Abstract
Background:
Sleep disturbance is a common complaint of cancer patients and is well-established in both pain conditions and Posttraumatic Stress Disorder (PTSD). An estimated one-third of cancer patients develop symptoms of PTSD at some point in their treatment. However, few studies have evaluated the contributions of PTSD and sleep disturbance to pain processes in cancer populations. The current study used mediation models to test the hypothesis that sleep disturbance would mediate relationships between PTSD symptoms and pain intensity and PTSD symptoms and pain interference in a sample of cancer patients.
Methods:
A cross-sectional, retrospective chart review was conducted of the Electronic Medical Records of 85 adult cancer patients (89.4% female; 59% White; 42% metastatic) who sought individual psychosocial support services at our institution.
Results:
PTSD symptoms, sleep disturbance, pain intensity, and pain-interference were all positively correlated (p’s < 0.01). Clinical levels of PTSD symptoms were reported by 30–60% of the sample. Even after controlling for metastatic disease, race and cancer type, sleep disturbance mediated the relationships between PTSD symptoms and pain intensity (B = 0.27; 95% C.I. [0.10, 0.44]) and PTSD symptoms and pain-related interference (B = 0.58; 95% C.I. [0.28, 0.87]).
Conclusions:
The relationships among PTSD symptoms, pain intensity, and pain interference could be explained by co-occurring sleep disturbance. Given the high frequency of PTSD symptoms among cancer patients and PTSD’s known links to sleep problems and pain, clinicians should be attentive to the role that traumatogenic processes may play in eliciting sleep and pain-related complaints among cancer patients.
Keywords: cancer, sleep disturbance, PTSD, pain intensity, pain interference, mediation
BACKGROUND
The diagnosis and treatment of cancer can be a psychologically traumatic experience for many patients given that it is a life-threatening illness and many of its treatments may involve significant violation of bodily integrity or also be considered life-threatening (i.e., surgery, immune-suppressing chemotherapy). In 2015, the National Cancer Institute1 reported that nearly one-third of cancer patients develop symptoms of Posttraumatic Stress Disorder (PTSD) during their treatment, including intrusive trauma-related thoughts, avoidance of trauma-related reminders, and hypervigilance and heightened arousal2. In addition to the emotional toll, cancer treatment and the disease itself may also be physically painful3. The American Cancer Society estimates that 80% of patients with advanced cancer experience moderate to severe pain4. Emotional distress and pain are co-morbid in non-cancer populations as well, and studies estimate that 20–70% of individuals with PTSD report pain-related complaints5.
Sleep disturbance is a common factor of both PTSD6 and pain conditions7, and there is increasing attention to this common complaint of patients with cancer8. In PTSD, sleep disturbances represent two diagnostic criteria of the disorder, and include trouble falling and/or staying asleep and intrusive, distressing dreams2. Similarly, pain-related conditions and sleeping difficulties frequently co-occur. Although some studies have highlighted bidirectional pathways between sleep and pain9, longitudinal research in diverse chronic pain populations has implicated sleep disturbance as the probable driver of daily pain and pain-related dysfunction, and not vice versa7.
Despite the increased awareness of PTSD-related concerns in cancer patients and the documented incidence of cancer-related pain and sleep problems, the majority of studies that have examined the co-occurrence of PTSD, pain and sleep problems have been conducted in veteran populations10–12. These studies with veterans have yielded results that are consistent with the results of Mystakidou and colleagues13 who found that, among a sample of advanced stage cancer patients, poor sleep quality was linked with both increased pain and more severe symptoms of PTSD.
Although previous research has begun to assess the associations of sleep disturbance, PTSD, and pain among cancer patients, additional investigation is warranted. In particular, determining the mechanisms of overlap among these variables may have implications for effective PTSD screening and treatment options for pain and sleep problems in cancer patients as well as understanding the complex etiology of pain and pain interference (i.e., pain that interferes with daily functioning) in cancer patient populations. In order to assess potential mediating pathways of pain, this study utilized mediation modeling (a statistical approach combining theory and data analysis) to ascertain whether the association of PTSD symptoms with pain intensity and pain interference could be explained or mediated by co-occurring sleep disturbance in a sample of cancer patients. Given the lack of longitudinal data on pain and sleep problems in cancer patient populations, we generalized from the chronic pain literature to hypothesize that positive relationships among PTSD symptoms, pain intensity, and pain interference would be accounted for by co-occurring sleep disturbance.
METHOD
Participants and Procedure.
This retrospective chart review study was approved by the institutional review board of our academic medical center. Because there was no face-to-face interaction with participants or storage of participant Personal Health Information, this study was exempt from collecting informed consent. Records reviewed were from a convenience sample of 85 adult, patients in active cancer treatment who self-referred for psychosocial support from a Licensed Clinical Psychologist at our medical center’s Psychosocial Oncology clinic between June 2014 and June 2015. From the larger pool of potential patient records to include from this timeframe, children (18 and younger) and those who were not administered primary study measures (see below) were excluded.
Measures
Demographic and Medical Information.
All diagnostic and demographic information (i.e., age, gender, race/ethnicity, cancer type, metastatic status, receipt of chemotherapy and radiation therapy,) was obtained from review of participants’ Electronic Medical Records (EMR).
PTSD CheckList-Civilian Version (PCL-C)14.
PTSD symptoms were assessed with the PCL-C, a 17-item measure that assesses the degree to which respondents have experienced symptoms of re-experiencing, avoidance/numbing, and hyperarousal symptoms associated with PTSD. Respondents rate the extent to which they have been distressed by each symptom over the past month on a Likert scale from 1 (Not at all) to 5 (Extremely). Total scores range from 17–85 with higher scores indicating greater distress. Suggested cut-off scores of 30 and 45 denote clinical levels of PTSD symptomology in HMO (Health Maintenance Organization) samples and acute trauma populations, respectively15. Prior to analyses, the two sleep items from the PCL-C (having trouble falling/staying asleep and having distressing dreams) were subtracted from PCL-C total scores in order to prevent overlap with sleep items from the PROMIS-29 measure16.
Patient-Reported Outcomes Measurement Information System (PROMIS-29)15.
Pain and sleep disturbance were assessed with the PROMIS-29, a 29-item inventory assessing distress and functionality over the previous seven days across seven domains including Depression, Anxiety, Physical Function, Pain Interference, Fatigue, Sleep Disturbance and Social Role Activity/Participation.
Sleep Disturbance was assessed with the four sleep-related items from the PROMIS-29. Respondents were asked to provide an overall rating of their sleep quality (1 [Very Poor] to 5 [Very Good]) and rate the degree to which their sleep had been refreshing, if they had any problems with their sleep, or if they had any difficulty falling asleep (from 1 [Not at All] to 5 [Very Much]). After reverse coding sleep quality ratings, total raw scores were calculated and converted into T-scores based on normed standard scores developed for the PROMIS-29 (with higher T-scores indicating greater sleep disturbance).
Pain Interference was assessed with the four pain-interference items from the PROMIS-29. Respondents were asked to rate the degree to which pain had interfered with their ability to complete day-to-day activities, work around the home, engage in social activities and complete household chores [on a Likert-scale from 1 (Not at All) to 5 (Very Much)]. Total raw scores were converted into T-scores based on normed standard scores developed for the PROMIS-29 (with higher T-scores indicating greater pain-related interference). The one-item Pain Intensity question was interpreted as a raw score, based on PROMIS-29 scoring guidelines, and asked respondents to provide an average rating of pain on an 11-point Likert scale (0 [No Pain] - 10 [Worst Imaginable Pain]).
Data Analytic Strategy
Descriptive statistics and bivariate correlations were initially conducted to determine the strength and direction of relationships among primary study variables and covariates. Age, metastatic status, cancer type (i.e., hematologic-based cancer vs. solid tumor), chemotherapy, and radiation therapy were selected as covariates on the theoretical grounds that advanced age, disease severity and active cancer treatment may impose a differential impact on sleep and pain outcomes.
Relationships among sleep disturbance, PTSD symptoms, pain intensity, and pain interference were then assessed with path analysis, a methodology that posits and statistically tests a theory-driven model. The path analyses were conducted with bootstrapped mediation models controlling for covariates of metastatic disease and African-American race which were indicated as significantly related to primary study variables at the zero-order level17. In brief, mediation testing and path analysis represent an empirical method that integrates theory and data analysis. Mediation is said to occur when the association between an independent variable (i.e., PTSD) and dependent variable (i.e. Pain) is explained by an intervening or mediating third variable (i.e., Sleep Disturbance). Whereas other analyses may adjust for potential confounders of a relationship to remove nuisance variation or noise, mediation is useful for considering whether a third variable or mediator may participate in a cascade of influence from one variable to the next. Beyond evaluating the hypothesized model that sleep disturbance would function as a mediator of the relationship between PTSD symptoms and pain intensity and the relationship between PTSD symptoms and pain interference, we also evaluated a plausible alternative model that linked sleep disturbance to pain intensity and pain interference via PTSD symptoms.
RESULTS
Sample Characteristics.
As noted in Table 1, participants were predominantly female (89.3%) and approximately 52 years old (SD = 13). Most identified as White (58.8%), followed by Black/African American (32.9%) and other (8.3%). Approximately 11% of the sample was diagnosed with a hematologic-based cancer (e.g. lymphoma, leukemia) and the most common solid tumor cancers were breast cancer (49.4%) and lung cancer (16.5%). Just under half (42.4%) of patients had metastatic disease. Nearly all patients were on chemotherapy (85.9%) and two-thirds were on concomitant radiation therapy (65.9%).
Table 1.
Sample and Descriptive Characteristics
| Sample (n = 85) |
|
|---|---|
| Agea | 52.29 (13.62) |
| Genderb | |
| Female | 76 (89.4%) |
| Male | 9 (10.6%) |
| Raceb | |
| White | 50 (58.8%) |
| African American | 28 (32.9%) |
| Other | 7 (8.3%) |
| Disease Typeb | |
| Solid Tumor | 75 (88.2%) |
| Breast Cancer | 42 (49.4%) |
| Lung Cancer | 14 (16.5%) |
| Carcinoma | 12 (14.1%) |
| Sarcoma | 2 (2.4%) |
| Other solid tumor | 5 (5.9%) |
| Hematologic Cancer | 10 (11.8%) |
| Leukemia | 3 (3.5%) |
| Lymphoma | 3 (3.5%) |
| Myeloma | 4 (4.7%) |
| Metastatic Statusb | |
| Metastatic Disease | 36 (42.4%) |
| Non-Metastatic | 49 (57.6%) |
| Cancer Treatment Modality | |
| Chemotherapyb | 73 (85.9%) |
| Radiation therapyb | 56 (65.9%) |
| Primary Study Variablesa | |
| PCL-C total score (PTSD Symptoms) | 35.86 (14.47) |
| PROMIS-29 Sleep Disturbance T-Score | 54.30 (3.4) |
| PROMIS-29 Pain Intensity rating | 4.71 (3.04) |
| PROMIS-29 Pain Interference T-Score | 58.50 (1.8) |
Note. M (SD);
n (%).
Based on PCL-C total scores (with sleep items included; M = 38.06, SD = 14.62), 64% of the sample endorsed clinical levels of PTSD symptoms (i.e., clinical cutoff PCL-C total score of 30) and 34% fell above the clinical cutoff score of 45 for a probable PTSD diagnosis15. The average PCL-C score (without sleep items) was 35.31 (SD = 13.99). The sample scored slightly above the general population on the PROMIS-29 sleep disturbance scale (T-Score = 54.30, SE = 3.40) and nearly one standard deviation above the general population on the pain interference scale (T-Score = 58.50, SE = 1.80). The average pain intensity rating was a 4.80 (SD = 3.04), which is consistent with previously reported pain intensity ratings from patients in active cancer treatment18.
As noted in Table 2, bivariate correlations indicated that the PCL-C total score (without sleep items), sleep disturbance score, pain intensity score, and pain interference score were all positively related to each other (all p’s < 0.01). With regard to relationships between covariates and primary study variables, metastatic disease was negatively related to PCL-C total scores and African American race was positively related to pain intensity and pain interference scores (all p’s < 0.01). Accordingly, African American race and metastatic status were entered as dichotomous covariates in subsequent mediation models. In addition, although hematologic cancer status was not significantly related to primary study variables at the zero-order level, it was entered as a dichotomous covariate in order to control for differences in metastatic status between solid tumor cancers and hematologic cancers.
Table 2.
Pearson and Spearman Correlations among Study Variable and Covariates.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pain Intensity | 1 | |||||||||
| 2 | Pain Interference | .87** | 1 | ||||||||
| 3 | Sleep Disturbance | .48** | .55** | 1 | |||||||
| 4 | PTSD Symptoms | .37** | .37* | .40** | 1 | ||||||
| 5 | Age | .04 | .10 | −.05 | −.07 | 1 | |||||
| 6 | Female | .02 | .10 | .13 | .14 | −.08 | 1 | ||||
| 7 | African American | .33** | .35** | .15 | .16 | .13 | .16 | 1 | |||
| 8 | Chemotherapy | −.08 | −.05 | .02 | .08 | .01 | −.14 | −.08 | 1 | ||
| 9 | Radiation therapy | −.01 | −.01 | −.01 | −.05 | .16 | .16 | −.02 | .07 | 1 | |
| 10 | Metastatic Status | .01 | −.04 | −.04 | −.32** | .24* | −.20 | .11 | .21 | .17 | 1 |
| 11 | Hematologic Cancer | .11 | .04 | .04 | .07 | −.14 | −.23* | −.18 | .15 | −.51** | −.31** |
Note. p < .05;
p < .01;
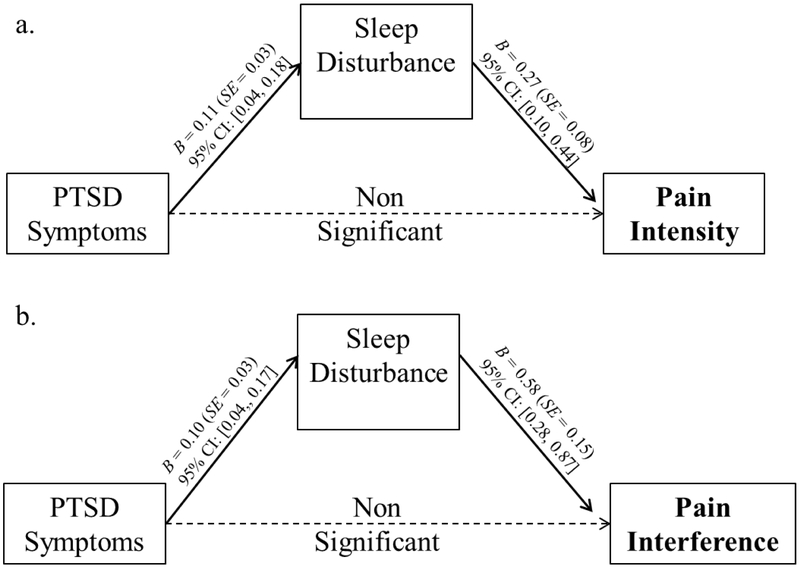
Path analyses of our hypothesized models indicated that sleep disturbance scores mediated the association between PCL-C total scores (without sleep items) and pain intensity scores (R2=0.40) and the association between PCL-C total scores (without sleep items) and pain interference scores (R2=0.47; Figure 1). These effects were maintained even after controlling for metastatic disease, African American race and hematologic cancer type. Of these covariates, only African American race remained significantly linked with pain intensity (B = 2.08, SE = .69) and pain interference (B = 4.93, SE = 1.23).
Figure 1.
Unstandardized regression coefficients and standard errors from our hypothesized model: the relationship between a) PTSD symptoms and pain intensity as mediated by sleep disturbance and b) PTSD symptoms and pain interference as mediated by sleep disturbance. Standardized indirect effect of sleep disturbance on pain intensity = 0.14, SE = 0.06, 95% C.I. [0.04, 0.28]. Standardized indirect effect of sleep disturbance on pain interference = 0.16, SE = 0.06, 95% C.I. [0.05, 0.29].
Note. For clarity, pathways and values of covariates (i.e., metastatic status, African American race, and hematologic cancer type) not pictured. As noted in Table 1, PTSD symptoms were significantly corelated with pain intensity and pain interference at the zero-order level (p < .05). However, after accounting for the effects of sleep disturbance, the relationship between PTSD symptoms and pain intensity and the relationship between PTSD symptoms and pain interference were no longer significant. Thus, sleep disturbance was a significant mediator of those relationships.
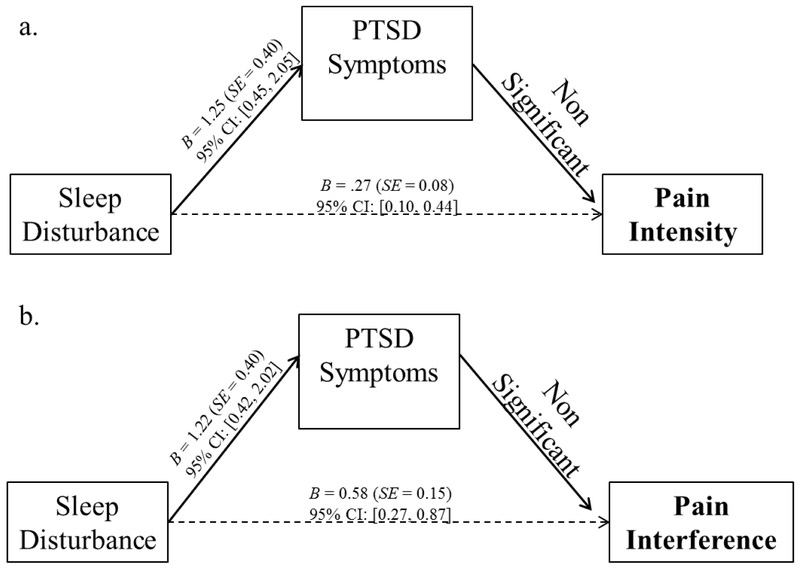
Evaluation of our alternative mediation model (Figure 2) indicated that PCL-C total scores (without sleep items) was not a significant mediator of the association between sleep disturbance scores and pain intensity scores or the association between sleep disturbance scores and pain interference scores.
Figure 2.
Unstandardized regression coefficients and standard errors from our alternative model: the relationship between a) Sleep disturbance and pain intensity as mediated by PTSD Symptoms and b) Sleep disturbance and pain interference as mediated by PTSD Symptoms. Standardized indirect effect of PTSD symptoms on pain intensity = 0.15, SE = 0.65, 95% C.I. [−0.01, 0.19]. Standardized indirect effect of PTSD symptoms on pain interference = 0.06, SE = 0.05, 95% C.I. [−0.02, 0.18].
Note. For clarity, pathways and values of covariates (i.e., metastatic status, African American race, and hematologic cancer type) not pictured. As noted in Table 1, sleep disturbance was significantly correlated with pain intensity and pain interference at the zero-order level (p < .05).After accounting for the effects of PTSD symptoms, the significant relationship between sleep disturbance and pain intensity and the relationship between sleep disturbance and pain interference were still significant (p < .05). Thus, PTSD symptoms did not significantly mediate those relationships.
DISCUSSION
Approximately 30–60% of the current sample of cancer patients reported clinical levels of PTSD symptoms, which is higher than the 2015 estimate by the NCI1 and may be accounted for by the selection of patients receiving psychosocial oncology services. Pain and sleep-related complaints were also common in the current sample. Mediation analyses of our hypothesized and alternative models indicated that the relationships between PTSD symptoms, pain intensity, and pain interference could be explained in part by co-occurring sleep disturbance. One possibility is that intrusive trauma-related thoughts and memories may lead to pre-sleep arousal which may negatively impact overall sleep quality and duration. The degradation in sleep quality and duration may, in turn, deplete resources needed to effectively manage pain. Anxious and traumatized patients may also be more inclined to attend to and make catastrophic interpretations of physical ailments and pain and avoid activity that they believe will increase their anxiety and/or their pain19. These processes may then culminate in activity restriction and poorer overall quality of life.
Although prior studies have highlighted bidirectional pathways between sleep and pain9 and sleep and PTSD20, our findings are consistent with past chronic pain research that posits sleep disturbance as the potential driver of daily pain and pain-related dysfunction7. To that end, the present findings are consistent with existing longitudinal models that link poor sleep to pain through cognitive, affective and behavioral pathways21. Importantly, although our findings map on to the directional pathways between sleep disturbance and pain processes evinced in chronic pain populations, additional, longitudinal studies with cancer patient populations are needed to validate the directional pathways between sleep disturbance and pain reported here.
Of note, African American race and metastatic status were identified as key contributing factors to the relationships among PTSD symptoms, sleep disturbance, and pain. Participants who identified as African American had higher pain intensity and pain-related interference than participants from other racial backgrounds. These findings are consistent with documentation of worse self-reported pain outcomes among African Americans as compared to other racial groups22 and warrant additional research on the mechanisms underlying these race-related differences in cancer patient populations. Although these patients were selected based on their engagement in psychosocial oncology services, racial and ethnic minorities and other marginalized groups may face barriers to accessing supportive oncology services.
In addition, metastatic status was found to be negatively related to PTSD symptoms, suggesting that, as compared those with metastatic disease, individuals without metastatic disease experienced more severe symptoms of PTSD. Although this finding may appear counterintuitive, the literature regarding the relationship between disease staging and PTSD symptomology is decidedly mixed. Some studies have reported that PTSD may be more common among cancer patients with later-stage disease vs. earlier stages23, however, the majority of studies that have examined disease stage in relation to PTSD are skewed towards earlier stage diseases24. Furthermore, many studies outright exclude patients with metastatic disease25–29. Thus, our finding contributes to the literature metastatic disease processes and PTSD symptoms and may indicate that patients with non-metastatic disease are at a particularly high risk for experiencing severe symptoms of PTSD.
These results should be evaluated in the context of the current study’s limitations. Data were cross-sectional, thus, our mediation models need validation in prospective, longitudinal study designs to determine whether sleep disturbance is a primary mechanism underlying the relationship between PTSD symptoms and pain in cancer patients. In addition, the relationship between PTSD and depression is complex, with the two disorders so intertwined30 that the DSM-52 has integrated symptoms of negative mood states (i.e., anhedonia, negativistic thinking, and negative emotionality) into the new nosology. We acknowledge the considerable overlap between depression and PTSD and recognize that future studies would be needed to ascertain whether a pre-existing or co-occurring diagnosis of depression alters the relationships reported here. Finally, data were collected via self-report from a small, mostly female, sample of patients in active cancer treatment who were receiving outpatient psychotherapy services. Although data may be of clinical relevance, they may not represent the diverse experiences of all cancer patients.
This study offers additional support for the high frequency of PTSD symptoms, sleep problems and pain related complaints among cancer patients. Furthermore, our study highlights important qualitative differences in PTSD symptomology based on metastatic disease status, and the role of sleep disturbance as a mechanism linking PTSD symptoms to reports of pain. Currently, many psychosocial interventions for cancer patients target individual symptom clusters (i.e., pain or sleep disturbance). Appropriate screening for PTSD and trauma-related distress may aid in treatment planning to address multiple symptoms simultaneously in cancer patients, particularly with regard to metastatic disease status. Thus, clinicians who provide psychosocial treatments to cancer patients should be attentive to the role that traumatogenic processes may play in the context of sleep and pain-related complaints.
Footnotes
Declaration of Conflicts of Interest
None of the authors have any conflicts to disclose.
REFERENCES
- 1.National Cancer Institute, 2015. Cancer-Related Post-traumatic Stress (PDQ®) Prevalence. http://www.cancer.gov/cancertopics/pdq/supportivecare/post-traumatic-stress/HealthProfessional/page2. Accessed December 27, 2016.
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013. May 22. [Google Scholar]
- 3.Van den Beuken-van Everdingen MH, De Rijke JM, Kessels AG, Schouten HC, Van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Annals of oncology. 2007. September 1;18(9):1437–49. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts and Figures 2017. Atlanta: American Cancer Society; 2017. [Google Scholar]
- 5.Beck JG, Clapp JD. A different kind of co-morbidity: understanding posttraumatic stress disorder and chronic pain. Psychol Trauma. 2011;3(2):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox RC, Tuck BM, Olatunji BO. Sleep Disturbance in Posttraumatic Stress Disorder: Epiphenomenon or Causal Factor?. Current psychiatry reports. 2017. April 1;19(4):22. [DOI] [PubMed] [Google Scholar]
- 7.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep medicine reviews. 2004. April 30;8(2):119–32. [DOI] [PubMed] [Google Scholar]
- 8.Sharma N, Hansen CH, O’Connor M, Thekkumpurath P, Walker J, Kleiboer A, Murray G, Espie C, Storey D, Sharpe M. Sleep problems in cancer patients: prevalence and association with distress and pain. Psycho-Oncology. 2012. September 1;21(9):1003–9. [DOI] [PubMed] [Google Scholar]
- 9.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. The Journal of Pain. 2013. December 31;14(12):1539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang KP, Veazey-Morris K, Andrasik F. Exploring the role of insomnia in the relation between PTSD and pain in veterans with polytrauma injuries. The Journal of head trauma rehabilitation. 2014. January 1;29(1):44–53 [DOI] [PubMed] [Google Scholar]
- 11.Powell MA, Corbo V, Fonda JR, Otis JD, Milberg WP, McGlinchey RE. Sleep quality and reexperiencing symptoms of PTSD are associated with current pain in US OEF/OIF/OND Veterans with and without mTBIs. Journal of traumatic stress. 2015. August 1;28(4):322–9. [DOI] [PubMed] [Google Scholar]
- 12.Lamotte AD, Taft CT, Weatherill RP, Casement MD, Creech SK, Milberg WP, Fortier CB, McGlinchey RE. Sleep problems and physical pain as moderators of the relationship between PTSD symptoms and aggression in returning veterans. Psychological trauma: theory, research, practice, and policy. 2017. January;9(1):113. [DOI] [PubMed] [Google Scholar]
- 13.Mystakidou K, Parpa E, Tsilika E, Gennatas C, Galanos A, Vlahos L. How is sleep quality affected by the psychological and symptom distress of advanced cancer patients? Palliative Medicine. 2009. January;23(1):46–53. [DOI] [PubMed] [Google Scholar]
- 14.Weathers FW, Litz BT, Herman D, Huska J, Keane T. The PTSD checklist-civilian version (PCL-C). Boston, MA: National Center for PTSD; 1994. [Google Scholar]
- 15.Walker EA, Newman E, Dobie DJ, et al. Validation of the PTSD checklist in an HMO sample of women. Gen Hosp Psychiatry 2002; 24(6): 375–380. [DOI] [PubMed] [Google Scholar]
- 16.Measures Health, 2016. Patient Reported Outcomes Measurement Information System (PROMIS). http://www.healthmeasures.net/index.php. Accessed August 17, 2017.
- 17.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; 2013. [Google Scholar]
- 18.Li J, Sheng S, Zhang K, Liu T. Pain Analysis in Patients with Pancreatic Carcinoma: Irreversible Electroporation versus Cryoablation. BioMed research international. 2016. December 15;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerhart JI, Hall BJ, Russ EU, Canetti D, Hobfoll SE. Sleep disturbances predict later trauma-related distress: cross-panel investigation amidst violent turmoil. Health psychology. 2014. April;33(4):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013. July 1;36(7):1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerhart JI, Burns JW, Post KM, Smith DA, Porter LS, Burgess HJ, Schuster E, Buvanendran A, Fras AM, Keefe FJ. Relationships Between Sleep Quality and Pain-Related Factors for People with Chronic Low Back Pain: Tests of Reciprocal and Time of Day Effects. Annals of Behavioral Medicine. 2016. November 14:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HJ, Yang GS, Greenspan JD, Downton KD, Griffith KA, Renn CL, Johantgen M, Dorsey SG. Racial and ethnic differences in experimental pain sensitivity: systematic review and meta-analysis. Pain. 2017. February 1;158(2):194–211. [DOI] [PubMed] [Google Scholar]
- 23.Arnaboldi P, Riva S, Crico C, Pravettoni G. A systematic literature review exploring the prevalence of post-traumatic stress disorder and the role played by stress and traumatic stress in breast cancer diagnosis and trajectory. Breast Cancer: Targets and Therapy. 2017;9:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurevich M, Devins GM, Rodin GM. Stress response syndromes and cancer: conceptual and assessment issues. Psychosomatics 2002;43:259–281. [DOI] [PubMed] [Google Scholar]
- 25.Voigt V, Neufeld F, Kaste J, Bühner M, Sckopke P, Wuerstlein R, Hellerhoff K, Sztrókay‐Gaul A, Braun M, Koch FE, Silva‐Zürcher E. Clinically assessed posttraumatic stress in patients with breast cancer during the first year after diagnosis in the prospective, longitudinal, controlled COGNICARES study. Psycho‐Oncology. 2017. January 1;26(1):74–80. [DOI] [PubMed] [Google Scholar]
- 26.Shelby RA, Golden-Kreutz DM, Andersen BL. PTSD diagnosis, subsyndromal symptoms, and comorbidities contribute to impairment for breast cancer survivors. J Trauma Stress. 2008;21(2):165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hegel MT, Moore CP, Collins ED, Kearing S, Gillock KL, Riggs RL, Clay KF, Ahles TA Distress, psychiatric syndromes, and impairment of function in women with newly diagnosed breast cancer. Cancer. 2006. December 15; 107(12):2924–31. [DOI] [PubMed] [Google Scholar]
- 28.lkhyatt MK, Elham Kh, Abdullah EK, Ibraim RH, Anee BA, Raho JA. Post-traumatic stress in women with breast cancer. J Med J. 2012;46(4):315–319. [Google Scholar]
- 29.Yang YL, Liu L, Li MY, Shi M, Wang L. Psychological disorders and psychosocial resources of patients with newly diagnosed bladder and kidney cancer: A cross-sectional study. PloS one. 2016. May 18;11(5):e0155607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flory JD, Yehuda R. Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues in clinical neuroscience. 2015. June;17(2):141. [DOI] [PMC free article] [PubMed] [Google Scholar]