Abstract
Background:
One of the most clinically significant current discussions is the optimal pancreaticojejunostomy (PJ) technique for pancreaticoduodenectomy (PD). We performed a meta-analysis to compare duct-to-mucosa and invagination techniques for pancreatic anastomosis after PD.
Methods:
A systematic search of PubMed, Embase, Web of Science, the Cochrane Central Library, and ClinicalTrials.gov up to June 1, 2018 was performed. Randomized controlled trials (RCTs) comparing duct-to-mucosa versus invagination PJ were included. Statistical analysis was performed using RevMan 5.3 software.
Results:
Eight RCTs involving 1099 patients were included in the meta-analysis. The rate of postoperative pancreatic fistula (POPF) was not significantly different between the duct-to-mucosa PJ (110/547, 20.10%) and invagination PJ (98/552, 17.75%) groups in all 8 studies (risk ratio, 1.13; 95% CI, 0.89–1.44; P = .31). The subgroup analysis using the International Study Group on Pancreatic Fistula criteria showed no significant difference in POPF between duct-to-mucosa PJ (97/372, 26.08%) and invagination PJ (78/377, 20.68%). No significant difference in clinically relevant POPF (CR-POPF) was found between the 2 groups (55/372 vs 40/377, P = .38). Additionally, no significant differences in delayed gastric emptying, post-pancreatectomy hemorrhage, reoperation, operation time, or length of stay were found between the 2 groups. The overall morbidity and mortality rates were not significantly different between the 2 groups.
Conclusion:
The duct-to-mucosa technique seems no better than the invagination technique for pancreatic anastomosis after PD in terms of POPF, CR-POPF, and other main complications. Further studies on this topic are therefore recommended.
Keywords: duct-to-mucosa, invagination, meta-analysis, pancreatoduodenectomy, systematic review
1. Introduction
Pancreaticoduodenectomy (PD) is a complex, high-risk standard surgical procedure that is indicated primarily for periampullary diseases. Central to the entire discipline of PD are postoperative mortality and morbidity. Although operative mortality in patients undergoing PD has decreased, the incidence of postoperative morbidity remains high at 40% to 50%.[1–6] Postoperative pancreatic fistula (POPF) is the most common complication, with rates ranging from 5% to 30% in previous studies.[7,8] Many methods have been described to decrease the risk of POPF, including the use of medications (prophylactic octreotide,[9,10] sealants[11]), prophylactic pancreatic stenting,[12] and improvements in pancreatic reconstruction techniques.[1,2] The most commonly used pancreatic reconstruction techniques are pancreaticogastrostomy (PG) and pancreaticojejunostomy (PJ). Several methods of PJ currently exist, the 2 most common of which are duct-to-mucosa PJ and invagination PJ. In the past few decades, many studies have assessed the safety and efficacy of these 2 methods.[13–15] A major advantage of duct-to-mucosa PJ is that it allows drainage of the main duct into the intestine. Many previous studies have shown a lower incidence of pancreatic fistula after duct-to-mucosa PJ than invagination PJ.[16–19] Therefore, duct-to-mucosa PJ is one of the most widely used PJ methods. Theoretically, however, duct-to-mucosa PJ cannot provide drainage of minor ducts and may require higher-level technology. A previous study demonstrated that invagination PJ could reduce the rate of POPF.[20] However, a recent randomized controlled trial (RCT) showed that invagination PJ was not associated with a lower rate of POPF but was instead associated with a decreased severity of POPF.[15] One of the most clinically significant current discussions is the optimal PJ technique for PD. Increasingly more RCTs have been performed or are ongoing. The aim of this study was to compare the clinical outcomes of duct-to-mucosa PJ and invagination PJ.
2. Materials and methods
2.1. Search strategy
Two researchers (TL and YXL) independently conducted a comprehensive and systematic search of PubMed, Embase, Web of Science, the Cochrane Central Library, and ClinicalTrials.gov up to June 2018. English search terms included but were not limited to the following: pancreatoduodenectomy, PD, PJ, duct-to-mucosa, and invagination. The search was limited initially to publications of RCTs. The references of the articles identified after the initial search were also manually reviewed. This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
2.2. Inclusion and exclusion criteria
The following inclusion criteria were applied: the RCT must have compared the clinical outcomes between duct-to-mucosa PJ and invagination PJ after PD. The participants must have had a clinical diagnosis of POPF. The study must have provided adequate data on the clinical outcomes.
We excluded studies that were non-RCTs, retrospective studies, review articles, case reports, abstract, editorials, and letters to the editor; were repeatedly published by the same author or agency; and had insufficient data on outcome measures.
2.3. Clinical outcomes of interest
The primary outcomes were the incidence of POPF and clinically relevant POPF (CR-POPF) after PD. The other outcomes were delayed gastric emptying (DGE), post-pancreatectomy hemorrhage (PPH), reoperation, morbidity, mortality, operation time, and length of stay (LOS).
2.4. Data extraction
Two reviewers (YXC and BW) independently extracted the following original data from the literature and entered it onto a standardized form: first author, year of publication, study period, and country where the study took place; sample size, types of PJ, texture and diameter of the pancreas, and definition of POPF. If necessary, the author or authors of the study were contacted to obtain the necessary data. Conflicts in data abstraction were resolved by consensus and by referring to the original article.
2.5. Quality assessment
The authors independently assessed the quality of the literature in accordance with the Cochrane Collaboration Handbook.[21] The scoring system included the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of the results assessment, incomplete data of the results, selective reporting, and other sources of bias.
2.6. Statistical analysis
All included data were assessed using Review Manager (RevMan) version 5.3 software (Cochrane Informatics and Knowledge Management Department, Copenhagen, Kongeriget Danmark). The risk ratio (RR) and 95% confidence interval (CI) were used for dichotomous outcomes. Publication bias was evaluated by the chi-squared test and funnel plots. Heterogeneity among studies was evaluated by the chi-squared test. A two-tailed P value of <.05 was considered statistically significant.
2.7. Ethics statement
This study was a secondary analysis regarding human subject data published in the public domain; thus, no ethical approval was required.
3. Results
3.1. Selected studies and characteristics of the trials
Based on our search criteria, we yielded a total of 487 papers from the respective search engines, of which 320 duplicate articles were excluded. The remaining 159 studies were retrieved for assessment of their titles and abstracts, leaving 8 articles that met the inclusion criteria. Finally, 8 RCTs involving 1099 participants were included in the meta-analysis.[13–15,20,22–25] A detailed flowchart of the selection process is depicted in Fig. 1.
Figure 1.
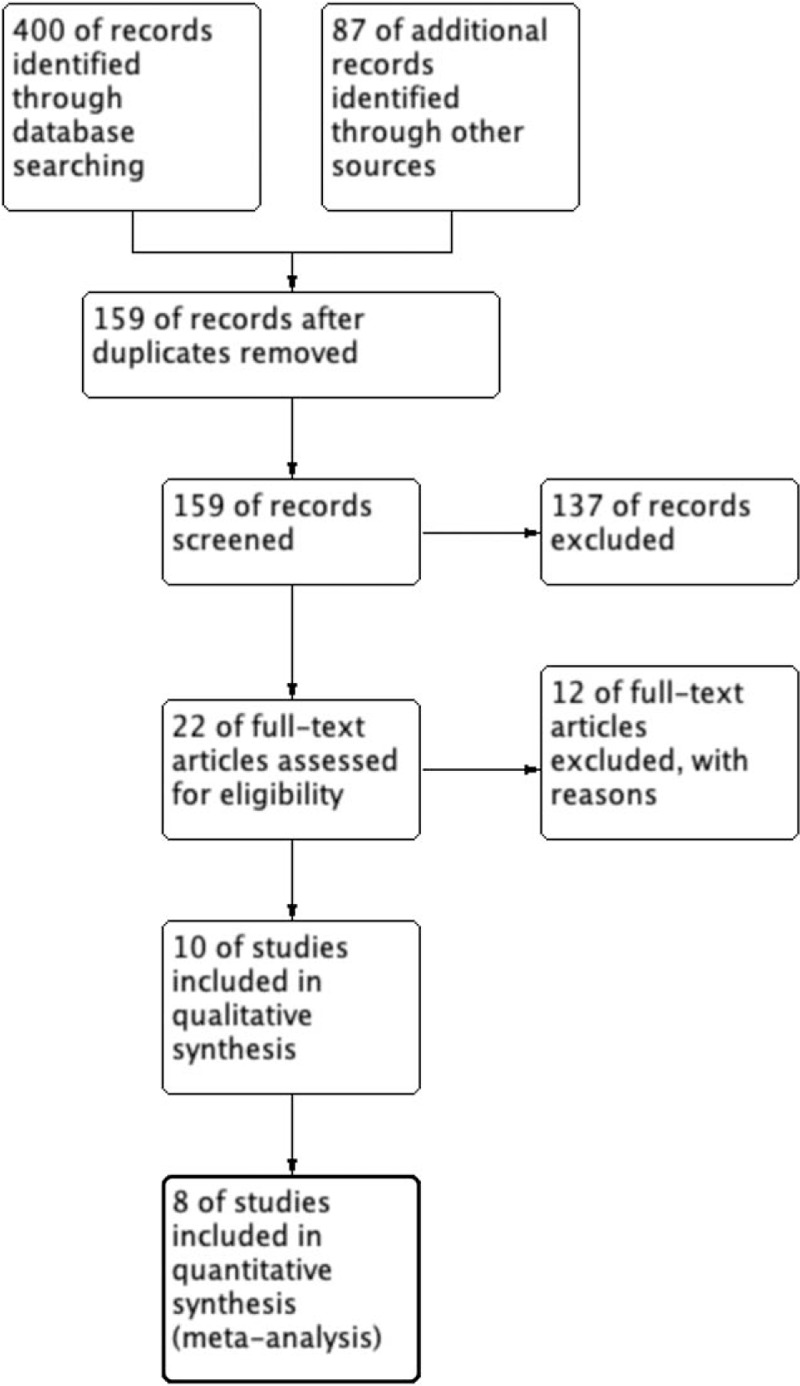
Flow diagram of the published articles evaluated for inclusion in this meta-analysis.
The 1099 patients were divided into the duct-to-mucosa PJ group (n = 547) and invagination group (n = 552). The sample sizes ranged from 92 to 197, and the incidence rate of POPF varied from 3.5% to 32.0%. Of these studies, 5 trials[15,20,22,24,25] provided POPF data using the definition established by the International Study Group on Pancreatic Fistula (ISGPF), and 3 studies[13,14,23] used different definitions of POPF. Data regarding the pancreatic texture were provided in 6 studies,[13,15,22,24,25] and the diameter of the pancreatic duct was provided in 5 studies.[15,20,22,24,25]Table 1 shows the main characteristics of the studies included in this meta-analysis, and Table 2 shows the definitions of POPF used in the studies. Figure 2 presents an consensus risk-of bias assessment of the included studies.
Table 1.
Characteristics of the included studies.
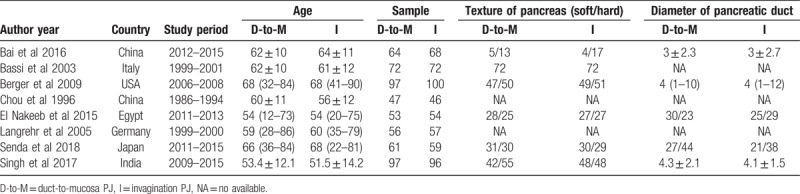
Table 2.
Definition of POPF.
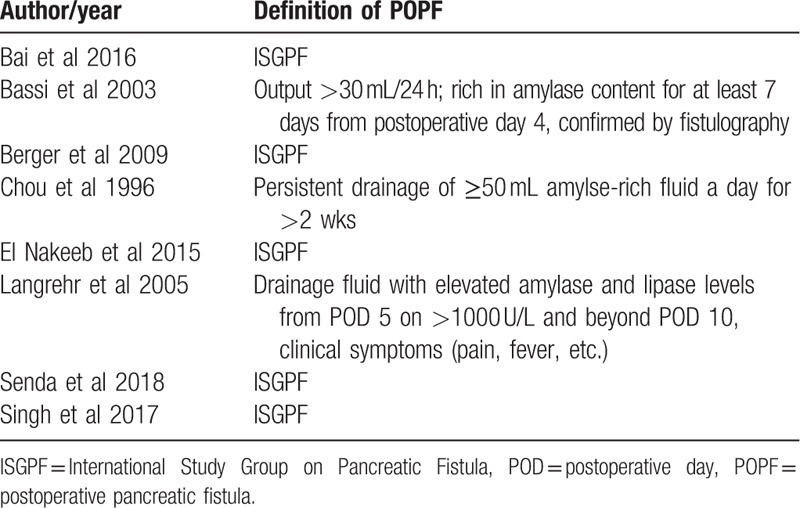
Figure 2.
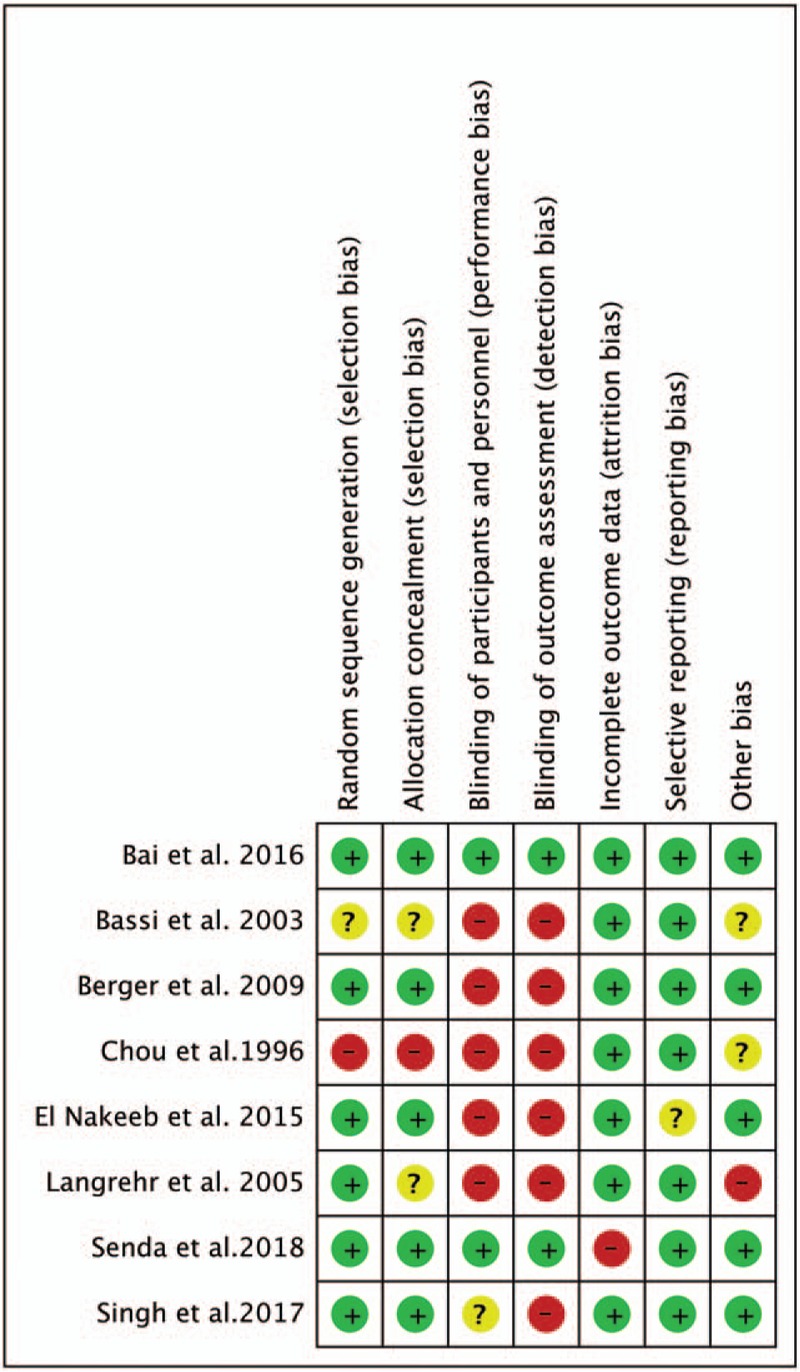
Consensus risk-of-bias assessment of the included studies. Green, low risk; yellow, unclear; red, high risk.
3.2. POPF
All 8 trials involving 1099 participants were pooled to compare the incidence of POPF after PD. There were no significant differences between the duct-to-mucosa PJ group (20.1%) and invagination PJ group (17.75%) (RR, 1.13; 95% CI, 0.89–1.44; P = .31) (Fig. 3 A). Five studies involving 661 samples using the ISGPF definition showed that there were no significant differences between the duct-to-mucosa group and invagination group (RR, 1.26; 95% CI, 0.97–1.63; P = .08) (Fig. 3 A).
Figure 3.
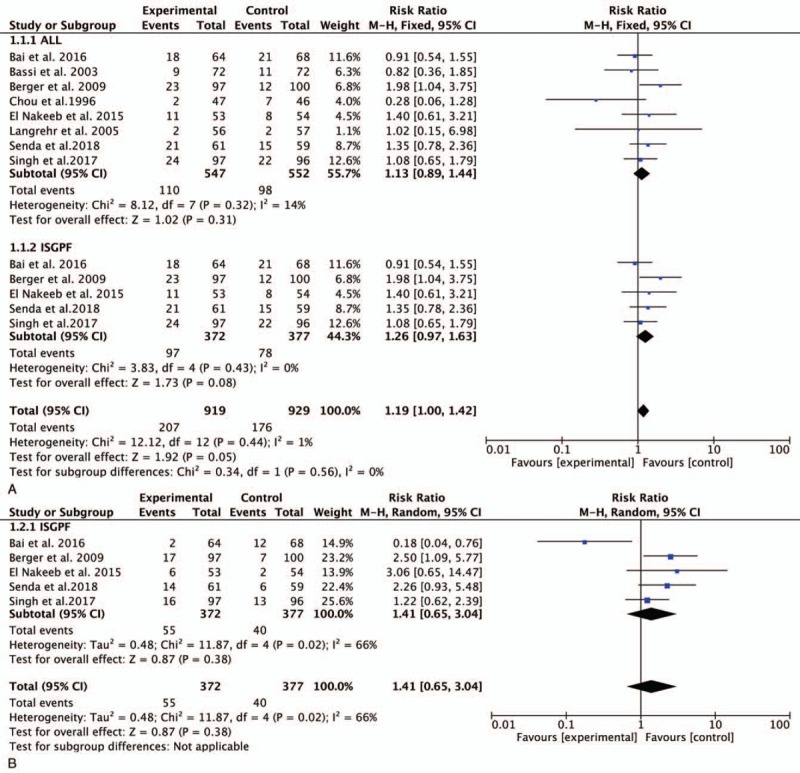
Forest plot of the meta-analysis comparing duct-to-mucosa PJ and invagination PJ with respect to (A) POPF, (B) CR-POPF, (C) DGE, (D) PPH, and (E) reoperation. CR-POPF = clinically relevant POPF, DGE = delayed gastric emptying, PJ = pancreaticojejunostomy, POPF = postoperative pancreatic fistula, PPH = post-pancreatectomy hemorrhage.
Figure 3 (Continued).
Forest plot of the meta-analysis comparing duct-to-mucosa PJ and invagination PJ with respect to (A) POPF, (B) CR-POPF, (C) DGE, (D) PPH, and (E) reoperation. CR-POPF = clinically relevant POPF, DGE = delayed gastric emptying, PJ = pancreaticojejunostomy, POPF = postoperative pancreatic fistula, PPH = post-pancreatectomy hemorrhage.
3.3. CR-POPF
Five trials provided data regarding CR-POPF in accordance with the ISGPF criteria. The pooled data demonstrated no statistically significant difference between the duct-to-mucosa PJ and invagination PJ groups (RR, 1.14; 95% CI, 0.65–3.04; P = .38) (Fig. 3 B).
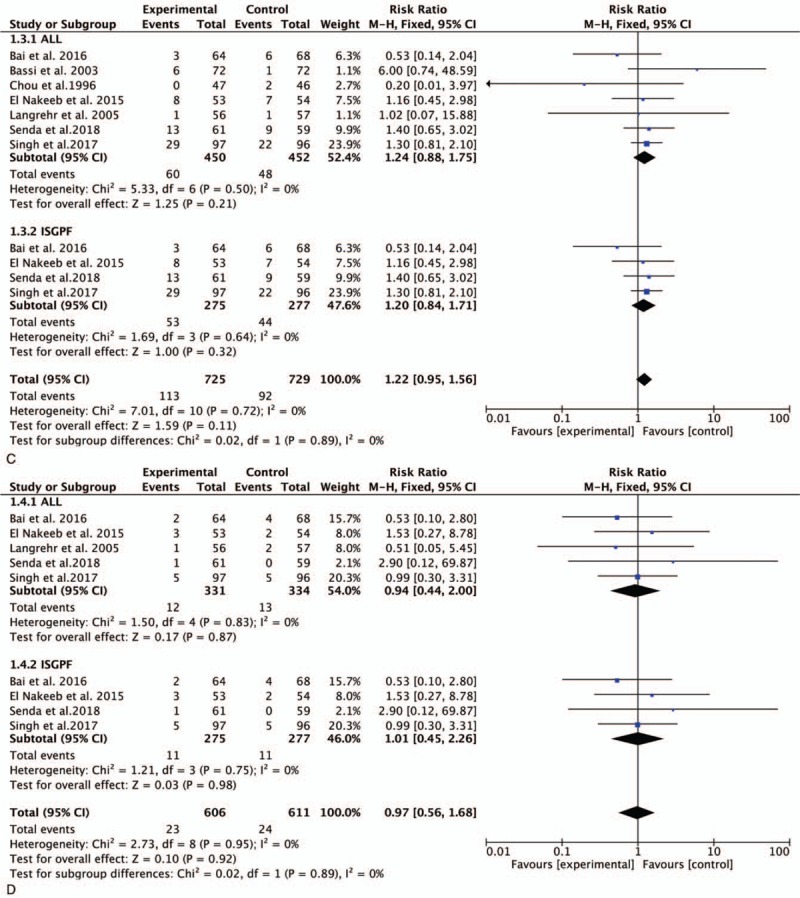
3.4. DGE
We calculated the pooled estimates using a random-effects model (I2 = 0%). Data regarding DGE were provided in 7 studies with 60 of 450 patients in the duct-to-mucosa PJ group and 48 of 452 in the invagination PJ group. There was no significant difference between the 2 groups (RR, 1.24; 95% CI, 0.88–1.76; P = .22) (Fig. 3 C). In accordance with the ISGPF criteria, no significant difference was shown in the meta-analysis (RR, 1.22; 95% CI, 0.85–1.75; P = .27) (Fig. 3 C).
3.5. PPH
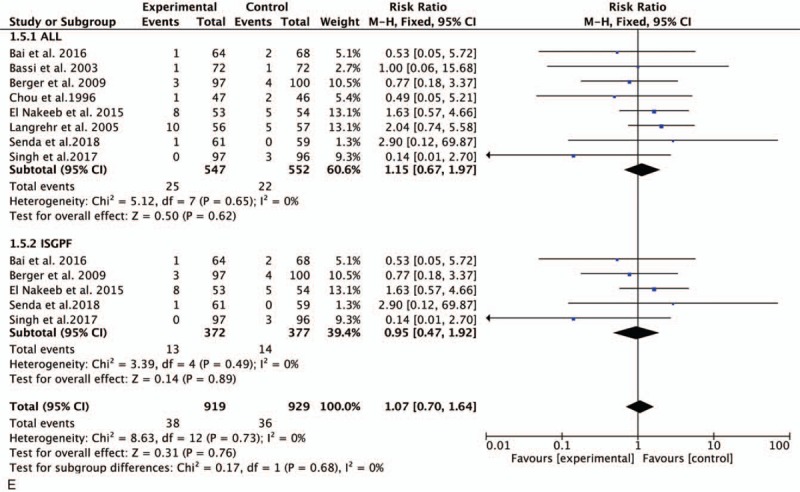
In a comparison of the incidence of PPH, we found that 5 studies reported the clinical outcome of interest. The incidence of PPH in the duct-to-mucosa and invagination PJ groups is presented in Fig. 3 D. This meta-analysis demonstrated no significant difference between the 2 PJ techniques (RR, 0.94; 95% CI, 0.44–2.00; P = .87) (Fig. 3 D). After stratifying the patients according to the definition of PPH, duct-to-mucosa PJ was not superior to invagination PJ (RR, 1.01; 95% CI, 0.45–2.26; P = .98) (Fig. 3 D).
3.6. Reoperation
All 8 studies reported the rates of reoperation. No significant difference was found between the 2 groups (RR, 1.15; 95% CI, 0.67–0.1.97; P < .62) (Fig. 3 E). Similar results were obtained in the ISGPF analysis (RR, 0.95; 95% CI, 0.47–1.92; P = .89) (Fig. 3 E).
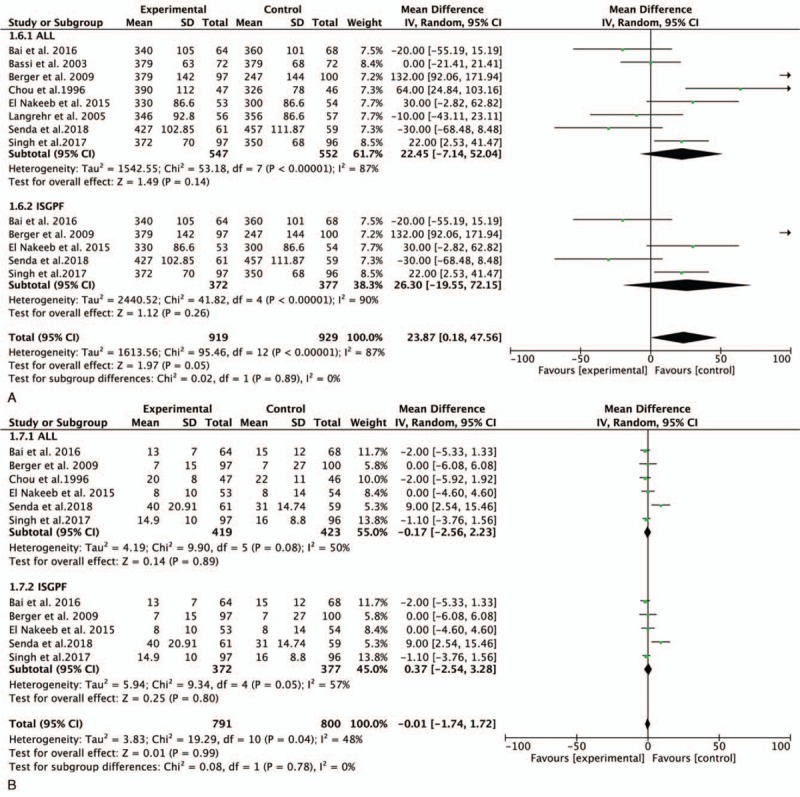
3.7. Operation time
Data on the operation time were reported in all trials. However, there was no significant difference between the 2 groups in the meta-analysis (mean difference [MD], 22.45; 95% CI,– 7.14–52.04; P = .14). The meta-analysis of studies performed in accordance with the ISGPF criteria demonstrated no significant difference between the 2 groups (MD, 26.30; 95% CI, –19.55–72.15; P = .26) (Fig. 4 A).
Figure 3 (Continued).
Forest plot of the meta-analysis comparing duct-to-mucosa PJ and invagination PJ with respect to (A) POPF, (B) CR-POPF, (C) DGE, (D) PPH, and (E) reoperation. CR-POPF = clinically relevant POPF, DGE = delayed gastric emptying, PJ = pancreaticojejunostomy, POPF = postoperative pancreatic fistula, PPH = post-pancreatectomy hemorrhage.
Figure 4.
Forest plot of the meta-analysis comparing duct-to-mucosa PJ and invagination PJ with respect to the (A) operation time, (B) LOS, (C) morbidity, and (D) mortality. LOS = length of stay, PJ = pancreaticojejunostomy.
Figure 4 (Continued).
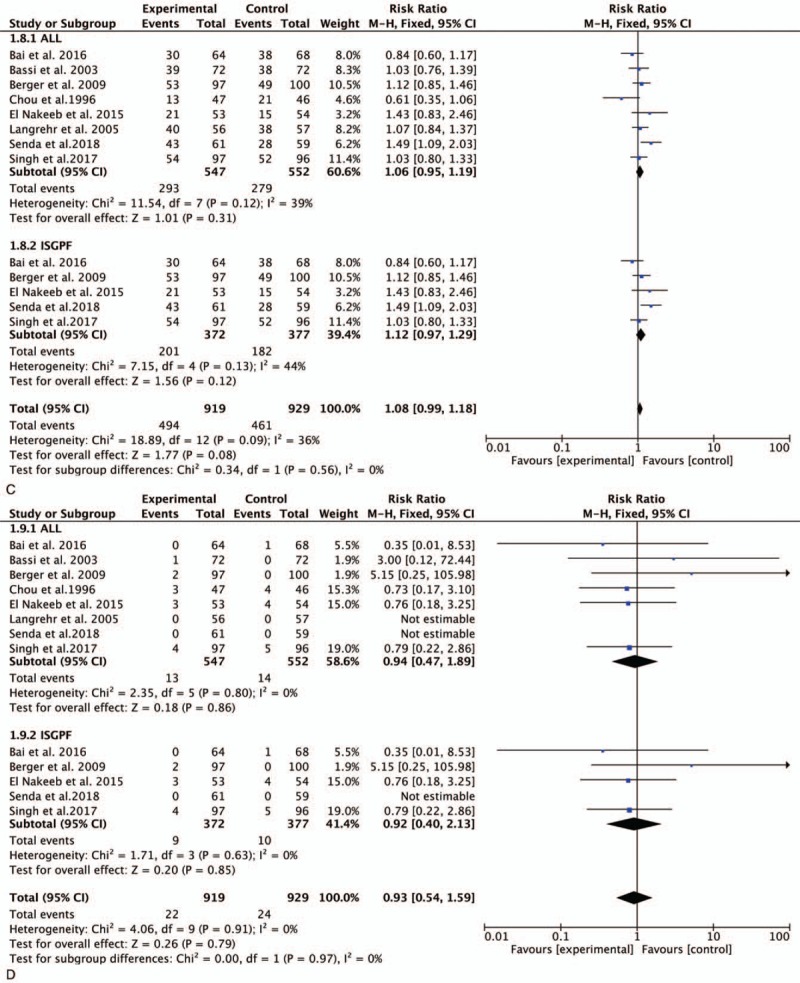
Forest plot of the meta-analysis comparing duct-to-mucosa PJ and invagination PJ with respect to the (A) operation time, (B) LOS, (C) morbidity, and (D) mortality. LOS = length of stay, PJ = pancreaticojejunostomy.
3.8. LOS
Six studies involving 842 patients (419 in the duct-to-mucosa PJ group and 423 in the invagination PJ group) were pooled to compare the LOS. There was no significant difference between the 2 groups (MD, –0.17; 95% CI, –2.56–2.23; P = .89). Among studies using the ISGPF definition, no significant difference was found between the 2 groups (MD, 0.37; 95% CI, –2.54–3.28; P = .80) (Fig. 4 B).
3.9. Morbidity
All trials provided data regarding morbidity. The meta-analysis demonstrated no significant difference between the 2 groups (RR, 1.06; 95% CI, 0.95–1.19; P = .31) (Fig. 4 D). Analysis of morbidity according to the ISGPF criteria revealed no significant difference between the 2 techniques (RR, 1.12; 95% CI, 0.97–1.29; P = .12) (Fig. 4 D).
3.10. Mortality
The analysis of mortality was performed using a random-effects model (I2 = 0%). All studies provided data on mortality rates among 1099 patients. The meta-analysis demonstrated no significant difference between the 2 groups (RR, 0.94; 95% CI, 0.47–1.89; P = .86) (Fig. 4 E). Analysis according to the ISGPF criteria did not change the result (RR, 0.92; 95% CI, 0.40–2.13; P = .85) (Fig. 4 E).
4. Discussion
The optimal reconstruction technique for PJ after PD remains controversial. In the present study, duct-to-mucosa PJ did not seem to be superior to invagination PJ in terms of POPF and CR-POPF. No significant differences in DGE, PPH, or the main clinical outcomes were found between the 2 groups.
The most effective pancreatic construction technique has been debated in many studies.[2,26,27] Two major techniques performed universally are PG and PJ. Although many studies have compared PG with PJ, the best way to reconstruct the pancreas has not been determined.[28–30] PJ is the most commonly used method to restore the pancreatic anastomosis, and its main advantage is that it is more physiological. The surgical techniques of PJ are duct-to-mucosa, invagination, and binding PJ. Binding PJ was proposed in 2002 by Peng et al.[31] Some studies have proposed that binding PJ may reduce the incidence of POPF.[27,32] In the European population, however, binding PJ did not reduce the incidence of POPF.[33] Few clinical studies have been performed to evaluate binding PJ, and the technique may still need some modifications. Therefore, assessment of this technique is not within the scope of the present study.
As mentioned above, the 2 most widely used PJ methods are duct-to-mucosa PJ and invagination PJ. A primary concern of PD is POPF. POPF can lead to intra-abdominal abscess formation, DGE, PPH, and increased morbidity. The occurrence of POPF is multifactorial, and studies have shown that it may be related to obesity, the pancreatic texture, the pancreatic duct diameter, and pancreas reconstruction.[34–38] Previously published studies of the effect of pancreatic reconstruction on POPF are not consistent. The main advantage of duct-to-mucosa PJ is that it assures drainage of the main duct into the intestine. Previous studies involving animals and humans suggest that duct-to-mucosa PJ is associated with a lower incidence of POPF than is invagination PJ.[17–19,39] In the present meta-analysis, 7 studies demonstrated that invagination PJ was associated with a lower incidence of POPF. However, minor ducts and a soft pancreas make duct-to-mucosa PJ difficult. In contrast to duct-to-mucosa PJ, invagination PJ allows for easier reconstruction and has advantages in patients with a soft pancreas. Some studies, including an RCT, demonstrated that invagination PJ was associated with a lower incidence of POPF. Nevertheless, the conclusions of previous relevant research regarding the effects of duct-to-mucosa PJ and invagination PJ on the development of POPF remain controversial.[40–42] A major strength of the present study is the inclusion of 2 recent RCTs. Nonetheless, the results of previous studies are conflicted.
In previous studies, a major source of heterogeneity was the difference in the definition of pancreatic fistula. Before 2005, the definition of POPF was variable among individual studies. The ISGPF organized experts from well-known European, Japanese, Australian, North American, and South American centers in 2005 to establish the definition and classification system of pancreatic fistula.[43] However, this only included 5 RCTs that applied the definition of POPF established by the ISGPF. In the ISGPF system, Grade B/C POPF is defined as clinically relevant POPF and requires more positive clinical intervention. In the ISGPF criteria, Grade A POPF has no impact on the clinical process. The meta-analysis of 5 RCTs showed no significant difference between duct-to-mucosa and invagination PJ in terms of CR-POPF. However, previous studies have provided conflicting results for this outcome. A meta-analysis involving 5 RCTs showed that invagination PJ appears to reduce CR-POPF rates.[42] However, the original study included in this study did not adopt a unified definition of POPF. Similar to our study, the study conducted by Kilambi and Singh[40] showed that duct-to-mucosa PJ does not appear to be better than invagination PJ. Our study's strength lies in incorporating the latest and most comprehensive RCTs. The study reported by Han et al[44] was excluded because it was published in Chinese and did not provide enough data. A recent trial published by Bai et al[15] demonstrated that duct-to-mucosa PJ seems to be better than invagination PJ. One of the factors that affects the development of POPF is the pancreatic texture. An RCT of patients with a soft pancreas conducted by Senda et al[24] showed that invagination PJ was associated with lower rates of POPF and CR-POPF. Retrospective studies have shown that invagination PJ is more suitable for patients with soft pancreatic tissue and a smaller pancreatic duct diameter.[45,46] According to a position statement by the ISGPF, no specific technique can reduce the incidence of POPF and CR-POPF.[47] Future studies on this topic are therefore recommended.
DGE, which is usually not life-threatening, can increase patient discomfort, LOS, and medical costs. As with POPF, the definition of DGE varies among studies. POPF can increase the incidence of DGE. The DGE rates in the studies of the present meta-analysis ranged from 1.7% to 26.4%, and the rates between the 2 groups were similar. PPH is one consequence of CR-POPF; however, the definition of PPH varies among previous studies. The I2 test of the RCTs in the present analysis showed no heterogeneity (I2 = 0) among all studies and among studies using the ISGPF definition. Severe complications including severe POPF, bleeding, and abscess formation may require reoperation. The incidence of reoperation is an indicator for evaluating the safety of the PJ method. No significant difference in the reoperation rate was found in our meta-analysis. The overall rates of morbidity and mortality also vary among different studies. Similar to previous RCTs and meta-analyses,[48,49] the morbidity and mortality rates were not significantly differences between the 2 groups.
The indications for PD in the included trials were heterogeneous. The most common indication was malignant disease. Few studies to date have focused on the long-term effects of tumors and the differences in residual pancreatic function between the 2 anastomotic methods. Studies have shown that catheter-to-mucosal anastomosis may cause catheter obstruction, leading to insufficient pancreatic function.
This meta-analysis had 2 main limitations. First, the details of the duct-to-mucosa and invagination techniques were variable among previous studies. Second, the usefulness of external stents and somatostatin and the patients’ clinical characteristics showed heterogeneity. Give these limitations, further RCTs on this topic are required.
5. Conclusion
Our study showed that duct-to-mucosa PJ is comparable with invagination PJ in terms of POPF, CR-POPF, and other main outcomes. Considering the above-mentioned limitations, high-quality RCTs are necessary in the future.
Acknowledgment
The authors thank Angela Morben, DVM, ELS, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author contributions
Conception and design of the study: Yunxiao Lyu. Studies selection: Ting Li and Yunxiao Lyu. Data extraction: Yunxiao Cheng and Bin Wang. Statistical analyses: Yunxiao Lyu and Sicong Zhao. Wrote the paper: Ting Li. The paper was revised and approved by Yunxiao Lyu.
Conceptualization: Yunxiao Lyu.
Data curation: Ting Li, Bin Wang, Yunxiao Cheng.
Investigation: Yunxiao Lyu.
Software: Yunxiao Lyu, Sicong Zhao.
Writing – original draft: Yunxiao Lyu, Ting Li.
Writing – review & editing: Yunxiao Lyu.
Yunxiao Lyu orcid: 0000-0002-2795-9698
Footnotes
Abbreviations: DGE = delayed gastric emptying, ISGPF = international study group on pancreatic fistula, LOS = length of stay, MD = mean difference, PD = pancreaticoduodenectomy, PG = pancreaticogastrostomy, PJ = pancreaticojejunostomy, POD = postoperative day, POPF = postoperative pancreatic fistula, PPH = post-pancreatectomy hemorrhage, RCT = randomized controlled trial.
Availability of data and materials: As this is a systematic review and meta-analysis, all included studies and the related results in our paper are listed in the reference and manuscript.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
All authors have declared that no competing interests exist. And all data and materials in this work were available from publications.
References
- [1].Figueras J, Sabater L, Planellas P, et al. Randomized clinical trial of pancreaticogastrostomy versus pancreaticojejunostomy on the rate and severity of pancreatic fistula after pancreaticoduodenectomy. Br J Surg 2013;100:1597–605. [DOI] [PubMed] [Google Scholar]
- [2].Topal B, Fieuws S, Aerts R, et al. Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy for pancreatic or periampullary tumours: a multicentre randomised trial. Lancet Oncol 2013;14:655–62. [DOI] [PubMed] [Google Scholar]
- [3].Addeo P, Delpero JR, Paye F, et al. Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HPB (Oxford) 2014;16:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Muscari F, Suc B, Kirzin S, et al. Risk factors for mortality and intra-abdominal complications after pancreatoduodenectomy: multivariate analysis in 300 patients. Surgery 2006;139:591–8. [DOI] [PubMed] [Google Scholar]
- [5].Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 2006;10:1199–211. [DOI] [PubMed] [Google Scholar]
- [6].Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997;226:248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bassi C, Butturini G, Molinari E, et al. Pancreatic fistula rate after pancreatic resection. The importance of definitions. Dig Surg 2004;21:54–9. [DOI] [PubMed] [Google Scholar]
- [8].Schäfer M, Müllhaupt B, Clavien PA. Evidence-based pancreatic head resection for pancreatic cancer and chronic pancreatitis. Ann Surg 2002;236:137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kollmar O, Moussavian MR, Richter S, et al. Prophylactic octreotide and delayed gastric emptying after pancreaticoduodenectomy: results of a prospective randomized double-blinded placebo-controlled trial. Eur J Surg Oncol 2008;34:868–75. [DOI] [PubMed] [Google Scholar]
- [10].Graham JA, Johnson LB, Haddad N, et al. A prospective study of prophylactic long-acting octreotide in high-risk patients undergoing pancreaticoduodenectomy. Am J Surg 2011;201:481–5. [DOI] [PubMed] [Google Scholar]
- [11].Lillemoe KD, Cameron JL, Kim MP, et al. Does fibrin glue sealant decrease the rate of pancreatic fistula after pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg 2004;8:766–72. [DOI] [PubMed] [Google Scholar]
- [12].Motoi F, Egawa S, Rikiyama T, et al. Randomized clinical trial of external stent drainage of the pancreatic duct to reduce postoperative pancreatic fistula after pancreaticojejunostomy. Br J Surg 2012;99:524–31. [DOI] [PubMed] [Google Scholar]
- [13].Bassi C, Falconi M, Molinari E, et al. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery 2003;134:766–71. [DOI] [PubMed] [Google Scholar]
- [14].Chou FF, Sheen-Chen SM, Chen YS, et al. Postoperative morbidity and mortality of pancreaticoduodenectomy for periampullary cancer. Eur J Surg 1996;162:477–81. [PubMed] [Google Scholar]
- [15].Bai X, Zhang Q, Gao S, et al. Duct-to-mucosa vs invagination for pancreaticojejunostomy after pancreaticoduodenectomy: a prospective, randomized controlled trial from a single surgeon. J Am Coll Surg 2016;222:10–8. [DOI] [PubMed] [Google Scholar]
- [16].Zhang JL, Xiao ZY, Lai DM, et al. Comparison of duct-to-mucosa and end-to-side pancreaticojejunostomy reconstruction following pancreaticoduodenectomy. Hepatogastroenterology 2013;60:176–9. [DOI] [PubMed] [Google Scholar]
- [17].You D, Jung K, Lee H, et al. Comparison of different pancreatic anastomosis techniques using the definitions of the International Study Group of Pancreatic Surgery: a single surgeon's experience. Pancreas 2009;38:896–902. [DOI] [PubMed] [Google Scholar]
- [18].Strasberg SM, Drebin JA, Mokadam NA, et al. Prospective trial of a blood supply-based technique of pancreaticojejunostomy: effect on anastomotic failure in the Whipple procedure. J Am Coll Surg 2002;194:746–58. discussion 759-60. [DOI] [PubMed] [Google Scholar]
- [19].Greene BS, Loubeau JM, Peoples JB, et al. Are pancreatoenteric anastomoses improved by duct-to-mucosa sutures? Am J Surg 1991;161:45–9. discussion 49-50. [DOI] [PubMed] [Google Scholar]
- [20].Berger AC, Howard TJ, Kennedy EP, et al. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual-institution trial. J Am Coll Surg 2009;208:738–47. discussion 747-739. [DOI] [PubMed] [Google Scholar]
- [21].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].El Nakeeb A, El Hemaly M, Askr W, et al. Comparative study between duct to mucosa and invagination pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized study. Int J Surg 2015;16(pt A):1–6. [DOI] [PubMed] [Google Scholar]
- [23].Langrehr JM, Bahra M, Jacob D, et al. Prospective randomized comparison between a new mattress technique and Cattell (duct-to-mucosa) pancreaticojejunostomy for pancreatic resection. World J Surg 2005;29:1111–9. discussion 1120-1111. [DOI] [PubMed] [Google Scholar]
- [24].Senda Y, Shimizu Y, Natsume S, et al. Randomized clinical trial of duct-to-mucosa versus invagination pancreaticojejunostomy after pancreatoduodenectomy. Br J Surg 2018;105:48–57. [DOI] [PubMed] [Google Scholar]
- [25].Singh AN, Pal S, Mangla V, et al. Pancreaticojejunostomy: does the technique matter? A randomized trial. J Surg Oncol 2018;117:389–96. [DOI] [PubMed] [Google Scholar]
- [26].Zhang L, Li Z, Wu X, et al. Sealing pancreaticojejunostomy in combination with duct parenchyma to mucosa seromuscular one-layer anastomosis: a novel technique to prevent pancreatic fistula after pancreaticoduodenectomy. J Am Coll Surg 2015;220:e71–7. [DOI] [PubMed] [Google Scholar]
- [27].Peng SY, Wang JW, Lau WY, et al. Conventional versus binding pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg 2007;245:692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Crippa S, Cirocchi R, Randolph J, et al. Pancreaticojejunostomy is comparable to pancreaticogastrostomy after pancreaticoduodenectomy: an updated meta-analysis of randomized controlled trials. Langenbecks Arch Surg 2016;401:427–37. [DOI] [PubMed] [Google Scholar]
- [29].Perivoliotis K, Sioka E, Tatsioni A, et al. Pancreatogastrostomy versus pancreatojejunostomy: an up-to-date meta-analysis of RCTs. Int J Surg Oncol 2017;2017:7526494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wente MN, Shrikhande SV, Müller MW, et al. Pancreaticojejunostomy versus pancreaticogastrostomy: systematic review and meta-analysis. Am J Surg 2007;193:171–83. [DOI] [PubMed] [Google Scholar]
- [31].Peng S, Mou Y, Cai X, et al. Binding pancreaticojejunostomy is a new technique to minimize leakage. Am J Surg 2002;183:283–5. [DOI] [PubMed] [Google Scholar]
- [32].Kim JM, Hong JB, Shin WY, et al. Preliminary results of binding pancreaticojejunostomy. Korean J Hepatobiliary Pancreat Surg 2014;18:21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Casadei R, Ricci C, Silvestri S, et al. Peng's binding pancreaticojejunostomy after pancreaticoduodenectomy. An Italian, prospective, dual-institution study. Pancreatology 2013;13:305–9. [DOI] [PubMed] [Google Scholar]
- [34].Reid-Lombardo KM, Farnell MB, Crippa S, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1.507 patients: a report from the Pancreatic Anastomotic Leak Study Group. J Gastrointest Surg 2007;11:1451–8. discussion 1459. [DOI] [PubMed] [Google Scholar]
- [35].Kollmar O, Moussavian MR, Bolli M, et al. Pancreatojejunal leakage after pancreas head resection: anatomic and surgeon-related factors. J Gastrointest Surg 2007;11:1699–703. [DOI] [PubMed] [Google Scholar]
- [36].Rault A, SaCunha A, Klopfenstein D, et al. Pancreaticojejunal anastomosis is preferable to pancreaticogastrostomy after pancreaticoduodenectomy for longterm outcomes of pancreatic exocrine function. J Am Coll Surg 2005;201:239–44. [DOI] [PubMed] [Google Scholar]
- [37].El Nakeeb A, Sultan AM, Salah T, et al. Impact of cirrhosis on surgical outcome after pancreaticoduodenectomy. World J Gastroenterol 2013;19:7129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].El Nakeeb A, Hamed H, Shehta A, et al. Impact of obesity on surgical outcomes post-pancreaticoduodenectomy: a case-control study. Int J Surg 2014;12:488–93. [DOI] [PubMed] [Google Scholar]
- [39].Tani M, Onishi H, Kinoshita H, et al. The evaluation of duct-to-mucosal pancreaticojejunostomy in pancreaticoduodenectomy. World J Surg 2005;29:76–9. [DOI] [PubMed] [Google Scholar]
- [40].Kilambi R, Singh AN. Duct-to-mucosa versus dunking techniques of pancreaticojejunostomy after pancreaticoduodenectomy: Do we need more trials? A systematic review and meta-analysis with trial sequential analysis. J Surg Oncol 2018;117:928–39. [DOI] [PubMed] [Google Scholar]
- [41].Wang W, Zhang Z, Gu C, et al. The optimal choice for pancreatic anastomosis after pancreaticoduodenectomy: a network meta-analysis of randomized control trials. Int J Surg 2018;57:111–6. [DOI] [PubMed] [Google Scholar]
- [42].Hua J, He Z, Qian D, et al. Duct-to-mucosa versus invagination pancreaticojejunostomy following pancreaticoduodenectomy: a systematic review and meta-analysis. J Gastrointest Surg 2015;19:1900–9. [DOI] [PubMed] [Google Scholar]
- [43].Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- [44].Han JM, Wang XB, Quan ZF, et al. Duct-to-mucosa anastomosis and incidence of pancreatic fistula following pancreaticoduodenectomy. J Med Postgrad 2009;22:961–4. [Google Scholar]
- [45].Marcus SG, Cohen H, Ranson JH. Optimal management of the pancreatic remnant after pancreaticoduodenectomy. Ann Surg 1995;221:635–45. discussion 645-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hosotani R, Doi R, Imamura M. Duct-to-mucosa pancreaticojejunostomy reduces the risk of pancreatic leakage after pancreatoduodenectomy. World J Surg 2002;26:99–104. [DOI] [PubMed] [Google Scholar]
- [47].Shrikhande SV, Sivasanker M, Vollmer CM, et al. Pancreatic anastomosis after pancreatoduodenectomy: a position statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2017;161:1221–34. [DOI] [PubMed] [Google Scholar]
- [48].Huttner FJ, Klotz R, Ulrich A, et al. Antecolic versus retrocolic reconstruction after partial pancreaticoduodenectomy. Cochrane Database Syst Rev 2016;9:Cd011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Miyazaki Y, Kokudo T, Amikura K, et al. Age does not affect complications and overall survival rate after pancreaticoduodenectomy: single-center experience and systematic review of literature. Biosci Trends 2016;10:300–6. [DOI] [PubMed] [Google Scholar]


