Supplemental Digital Content is available in the text
Keywords: accommodative intraocular lenses, cataract, meta-analysis, monofocal intraocular lenses, systematic review
Abstract
Introduction:
We performed a systematic review and meta-analysis to evaluate whether accommodative intraocular lenses (AC-IOLs) are superior for cataract patients compared with monofocal IOLs (MF-IOLs).
Methods:
Pubmed, Embase, Cochrane library, CNKI, and Wanfang databases were searched through in August 2018 for AC-IOLs versus MF-IOLs in cataract patients. Studies were pooled under either fixed-effects model or random-effects model to calculate the relative risk (RR), weighted mean difference (WMD), or standard mean difference (SMD) and their corresponding 95% confidence interval (CI). Distance-corrected near visual acuity (DCNVA) was chosen as the primary outcome. The secondary outcomes were corrected distant visual acuity (CDVA), pilocarpine-induced IOL shift, contrast sensitivity, and spectacle independence.
Results:
Seventeen studies, involving a total of 1764 eyes, were included. Our results revealed that AC-IOLs improved DCNVA (SMD = −1.84, 95% CI = −2.56 to −1.11) and were associated with significantly greater anterior lens shift than MF-IOLs (WMD = −0.30, 95% CI = −0.37 to −0.23). Furthermore, spectacle independence was significantly better with AC-IOLs than with MF-IOLs (RR = 3.07, 95% CI = 1.06–8.89). However, there was no significant difference in CDVA and contrast sensitivity between the 2 groups.
Conclusion:
Our study confirmed that AC-IOLs can provide cataract patients with DCNVA and result in more high levels of spectacle independence than MF-IOLs. Further studies with larger data set and well-designed models are required to validate our findings.
1. Introduction
Cataract is the leading cause of visual impairment and blindness among elderly throughout the world.[1] With the rapidly aging of the population, cataracts are becoming a major social problem in the global scale. Apart from age, other factors such as exposure to sunlight, alcohol consumption, smoking, and some drugs have been reported to increase cataract risk.[2,3] In 2011, China had the largest number with 8 million blind and 75 million visually impaired individuals. At present, cataract is a major cause of visual disability in China.[4,5]
Cataract surgery can effectively restore visual clarity and distance vision. The design of the traditional monofocal intraocular lenses (MF-IOLs) with a single fixed focal length can provide excellent distance vision, the MF-IOL's limited depth of focus means that they cannot provide clear vision at both distance and near.[6,7] Functional near-vision is indispensable in modern society because it requires a lot of near tasks in daily life. For example, loss of reading ability can significantly reduce a person's quality of life.[8,9] Patients with traditional MF-IOLs usually require glasses during computer work or reading.[10] Accommodating IOLs (AC-IOLs) were designed to move along the visual axis to provide near, intermediate, and distance vision in pseudophakic patients.[11,12] AC-IOLs were developed with the purpose of providing some adjusting capacity and some functional near-vision after cataract extraction. AC-IOLs provide useful near-vision without glasses while maintaining good distance vision.[11,12]
Functional assessment of AC-IOLs versus MF-IOLs in cataract surgery has been investigated by 2 previous meta-analyses.[13,14] However, the results from these meta-analyses remain inconclusive and conflicting. Moreover, spectacle independence was not evaluated by systematic synthesis in the previous meta-analyses owing to the limited number of available studies. Therefore, we performed an update systematic review and meta-analysis to provide a comprehensive assessment of the visual outcomes of AC-IOLs compared with MF-IOLs after cataract surgery.
2. Methods
The present meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.[15] All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
2.1. Search strategy
We searched for relevant studies up to August 2018 through the PubMed, Embase Cochrane Library, CNKI, and Wanfang databases with the following terms and their combinations: “cataract,” “intraocular lenses,” “lens implantation,” and “accommodative” (Table S1). Two reviewers were independently performed database search and all disagreements about eligibility were resolved through discussion. We did not restrict the publication date and language. All scanned abstracts, studies, and citations were reviewed. Moreover, references of the retrieved manuscripts were also manually cross-searched for further relevant publications.
2.2. Selection criteria
The studies had to meet the following criteria to be eligible for inclusion in the present meta-analysis: enrolled cataract patients; provide ≥2 comparison groups, one group received AC-IOLs, another group received MF-IOLs; provide outcomes: distance-corrected near visual acuity (DCNVA), corrected distant visual acuity (CDVA), pilocarpine-induced IOL shift, contrast sensitivity, and spectacle independence. If multiple studies from the same population were identified, we included the one that provides more relevant information.
2.3. Data extraction and quality assessment
Two investigators independently extracted the characteristics of the included studies. Any disagreement was subsequently resolved by discussion with the third author. The following information was extracted from each article: first author, year of publication, country, sex, mean age, duration of follow-up, study design, the type of AC-IOLs and MF-IOLs, outcomes assessed. We evaluate the quality of randomized controlled trials (RCTs) with the Cochrane Collaboration's tool for assessing risk of bias.[16] We used the Newcastle-Ottawa Scale for assessing the quality of cohort studies, the full score was 9 stars, and the high-quality study was defined as a study with ≥7 stars.[17]
2.4. Statistical analysis
We calculated the weighted mean difference (WMD)/standard mean difference (SMD) and 95% confidence intervals (CIs) for the continuous data, and calculate the risk ratio (RR) and 95% CIs for dichotomous data. When all studies used the same tool to measure the same outcome, WMD was used; when the different studies used different scales/tools to assess outcomes, SMD was used. The heterogeneity across each effect size was evaluated with Q-statistics and the I2 index.[18]I2 >50% indicated that the heterogeneity was statistically significant. Thus, the random-effects model[19] was used to perform the analysis. Otherwise, we computed the summary effect using the fixed-effects model.[20] Subgroup analyses were performed according to the type of AC-IOLs (1CU, Crystalens HD, or other), study design (RCT or non-RCT), and follow-up time (follow-up time <12 months or ≥12 months). Sensitivity analysis by omitting a single study in each turn was performed to assess the relative influence of each study on the pooled estimate. Visual inspections of funnel plots and the Egger and Begg tests were used to evaluate publication bias.[21] Trim and fill analysis was applied if publication bias was detected[22] An article[23] investigated AC-IOLs in 2 different groups (1CU IOL implantation and AT-45 IOL implantation) and the data were analyzed separately for each group, so we analyzed them as 2 studies. All statistical analyses were performed using STATA Software (version 12.0; StataCorp, College Station, TX). All P values were 2-sided, and the level of significance was set at <.05.
3. Results
3.1. Characteristics of the studies
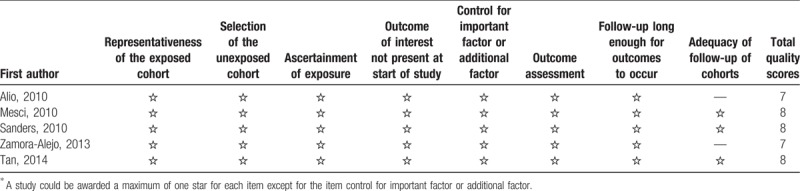
Our initial database searches and manual search retrieved a total of 412 articles. After duplicate removal and abstract/title reviewed, 32 articles were eligible for full-text review. Of these, 15 were further excluded, leaving a total of 17 articles eligible to be included in the present meta-analysis. Finally, 17 studies[23–38] with 1764 eyes were incorporated into the current meta-analysis. The flow chart of selection of studies and reasons for exclusion is presented in Figure 1. The main characteristics of the eligible studies are shown in Table S2. The studies were performed in various countries, and the study size ranged from 23 to 670 eyes. The mean age of patient ranged from 61.1 to 75.9 years. Twelve RCTs and 5 cohort studies were included in our study. A summary of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias identified in each individual RCT is shown in Figure 2. All of the included RCTs studies showed moderate and high quality with acceptable and moderate risk of bias. The main features of the eligible study are shown in Table S1. Methodological quality of cohort studies included in the meta-analysis is shown in Table 1. The quality of the cohort studies included in the meta-analysis was generally high; 2 studies had 8 stars, and 2 studies had 7 stars.
Figure 1.
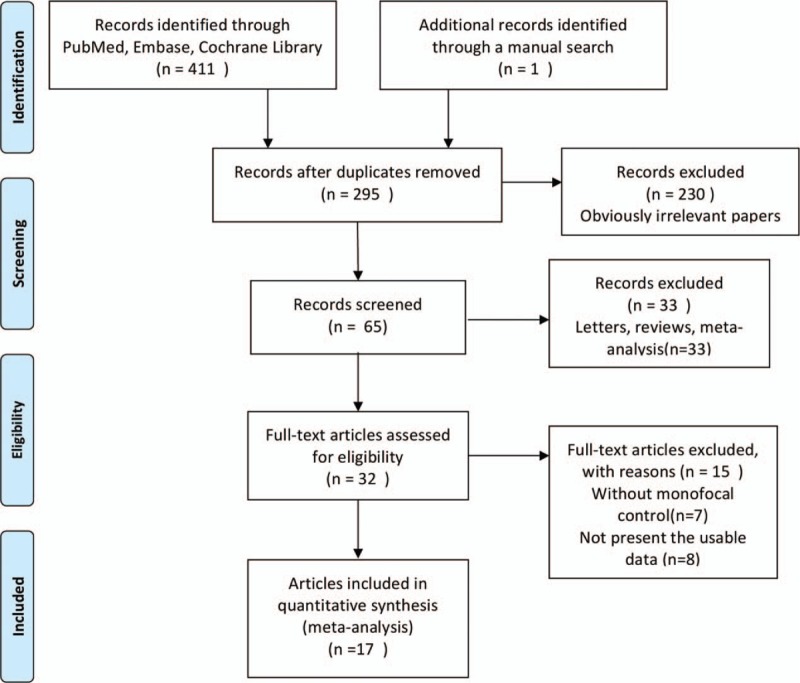
Flow diagram of studies identification.
Figure 2.
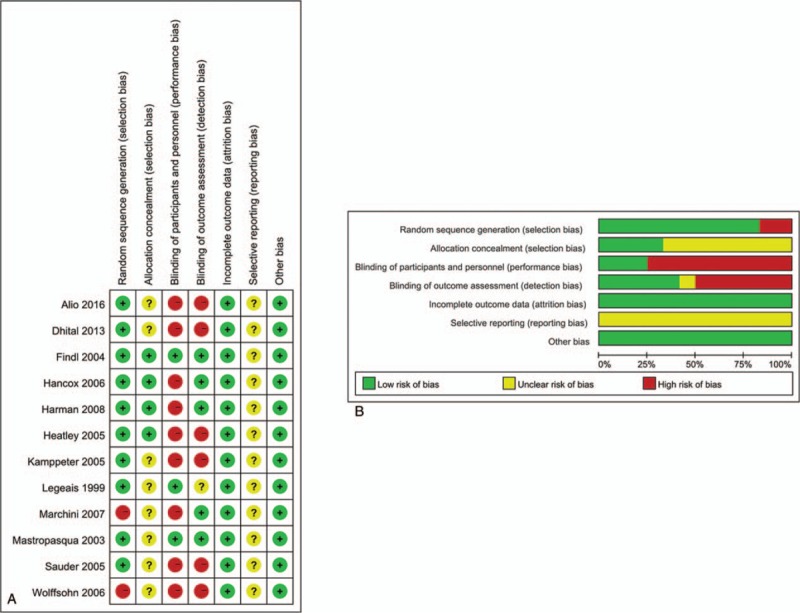
Risk of bias assessments for the randomized trials included in the meta-analysis. (A) Risk of bias summary; (B) risk of bias graph. +: low risk of bias; ?: unclear risk of bias; −: high risk of bias.
Table 1.
Methodological quality of observational studies included in the meta-analysis∗.
3.1.1. Distance-corrected near visual acuity
Fourteen studies[23,25–28,30–33,35–38] with 1694 eyes were included in analysis of DCNVA. The pooled results showed that AC-IOLs improved DCNVA more than MF-IOLs (SMD = −1.84, 95% CI = −2.56 to −1.11, Pheterogeneity < 0.001, I2 = 94.2%) (Fig. 3A).
Figure 3.

Forest plots showing the effect of accommodating IOLs versus monofocal IOLs in patients with cataract. (A) DCNVA; (B) CDVA; (C) pilocarpine-induced IOL shift; (D) contrast sensitivity; (E) spectacle independence.
3.1.2. Corrected distant visual acuity
Ten trials[25,27,28,30–33,36–38] with 747 eyes were included in analysis of CDVA. No significant difference between the 2 groups (WMD = 0.03, 95% CI = −0.01 to 0.06, Pheterogeneity < 0.001, I2 = 76.1%; Fig. 3B) was observed, which indicates that the AC-IOLs and MF-IOLs were not significantly different in terms of CDVA.
3.1.3. Pilocarpine-induced IOL shift
Five studies[23,24,26,28,29] with 340 eyes were included in analysis of pilocarpine-induced IOL shift. The pooled results showed that AC-IOLs were associated with significantly greater anterior lens shift than MF-IOLs (WMD = −0.30, 95% CI = −0.37 to −0.23, Pheterogeneity = 0.047, I2 = 55.5%) (Fig. 3C).
3.1.4. Contrast sensitivity
Four trials[28,30,31,37] with 251 eyes were included in analysis of contrast sensitivity. The results are shown in Figure 3D. No significant difference between the 2 groups (SMD = −0.19, 95% CI = −0.45 to 0.06, Pheterogeneity = 0.670, I2 = 0%) was observed, which indicates that the AC-IOLs and MF-IOLs were not significantly different in terms of contrast sensitivity.
3.1.5. Spectacle independence
Four studies[31,33,34,37] with 1023 eyes were included in analysis of spectacle independence. The pooled results showed that spectacle independence was significantly better with AC-IOLs than with MF-IOLs (RR = 3.07, 95% CI = 1.06–8.89, Pheterogeneity = 0.007, I2 = 75.5%) (Fig. 3E).
3.2. Subgroup analyses
Subgroup analyses were performed according to the type of AC-IOLs (1CU, Crystalens HD, or other), study design (RCT or non-RCT), and follow-up time (follow-up time <12 months or ≥12 months). The results from subgroup analyses were quite consistent with the overall results for distance-corrected near visual acuity, corrected distant visual acuity, pilocarpine-induced IOL shift, and contrast sensitivity. However, in the subgroup analyses by the type of AC-IOLs, a significant different was observed in 1CU group (Table S3) for spectacle independence. All subgroup results are summarized in Table S3.
3.3. Sensitivity analysis
Sensitivity analysis by omitting a single study in each turn revealed that the overall results were free from the influence of a single study (Fig. 4).
Figure 4.
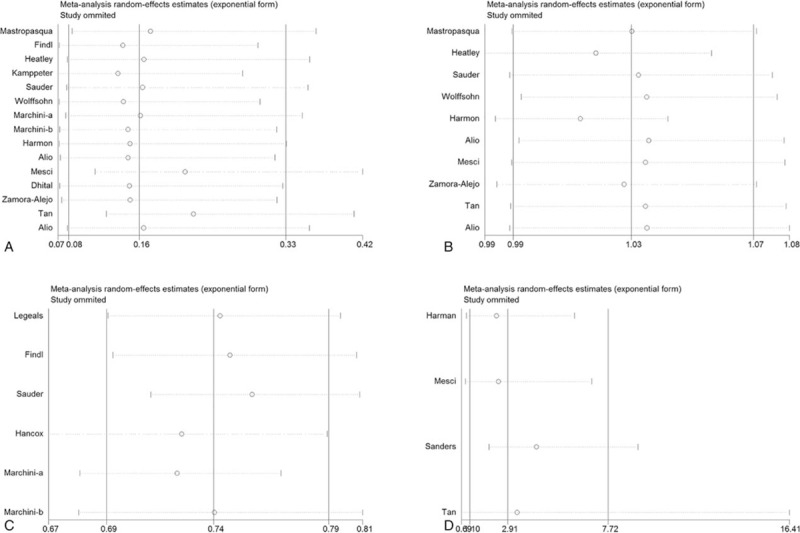
Sensitivity analysis of the effect of accommodating IOLs versus monofocal IOLs in patients with cataract. (A) DCNVA; (B) CDVA; (C) pilocarpine-induced IOL shift; (D) spectacle independence.
3.4. Publication bias
The Begg and Egger regression test showed no significant publication bias in analyses of DCNVA (Begg test P = .373; Egger test P = .109) (Fig. 5A) and CDVA (Begg test P = .283; Egger test P = .837) (Fig. 5B).
Figure 5.
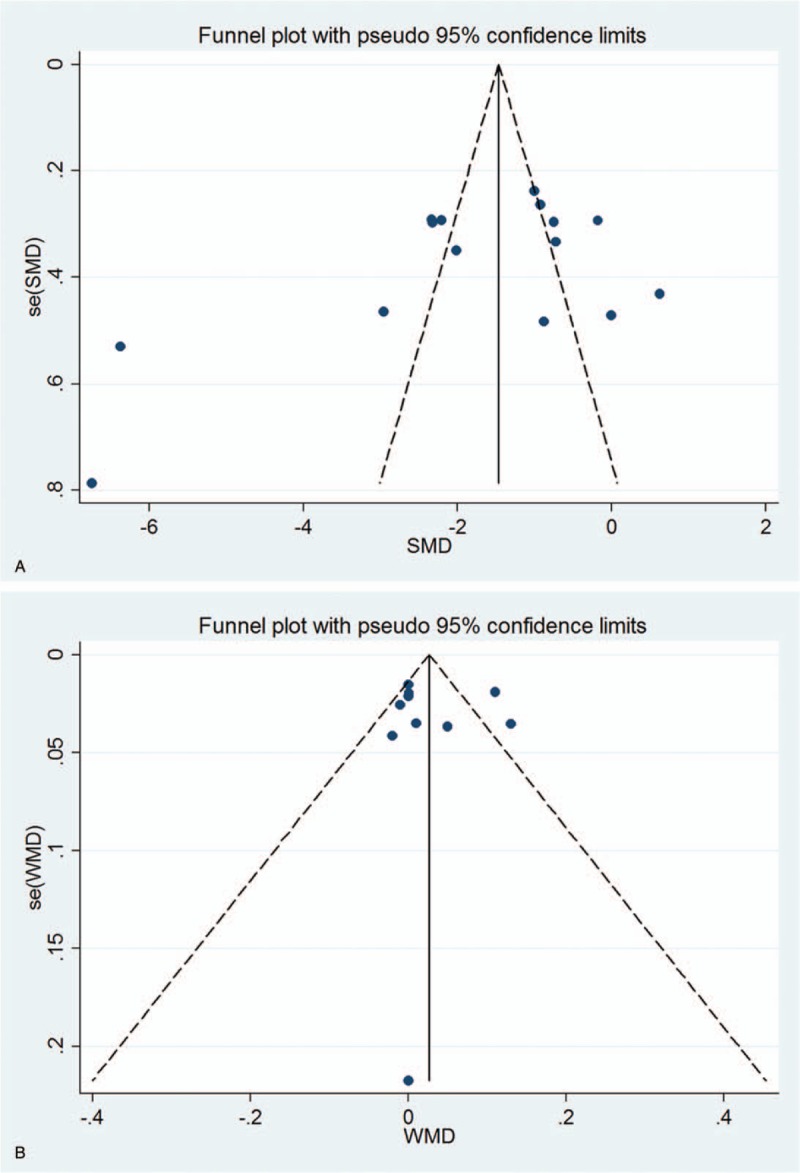
Funnel plot for publication bias test. Each point represents a separate study for the indicated association. (A) DCNVA; (B) CDVA.
4. Discussion
The present systematic review and meta-analysis examined RCTs and cohort studies to compare the postoperative visual performances of AC-IOLs and MF-IOLs. Our results revealed that AC-IOLs improved DCNVA and associated with significantly greater anterior lens shift than MF-IOLs. Furthermore, spectacle independence was significantly better with AC-IOLs than with MF-IOLs. However, there was no significant difference in CDVA and contrast sensitivity between the 2 groups.
DCNVA improved significantly with both groups in our study. The results of our study were consistent with 2 previous meta-analyses,[13,14] which also found slight to moderate improvement in DCNVA with AC-IOLs over MF-IOLs. However, we noted a significant statistical heterogeneity between the trials. The mixture of study designs (unilateral versus bilateral intervention) could be a cause for heterogeneity between the trials. Most participants received the same intervention to both eyes but some participants only received an intervention to one eye. A possible influence on accommodation amplitude is the patient's age. In our study, most of patients were >60 years and had a subjective amplitude of accommodation less than the young patients. In contrast, the objective accommodation amplitude seemed to correlate poorly with age. Contrast sensitivity represents a person's ability to distinguish objects with fuzzy boundaries. As previously reported, contrast sensitivity decreases with age.[39,40] The higher contrast sensitivity in younger patients might contribute to deeper depth of field, resulting in a wider range of subjective accommodation of amplitude. In this study, we discovered that the contrast sensitivity of AC-IOLs was not significantly different from that of MF-IOLs.
Functional assessment of AC-IOLs versus MF-IOLs in cataract surgery has been investigated by previous meta-analyses.[13,14] Our results also differ from previous meta-analysis because of the additional studies included. Recently, Ong et al[14] conducted a meta-analysis, which involved 256 eyes from 5 studies. Compared with Ong's work, we identified 12 additional eligible studies[24–26,28,30,32–38] and our study involved 1764 eyes from 17 studies. Our study also reported pilocarpine-induced IOL shift that was not reported in meta-analysis by Ong et al and found that AC-IOLs were associated with significantly greater anterior lens shift than MF-IOLs. A previous meta-analysis conducted by Takakura et al[13] was limited as only 12 studies were included. Compared with meta-analysis by Takura et al, we were able to include 7 additional eligible studies.[32–38] Therefore, we were able to perform more comprehensive analyses; for example, Takakura et al only analyzed one study in analysis spectacle independence, whereas we identified 3 additional eligible studies and found that spectacle independence was significantly better with AC-IOLs than with MF-IOLs.
Some limitations should be noticed in this meta-analysis: first, the different follow-up time periods and the insufficient reporting of postoperative adverse visual events may have caused selection bias. Second, significant heterogeneity was observed across trials, suggesting that the results from the present meta-analysis should be treated with caution. Different patient selection criteria, surgery protocols, and accommodating IOL models are possible explanations for the heterogeneity. Finally, this limitation of small sample size, which raises concerns about the power to detect a statistically significant effect.
In conclusion, despite the limitations of this meta-analysis, our study confirmed that AC-IOLs can provide cataract patients with excellent DCNVA and result in more high levels of spectacle independence than MF-IOLs. Further studies with larger data set and well-designed models are required to validate our findings.
Author contributions
Investigation: Hongwei Zhou, Chongyan Zhu, Wenya Xu, Fang Zhou.
Methodology: Hongwei Zhou, Chongyan Zhu, Wenya Xu, Fang Zhou.
Validation: Hongwei Zhou.
Visualization: Hongwei Zhou.
Writing – original draft: Hongwei Zhou, Chongyan Zhu, Wenya Xu, Fang Zhou.
Writing – review and editing: Hongwei Zhou, Chongyan Zhu, Wenya Xu, Fang Zhou.
Supplementary Material
Footnotes
Abbreviations: AC-IOLs = accommodating IOLs, AC-IOLs = accommodative intraocular lenses, CDVA = corrected distant visual acuity, CI = confidence interval, DCNVA = distance-corrected near visual acuity, MF-IOLs = monofocal intraocular lenses, MF-IOLs = monofocal IOLs, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analysis, RCTs = randomized controlled trials, RR = relative risk, SMD = standard mean difference, WMD = weighted mean difference.
HZ and WX equally contributed to this work.
The study was supported by Huai’an Natural Science Research Project (Fund Number: HAB201738).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol 2012;96:614–8. [DOI] [PubMed] [Google Scholar]
- [2].Tarwadi KV, Agte VV. Interrelationships between nutritional status, socioeconomic factors, and lifestyle in Indian cataract patients. Nutrition 2011;27:40–5. [DOI] [PubMed] [Google Scholar]
- [3].Wu R, Wang JJ, Mitchell P, et al. Smoking, socioeconomic factors, and age-related cataract: the Singapore Malay Eye study. Arch Ophthalmol 2010;128:1029–35. [DOI] [PubMed] [Google Scholar]
- [4].Xu L, Cui T, Yang H, et al. Prevalence of visual impairment among adults in China: the Beijing Eye Study. Amer J Ophthalmol 2006;141:591–3. [DOI] [PubMed] [Google Scholar]
- [5].Huang S, Zheng Y, Foster PJ, et al. Prevalence and causes of visual impairment in Chinese adults in urban southern China. Arch Ophthalmol 2009;127:1362–7. [DOI] [PubMed] [Google Scholar]
- [6].Becker KA, Martin M, Rabsilber TM, et al. Prospective, non-randomised, long term clinical evaluation of a foldable hydrophilic single piece intraocular lens: Results of the Centerflex FDA study. Br J Ophthalmol 2006;90:971–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lehmann R, Waycaster C, Hileman K. A comparison of patient-reported outcomes from an apodized diffractive intraocular lens and a conventional monofocal intraocular lens. Curr Med Res Opin 2006;22:2591–602. [DOI] [PubMed] [Google Scholar]
- [8].Alio JL, Radner W, Plaza-Puche AB, et al. Design of short Spanish sentences for measuring reading performance: Radner-Vissum test. J Cataract Refract Surg 2008;34:638–42. [DOI] [PubMed] [Google Scholar]
- [9].Uusitalo RJ, Brans T, Pessi T, et al. Evaluating cataract surgery gains by assessing patients’ quality of life using the VF-7. J Cataract Refract Surg 1999;25:989–94. [DOI] [PubMed] [Google Scholar]
- [10].Leyland M, Zinicola E. Multifocal versus monofocal intraocular lenses in cataract surgery: a systematic review. Ophthalmology 2003;110:1789–98. [DOI] [PubMed] [Google Scholar]
- [11].Küchle M, Langenbucher A, Gusek-Schneider GC, et al. Erste Ergebnisse der Implantation einer neuen, potenziell akkommodierbaren Hinterkammerlinse - eine prospektive Sicherheitsstudie123. Klin Monatsbl Augenheilkd 2001;218:603–8. [DOI] [PubMed] [Google Scholar]
- [12].Cumming JS, Slade SG, Chayet A. Clinical evaluation of the model AT-45 silicone accommodating intraocular lens: results of feasibility and the initial phase of a Food and Drug Administration clinical trial. Ophthalmology 2001;108:2005. [DOI] [PubMed] [Google Scholar]
- [13].Takakura A, Iyer P, Adams JR, et al. Functional assessment of accommodating intraocular lenses versus monofocal intraocular lenses in cataract surgery: metaanalysis. J Cataract Refract Surg 2010;36:380–8. [DOI] [PubMed] [Google Scholar]
- [14].Ong HS, Evans JR, Allan BD. Accommodative intraocular lens versus standard monofocal intraocular lens implantation in cataract surgery. Cochrane Database Syst Rev 2014;5:Cd009667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [16].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [18].Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–6. [DOI] [PubMed] [Google Scholar]
- [19].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [20].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [21].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method. Biometrics 2000;56:455–63, 459. [DOI] [PubMed] [Google Scholar]
- [23].Marchini G, Mora P, Pedrotti E, et al. Functional assessment of two different accommodative intraocular lenses compared with a monofocal intraocular lens. Ophthalmology 2007;114:2038–43. [DOI] [PubMed] [Google Scholar]
- [24].Legeais JM, Werner L, Werner L, et al. Pseudoaccommodation: BioComFold versus a foldable silicone intraocular lens. J Cataract Refract Surg 1999;25:262–7. [DOI] [PubMed] [Google Scholar]
- [25].Mastropasqua L, Toto L, Nubile M, et al. Clinical study of the 1CU accommodating intraocular lens. J Cataract Refract Surg 2003;29:1307–12. [DOI] [PubMed] [Google Scholar]
- [26].Findl O, Kriechbaum K, Menapace R, et al. Laserinterferometric assessment of pilocarpine-induced movement of an accommodating intraocular lens: a randomized trial. Ophthalmology 2004;111:1515–21. [DOI] [PubMed] [Google Scholar]
- [27].Heatley CJ, Spalton DJ, Hancox J, et al. Fellow eye comparison between the 1CU accommodative intraocular lens and the Acrysof MA30 monofocal intraocular lens. Am J Ophthalmol 2005;140:207–13. [DOI] [PubMed] [Google Scholar]
- [28].Sauder G, Degenring RF, Kamppeter B, et al. Potential of the 1 CU accommodative intraocular lens. Br J Ophthalmol 2005;89:1289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hancox J, Spalton D, Heatley C, et al. Objective measurement of intraocular lens movement and dioptric change with a focus shift accommodating intraocular lens. J Cataract Refract Surg 2006;32:1098–103. [DOI] [PubMed] [Google Scholar]
- [30].Wolffsohn JS, Naroo SA, Motwani NK, et al. Subjective and objective performance of the Lenstec KH-3500 “accommodative” intraocular lens. Br J Ophthalmol 2006;90:693–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Harman FE, Maling S, Kampougeris G, et al. Comparing the 1CU accommodative, multifocal, and monofocal intraocular lenses: a randomized trial. Ophthalmology 2008;115:993–1001.e2. [DOI] [PubMed] [Google Scholar]
- [32].Alio JL, Pinero DP, Plaza-Puche AB. Visual outcomes and optical performance with a monofocal intraocular lens and a new-generation single-optic accommodating intraocular lens. J Cataract Refract Surg 2010;36:1656–64. [DOI] [PubMed] [Google Scholar]
- [33].Mesci C, Erbil HH, Olgun A, et al. Visual performances with monofocal, accommodating, and multifocal intraocular lenses in patients with unilateral cataract. Am J Ophthalmol 2010;150:609–18. [DOI] [PubMed] [Google Scholar]
- [34].Sanders DR, Sanders ML. US FDA clinical trial of the Tetraflex potentially accommodating IOL: comparison to concurrent age-matched monofocal controls. J Refract Surg 2010;26:723–30. [DOI] [PubMed] [Google Scholar]
- [35].Dhital A, Spalton DJ, Gala KB. Comparison of near vision, intraocular lens movement, and depth of focus with accommodating and monofocal intraocular lenses. J Cataract Refract Surg 2013;39:1872–8. [DOI] [PubMed] [Google Scholar]
- [36].Zamora-Alejo KV, Moore SP, Parker DG, et al. Objective accommodation measurement of the Crystalens HD compared to monofocal intraocular lenses. J Refract Surg 2013;29:133–9. [DOI] [PubMed] [Google Scholar]
- [37].Tan N, Zheng D, Ye J. Comparison of visual performance after implantation of 3 types of intraocular lenses: accommodative, multifocal, and monofocal. Eur J Ophthalmol 2014;24:693–8. [DOI] [PubMed] [Google Scholar]
- [38].Alio JL, Simonov A, Plaza-Puche AB, et al. Visual outcomes and accommodative response of the Lumina Accommodative Intraocular Lens. Am J Ophthalmol 2016;164:37–48. [DOI] [PubMed] [Google Scholar]
- [39].McGrath C, Morrison JD. The effects of age on spatial frequency perception in human subjects. Q J Exp Physiol 1981;66:253–61. [DOI] [PubMed] [Google Scholar]
- [40].Nomura H, Ando F, Niino N, et al. Age-related change in contrast sensitivity among Japanese adults. Jpn J Ophthalmol 2003;47:299–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


