Abstract
Rationale:
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening disease characterized by an excessive systemic inflammatory response. HLH is classified as primary or secondary, where the latter may occur in association with many infections. However, no case of HLH has been previously associated with group b streptococcus (GBS) sepsis.
Patient concerns:
We present a fatal case of HLH in a 5-year-old girl with GBS sepsis.
Diagnosis:
The present patient met 5 of the HLH criteria: fever, splenomegaly, bicytopenia, hypertriglyceridemia and/or hypofibrinogenemia, and hyperferritinemia. GBS was identified in 2 sets of peripheral blood bacterial cultures.
Interventions:
Empirical antibiotics, inotropes, and immunoglobulins were administered.
Outcomes:
The clinical course of the patient was fulminant and the patient died of septic shock 10 hours after admission to the hospital.
Lessons:
We suggest GBS infection can cause HLH and early awareness of HLH associated with GBS infection and proper effective treatment are necessary to reduce mortality.
Keywords: group B streptococcus, hemophagocytic lymphohistiocytosis, septic shock
1. Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a syndrome characterized by an excessive systemic inflammatory response.[1] HLH may have genetic causes or be associated with a variety of medical conditions, including infections, autoimmune diseases, malignancies, immunodeficiencies, and hematopoietic stem cell and organ transplantations.[1] Infection-associated HLH has been identified in immunodeficient patients, but most cases occur in previously healthy people.[2] Among the infectious agents that trigger HLH, viruses are the primary pathogens causing HLH, where Epstein-Barr virus (EBV) is the most common trigger.[3] Additionally, other pathogens have been associated with HLH, including bacteria, fungus, and parasites.[2] Several studies have reported bacterial infection-associated HLH,[4–9] but none associated with invasive group B streptococcus (GBS) infection. Herein, we report a fatal case of HLH associated with GBS sepsis that occurred in a previously healthy child. This study was approved by Institutional Review Board of the Chung-Ang University Hospital, and the informed consent was waived due to the retrospective collection of data which secured the anonymity of the patient.
2. Case report
A 5-year-old girl was admitted to the emergency room with fever and cyanosis. She had no specific past or familial medical history. Fever and myalgia had started 2 days prior and then vomiting, cyanosis, and bilateral leg swelling occurred 2 hours prior to admission to the emergency room. Vital signs consisted of a heart rate of 170 beats per minute, body temperature of 38 °C, respiratory rate of 50 times/min, and oxygen saturation of 85%; blood pressure was not measured. Upon physical examination, the patient had splenomegaly, systemic cyanosis, and bilateral leg edema. Hematologic studies revealed a leukocyte count of 18,050 cells/μL (reference value, 4000–12,000 cells/μL) with 20% band neutrophils, hemoglobin concentration of 12.2 g/dL (reference value, 11.5–14.5 g/dL), and platelet count of 73,000 platelets/μL (reference value, 150,000–400,000 platelets/μL). Peripheral blood smears showed leukoerythroblastosis, neutrophilic leukocytosis with a shift to the left in terms of maturation, and thrombocytopenia. Blood chemistries included levels of C-reactive protein of 410 mg/L (reference value, 0.5–10.0 mg/L), procalcitonin of 40.36 ng/mL (reference value, 0–0.5 ng/mL), aspartate aminotransferase of 319 IU/L (reference value, 15–50 IU/L), alanine aminotransferase 99 IU/L (reference value, 5–45 IU/L), total bilirubin of 4.7 mg/dL (reference value, 0.2–1.2 mg/dL), direct bilirubin of 3.6 mg/dL (reference value, 0–0.3 mg/dL), and blood urea nitrogen of 75 mg/dL (reference value, 5–18 mg/dL), creatinine of 2.7 mg/dL (reference value, 0.1–0.6 mg/dL). Other laboratory abnormalities included a triglyceride level of 655 mg/dL (reference value, 30–86 mg/dL) and fibrinogen level of 50 mg/dL (reference value, 200–400 mg/dL). Bilateral pulmonary congestion was observed by chest x-ray.
The patient had respiratory distress that required intubation and mechanical ventilator support. Due to septic shock, the patient was treated with empirical antibiotics (teicoplanin and meropenem) after obtaining 2 sets of peripheral blood bacterial cultures and administering intravenous hydration and inotropes (20 μg/kg/min dopamine, 20 μg/kg/min dobutamine, 1 mg/kg/min epinephrine, and 1 μg/kg/min norepinephrine). Glucocorticoids, including dexamethasone, were not administered.
Five hours after admission to the hospital, a blood test showed anemia and thrombocytopenia (Table 1). Packed red blood cells, platelets, and fresh frozen plasma were transfused into the patient and 0.5 g/kg intravenous immunoglobulin was administered to manage septic shock. Despite this, hypotension was sustained and cardiac arrest occurred 10 hours after admission to the hospital. Despite 1 hour of cardiopulmonary resuscitation, the patient died of septic shock.
Table 1.
Blood laboratory findings.
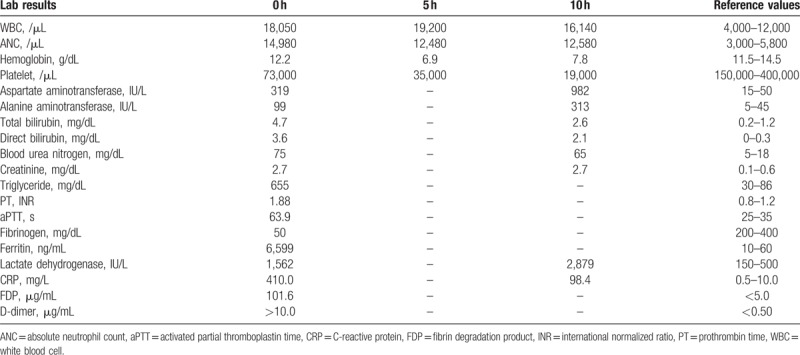
After the patient died, serum ferritin levels were determined and serologic and culture studies were conducted. The ferritin level was 6599 ng/mL (reference value, 10–60 ng/mL). Serology for hepatitis A, B, and C viruses was negative. EBV viral capsid antigen IgM/IgG antibody, EBV nuclear antigen IgM/IgG antibody, and quantitative polymerase chain reaction for EBV in the serum were negative. Mycoplasma pneumoniae IgM antibody was negative. Nasal swab culture for methicillin-resistant Staphylococcus aureus was negative. Only GBS was identified in the 2 sets of peripheral blood bacterial cultures. Based on antibiotic susceptibility testing, GBS isolated from the patient showed susceptibility to penicillin-G, ampicillin, clindamycin, erythromycin, cefotaxime, and vancomycin. HLH was diagnosed after the patient died, therefore the other studies for diagnosis of HLH, including genetic studies, bone marrow biopsy, and assessments of levels of natural killer (NK) cell activity and the soluble CD25, were not performed.
3. Discussion
HLH is a pathologic immune response characterized by hyperinflammation.[1] The first case of HLH was reported in 1952 by Farquhar and Claireaux,[10] who referred to this syndrome as familial hemophagocytic reticulocytosis. HLH can be classified as either primary or secondary. Primary HLH includes familial HLH and several primary immune deficiencies, which may involve immune defects associated with genetic mutations for NK cells and cytotoxic T lymphocytes.[2] Despite activation of NK cells and cytotoxic T lymphocytes, these cytotoxic immune cells fail to kill infected target cells due to defects in granule-dependent cytotoxicity, which leads to ongoing pathologic immune activation. In this process, levels of many cytokines, including interleukin (IL)-1, IL-6, tumor necrosis factor-alpha, interferon-gamma, IL-12, and IL-18, are increased and macrophages are activated.[11] Prolonged fever, splenomegaly, cytopenia, increased triglyceride synthesis, hyperfibrinolysis, and/or hyperferritinemia, increased CD25 levels, decreased or absent NK cell activity, and hemophagocytosis by macrophages in the bone marrow, cerebral spinal fluid, or lymph nodes are observed in patients with HLH.[1] By contrast, secondary (i.e., acquired) HLH is associated with infections, autoimmune diseases, malignancies, and immunodeficiencies without known genetic mutations for HLH.[12] These infections include those caused by viruses, bacteria, fungi, and parasites, where EBV-associated HLH is a major form of secondary HLH.[13] Among bacterial infections, M pneumoniae,[4,5]Listeria monocytogenes,[6]Leptospira spp.,[7]Brucella spp.,[9] and Streptococcus pneumoniae[14] are associated with HLH.
Various bacterial infections have been reported to be associated with HLH. However, no cases of HLH associated with GBS sepsis in a child have been reported previously. The present patient met 5 of the HLH criteria: fever, splenomegaly, bicytopenia, hypertriglyceridemia and/or hypofibrinogenemia, and hyperferritinemia. Unfortunately, we were unable to evaluate whether the patient had a genetic mutation associated with HLH or another immunodeficiency. However, only GBS was identified in 2 sets of blood cultures during microbiological analysis. Therefore, we conclude that GBS may have triggered HLH in the patient.
GBS, also known as Streptococcus agalactiae, is a major cause of neonatal invasive disease, but administration of prophylactic intrapartum antibiotics has led to a substantial decline in the incidence of GBS infection in neonates.[15] GBS infection is rare in children over 1 year of age.[16–18] Phares et al[17] reported that the incidence of invasive GBS infection in the United States was 0.22 per 100,000 in children aged 1 to 14 years compared with 7.2 per 100,000 in the total population. Additionally, 40% of GBS-infected patients aged 1 to 14 years had at least 1 underlying disease, such as a neurologic disorder, immunosuppression, asthma, malignancy, or renal disease.[17] In our case, when antibiotic susceptibility for GBS was assessed, the patient received the appropriate antibiotics. However, the clinical course of this case was fatal, which might be due to HLH following GBS sepsis. Therefore, when septic shock with an unusual course and clinical findings such as splenomegaly are present, the possibility of HLH should be considered and appropriate treatment should be performed without delay.
4. Conclusion
We presented a rare fatal case of HLH associated with GBS sepsis in a 5-year-old child. We suggest GBS infection may have caused HLH and early awareness of HLH associated with GBS infection and proper effective treatment are necessary to reduce mortality.
Author contributions
Writing – original draft: Young Bae Choi.
Writing – review & editing: Dae Yong Yi.
Footnotes
Abbreviations: C = Celsius, EBV = Epstein-Barr virus, GBS = group b streptococcus, HLH = hemophagocytic lymphohistiocytosis, IL = interleukin, NK = natural killer.
Funding: Not applicable.
Ethics approval and consent to participate: This study was conducted with the approval from the Institutional Review Board of the Chung-Ang University Hospital. Informed consent was waived by the IRB (1712–018–16129).
The authors declare no conflicts of interest.
References
- [1].Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med 2012;63:233–46. [DOI] [PubMed] [Google Scholar]
- [2].Janka GE, Lehmberg K. Hemophagocytic syndromes-an update. Blood Rev 2014;28:135–42. [DOI] [PubMed] [Google Scholar]
- [3].Filipovich AH, Chandrakasan S. Pathogenesis of hemophagocytic lymphohistiocytosis. Hematol Oncol Clin North Am 2015;29:895–902. [DOI] [PubMed] [Google Scholar]
- [4].Ishida Y, Hiroi K, Tauchi H, et al. Hemophagocytic lymphohistiocytosis secondary to Mycoplasma pneumoniae infection. Pediatr Int 2004;46:174–7. [DOI] [PubMed] [Google Scholar]
- [5].Hibino M, Sato S, Shimizu T, et al. Hemophagocytic lymphohistiocytosis secondary to Mycoplasma pneumoniae infection without pneumonia. Intern Med 2014;53:1679–83. [DOI] [PubMed] [Google Scholar]
- [6].Lambotte O, Fihman V, Poyart C, et al. Listeria monocytogenes skin infection with cerebritis and haemophagocytosis syndrome in a bone marrow transplant recipient. J Infect 2005;50:356–8. [DOI] [PubMed] [Google Scholar]
- [7].Niller HH. Myelodysplastic syndrome (MDS) as a late stage of subclinical hemophagocytic lymphohistiocytosis (HLH): a putative role for Leptospira infection. A hypothesis. Acta Microbiol Immunol Hung 2010;57:181–9. [DOI] [PubMed] [Google Scholar]
- [8].Harris P, Dixit R, Norton R. Coxiella burnetii causing haemophagocytic syndrome: a rare complication of an unusual pathogen. Infection 2011;39:579–82. [DOI] [PubMed] [Google Scholar]
- [9].Karakukcu M, Patiroglu T, Ozdemir MA, et al. Pancytopenia, a rare hematologic manifestation of brucellosis in children. J Pediatr Hematol Oncol 2004;26:803–6. [PubMed] [Google Scholar]
- [10].Farquhar JW, Claireaux AE. Familial haemophagocytic reticulosis. Arch Dis Child 1952;27:519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bode SF, Lehmberg K, Maul-Pavicic A, et al. Recent advances in the diagnosis and treatment of hemophagocytic lymphohistiocytosis. Arthritis Res Ther 2012;14:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ishii E. Hemophagocytic lymphohistiocytosis in children: pathogenesis and treatment. Front Pediatr 2016;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rosado FG, Kim AS. Hemophagocytic lymphohistiocytosis: an update on diagnosis and pathogenesis. Am J Clin Pathol 2013;139:713–27. [DOI] [PubMed] [Google Scholar]
- [14].Birlutiu V, Birlutiu RM. Sepsis due to Streptococcus pneumoniae associated with secondary hemophagocytic lymphohistiocytosis in a splenectomized patient for spherocytosis: a case report. Medicine (Baltimore) 2017;96:e7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schrag SJ, Zywicki S, Farley MM, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med 2000;342:15–20. [DOI] [PubMed] [Google Scholar]
- [16].Gomez B, Hernandez-Bou S, Garcia-Garcia JJ, et al. Bacteremia in previously healthy children in emergency departments: clinical and microbiological characteristics and outcome. Eur J Clin Microbiol Infect Dis 2015;34:453–60. [DOI] [PubMed] [Google Scholar]
- [17].Phares CR, Lynfield R, Farley MM, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA 2008;299:2056–65. [DOI] [PubMed] [Google Scholar]
- [18].Hosoda A, Gatayama R, Moriyama S, et al. The first case of recurrent ultra late onset group B streptococcal sepsis in a 3-year-old child. IDCases 2017;7:16–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


