Abstract
Rationale:
Hypercoagulability and pregnancy morbidity are hallmarks of the antiphospholipid syndrome (APS). Catastrophic antiphospholipid syndrome (CAPS) is a potentially life-threatening omplication of APS, with widespread acute thrombotic microangiopathy (TMA) that can be precipitated by pregnancy and delivery and result in multiorgan damage. Unrestrained activation of the complement cascade is involved, favoring endothelial activation, tissue factor expression by leukocytes, and platelet aggregation. The complement block, which interrupts this amplification cycle, could prevent CAPS in patients with early TMA who face precipitating events.
Patient concerns:
We present a nulliparous pregnant woman with APS at the 30+6 week of gestation who has developed thrombocytopenia, intravascular hemolysis, elevated creatinine, proteinuria, and hematuria.
Diagnoses:
These featurs were compatible with the diagnosis of CAPS. Consensually, serum C3 protein levels were rapidly decreasing, reflecting complement consumption.
Interventions:
She was treated with eculizumab, a humanized monoclonal antibody against C5 that prevents the formation of the complement membrane attack complex.
Outcomes:
Laboratory parameters improved and the patient did not develop thrombosis or detectable organ/tissue damage. The patient safely delivered by cesarean section at week 32 of gestation a healthy 1640 g male infant. After 5 days, she received additional eculizumab, with complete resolution of the clinical condition. Low complement activity was detectable in the infant blood for a week after delivery. No infectious complication occurred.
Lessons:
Inhibition of the terminal complement activation is safe and might be effective in patients with APS developing early TMA, enabling safe delivery and preventing thrombotic events both in the mother and in the newborn.
Keywords: antiphospholipid syndrome, complement, pregnancy, thrombotic microangiopathy
1. Introduction
Antiphospholipid syndrome (APS) is a systemic autoimmune disease characterized by hypercoagulability. APS features comprise arterial, venous and microvascular thrombosis, pregnancy morbidity, and persistent evidence of antiphospholipid antibodies (aPL Abs), such as anticardiolipin (aCL), antiβ2glicoprotein I (aβ2GPI), and lupus anticoagulant (LA).[1] Catastrophic antiphospholipid syndrome (CAPS) is a severe acute complication characterized by multiple organ damage and failure due to widespread thrombotic microvascular angiopathy (TMA) associated with high mortality in pregnant patients (up to one-third of patients).[2,3] Initiating and/or precipitating factors in pregnant patients include infection, surgery, bleeding, delivery, and puerperium. Early diagnosis and aggressive therapy are essential.[3,4]
The mechanisms by which APS is mediated are not fully understood because patients with persistent aPL may remain asymptomatic for decades. Thus, a “second hit” might be required to initiate thrombosis.[5] Pregnancy itself represents a hypercoagulable state and patients with triple positivity for aPL (aCL, aβ2GPI and LA) and a history of thrombosis and pregnancy complications are at the highest risk of developing CAPS.[6]
Randomized clinical trials to guide the treatment of CAPS are missing.[7] Pregnant patients with CAPS receive unfractionated heparin, steroids, and plasma exchange or intravenous immunoglobulins.[7] However, outcomes are often disappointing. Early intervention before microvascular thrombosis and organ failure would be a fundamental goal in pregnant patients, particularly so because the delivery itself represents a further hit, capable of precipitating the clinical situation and associated with adverse outcomes, and the risk of catastrophic episodes is markedly higher during the postpartum period.[3]
Complement is involved in microvascular thrombosis, possibly because products of the complement activation/membrane attack complex amplify and sustain platelet and endothelial activation.[8] Accordingly, agents that inhibit complement activation might play a role in the management of CAPS.[9–11] Patients with APS and triple APL positivity with suspicious laboratory findings represent a challenge for the clinician, as deterioration can occur abruptly when CAPS develops.[1,3] Here, we show that eculizumab administered before multiorgan thrombosis was safe and effective in a patient at a high risk of CAPS with features of TMA, allowing safe delivery and uneventful puerperium.
2. Case report
A pregnant (30+6 week of gestation, wg) 33-year-old nulliparous woman diagnosed with APS was admitted to the emergency room for active bleeding. She had suffered pulmonary embolism at the age of 21 years. At that time, heterozygous factor V Leiden mutation and persistent triple aPL positivity (aCL, aβ2GPI, and LA) were identified. She had since been on oral anticoagulant therapy. At the age of 29 years, in combination with pregnancy, warfarin was replaced by low molecular weight heparin (LMWH), adjusted up to 100 IU/kg twice daily, low-dose aspirin (LDA, 100 mg daily), and hydroxychloroquine (HCQ, 300 mg daily). Despite treatment, she suffered 2 early miscarriages. She was treated with rituximab at the age of 31, and 5 months later (December 2016), the patient became pregnant. Treatment with LMWH, LDA, and HCQ was continued during pregnancy.
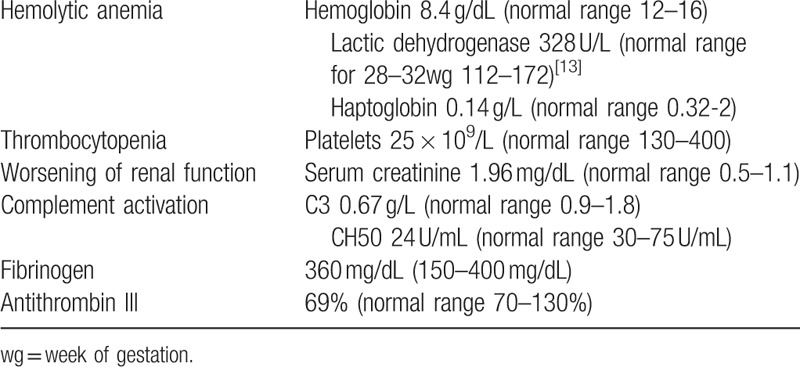
The pregnancy was uneventful up to 30+6 wg, when the patient suffered acute hemorrhage from a vulvar angiokeratoma (day 0, Fig. 1). Up to now, blood test and complement level were normal. After the bleeding, blood tests revealed mild anemia (hemoglobin, Hb, 10.8 g/dL), new-onset thrombocytopenia (platelet count 87 × 109/L), mild renal impairment (serum creatinine 1.27 mg/dl and proteinuria 1.34 g/24 hours), and mild complement consumption (C3 0.83 g/L). Blood pressure, liver enzymes, and coagulation tests were in the normal range. In less than a week, hemolytic anemia (Hb 8.4 g/dL, lactic dehydrogenase, LDH, 328 U/L, haptoglobin 0.14 g/L), thrombocytopenia (platelet counts 60 to 25 × 109/L), and renal function (serum creatinine 1.96–2.58 mg/dL) progressively worsened, with reduced complement levels and activity (C3 0.67 g/L, CH50 activity 24 U/mL). Fibrinogen levels were normal and D-dimer levels moderately increased (up to 2.06 mg/L), but antithrombin activity dropped to 69% of normal. Continuous infusion of antithrombin III concentrate aiming at plasma levels ≥ 100% was started (1.7 IU/kg/h). We infused intravenous eculizumab 600 mg (day 7, Fig. 1); duration of the infusion was 45 minutes. Prophylactic antibiotic for meningococcal infection was started and continued for 2 weeks after meningococcal vaccination.[12] At 32+1 wg, the patient underwent a cesarean section because of the fall of the platelet count till 25 × 109/L, giving birth to a healthy infant (1640 g, APGAR 4/7) (day 9, Table 1 and Fig. 1). [13] Eculizumab infusion was repeated at day 14 (Fig. 1), after 1 week from the previous drug administration. The platelet count improved rapidly until normalization, which occurred 6 days after the second infusion (day 20). Renal function and Hb levels also underwent rapid stabilization (creatinine 1.53 mg/dL and Hb 10.5 g/dL, at day 20). At this time, C3 levels were within normal limits, and at day 27 also, CH50 activity was normal (Fig. 1). The mother did not develop any complication until the end of the follow-up period (9 months). There were no complications in the newborn until the end of the follow-up period (9 months of age). In the newborn, we observed only mild and transient thrombocytopenia (88 × 109/L platelets at day 14 with spontaneous normalization to 191 × 109/L at day 20), a transient inhibition of CH50 with low C3 (0.66 g/L), and spontaneous normalization within day 20.
Figure 1.
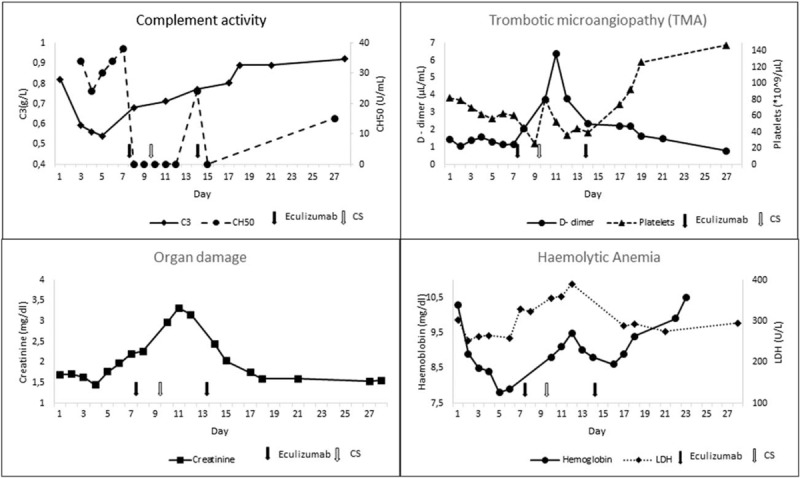
Course of laboratory parameters from day 0 (acute hemorrhage) till follow-up (eculizumab infusion at day 7 and 14; cesarean section at day 9). LDH = lactic dehydrogenase, TMA = thrombotic microangiopathy.
Table 1.
Laboratory features of the patient at delivery.
Consent for eculizumab use was obtained from the patient. Written consent was obtained from the patient to publish this study.
3. Discussion
Our patient has had previous thrombotic events, a history of abortions, and triple persistent positivity for aPL. After an acute hemorrhage in third trimester of pregnancy, despite previous treatment with rituximab and current treatment with HCQ and with LMWH at therapeutic dosages along with LDA, the patient abruptly developed microangiopathic hemolytic anemia, renal insufficiency, and thrombocytopenia. The clinical situation was extremely unstable with a high risk of thrombotic and hemorrhagic complications associated with delivery and puerperium. Signs reflecting multiorgan damage were missing, suggesting that inhibition of amplificatory circuits could be an effective strategy to protect the mother and the fetus. To control thrombin generation, we administered antithrombin concentrate to the patient. However, laboratory parameters worsened and we focused on complement, as the patient had clear laboratory evidence of C3 activation and consumption, while the C4 levels remained normal. Even small amounts of the C5b-C9 complex generated downstream of C3 activation and cleavage are sufficient to trigger the activation and death of endothelial cells, with expression of tissue factor, disruption of the endothelial layer and exposure of the subendothelial matrix, platelet aggregation, and further activation of the blood coagulation cascade.[5] Eculizumab has been shown to bind to C5 with high affinity, preventing its cleavage and restricting the generation of the membrane activation (C5b-C9) complex. Its use in obstetrical APS has been discussed.[14] Eculizumab has been used in pregnant patients with paroxysmal nocturnal hemoglobinuria[15] and hemolytic uremic syndrome even in the context of pregnancy[16–18] with excellent safety profiles in both mothers and newborns.[19] A short-term treatment with the antibody has been preventively used to protect a pregnant patient with severe APS from the risks associated with delivery and puerperium.[11] No complications developed and analysis carried out revealed that eculizumab does not cross the placental barrier.[11] In the case we describe here, the newborn had a relatively low complement activity. Low levels of most complement components, frequently detected in premature infants independently of eculizumab, might account for the results.[11]
4. Conclusion
The eculizumab administered during pregnancy may be a useful treatment for the mother and the newborn in case-selected patients. If there is laboratory evidence of CAPS but tissue damage associated with TMA has not yet given clear clinical signs, eculizumab represents a valid addition to the current treatment regimen, which can prevent the rapid deterioration of clinical conditions and protect the patient and the child during delivery and the postdelivery phases.
Author contributions
Conceptualization: Patrizia Rovere-Querini, Giorgio Slaviero, Armando D’Angelo, Susanna Rosa, Maria Teresa Castiglioni.
Data curation: Valentina Canti, Roberta Erra, Esperia Bianchi.
Formal analysis: Patrizia Rovere-Querini.
Writing - original draft: Valentina Canti, Roberta Erra, Esperia Bianchi.
Writing - review & editing: Patrizia Rovere-Querini, Massimo Candiani, Maria Teresa Castiglioni.
Footnotes
Abbreviations: aβ2GPI = antiβ2glicoprotein I, aCL = anticardiolipin, aPL Abs = antiphospholipid antibodies, APS = antiphospholipid syndrome, CAPS = catastrophic antiphospholipid syndrome, Hb = hemoglobin, HCQ) = hydroxychloroquine, LA = lupus anticoagulant, LDA = low-dose aspirin, LDH = lactic dehydrogenase, LMWH = low molecular weight heparin, TMA = thrombotic microangiopathy.
The authors report no conflicts of interest.
References
- [1].Cervera R, Piette JC, Font J, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum 2002;46:1019–27. [DOI] [PubMed] [Google Scholar]
- [2].Ortega-Hernandez OD, Agmon-Levin N, Blank M, et al. The physiopathology of the catastrophic antiphospholipid (Asherson's) syndrome: compelling evidence. J Autoimmun 2009;32:1–6. [DOI] [PubMed] [Google Scholar]
- [3].Hoayek JG, Moussa HN, Rehman HA, et al. Catastrophic antiphospholipid syndrome in pregnancy, a diagnosis that should not be missed. J Matern Fetal Neonatal Med 2016;29:3950–5. [DOI] [PubMed] [Google Scholar]
- [4].Espinosa G, Rodriguez-Pinto I, Cervera R. Catastrophic antiphospholipid syndrome: an update. Panminerva Med 2017;59:254–68. [DOI] [PubMed] [Google Scholar]
- [5].Rodriguez-Pinto I, Espinosa G, Cervera R. Catastrophic APS in the context of other thrombotic microangiopathies. Curr Rheumatol Rep 2015;17:482. [DOI] [PubMed] [Google Scholar]
- [6].Cervera R, Rodriguez-Pinto I, Colafrancesco S, et al. 14th international congress on antiphospholipid antibodies task force report on catastrophic antiphospholipid syndrome. Autoimmun Rev 2014;13:699–707. [DOI] [PubMed] [Google Scholar]
- [7].Silver RM. Catastrophic antiphospholipid syndrome and pregnancy. Semin Perinatol 2018;42:26–32. [DOI] [PubMed] [Google Scholar]
- [8].Barratt-Due A, Floisand Y, Orrem HL, et al. Complement activation is a crucial pathogenic factor in catastrophic antiphospholipid syndrome. Rheumatology (Oxford) 2016;55:1337–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lonze BE, Singer AL, Montgomery RA. Eculizumab and renal transplantation in a patient with CAPS. N Engl J Med 2010;362:1744–5. [DOI] [PubMed] [Google Scholar]
- [10].Shapira I, Andrade D, Allen SL, et al. Brief report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum 2012;64:2719–23. [DOI] [PubMed] [Google Scholar]
- [11].Gustavsen A, Skattum L, Bergseth G, et al. Effect on mother and child of eculizumab given before caesarean section in a patient with severe antiphospholipid syndrome: a case report. Medicine (Baltimore) 2017;96:e6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Loirat C, Fakhouri F, Ariceta G, et al. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol 2016;31:15–39. [DOI] [PubMed] [Google Scholar]
- [13].Larsson A, Palm M, Hansson LO, et al. Reference values for clinical chemistry tests during normal pregnancy. BJOG 2008;115:874–81. [DOI] [PubMed] [Google Scholar]
- [14].Mekinian A, Kayem G, Cohen J, et al. Obstetrical APS: is there a place for additional treatment to aspirin-heparin combination? Gynecol Obstet Fertil Senol 2017;45:37–42. [DOI] [PubMed] [Google Scholar]
- [15].Kelly RJ, Hochsmann B, Szer J, et al. Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med 2015;373:1032–9. [DOI] [PubMed] [Google Scholar]
- [16].Demir E, Yazici H, Ozluk Y, et al. Pregnant woman with atypical hemolytic uremic syndrome delivered a healthy newborn under eculizumab treatment. Case Rep Nephrol Dial 2016;6:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gately R, San A, Kurtkoti J, et al. Life-threatening pregnancy-associated atypical haemolytic uraemic syndrome and its response to eculizumab. Nephrology (Carlton) 2017;22(suppl 1):32–5. [DOI] [PubMed] [Google Scholar]
- [18].Ardissino G, Wally Ossola M, Baffero GM, et al. Eculizumab for atypical hemolytic uremic syndrome in pregnancy. Obstet Gynecol 2013;122:487–9. [DOI] [PubMed] [Google Scholar]
- [19].Hallstensen RF, Bergseth G, Foss S, et al. Eculizumab treatment during pregnancy does not affect the complement system activity of the newborn. Immunobiology 2015;220:452–9. [DOI] [PubMed] [Google Scholar]


