Abstract
Background:
Patients with metastatic colorectal cancer (mCRC) often suffer from progressive disease despite previous therapy. It has been a great challenge for those patients. In 2012, regorafenib was approved for mCRC. In this meta-analysis, we aimed to collect and present existing data to explorethe clinical use of regorafenib.
Methods:
The online electronic databases, such as PubMed, Embase, and the Cochrane library, updated to November 2017 were systematically searched. Trials on the effectiveness of regorafenib in patients who suffer from treatment-refractory metastatic colorectal cancer were included, of which the main outcomes included 3 parameters: overall survival (OS), progression-free survival (PFS), and grade 3/4 AE.
Results:
Totally, 4 trials were included in this meta-analysis. The OD was significantly better with the use of regorafenib (OR = 0.78, 95%CI = 0.65–0.94, I2 = 69%, P = .008), and PFS (OR = 0.52, 95%CI = 0.34–0.79, I2 = 97%, P = .002). However, the most common toxicities occurred more frequently in the regorafenib group than the control group (OR = 3.73, 95%CI = 1.68–8.28, I2 = 79%, P = .001).
Conclusion:
Regorafenib demonstrates better efficacy and has manageable adverse-event profile for treatment-refractory mCRC. Considering the safety feature of regorafenib, further studies and clinical trials are warranted to investigate the dosing of regorafenib and alternative approaches are needed to explore predictive biomarker fortherapy selection.
Keywords: meta-analysis, metastatic colorectal cancer, regorafenib
1. Introduction
Colorectal cancer (CRC), the third most commonly diagnosed cancer in humans, is one of the leading causes of cancer-related death in the world.[1] Patients harboring unresectable or metastatic CRC (mCRC) with standard treatment are offered anti-epidermal growth factor receptor (EGFR) antibodies,[4–6] antivascular endothelial growth factor (VEGF) antibodies,[2,3] and chemotherapy (fluoropyrimidines plus either irinotecan or oxaliplatin) typically in the case of RAS wild-type tumors. Novel drugs have been developed for mCRC, and have been shown to extend median overall survival (OS) to 30 months versus first-line chemotherapy.[7–9]
Nevertheless, few other therapeutic options can be offered to treatment-refractory patients who develop metastases and do not respond to the above mentioned drugs. Therefore, alternative therapeutic approaches are in great demand for patients with progressive diseases even who have exhausted all current available standard treatments. Previous studies have shown that patients harboring mCRC with disease progression after standard chemotherapy exhibited longer OS with the use of regorafenib versus placebo.
Regorafenib is an oral small-molecule multikinase inhibitor, which targets drug to inhibit angiogenesis and apoptosis.[10] In the CORRECT trial (regorafenib monotherapy for patients with mCRC who were previously treated), regorafenib and placebo were compared in patients with mCRC who were unable to tolerate standard therapies or refractory to all current standard chemotherapies.[11] Regorafenib resulted in significantly longer progression-free survival (PFS) and OS. On the basis of the results of CORRECT, the US Food and Drug Administration approved regorafenib on September 2012 to treat patients with refractory mCRC.[12]
In order to make a more rational choice of treatment for treatment-refractory mCRC patients, we performed the current meta-analysis to pool controlled trials with regorafenib and analyze both the efficacy and toxicity of regorafenib.
2. Methods and materials
2.1. Ethical review
Ethical approval was waived because this study did not involve any human participants or animals.
2.2. Search strategy
Two investigators independently searched electronic databases, including PubMed, Embase, and the Cochrane library updated to November 2017. The process was established to find all articles with the keywords “metastatic colorectal cancer” AND “regorafenib,” and associated Medical Subject Heading (MeSH) terms were also used. The reference materials of included articles that dealt with the topic of interest were also manually searched to check for additional relevant publications.
2.3. Eligibility criteria
Studies to meet the following criteria should be included in the meta-analysis:[1] the studies were designed as randomized controlled trials (RCTs) and propensity score matching (PSM) controlled trials;[2] patients harboring treatment-refractory mCRC;[3] the outcomes included efficacy (PFS and OS) and toxicity (incidence of severe adverse effects), and ORs with corresponding 9 confidence intervals (CIs) were provided;[4] only full texts were included. Studies with complete information would be included from overlapped or duplicated data in multiple reports. Only English publications were eligible.
2.4. Eligibility assessment
The quality of the retrieved studies was assessed by 2 reviewers separately. The risk of bias items (ROBI) recommended by The Cochrane Handbook for Systematic Reviews of Interventions was used.
2.5. Data extraction
Two authors extracted the relevant data from individual studies separately. Disagreement was settled by discussion. The main categories were based on the following parameters from the eligible studies: family name of first author, year of publication, study design, number of patients, and end point of interests. We extracted the corresponding risk ratios (RRs) and odds ratios (ORs) to assess the association strength for dichotomous (severe adverse effect [SAE] rate [grade ≥ 3]) data and survival (PFS and OS), respectively, with the corresponding 95% CI.
2.6. Statistical analysis
In the pooled analysis, the end points of interest were PFS, OS, and AE. Additionally, using the method of inverse variance, the end point outcome was regarded as a weighted average of individual estimates of OR from included studies.
Based on heterogeneity, we performed a systematic analysis to explore the overall results across the included studies. Heterogeneity across studies was examined using the I2 statistic.[13] Studies were considered to have high, moderate, or low heterogeneity when I2 was >75%, 50–75%, or 25% to 50%, respectively.[14] We used the fixed-effects model when there was low heterogeneity among studies. In other cases, we used the random effects model. A P value <0.05 was considered statistically significant. Review Manager version 5.3 software (Revman; The Cochrane collaboration Oxford, United Kingdom) was used to perform further statistical analyses. Forest plots indicated the findings of our meta-analysis, and the Begg test and the Egger test were conducted for publication bias evaluation.
3. Results
3.1. Literature search process and characteristics of the included studies
In total, 254 studies were searched for evaluation initially. Based on the criteria described in the methods, 9 publications were identified, but several publications did not provide sufficient details of outcomes of 2 approaches. Therefore, a final total of 4 RCTs[15–17] compared regorafenib versus chemotherapy. The search process is described in Figure 1.
Figure 1.
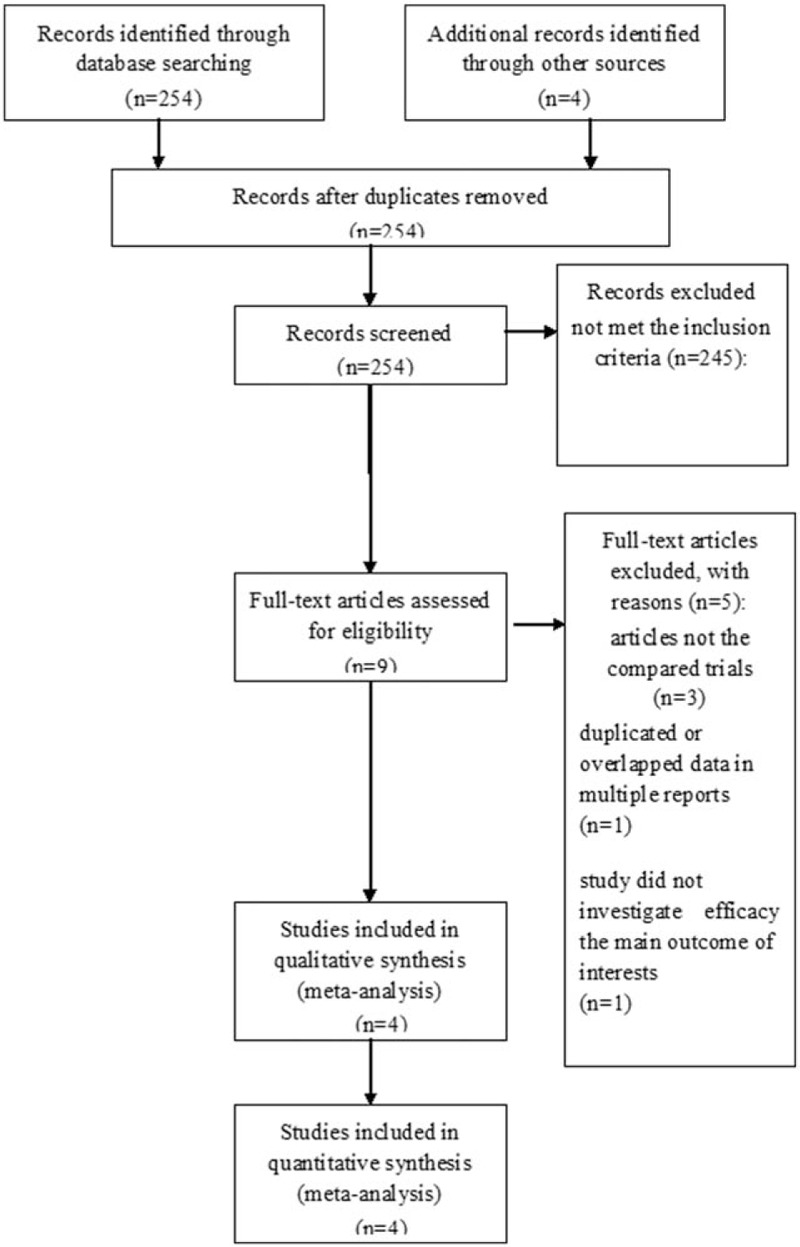
PRISMA flow chart of the selection process of eligible studies for pooling.
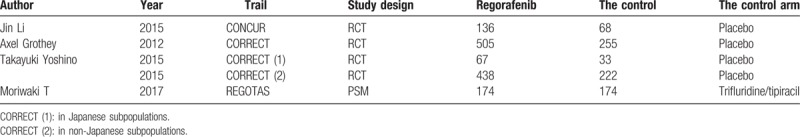
All the mentioned studies were based on moderate-to-high quality evidence. Table 1 describes the major characteristics of the qualified studies.
Table 1.
the primary characteristics of the eligible studies in more detail.
3.2. Clinical and methodological heterogeneity
3.2.1. Pooled analysis of PFS comparing regorafenib with the control group
Pooled PFS data from 4 studies[15–17] showed significant differences in PFS of patients in the regorafenib versus the control group (OR = 0.52, 95%CI = 0.34–0.79, P = .002) (Fig. 2).
Figure 2.
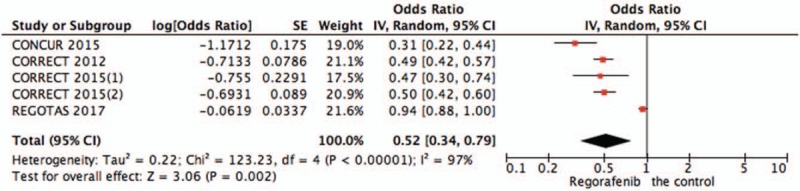
Pooled analysis of PFS comparing regorafenib with the control group. PFS = progression-free survival.
3.2.2. Pooled analysis of OS comparing regorafenib with the control group
Pooled OS data from all studies[15–17] showed that regorafenib significantly improved OS of patients versus chemotherapy (OR = 0.78,95%CI = 0.65–0.94, P = .008) (Fig. 3).
Figure 3.
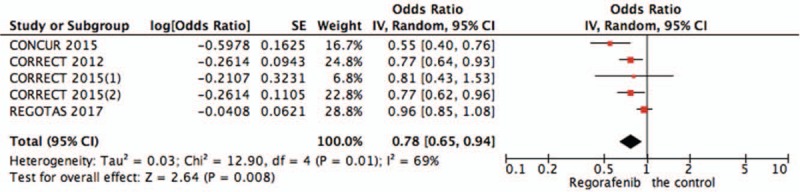
Pooled analysis of OS comparing regorafenib with the control group. OS = overall survival.
3.2.3. Pooled analysis of AEs comparing regorafenib with the control group
Systematic evaluations of AE data are shown in Figures 4–10. The most common toxicities occurred significantly more frequently in the regorafenib group than in the placebo group (OR = 3.73,95%CI = 1.68–8.28, P = .001) (Fig. 4). The most common treatment-emergent AEs were diarrhea (OR = 7.12,95%CI = 2.99–16.99, P < .00001) (Fig. 5), fatigue (OR = 1.96,95%CI = 1.27–3.04, P = .003) (Fig. 6), hand-foot skin reaction (OR = 38.60,95%CI = 12.23–121.80, P < .00001) (Fig. 7), thrombocytopenia (OR = 5.72,95%CI = 1.74–18.75, P = .004) (Fig. 8) and hypertension (OR = 7.34,95%CI = 3.28–16.41, P < .00001) (Fig. 9); the pooled data showed that the SAEs were more commonly reported in the regorafenib group. The AEs had no statistical significance only in anorexia with exclusion of the regorafenib group (OR = 1.17; 95% CI, 0.63–2.19; P = .62) (Fig. 10).
Figure 4.
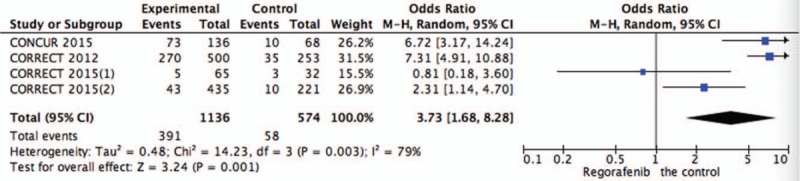
Pooled analysis of AEs comparing regorafenib with the control group. AEs = adverse effects.
Figure 10.
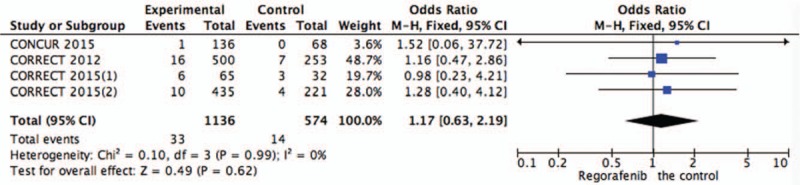
Pooled analysis of anorexia comparing regorafenib with the control group.
Figure 5.
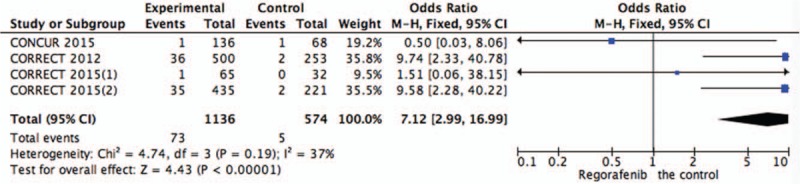
Pooled analysis of diarrhea comparing regorafenib with the control group.
Figure 6.
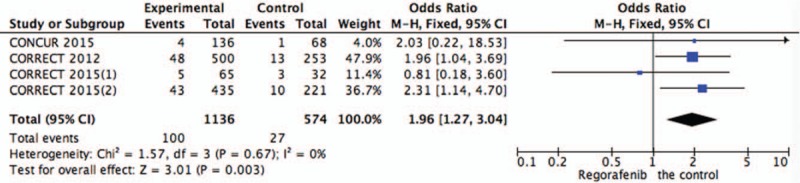
Pooled analysis of fatigue comparing regorafenib with the control group.
Figure 7.
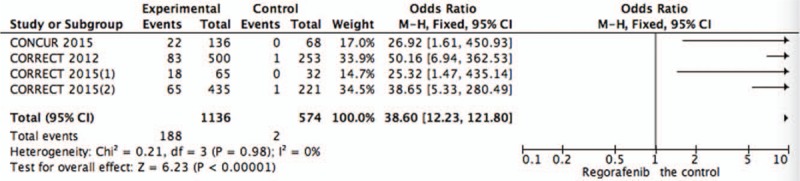
Pooled analysis of hand-foot skin reaction comparing regorafenib with the control group.
Figure 8.
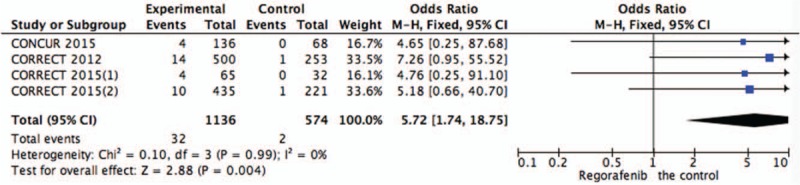
Pooled analysis of thrombocytopenia comparing regorafenib with the control group.
Figure 9.
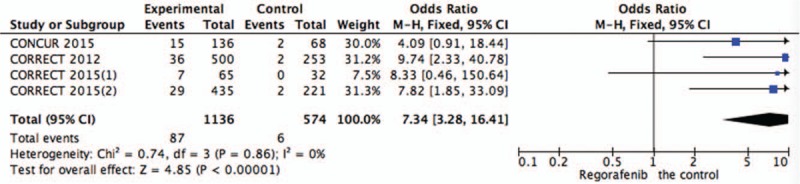
Pooled analysis of hypertension comparing regorafenib with the control group.
4. Discussion
Regorafenib, a multikinase inhibitor and orally administered drug, blocks the activity of several protein kinases linked to the tumor microenvironment (fibroblast growth factor receptor and platelet-derived growth factor receptor), oncogenesis (RAF1, KIT, RET, BRAF, and BRAF V600E) and angiogenesis (TIE2 and VEGF receptors 1–3).[10]
In 2012, regorafenib was approved to treat patients with CRC. Two prospective randomized trials have shown clinically benefits of regorafenib for patients with refractory mCRC.[18] Nevertheless, there was a debate regarding the clinical significance of regorafenib amongseveral authors due to its adverse effects.[19] Additionally, given that the moderate beneficial effects could be found in the trial setting, it is still uncertain for clinical practice to have similar outcomes. We specifically focused on the question through results aggregation from control trials of various countries.
Remarkably larger overall survival benefit had been noted in our trial. Cross-trial comparisons were needed with cautions with the unclear reasons for differences. Considering the different targeted treatments had been offered earlier, the regorafenib effect might be affected despite its independence of ethnic origin. It indicated that patients who had received targeted treatment (appendix) earlier may be exposed to the effect of post-study treatment in all subgroups. A moderate-to-high heterogeneity was presented in this meta-analysis. This heterogeneity reflected differences in patient populations in terms of ethnicity, previous treatment, and mutation statuses.
The commonly occurred adverse events of grade 3 or higher with the use of regorafenib were diarrhea, hypertension, fatigue, hand-foot skin reaction, and thrombocytopenia. Although a larger chance of adverse events was observed in the regorafenib group in comparison with the control group, most adverse events could be managed through reduction or discontinuance of the drug. The frequent occurrence of adverse events, calls for further investigation of regorafenib dosing. According to a recently published retrospective study, it remains unclear whether the adverse events associated with regorafenib treatment are dose- or time-dependent.[20] Therefore, looking into toxicity driven dosing is highly relevant considering the safety profile of regorafenib. Further work is needed to determine the biomarkers which may provide further tailoring of the therapy to obtain clinical benefit in a maximum way.[21]
Our data indicated that regorafenib could be regarded as a novel standard therapy for late-stage mCRC. However, several unsolved questions exist for further investigation. On one hand, the mechanism of regorafenib on how to activate in human CRC remains to be explored regardless of the available preclinical data in CRC models.[22,23] On the other hand, different responses could be gained with patients in different subgroups through regorafenib therapy. The validation and identification of biomarkers that are to refine the patient populations might obtain benefit from regorafenib. Further work is in great need to identify the subgroups of the current study.
In conclusion, the current evidence indicated that regorafenib conferred a survival benefit mCRC patients not responding to standard treatments. The AEs associated with regorafenib treatment frequently occurred. Considering the safety profile of regorafenib, further studies and clinical trials to investigate the dosing of regorafenib and alternative approaches are needed to explore molecular biomarkers for therapy selection.
Author contributions
Conceptualization: Reng-Hai Liu.
Data curation: Reng-Hai Liu.
Formal analysis: Wu-Song Xue.
Methodology: Wu-Song Xue.
Project administration: Si-Ye Men.
Resources: Si-Ye Men.
Writing – original draft: Wei Liu.
Writing – review & editing: Wei Liu.
Footnotes
Abbreviations: CIs = confidence intervals, mCRC = metastatic colorectal cancer, ORs = odds ratios, OS = overall survival, PFS = progression-free survival, PSM = propensity score matching, ROBI = risk of bias items, RRs = risk ratios, VEGF = vascular endothelial growth factor.
WSX and SYM contributed equally to this study.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42. [DOI] [PubMed] [Google Scholar]
- [3].Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013–9. [DOI] [PubMed] [Google Scholar]
- [4].Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–34. [DOI] [PubMed] [Google Scholar]
- [5].Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J ClinOncol 2010;28:4697–705. [DOI] [PubMed] [Google Scholar]
- [6].Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011–9. [DOI] [PubMed] [Google Scholar]
- [7].Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1065–75. [DOI] [PubMed] [Google Scholar]
- [8].Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609–18. [DOI] [PubMed] [Google Scholar]
- [9].Yamazaki K, Nagase M, Tamagawa H, et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol 2016;27:1539–46. [DOI] [PubMed] [Google Scholar]
- [10].Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245–55. [DOI] [PubMed] [Google Scholar]
- [11].Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. [DOI] [PubMed] [Google Scholar]
- [12].Shrikhande SV. FDA approves regorafenib (Stivarga) for GIST. Oncology 2013;27:164–164. [PubMed] [Google Scholar]
- [13].Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [14].Higgins J, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619–29. [DOI] [PubMed] [Google Scholar]
- [16].Yoshino T, Komatsu Y, Yamada Y, et al. Randomized phase III trial of regorafenib in metastatic colorectal cancer: analysis of the CORRECT Japanese and non-Japanese subpopulations. Invest New Drugs 2015;33:740–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moriwaki T, Fukuoka S, Taniguchi H, et al. Propensity score analysis of regorafenib versus trifluridine/tipiracil in patients with metastatic colorectal cancer refractory to standard chemotherapy (REGOTAS): a Japanese Society for Cancer of the Colon and Rectum Multicenter Observational Study. Oncologist 2017;23:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mercier J, Voutsadakis IA. A systematic review and meta-analysis of retrospective series of regorafenib for treatment of metastatic colorectal cancer. Anticancer Res 2017;37:5925–34. [DOI] [PubMed] [Google Scholar]
- [19].Garcia-Alfonso P, Feliu J, Garcia-Carbonero R, et al. Is regorafenib providing clinically meaningful benefits to pretreated patients with metastatic colorectal cancer? Clin Transl Oncol 2016;18:1072–81. [DOI] [PubMed] [Google Scholar]
- [20].Osawa H. Response to regorafenib at an initial dose of 120 mg as salvage therapy for metastatic colorectal cancer. Mol Clin Oncol 2017;6:365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Martinelli E, Sforza V, Cardone C, et al. Clinical outcome and molecular characterisation of chemorefractory metastatic colorectal cancer patients with long-term efficacy of regorafenib treatment. ESMO Open 2017;2:e000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zopf D, Scholz A, Fichtner I, et al. Regorafenib (BAY 73-4506): a broad-spectrum tumor deactivator with high combinability potential and antimetastasis activity. American Association for Cancer Research 102nd Annual Meeting; Orlando, FL, USA; April 2–5, 2011. 4262. [Google Scholar]
- [23].Schmieder R, Hoffmann J, Bhargava A, et al. Regorafenib (BAY 73-4506): antimetastatic activity in a mouse model of colorectal cancer. American Association for Cancer Research 103rd Annual Meeting; Chicago, IL, USA; March 31–April 4, 2012. 2337. [Google Scholar]


