Abstract
Objectives:
To understand why the incidence of population-based incidence of diverticulitis has increased over time, we studied temporal changes in age, BMI, and diverticulitis in Olmsted County, Minnesota.
Patients and Methods:
We compared the body mass index (BMI) of 2,967 diverticulitis patients from January 1, 1980, through December 31, 2007 and 9,795 people without diverticulitis in Olmsted County. Since BMI is a surrogate for adipose tissue, computed tomographic estimations of abdominal fat content were compared between 381 diverticulitis cases and 381 age- and sex-matched controls.
Results:
Between 1980 and 2007, the prevalence of obesity increased from 12% to 49% in the population and 19% to 40% (P<.001) in diverticulitis. Temporal trends in age, BMI, and the increased incidence of diverticulitis in people with normal BMI, respectively, accounted for 48%, 47%, and 20% of corresponding trends in diverticulitis. The secular decline in the proportion of people with normal BMI was partly offset by an increased incidence of diverticulitis in such people. In the case-control study, BMI was greater (P=.001) in cases than controls. However, after incorporating abdominal visceral (OR, 2.4; 95% CI, 1.6-3.7) and subcutaneous fat (OR 2.9; 95% CI, 1.7-5.2) content, which were both associated with diverticulitis, BMI was associated with lower risk (OR, 0.75; 95% CI, 0.7-0.8) of diverticulitis.
Conclusions:
Aging, increasing obesity, and the increased incidence of diverticulitis in people with normal BMI account for the temporal increase in diverticulitis. Rather than BMI per se, increased abdominal visceral and subcutaneous fat are independently associated with diverticulitis.
Keywords: diverticulosis, visceral adipose tissue, population
Summary:
The incidence of diverticulitis, which is among the most common gastrointestinal diagnosis in hospitalized patients, has increased markedly since 2000. This study suggests that aging, increasing obesity, and the increased incidence of diverticulitis in people with normal BMI account for the temporal increase in diverticulitis.
Background
In the United States, the most common gastrointestinal tract diagnoses among hospitalized patients are diverticulitis and diverticular hemorrhage.1 From 1998 through 2005, the number of patients who were admitted for diverticulitis or underwent elective surgery related to diverticulitis increased by more than 25% overall and by more than 70% among younger patients (<45 years old).2 In our previous population-based study from Olmsted County, Minnesota, we observed a statistically significant increase in the incidence of diverticulitis per 100,000 person-years over 28 years (115 in 1980-1989; 123 in 1990-1999; and 188 in 2000-2007).3 In that study, the increased incidence was partly explained by increased utilization of computed tomography (CT) of the abdomen over time to investigate abdominal pain.3 Temporal trends in aging 4 and obesity,5 may also explain the increasing incidence of diverticulitis. However, the increased incidence of diverticulitis over time was most pronounced in people younger than 50 years.3
Between 1980 and 2006, the US prevalence of obesity increased from 15% to 34%.5 A meta-analysis observed that obesity and physical activity were associated with an increased and reduced risk of diverticular disease respectively.6 However, most studies in this meta-analysis relied on self-reported weight and only 2 studies specifically addressed diverticulitis rather than diverticular disease in general. Among 47,228 health professionals in a prospective cohort study,7 the risks of self-reported diverticulitis and diverticular bleeding were highest among those with high body mass index (BMI), waist circumference, or waist-to-hip ratio. Age and BMI were risk factors for hospitalization for diverticulitis in a Norwegian population.8
While these studies suggest there is an association between BMI and diverticulitis, several questions remain since previous studies that evaluated the link between BMI and diverticulitis relied upon self-reported diverticulitis 7 or diverticulitis requiring hospitalization.8 Since abdominal pain related to irritable bowel syndrome may be misattributed to diverticulitis, a clinical assessment may increase the specificity of a diagnosis of diverticulitis.9 Inpatient series exclude most patients with uncomplicated disease, who are treated as outpatients.10 Self-reported BMI may not be accurate. In other studies (e.g., among 2,222 patients with asymptomatic diverticulosis in a US Department of Veterans Affairs system), BMI was not a risk factor for diverticulitis.11 Finally, all these studies used BMI. However, BMI is an imperfect surrogate marker for obesity because the correlation between BMI and visceral adipose tissue, which is the proximate risk factor for metabolic abnormalities (eg, insulin resistance and glucose intolerance) and inflammation, is imperfect.12
A better understanding of the risk factors for diverticulitis may be helpful for reversing these temporal trends and planning future health care needs, particularly as the population ages. Following through on our previous population-based study,3 we analyzed incidence trends from 1980 through 2007 as a function of age, sex, and BMI. Thereafter, we assessed the contribution of abdominal visceral and subcutaneous fat to diverticulitis through a case-control study. The primary aims were to evaluate the contribution of temporal trends in age, BMI, and the BMI-specific incidence of diverticulitis to temporal trends in incident diverticulitis.
Methods
Setting
Through the population-based data resources of the Rochester Epidemiology Project (REP), we identified individuals who were diagnosed with diverticulitis while resident in Olmsted County, Minnesota, from January 1, 1980, through December 31, 2007.13 Patients were excluded from the study if they did not allow research use of their medical records. This project was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards.
As described elsewhere,14 residents of Olmsted County receive nearly all their medical care at the outpatient and inpatient facilities of Mayo Clinic and Olmsted Medical Center, and population-based epidemiologic research is possible because medical records with indexed diagnoses have been maintained for more than 100 years. Because the REP also indexes medical records of county residents’ other providers, the epidemiology of several medical conditions can be documented.15
Identification of Patients and Review of Case Records
This study includes all Olmsted County patients diagnosed with diverticulitis between January 1980 and December 2007. Diagnoses were established with the Berkson Coding System from January 1, 1980, through December 31, 1987, and the International Classification of Diseases (ICD), Ninth Revision, Clinical Modification (ICD-9-CM) codes from January 1, 1988, through December 31, 2007.3 In a subset of patients, the medical record diagnosis was confirmed by physician review of medical records. All 100 randomly selected patients satisfied American Society of Colorectal Surgeons clinical criteria for sigmoid diverticulitis,16 i.e., acute-onset abdominal pain and left lower quadrant tenderness on examination; however, surgery disclosed acute appendicitis in 1 patient. In addition, the diagnosis was confirmed by review in all 839 patients with complicated or recurrent diverticulitis or who required surgery for diverticulitis.3 The medical records of all patients were reviewed to obtain the BMI on the closest date before or after the diagnosis of diverticulitis.
Obesity Trends in Olmsted County
To estimate the contribution of temporal trends in obesity in the overall population to incident diverticulitis, we extracted the age, sex, and BMI for 9,795 residents who participated in 5 population-based studies from Olmsted County conducted from 1980 through 2004.17 Data from 2009 through 2014 were obtained from the Mayo Clinic records of 92,824 unique Olmsted County residents who had 273,271 visit-year values over that time. (Among residents in whom the BMI was measured more than once in a given year, a yearly mean was used.) Population-based data for 2004-2009 were derived by interpolation. Then, these data sets were combined to estimate the age- and sex-specific BMI distribution for every year of age and every calendar year in the Olmsted County population from 1980 through 2014. These model-based estimates of the population BMI were integrated with the Olmsted County census data to construct an Olmsted County population database stratified by age, sex, calendar year, and BMI category (ie, <25, 25 to <30, and ≥30 kg/m2). This population was then merged with the diverticulitis incidence data set that was also stratified by the same 4 variables from 1980 through 2007.
Case-Control Study of Abdominal Visceral and Subcutaneous Fat and Diverticulitis
During the index episode, 897 of 3,222 patients had a CT scan of the abdomen, which were reviewed by an experienced radiologist (J.G.F.) using standardized criteria 3 to diagnose diverticulitis and complications. From another population-based cohort, 381 healthy persons were identified who had a CT scan of the abdomen for an epidemiological study of osteoporosis but no lifetime history of diverticulitis (ie, controls).18 A case-control study was conducted with 381 case-control pairs; cases and controls were matched by age (±5 years), sex, and date of CT scan (±5 years). The area of abdominal visceral and subcutaneous fat at the L3 vertebral level was measured with a validated in-house software program that automatically calculated and placed 3 boundary lines between external air and subcutaneous fat (boundary 1), between subcutaneous fat and abdominal wall/paraspinal muscles (boundary 2), and between abdominal wall/paraspinal muscles and visceral fat (boundary 3) (Figure 1).19 The same reviewer manually corrected the boundaries using the mouse-computer interface as necessary. The software identified attenuation values from −190 through −30 Hounsfield units between boundaries 1 and 2 and between boundaries 2 and 3 to measure abdominal subcutaneous and visceral fat, respectively. Between boundaries 2 and 3, the software automatically created masks for colonic content. Visceral fat excluded bowel content. Visceral fat and subcutaneous fat areas were divided by the square of patient height (in meters) to calculate corresponding indices.
Figure 1.
Relationship Between Body Mass Index (BMI) and Adipose Tissue Volume. Panels A and B show representative computed tomographic scans in a case (A) and matched control (B) with BMI of 28.6 and 31.9 kg/m2, respectively. Boundaries 1, 2, and 3 (described in the text) are shown with thickened white lines to facilitate review. Visceral adipose tissue (filled circles, panel A) and subcutaneous adipose tissue (stars, panel B) are indicated. While the BMI was greater in the control, there was much more visceral fat in the case. Panels C and D show the relationships between BMI, visceral fat, and subcutaneous fat in cases and controls. Spearman correlation coefficients for case-control differences in BMI versus corresponding differences in visceral and subcutaneous fat were 0.72 (P<.001) and 0.78 (P<.001).
Statistical Analysis
Incidence
The incidence rate of diverticulitis was calculated using incident cases as the numerator and the entire at-risk population of Olmsted County as the denominator (ie, the numbers of age-, sex-, and BMI-specific persons) was derived from decennial census data (every decade from 1980-2010) with linear interpolation between census years, combined with the BMI population model described above.20 Estimates of standard error and 95% CI assumed that the incidence episodes followed a Poisson distribution.20 Temporal trends in the incidence rates were analyzed using Poisson regression models with a logarithmic link function and log (population) offset term that included age, sex, and calendar period alone and together with BMI categories as the X variables, and the counts of incidence events in each age-, sex-, and calendar-year–BMI bin as the Y variable. We considered both “main effects models,” which considered only the main effects of each X variable, and models that added 2-way interactions between each X variable and BMI category.
Attributable Risk
To estimate the contributions of temporal trends in age, BMI, and the BMI-specific diverticulitis incidence rate to temporal trends in incidence of diverticulitis (ie, attributable risk), Poisson models were applied to 4 simulated population data sets in which 1 or more of these variables was not allowed to change from 1980 through 2007. The risk attributable to these factors was estimated by comparing the incidence of diverticulitis among these simulated data sets.
Case-Control Study
Multivariable conditional logistic regression models of the matched case-control pairs were used to evaluate the contribution of normalized visceral fat and subcutaneous fat to incident diverticulitis.
Results
A total of 3,222 patients had an initial (index) diagnosis of diverticulitis from January 1980 through December 2007. Of these, 3,190 patients had provided research authorization (56% were women, and the mean±SD age was 62±16 years). As noted previously,3 the incidence of diverticulitis was 115 for the 1980-1989 period (per 100,000 person-years sex- and age-adjusted to the 2000 US white age distribution). For 1990-1999, it was 123 (a 7% increase) (P<.001), and for 2000-2007, it was 188 (an additional 53% increase) (P<.001).
The BMI was available for 2,980 of the 3,190 patients (93%), within 38±69 days of the date of index diverticulitis). The proportion of patients for whom the BMI was not available was greater in more recent calendar years (P<.001) and for people who were younger at the index episode of diverticulitis (P<.001). However, this was not related to sex (P=.06). Of these 2,980 patients, 2,967 had a healthcare encounter after the diagnosis of diverticulitis and constitute the primary data set for this study.
Temporal Trends in Obesity in Olmsted County
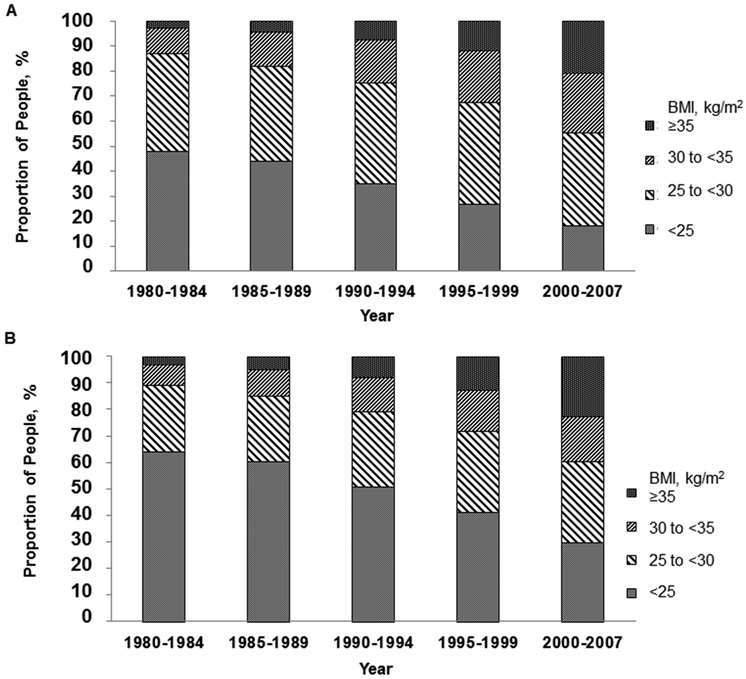
The proportion of Olmsted County residents who were obese increased from 12% in 1980 to 49% in 2007 (P<.001) (Figure 2). Among men, 13% had a BMI of at least 30 kg/m2 in 1980, increasing to 44.9% in 2007 (P<.001). Among women, the corresponding percentages were 11% and 40% (P<.001).
Figure 2.
Temporal Trends in Body Mass Index (BMI), Olmsted County, Minnesota, 1980-2007. A, Men. B, Women. Over time, the proportion of people who had a normal BMI declined while the proportion that were overweight or obese increased.
Relationship between Diverticulitis and BMI: Effects of Age, Sex, and Calendar Year
Among patients with diverticulitis, the proportion of obese patients increased, even after adjusting for age and sex, from 19% in 1980 to 40% in 2007 (P<.001).
Model 1 (Table 1), which only includes the main effects, suggests that the risk of diverticulitis was substantially greater in older people, men, and overweight and obese individuals and also increased over time.
Table 1.
Multivariable Models for Incident Diverticulitis
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Variable | Main effects | Interaction Sex*BMI |
Interaction Age*BMI |
Interaction Calendar year*BMI |
| Men, versus women |
1.75 (1.62 - 1.88) | 5.24 (4.50, 6.10) | 1.76 (1.63, 1.90) | 1.75 (1.63, 1.89) |
| Age, per decade | 1.80 (1.77 - 1.83) | 1.81 (1.78, 1.84) | 1.98 (1.92, 2.05) | 1.81 (1.77 - 1.84) |
| Calendar year, per decade | 1.06 (1.01 – 1.11) | 1.060 (1.01, 1.1) | 1.06 (1.01, 1.1) | 1.34 (1.22, 1.46) |
| BMI (kg/m2) | ||||
| < 25 | 1.0 (Reference group) | - | - | - |
| 25-30 | 1.23 (1.12, 1.35) | - | - | - |
| > 30 | 1.53 (1.39, 1.68) | - | - | - |
| BMI (kg/m2) | ||||
| <25 | 1.0 (Reference group) | 1.0 (Reference group) | 1.0 (Reference group) | |
| 25-30 | 0.20 (0.17, 0.24) | 0.90 (0.86, 0.94) | 0.76 (0.68, 0.85) | |
| >30 | 0.25 (0.21, 0.30) | 0.85 (0.81, 0.89) | 0.67 (0.60, 0.76) |
All values are multivariate IRR (95% CI)
Thereafter, 3 models incorporated interaction terms to assess if the relationship between BMI and diverticulitis were influenced by sex (Model 2), age (Model 3), and time (Model 4). Model 2 (Sex*BMI interaction), suggests that overall, men had an increased risk of diverticulitis (IRR 5.24, 95% CI 4.5 – 6.1). However, compared to people with a BMI < 25 kg/m2, this incremental risk was lower among overweight (IRR 0.20, 95% CI 0.17 – 0.24) and obese (IRR 0.25, 95% CI 0.21 – 0.30) people.
Model 3 (Age*BMI interaction model), suggests that older people had an increased risk of diverticulitis (IRR 1.98, 95% CI 1.92 – 2.05). However, compared to people with a BMI < 25 kg/m2, this age-related risk was slightly but significantly lower in overweight (IRR 0.90, 95% CI 0.86 – 0.94) and obese people (IRR 0.85, 95% CI 0.81 –0.89).
In Model 4 (Calendar year*BMI interaction model), the main effects suggest that the incidence of diverticulitis increased over time (IRR 1.34 per decade, 95% CI 1.22 – 1.46). The interaction terms suggest that compared to people with a BMI < 25 kg/m2, this increase, over time, was lower in overweight (IRR 0.76, 95% CI 0.68 – 0.85) and obese people (IRR 0.67, 95% CI 0.60 – 0.76). Consequently, the incidence rates among BMI categories converged in 2007 (Figure 3).
Figure 3.
Incidence Rate Ratios (IRRs) for Diverticulitis in Body Mass Index (BMI) Categories, Olmsted County, Minnesota, 1980-2007. In 1980, compared to the reference group IRR (ie, women with a BMI <25 kg/m2), the IRRs were 2.8 and 4.9, respectively, for women and men with a BMI of 30 kg/m2 or more. Over time, the IRRs converged among BMI categories in women and separately in men.
In summary, the effects of sex, age, and calendar period on incident diverticulitis were most pronounced in people with a BMI < 25 kg/m2.
Relative Contribution of Temporal Trends in Age, BMI, and BMI-Specific Incidence Rates to Temporal Trends in the Incidence of Diverticulitis
The contribution of BMI to corresponding trends in diverticulitis is a product of 2 factors, i.e., temporal trends in the distribution of BMI per se and temporal trends in the risk of diverticulitis for a given BMI. Simulations were used to evaluate the relationship between trends in these factors and age on temporal trends in diverticulitis.
The BMI-specific incidence of diverticulitis increased over time after 1980 in people with a BMI < 25 kg/m2 (Table 1). Therefore, scenario 1 (Table 2) simulated a population in which the BMI-specific incidence of diverticulitis increased over time in people with a BMI less than 25 kg/m2 but fixed it, at the 1980 rate, in the other BMI categories. The distributions of BMI and age in the population were allowed to change as observed. In scenario 1, the incidence of diverticulitis per 100,000 people increased by 57.0 (ie, from 60.8 to 117.8) between 1980 through 2007, which is similar to the actual increase in the raw incidence of diverticulitis (ie, from 77.2 to 125.2) over the same period. Scenario 2 differs from Scenario 1 by fixing age distribution at the 1980 distribution. Scenario 3 differs from Scenario 1 by fixing the diverticulitis incidence rate in normal BMI at the 1980 level. Finally, scenario 4 fixes both the BMI distribution and the BMI-specific diverticulitis incidence rate at 1980 levels.
Table 2.
Relative Contributions of Temporal Trends in Age, BMI, and BMI-Specific Incidence Rates to Temporal Trends in the Incidence of Diverticulitis
| Raw Incidence, | Mean Incidence of Diverticulitis per 100,000 People |
||||
|---|---|---|---|---|---|
| Year | Mean (Range) | Scenario 1a | Scenario 2b | Scenario 3c | Scenario 4d |
| 1980 | 77.2 (59.3-95.0) | 60.8 | 60.8 | 60.8 | 60.8 |
| 1985 | 96.4 (77.0-115.7) | 68.1 | 65.0 | 64.5 | 63.5 |
| 1990 | 80.1 (63.1-97.1) | 77.6 | 72.0 | 70.9 | 65.4 |
| 1995 | 75.2 (59.4-90.9) | 89.9 | 78.4 | 80.9 | 69.9 |
| 2000 | 102.3 (84.7-119.8) | 99.8 | 83.9 | 89.4 | 72.5 |
| 2007 | 125.2 (106.8-143.6) | 117.8 | 90.5 | 106. 4 | 79.6 |
| Increase from 1980 to 2007 |
… | 57.0 | 29.7 | 45.6 | 18.8 |
Abbreviation: BMI, body mass index.
Scenario 1 assumptions: age, actual; BMI, actual; BMI-specific incidence rate, actual for BMI <25 kg/m2 and fixed at the 1980 rate for BMI ≥25 kg/m2.
Scenario 2 assumptions: age, fixed at 1980 rate; BMI, actual; BMI-specific incidence rate, actual for BMI <25 kg/m2 and fixed at the 1980 rate for BMI ≥25 kg/m2.
Scenario 3 assumptions: age, actual; BMI, actual; BMI-specific incidence rate, fixed at the 1980 rate for all BMI categories.
Scenario 4 assumptions: age, actual; BMI, distribution among BMI categories in 1980 was used for all subsequent periods; BMI-specific incidence rate, fixed at the 1980 rate for all BMI categories.
A comparison of temporal trends among these 4 scenarios suggests how the following contribute to an increase in the incidence of diverticulitis over time: Age (ie, scenario 2 vs scenario 1) contributes 48% (ie, [57.0–29.7]/57.0); BMI (ie, [scenario 3 minus scenario 4] divided by scenario 1) contributes 47% ([45.6–18.8]/57.0), and the BMI-specific incidence of diverticulitis in people with normal-weight BMI (ie, scenario 3 vs scenario 1) contributes 20% ([57.0–45.6]/57.0).
Relationship Between Abdominal Visceral Fat Content and Diverticulitis in the Case-Control Study
The age and sex were, by design, similar between cases and controls (Table 3). In the univariate analysis, mean BMI was greater in cases (29.8±6.3 kg/m2) than controls (28.3±5.3 kg/m2) (P=.001). However, after adjusting for visceral and subcutaneous fat content in the multivariable model, higher BMI was associated with a lower risk of diverticulitis (OR, 0.8; 95% CI, 0.7-0.8). In this model, the normalized indices of visceral (OR, 2.4; 95% CI, 1.6-3.7) and subcutaneous fat (OR, 2.9; 95% CI, 1.7-5.2) were independently associated with an increased risk of diverticulitis. The interaction terms between these parameters and BMI were also significant: Increased visceral and subcutaneous fat content were associated with a higher risk of diverticulitis in people with a higher BMI.
Table 3.
Case-Control Assessment of Abdominal CT Parameters and Incident Diverticulitis
| Variable | Cases (n=381) | Controls (n=381) | Multivariable OR (95% CI) |
|---|---|---|---|
| Age, mean±SD, y | 60.0±16.7 | 59.1±16.8 | 1.1 (1.0-1.2) |
| Women, No. (%) | 202 (53) | 202 (53) | … |
| BMI, mean±SD, kg/m2 | 29.8±6.3 | 28.3±5.3 | 0.8 (0.7-0.8) |
| Visceral fat index, mean±SD | 63.5±36.5 | 49.0±25.2 | 2.4 (1.6-3.7)a |
| Subcutaneous fat index, mean±SD | 81.9±45.0 | 66.2±33.4 | 2.9 (1.7-5.2)a |
| Visceral fat index–BMIb | NA | NA | 1.6 (1.4-2.0)a |
| Subcutaneous fat index–BMIb | NA | NA | 1.6 (1.4-2.0)a |
Abbreviations: CT, computed tomographic; NA, not applicable; OR, odds ratio.
Normalized indices were used in the model.
Interaction of BMI with normalized indices.
Discussion
Compared with 1990–1999, the incidence of diverticulitis has increased by 50% in 2000–2007. Concomitantly, the prevalence of obesity in Olmsted County increased from 12% to 49% in the overall adult population and from 19% to 40% in patients with diverticulitis. At the same time, the Olmsted County population has aged; the proportion of individuals aged 45-64 and ≥ 65 years increased from respectively 16% and 10% in 1990 to 20% and 10% in 2000, and 25% and 12% in 2010.21 Age, BMI, and male sex are known risk factors for diverticulitis. Human aging is also characterized, in part, by a chronic, low-grade inflammation in various organs.22 However, the contributions of these risk factors to observed temporal trends in diverticulitis are unknown. By sequentially employing innovative approaches, we analyzed the contributions of age, BMI, and sex and their interactions to temporal trends in a large, truly population-based (i.e., outpatients and inpatients) cohort of people with physician-documented diverticulitis. These approaches include: an assessment of temporal trends in BMI in the Olmsted County population and separately in diverticulitis, simulated datasets to dissect the relative contributions of age and BMI to temporal trends in diverticulitis, and a case-control study to evaluate the contribution of visceral and subcutaneous fat to diverticulitis.
Aging, increasing obesity, and the increased incidence of diverticulitis in people with normal-weight BMI, respectively, accounted for approximately 48%, 47%, and 20% of the observed temporal increase in the incidence of diverticulitis. (Because of the overlapping effects of different risk factors, it is recognized that the cumulative estimated attributable risk can exceed 100% [ie, 115% in this instance]).23 Over time, the risk of diverticulitis increased markedly in people with a normal BMI but was unchanged in people with a BMI of 25-30 kg/m2 and declined in people with a BMI >30 kg/m2. Thus, the secular decline in the proportion of people with normal-weight BMI was partly offset by an increased incidence of diverticulitis in people with a normal-weight BMI. Older people and men also had an increased risk of diverticulitis. The interaction terms suggest that the incremental risks related to age and male sex were lower in overweight and obese people.
Why did the incidence of diverticulitis increase over time in people with a normal BMI? It is widely recognized that a subset of normal weight individuals express cardiometabolic abnormalities associated with being overweight or obese.24 Indeed, among 5440 participants of the National Health and Nutrition Examination Surveys (NHANES) from 1999-2004, approximately 24% of normal-weight adults had 2 or more metabolic abnormalities (i.e., elevated blood pressure, triglycerides, or glucose, decreased high density lipoprotein cholesterol (HDL-C) level, insulin resistance, or systemic inflammation). Moreover, the NHANES surveys observed that the BMI plateaued but waistlines, reflecting abdominal obesity, continued to increase in the first decade of the 21st century.25 That study and several others have observed that people with similar BMIs have various amounts of visceral adipose tissue.12, 26 In our case control study, the BMI was greater in cases than in controls. However, after adjusting for visceral and subcutaneous adipose tissue, a higher BMI was associated with a lower risk of diverticulitis, perhaps suggesting that muscle and bone mass protect against diverticulitis. Indeed, visceral and subcutaneous adipose tissue were independently associated with diverticulitis. Because adjusting for visceral and subcutaneous adipose tissue eliminated the BMI-related risk of diverticulitis, our data strongly suggest that the BMI-related risk of diverticulitis is mediated by visceral and subcutaneous adipose tissue. Absent an intervention targeted to reduce abdominal fat, these observations reflect an association rather than a cause-effect relationship. However, it seems implausible that diverticulosis predisposes to increased abdominal fat.
Previous studies have suggested that obesity is a risk factor for diverticulitis. Among 47,228 male health professionals, increased BMI, waist circumference, and waist-to-hip ratio were associated with an increased risk of self-reported diverticulitis over 18 years.7 A questionnaire-based study among US male health professionals aged 40 to 75 years27 and an analysis of hospitalizations for diverticular disease among men aged 47 to 55 years in a Swedish community28 also found positive associations between BMI and symptomatic diverticular disease (diverticulitis, diverticular bleeding, or nonspecific pain or bowel symptoms in patients with diverticulosis). By comparison, the present study was population-based, included adult men and women of all ages, and evaluated temporal trends rather than the point incidence of diverticulitis, which was diagnosed from medical records rather than self-report. The sample size (ie, nearly 3,000 patients) in this study is much larger than in previous studies.7, 27, 28
While increased visceral fat or central obesity is widely recognized to be pathologic,12, 26 increased subcutaneous fat is also associated with similar pathologic features, including global insulin resistance, a higher Framingham risk score, and greater expression of proinflammatory, lipogenic, and lipolytic genes.29 Increased abdominal visceral fat and, to a lesser extent, subcutaneous fat also independently predict the development of metabolic risk factors (eg, higher blood glucose) in the future.30 Visceral and subcutaneous adipose recruit macrophages that are activated by free fatty acids and release proinflammatory cytokines (eg, tumor necrosis factor [TNF]-α and interleukin [IL]-6), which can cause inflammation.31 Obesity-induced alterations in the colonic microbiome may also predispose persons to diverticulitis. Both obesity and diverticulitis32 are associated with alterations in the intestinal microbiome (eg, an increased ratio of Firmicutes to Bacteroidetes). Obesity is also associated with other risk factors for diverticulitis, such as low dietary fiber intake, red meat consumption, and physical inactivity.7 From a public health perspective, these observations suggest that actual or a surrogate measures (e.g., waist circumference, serum triglyceride levels) of visceral fat may be helpful to identify individuals with diverticulosis who have an increased risk of having incident diverticulitis.26 Since visceral adipose tissue is associated not only with metabolic syndrome and cardiovascular disease but also with esophageal inflammation, metaplasia and adenocarcinoma, perhaps waist circumference and fat (visceral and subcutaneous) should be routinely measured during physical examination and abdominal computed tomography respectively.33 Perhaps future trials (e.g., of aminosalicylates) 34 to prevent incident or recurrent diverticulitis should stratify treatment based on visceral adiposity. The methods used in this study may be applied to understand the contributions of aging and obesity in other age-related diseases such as arthirits, diabetes, and dementia.22
There were some limitations to this study. Patients were identified from an electronic database. The diagnosis of diverticulitis was confirmed by reviewing the medical records in 939 of 3222 patients. While complications and surgery related to diverticulitis were confirmed by physician review for all patients, abdominal pain related to irritable bowel syndrome may be misattributed to diverticulitis.9 The BMI was not available for 7% of patients with diverticulitis. Between 1980 and 2007, only 1.6% of Olmsted County residents had a BMI < 18.5 kg/m2, limiting our ability to analyze this group separately. Other putative risk factors for diverticulosis or diverticulitis (i.e., diet, bowel habits, and physical inactivity) were not evaluated. However, constipation and low dietary fiber content were not associated with diverticulosis in a recent study.35 In a population of 47,228 individuals, vigorous but not nonvigorous physical activity was associated with lower risk of diverticulitis.36 Although the emigration rate from Olmsted County is low, some patients may have received treatment for complications elsewhere without documentation of that treatment appearing in the local records. The rate of those occurrences would not be expected to have varied during the study period.
Conclusions
In conclusion, aging, increasing obesity, and the increased incidence of diverticulitis among people with normal-weight BMI accounted for the increased incidence of diverticulitis over time. Rather than BMI, it is increased visceral and subcutaneous adipose tissue that are independently associated with diverticulitis.
Acknowledgements
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr Bharucha was supported in part by grant R01 DK78924 from the National Institutes of Health, US Department of Health and Human Services. Dr Khosla was supported by grant R01 AR027065 from the National Institutes of Health, US Department of Health and Human Services.
Abbreviations
- BMI
body mass index
- CT
computed tomography
- OR
odds ratio
Footnotes
Portions of this manuscript have been published in: Bharucha et al. Am J Gastroenterol. 2015 Nov;110(11):1589-96.
Conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr Tae Hee Lee, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota..
Dr Pratyusha Tirumani Setty, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota..
Dr Gopanandan Parthasarathy, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota..
Dr Kent R. Bailey, Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota..
Dr Christina M. Wood-Wentz, Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota..
Dr Joel G. Fletcher, Department of Radiology, Mayo Clinic, Rochester, Minnesota..
Dr Naoki Takahashi, Department of Radiology, Mayo Clinic, Rochester, Minnesota..
Dr Sundeep Khosla, Division of Endocrinology, Diabetes, Metabolism, & Nutrition, Mayo Clinic, Rochester, Minnesota and Department of Physiology and Biomedical Engineering, Mayo Clinic, Rochester, Minnesota..
Dr Michael R. Moynagh, Department of Radiology, Mayo Clinic, Rochester, Minnesota..
Dr Alan R. Zinsmeister, Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota..
Dr Adil E. Bharucha, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota..
References
- 1.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–1187 e1171-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etzioni DA, Cannom RR, Ault GT, Beart RW Jr, Kaiser AM. Diverticulitis in California from 1995 to 2006: increased rates of treatment for younger patients. Am Surg. 2009;75(10):981–985. [PubMed] [Google Scholar]
- 3.Bharucha AE, Parthasarathy G, Ditah I, et al. Temporal Trends in the Incidence and Natural History of Diverticulitis: A Population-Based Study. Am J Gastroenterol. 2015;110(11):1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stollman N, Raskin JB. Diverticular disease of the colon. Lancet. 2004;363(9409):631–639. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087–2102. [DOI] [PubMed] [Google Scholar]
- 6.Aune D, Sen A, Leitzmann MF, Norat T, Tonstad S, Vatten LJ. Body mass index and physical activity and the risk of diverticular disease: a systematic review and meta-analysis of prospective studies. Eur J Nutr. 2017;09(09). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strate LL, Liu YL, Aldoori WH, Syngal S, Giovannucci EL. Obesity increases the risks of diverticulitis and diverticular bleeding. Gastroenterology. 2009;136(1):115–122.e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamal Talabani A, Lydersen S, Ness-Jensen E, Endreseth BH, Edna TH. Risk factors of admission for acute colonic diverticulitis in a population-based cohort study: The North Trondelag Health Study, Norway. World J Gastroenterol.22(48):10663–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longstreth GF, Tieu RS. Clinically Diagnosed Acute Diverticulitis in Outpatients: Misdiagnosis in Patients with Irritable Bowel Syndrome. Dig Dis Sci. 2016;61(2):578–588. [DOI] [PubMed] [Google Scholar]
- 10.Morris AM, Regenbogen SE, Hardiman KM, Hendren S. Sigmoid diverticulitis: a systematic review. JAMA. 2014;311(3):287–297. [DOI] [PubMed] [Google Scholar]
- 11.Shahedi K, Fuller G, Bolus R, et al. Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol. 2013;11(12):1609–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nature Reviews Endocrinology. 2015;11(2):90–100. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ, 3rd. History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. [DOI] [PubMed] [Google Scholar]
- 14.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ, 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57(3):284–294. [DOI] [PubMed] [Google Scholar]
- 17.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence.[Erratum appears in Circulation. 2006 Sep 12;114(11):e498]. Circulation. 2006;114(2):119–125. [DOI] [PubMed] [Google Scholar]
- 18.Riggs BL, Melton Iii LJ 3rd, Robb RA, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19(12):1945–1954. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi N, Sugimoto M, Psutka SP, Chen B, Moynagh MR, Carter RE. Validation study of a new semi-automated software program for CT body composition analysis. Abdominal Radiology. 2017;07(07). [DOI] [PubMed] [Google Scholar]
- 20.Bergstralh EJOKP, Chu CP, Beard CM, O'Fallon WM, Melton LJ III Calculating Incidence, Prevalence, And Mortality Rates For Olmsted County, Minnesota: An Update. Technical Report No. 49. Rochester: Mayo clinic; 1992. [Google Scholar]
- 21.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol.41(6):1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mau T, Yung R. Adipose tissue inflammation in aging. Exp Gerontol. 2017;18(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowe AK, Powell KE, Flanders WD. Why population attributable fractions can sum to more than one. Am J Prev Med. 2004;26(3):243–249. [DOI] [PubMed] [Google Scholar]
- 24.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med. 2008;168(15):1617–1624. [DOI] [PubMed] [Google Scholar]
- 25.Freedman DS, Ford ES. Are the recent secular increases in the waist circumference of adults independent of changes in BMI? Am J Clin Nutr. 2015;101(3):425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–1313. [DOI] [PubMed] [Google Scholar]
- 27.Aldoori WH, Giovannucci EL, Rimm EB, et al. Prospective study of physical activity and the risk of symptomatic diverticular disease in men. Gut. 1995;36(2):276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosemar A, Angeras U, Rosengren A. Body mass index and diverticular disease: a 28-year follow-up study in men. Dis Colon Rectum. 2008;51(4):450–455. [DOI] [PubMed] [Google Scholar]
- 29.Bays H Central obesity as a clinical marker of adiposopathy; increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132(17):1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooke AA, Connaughton RM, Lyons CL, McMorrow AM, Roche HM. Fatty acids and chronic low grade inflammation associated with obesity and the metabolic syndrome. Eur J Pharmacol. 2016;785(207-214). [DOI] [PubMed] [Google Scholar]
- 32.Feuerstein JD, Falchuk KR. Diverticulosis and Diverticulitis. Mayo Clin Proc. 2016;91(8):1094–1104. [DOI] [PubMed] [Google Scholar]
- 33.Singh S, Sharma AN, Murad MH, et al. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11(11):1399–1412.e1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter F, Alsayb M, Marshall JK, Yuan Y. Mesalamine (5-ASA) for the prevention of recurrent diverticulitis. Cochrane Database Syst Rev. 2017;10(CD009839). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peery AF, Sandler RS, Ahnen DJ, et al. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol. 2013;11(12):1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strate LL, Liu YL, Aldoori WH, Giovannucci EL. Physical activity decreases diverticular complications. Am J Gastroenterol. 2009;104(5):1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]