with different quality. Int J Radiat Biol. 2000;76:367–374. doi: 10.1080/095530000138709. [DOI] [PubMed] [Google Scholar]
- 38.Belli M, Cera F, Cherubini R, Haque AMI, Ianzini F, Moschini G, Sapora O, Simone G, Tabocchini MA, Tiveron P. Inactivation and mutation induction in V79 cells by low energy protons: re-evaluation of the results at the LNL facility. Int J Radiat Biol. 1993;63:331–337. doi: 10.1080/09553009314550441. [DOI] [PubMed] [Google Scholar]
- 39.Folkard M, Prise KM, Vojnovic B, Davies S, Roper MJ, Michael BD. The irradiation of V79 mammalian cells by protons with energies below 2MeV. Part I: experimental arrangement and measurement of cell survival. Int J Radiat Biol. 1989;56:221–237. doi: 10.1080/09553008914551401. [DOI] [PubMed] [Google Scholar]
- 40.Folkard M, Prise KM, Vojnovic B, Newman HC, Roper MJ, Michael BD. Inactivation of V79 cells by low-energy protons, deuterons and helium-3 ions. Int J Radiat Biol. 1996;69:729–738. doi: 10.1080/095530096145472. [DOI] [PubMed] [Google Scholar]
- 41.Perris A, Pialoglou P, Katsanos AA, Sideris EG. Biological effectiveness of low energy protons. I. Survival of Chinese hamster cells. Int J Radiat Biol. 1986;50:1093–1101. doi: 10.1080/09553008614551481. [DOI] [PubMed] [Google Scholar]
- 42.Paganetti H, Gerweck LE, Goitein M. The general relation between tissue response to x-radiation (a/b-values) and the relative biological effectineness (RBE) of protons: prediction by the Katz track-structure model. Int J Radiat Biol. 2000;76:985–998. doi: 10.1080/09553000050051007. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen S, Bassler N, Grzanka L, Swakon J, Olko P, Andreassen CN, Overgaard J, Alsner J, Sorensen BS. Differential gene expression in primary fibroblasts induced by proton and cobalt-60 beam irradiation. Acta Oncol. 2017;56:1406–1412. doi: 10.1080/0284186X.2017.1351623. [DOI] [PubMed] [Google Scholar]
- 44.Liu Q, Underwood TS, Kung J, Wang M, Lu HM, Paganetti H, Held KD, Hong TS, Efstathiou JA, Willers H. Disruption of SLX4-MUS81 function increases the relative biological effectiveness of proton radiation. Int J Radiat Oncol Biol Phys. 2016;95:78–85. doi: 10.1016/j.ijrobp.2016.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.George KA, Hada M, Chappell L, Cucinotta FA. Biological effectiveness of accelerated particles for the induction of chromosome damage: track structure effects. Radiat Res. 2013;180:25–33. doi: 10.1667/RR3291.1. [DOI] [PubMed] [Google Scholar]
- 46.Girdhani S, Sachs R, Hlatky L. Biological effects of proton radiation: what we know and don't know. Radiat Res. 2013;179:257–272. doi: 10.1667/RR2839.1. [DOI] [PubMed] [Google Scholar]
- 47.Giantsoudi D, Grassberger C, Craft D, Niemierko A, Trofimov A, Paganetti H. Linear energy transfer-guided optimization in intensity modulated proton therapy: feasibility study and clinical potential. Int J Radiat Oncol Biol Phys. 2013;87:216–222. doi: 10.1016/j.ijrobp.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grassberger C, Trofimov A, Lomax A, Paganetti H. Variations in linear energy transfer within clinical proton therapy fields and the potential for biological treatment planning. Int J Radiat Oncol Biol Phys. 2011;80:1559–1566. doi: 10.1016/j.ijrobp.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Indelicato DJ, Flampouri S, Rotondo RL, Bradley JA, Morris CG, Aldana PR, Sandler E, Mendenhall NP. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol. 2014;53:1298–1304. doi: 10.3109/0284186X.2014.957414. [DOI] [PubMed] [Google Scholar]
- 50.Peeler CR, Mirkovic D, Titt U, Blanchard P, Gunther JR, Mahajan A, Mohan R, Grosshans DR. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiother Oncol. 2016;121:395–401. doi: 10.1016/j.radonc.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giantsoudi D, Adams J, MacDonald SM, Paganetti H. Proton treatment techniques for posterior fossa tumors: xonsequences for linear energy transfer and dose-volume parameters for the brainstem and organs at risk. Int J Radiat Oncol Biol Phys. 2017;97:401–410. doi: 10.1016/j.ijrobp.2016.09.042. [DOI] [PubMed] [Google Scholar]
- 52.Fjaera LF, Li Z, Ytre-Hauge KS, Muren LP, Indelicato DJ, Lassen-Ramshad Y, Engeseth GM, Brydoy M, Mairani A, Flampouri S, Dahl O, Stokkevag CH. Linear energy transfer distributions in the brainstem depending on tumour location in intensity-modulated proton therapy of paediatric cancer. Acta Oncol. 2017;56:763–768. doi: 10.1080/0284186X.2017.1314007. [DOI] [PubMed] [Google Scholar]
- 53.Paganetti H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys Med Biol. 2012;57:R99–R117. doi: 10.1088/0031-9155/57/11/R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grassberger C, Paganetti H. Elevated LET components in clinical proton beams. Phys Med Biol. 2011;56:6677–6691. doi: 10.1088/0031-9155/56/20/011. [DOI] [PubMed] [Google Scholar]
- 55.Gridley DS, Pecaut MJ, Mao XW, Wroe AJ, Luo-Owen X. Biological effects of passive versus active scanning proton beams on human lung epithelial cells. Technol Cancer Res Treat. 2015;14:81–98. doi: 10.7785/tcrt.2012.500392. [DOI] [PubMed] [Google Scholar]
- 56.Paganetti H. Relating proton treatments to photon treatments via the relative biological effectiveness (RBE) – should we revise the current clinical practice? Int J Radiat Oncol Biol Phys. 2015;91:892–894. doi: 10.1016/j.ijrobp.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 57.Carabe A, Espana S, Grassberger C, Paganetti H. Clinical consequences of relative biological effectiveness variations in proton radiotherapy of the prostate, brain and liver. Phys Med Biol. 2013;58:2103–2117. doi: 10.1088/0031-9155/58/7/2103. [DOI] [PubMed] [Google Scholar]
- 58.Tilly N, Johansson J, Isacsson U, Medin J, Blomquist E, Grusell E, Glimelius B. The influence of RBE variations in a clinical proton treatment plan for a hypopharynx cancer. Phys Med Biol. 2005;50:2765–2777. doi: 10.1088/0031-9155/50/12/003. [DOI] [PubMed] [Google Scholar]
- 59.Frese MC, Wilkens JJ, Huber PE, Jensen AD, Oelfke U, Taheri-Kadkhoda Z. Application of constant vs. variable relative biological effectiveness in treatment planning of intensity-modulated proton therapy. Int J Radiat Oncol Biol Phys. 2011;79:80–88. doi: 10.1016/j.ijrobp.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 60.Dasu A, Toma-Dasu I. Impact of variable RBE on proton fractionation. Med Phys. 2013;40:011705. doi: 10.1118/1.4769417. [DOI] [PubMed] [Google Scholar]
- 61.Holloway RP, Dale RG. Theoretical implications of incorporating relative biological effectiveness into radiobiological equivalence relationships. Br J Radiol. 2013;86:20120417. doi: 10.1259/bjr.20120417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Withers HR, Thames HD, Jr, Hussey DH, Flow BL, Mason KA. Relative biological effectiveness (RBE) of 50 MV (Be) neutrons for acute and late skin injury. Int J Radiat Oncol Biol Phys. 1978;4:603–608. doi: 10.1016/0360-3016(78)90181-5. [DOI] [PubMed] [Google Scholar]
- 63.Jones B, Underwood TS, Dale RG. The potential impact of relative biological effectiveness uncertainty on charged particle treatment prescriptions. Br J Radiol. 2011;84(spec No 1):S61–S69. doi: 10.1259/bjr/36792876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones B, Wilson P, Nagano A, Fenwick J, McKenna G. Dilemmas concerning dose distribution and the influence of relative biological effect in proton beam therapy of medulloblastoma. Br J Radiol. 2012;85:e912–e918. doi: 10.1259/bjr/24498486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sethi RV, Giantsoudi D, Raiford M, Malhi I, Niemierko A, Rapalino O, Caruso P, Yock TI, Tarbell NJ, Paganetti H, MacDonald SM. Patterns of failure after proton therapy in medulloblastoma; linear energy transfer distributions and relative biological effectiveness associations for relapses. Int J Radiat Oncol Biol Phys. 2014;88:655–663. doi: 10.1016/j.ijrobp.2013.11.239. [DOI] [PubMed] [Google Scholar]
- 66.Fowler J, Chappell R, Ritter M. Is a/b for prostate tumors really low? Int J Radiat Oncol Biol Phys. 2001;50:1021–1031. doi: 10.1016/s0360-3016(01)01607-8. [DOI] [PubMed] [Google Scholar]
- 67.Giantsoudi D, Sethi RV, Yeap BY, Eaton BR, Ebb DH, Caruso PA, Rapalino O, Chen YL, Adams JA, Yock TI, Tarbell NJ, Paganetti H, MacDonald SM. Incidence of CNS Injury for a Cohort of 111 Patients Treated With Proton Therapy for Medulloblastoma: LET and RBE Associations for Areas of Injury. Int J Radiat Oncol Biol Phys. 2016;95:287–296. doi: 10.1016/j.ijrobp.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 68.Liu Q, Ghosh P, Magpayo N, Testa M, Tang S, Biggs P, Paganetti H, Efstathiou JA, Lu HM, Held KD, Willers H. Lung cancer cell line Screen Links Fanconi Anemia Pathway Defects to Increased Relative Biological Effectiveness of proton radiation. Int J Radiat Oncol Biol Phys. 2015;91:1081–1089. doi: 10.1016/j.ijrobp.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 69.Paganetti H. Relating the proton relative biological effectiveness to tumor control and normal tissue complication probabilities assuming interpatient variability in alpha/beta. Acta Oncol. 2017;56:1379–1386. doi: 10.1080/0284186X.2017.1371325. [DOI] [PubMed] [Google Scholar]
- 70.Grosse N, Fontana A, Hug EB, Lomax A, Coray A, Augsburger M, Paganetti H, Satori A, Pruschy M. Deficiency in homologous recombination renders mammalian cells more sensitive to proton versus photon irradiation. Int J Radiat Oncol Biol Phys. 2013;88:175–181. doi: 10.1016/j.ijrobp.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 71.Rostek C, Turner EL, Robbins M, Rightnar S, Xiao W, Obenaus A, Harkness TA. Involvement of homologous recombination repair after proton-induced DNA damage. Mutagenesis. 2008;23:119–129. doi: 10.1093/mutage/gem055. [DOI] [PubMed] [Google Scholar]
- 72.Fontana AO, Augsburger MA, Grosse N, Guckenberger M, Lomax AJ, Sartori AA, Pruschy MN. Differential DNA repair pathway choice in cancer cells after proton- and photon-irradiation. Radiother Oncol. 2015;116:374–380. doi: 10.1016/j.radonc.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 73.Satoh S, Inada T, Eguchi K, Tatsuzaki H, Kitagawa T, Hirokawa Y. Studies on relative biological effectiveness and therapeutic gain factor of high energy protons modulated for radiotherapy [in Japanese] Nippon Igaku Hoshasen Gakkai Zasshi. 1986;46:714–721. [PubMed] [Google Scholar]
- 74.Zlobinskaya O, Siebenwirth C, Greubel C, Hable V, Hertenberger R, Humble N, Reinhardt S, Michalski D, Roper B, Multhoff G, Dollinger G, Wilkens JJ, Schmid TE. The effects of ultra-high dose rate proton irradiation on growth delay in the treatment of human tumor xenografts in nude mice. Radiat Res. 2014;181:177–183. doi: 10.1667/RR13464.1. [DOI] [PubMed] [Google Scholar]
- 75.Urano M, Verhey LJ, Goitein M, Tepper JE, Suit HD, Mendiondo O, Gragoudas ES, Koehler A. Relative biological effectiveness of modulated proton beams in various murine tissues. Int J Radiat Oncol Biol Phys. 1984;10:509–514. doi: 10.1016/0360-3016(84)90031-2. [DOI] [PubMed] [Google Scholar]
- 76.Peacock J, Ashton A, Bliss J, Bush C, Eady J, Jackson C, Owen R, Regan J, Yarnold J. Cellular radiosensitivity and complication risk after curative radiotherapy. Radiother Oncol. 2000;55:173–178. doi: 10.1016/s0167-8140(00)00173-0. [DOI] [PubMed] [Google Scholar]
- 77.Russell NS, Grummels A, Hart AA, Smolders IJ, Borger J, Bartelink H, Begg AC. Low predictive value of intrinsic fibroblast radiosensitivity for fibrosis development following radiotherapy for breast cancer. Int J Radiat Biol. 1998;73:661–670. doi: 10.1080/095530098141915. [DOI] [PubMed] [Google Scholar]
- 78.Paganetti H, Goitein M. Biophysical modelling of proton radiation effects based on amorphous track models. Int J Radiat Biol. 2001;77:911–928. doi: 10.1080/09553000110066059. [DOI] [PubMed] [Google Scholar]
- 79.McMahon SJ, Schuemann J, Paganetti H, Prise KM. Mechanistic modelling of DNA repair and cellular survival following radiation-induced DNA damage. Sci Rep. 2016;6:33290. doi: 10.1038/srep33290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McMahon SJ, McNamara AL, Schuemann J, Paganetti H, Prise KM. A general mechanistic model enables predictions of the biological effectiveness of different qualities of radiation. Sci Rep. 2017;7:10790. doi: 10.1038/s41598-017-10820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilkens JJ, Oelfke U. A phenomenological model for the relative biological effectiveness in therapeutic proton beams. Phys Med Biol. 2004;49:2811–2825. doi: 10.1088/0031-9155/49/13/004. [DOI] [PubMed] [Google Scholar]
- 82.Wedenberg M, Lind BK, Hardemark B. A model for the relative biological effectiveness of protons: the tissue specific parameter alpha/beta of photons is a predictor for the sensitivity to LET changes. Acta Oncol. 2013;52:580–588. doi: 10.3109/0284186X.2012.705892. [DOI] [PubMed] [Google Scholar]
- 83.Resch AF, Landry G, Kamp F, Cabal G, Belka C, Wilkens JJ, Parodi K, Dedes G. Quantification of the uncertainties of a biological model and their impact on variable RBE proton treatment plan optimization. Phys Med. 2017;36:91–102. doi: 10.1016/j.ejmp.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 84.Unkelbach J, Paganetti H. Robust proton treatment planning: physical and biological optimization. Semin Radiat Oncol. 2018;28:88–96. doi: 10.1016/j.semradonc.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oden J, Eriksson K, Toma-Dasu I. Inclusion of a variable RBE into proton and photon plan comparison for various fractionation schedules in prostate radiation therapy. Med Phys. 2017;44:810–822. doi: 10.1002/mp.12117. [DOI] [PubMed] [Google Scholar]
- 86.Fager M, Toma-Dasu I, Kirk M, Dolney D, Diffenderfer ES, Vapiwala N, Carabe A. Linear energy transfer painting with proton therapy: a means of reducing radiation doses with equivalent clinical effectiveness. Int J Radiat Oncol Biol Phys. 2015;91:1057–1064. doi: 10.1016/j.ijrobp.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 87.Unkelbach J, Botas P, Giantsoudi D, Gorissen BL, Paganetti H. Reoptimization of intensity modulated proton therapy plans based on linear energy transfer. Int J Radiat Oncol Biol Phys. 2016;96:1097–1106. doi: 10.1016/j.ijrobp.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]