Abstract
Rationale:
Ovarian cancer has the poorest prognosis of the gynecological cancers. Early diagnosis and treatment are important, but early-stage ovarian carcinoma has nonspecific symptoms. Ultrasonography, computed tomography, magnetic resonance imaging, and serum CA-125 levels can be helpful but may not elucidate cases of diffuse peritoneal diseases mimicking carcinomatosis.
Patient concerns:
The patient had intermittent abdominal discomfort and dysuria. Abdominal-pelvic computed tomography findings were suspicious for peritoneal tuberculosis (TB) and a small cystic mass in the left ovary. The CA-125 values were normal.
Diagnoses:
She underwent laparoscopy for pathologic confirmation of tuberculous peritonitis and management of the ovary mass. Bilateral adnexectomy was performed. Histopathological examination of the surgical specimen revealed a serous ovarian carcinoma in her left ovary and salpinx.
Interventions:
Laparoscopic hysterectomy, pelvic lymphadenectomy, para-aortic nodal dissection, and omentectomy were carried out for staging evaluation.
Outcomes:
We encountered a rare case of ovary cancer stage IA serous ovarian carcinoma incidentally discovered by laparoscopy in a postmenopausal woman. She received adjuvant chemotherapy without relapse.
Lessons:
Peritoneal TB may mimic peritoneal or ovarian carcinoma, but the reverse case is rare. Hence, gynecologists should be careful in assessment of patients before treatment.
Keywords: incidental ovarian cancer, menopause, peritoneal tuberculosis
1. Introduction
The yearly incidence of ovarian cancer is 9 to 11 cases per 100,000 women in Western countries but is 5 to 7 cases per 100,000 women in Asia.[1] Ovarian cancer is the most common cause of death among patients with gynecologic cancers and requires cytoreductive surgery for adequate therapy. Despite advances in surgical technology and chemotherapy, a late diagnosis is the leading cause of mortality.[2] Although many tests are utilized, including gynecologic screening, assessment of cancer antigen 125 (CA-125) levels, ultrasonography, computed tomography (CT), and magnetic resonance imaging, these screening tests are only nominally effective for early detection because of their low sensitivity and specificity.[3]
Early ovarian cancer has nonspecific symptoms resembling those of less serious conditions. Thus, the diagnosis of ovarian cancer is challenging, since it is detected at an advanced stage via biopsy, requiring exploratory laparotomy or laparoscopy.[4] The differential diagnosis of peritoneal tuberculosis and ovarian cancer is difficult because of overlapping clinical and laboratory findings such as ascites, increased CA-125 levels, peritoneal thickening on ultrasound or CT images, and findings of intra-abdominal tumors.[5] Herein, we highlight a rare case of early-stage serous ovarian carcinoma incidentally discovered by laparoscopy in a postmenopausal woman with an initial suspicion of tuberculous peritonitis.
2. Case presentation
A 58-year-old woman visited the Department of Obstetrics and Gynecology at Cheonan Hospital, Soonchunhyang University College of Medicine, complaining of abdominal discomfort. Her obstetrical history included 2 term births, 0 preterm births, 0 abortions, 2 living children, and menopause beginning at age 52. Menarche was at age 16. Her surgical history included appendectomy 30 years previously and 2 Cesarean sections. She had no other relevant family history. Her general health was favorable with no specific findings during routine health screening 1 year earlier. She complained of urinary frequency and abdominal pain and had undergone treatment for suspected chronic pelvic inflammatory disease and cystitis in our hospital. At the time of admission, her systemic status was good with no apparent abnormalities. Her height was 144 cm, weight 45 kg, blood pressure 100/60 mmHg, heart rate 72 beats/min, respiratory rate 18 breaths/min, and body temperature 36.5 °C. No tenderness or rebound tenderness was observed on abdominal palpitation, and no specific symptoms were manifested excluding cervical motion tenderness on pelvic examination. Transvaginal ultrasonography revealed a 3 cm cystic mass in the left ovary with relatively clear margins and an irregular solid portion partially observed within the mass. Drug therapy (metronidazole and doxycycline for 14 days) was initiated to manage suspected pelvic inflammatory disease or a tubo-ovarian abscess. An abdomen-pelvis CT scan and blood and urine tests were performed, as the patient's abdominal discomfort persisted.
The abdomen-pelvis CT revealed a small amount of ascites in the pelvis, omental inflammation, peritoneal thickening, partial wall thickening in the ascending colon, suspected tuberculosis peritonitis, and a 3 cm cystic mass in the left ovary. There were no other findings in the uterus or in the right uterine adnexa (Fig. 1). Complete blood count (CBC), renal function, and liver function tests and electrocardiogram revealed no specific manifestations, and +1 leukocytes were found in a urine test. There was no clinical suspicion of tuberculosis on chest x-ray. A skin test with purified protein derivative was negative. Serum tumor marker tests revealed CA-125 9.88 IU/mL (normal: 0–35 IU/mL), CEA 1.74 ng/mL (normal: 0–5.0 ng/mL), and CA 19–9 7.41 U/mL (normal: 0–34 U/mL). The Department of Pulmonology was consulted, but no clinical signs or symptoms suspicious for pulmonary tuberculosis were detected. To confirm tuberculosis peritonitis, we performed diagnostic laparoscopy and bilateral adnexectomy.
Figure 1.
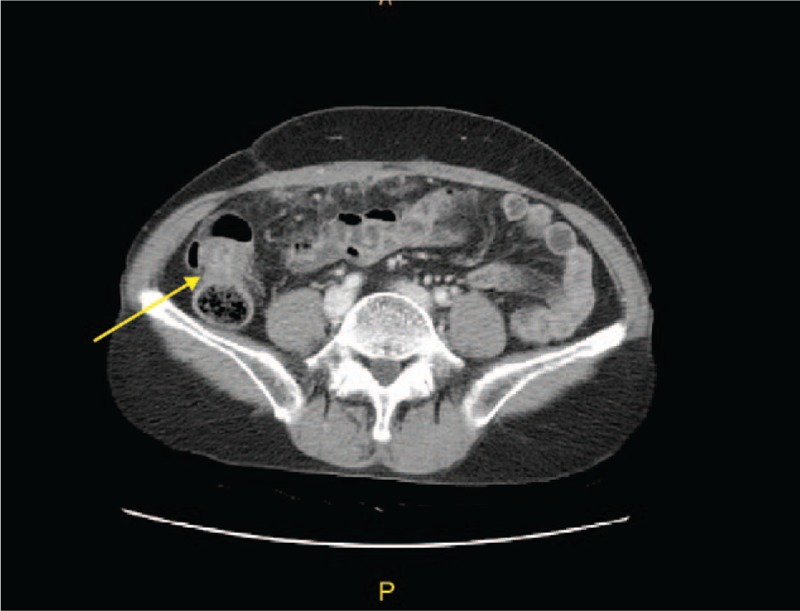
Contrast-enhanced CT image shows omental infiltration, peritoneal thickening, and ascending colon wall thickening. CT = computed tomography.
Diagnostic laparoscopy was conducted under general anesthesia (Fig. 2). Adhesiolysis was performed because of the extensive adhesions in the peritoneal cavity. The biopsy specimen was obtained by resecting a small portion of the greater omentum with severe adhesions for biopsy. Although no specific findings were observed in the uterus, extensive adhesions were present in the bilateral adnexa and cul-de-sac. No apparent signs were present in the right ovary or adnexa. However, a 3 to 4 cm mass within the left ovary caused adhesions involving the left fallopian tube. Bilateral adnexectomy was performed because a small ovarian cyst was found in the left postmenopausal ovary. Histological findings revealed infiltrating tumors around the left ovary and fallopian tube, and poorly differentiated tumor cells and severe dysplasia were detected at high magnification (Fig. 3A and B). Calcification was observed in the peripheral soft tissues and greater omentum around the right ovary and fallopian tube that were resected together with the left ovary, but no tumor cells were detected (Fig. 3C and D). The expression of tumors was positive for tissue-specific transcription factor, tumor suppressor protein (p16, p53), estrogen receptor, and Wilms’ tumor gene 1 but negative for transformation-related protein (p63) and GATA binding protein 3 on immunohistochemicalstaining (Fig. 3E and F). Tumor cell metastasis was not found in the uterus or dissected lymph nodes. The resected organs showed no inflammation or granuloma. Through histological and immunochemical assessment, the patient's ovarian cyst was diagnosed as high-grade serous carcinoma. After confirming the histopathology results, a detailed examination was conducted to evaluate for ovarian cancer metastasis, and there was no suspected metastatic cancer.
Figure 2.
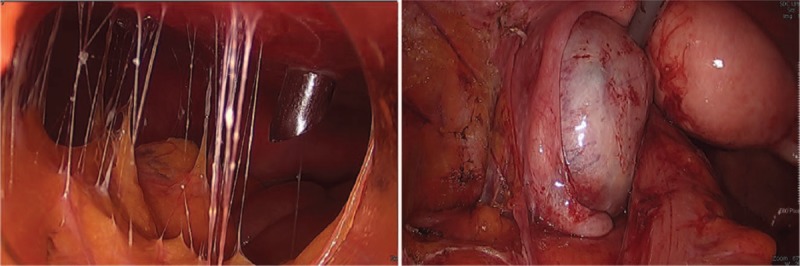
Laparoscopic findings show severe omental adhesions and a cystic mass in left ovary.
Figure 3.
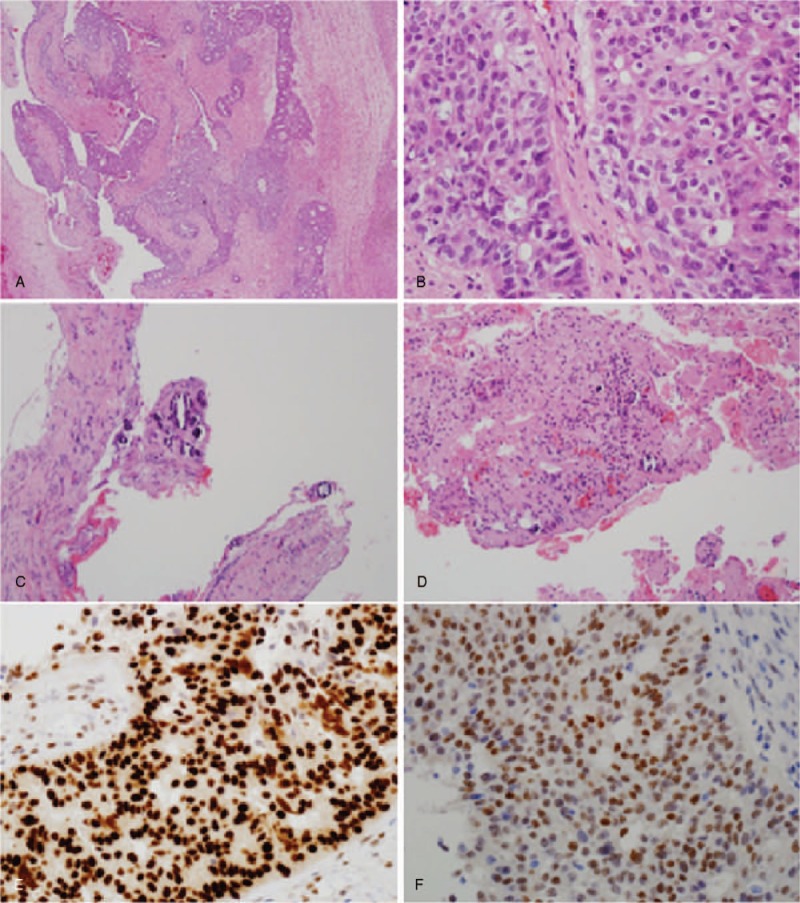
Pathological findings. (A) Left ovary and salpinx showed a tumor composed of infiltrative atypical glands (hematoxylin and eosin [H&E], ×40). (B) Left ovary and salpinx tumor cells exhibited moderate to severe pleomorphism and increased mitotic activity (H&E, ×400). (C, D) Paraovarian/parasalpingeal soft tissue and omentum showed small calcifications with no evidence of tumor cells (H&E, ×200). (E, F) Immunohistochemical staining revealed diffuse immunoreactivity for p53 and ER in tumor cells. ER = estrogen receptor, p53 = tumor suppressor protein.
Laparoscopic hysterectomy, pelvic lymphadenectomy, para-aortic nodal dissection, and omentectomy were carried out for staging evaluation. No lymph node metastases were found excluding the findings of the atrophic endometrium and chronic inflammation at the greater omentum. The patient was determined to have ovary cancer stage IA carcinoma categorized as a high-grade tumor, and she is currently undergoing adjuvant chemotherapy with Genexol and carboplatin. Currently, the patient continues to receive chemotherapy, and follow-up examination has not yet been conducted. The study was approved by the Institutional Review Board of Soonchunhyang University Cheonan Hospital. Written informed consent was obtained from the patient for publication of this case report and accompanying images.
3. Discussion
Correctly diagnosing ovarian carcinoma is difficult because of its nonspecific manifestation. The clinical features and radiological findings of peritoneal carcinomatosis can mimic tuberculosis peritonitis, and surgical exploration may be necessary for a definitive diagnosis.
The types of adnexal masses range from functional cysts to ovarian cancers, with malignancy rates of 4% to 24% in premenopausal women and 39% to 63% in postmenopausal women.[6] Early detection is essential, as the average 5-year survival rates of patients with ovarian cancer are 80% to 93% in patients with International Federation of Gynecology and Obstetrics stage I to II tumors and <30% in patients with stage III to IV tumors.[7,8] However, early detection is difficult since patients seldom exhibit disease-specific symptoms until the cancer becomes advanced.
The most common screening tests for ovarian cancer are serum CA-125 levels and transvaginal ultrasound. CA-125 is elevated in over 80% of women with advanced ovarian cancer. Its sensitivity and specificity for discriminating between malignant and benign tumors range from 50% to 100% in postmenopausal women with abdominal masses.[9] However, CA-125 levels can be elevated by inflammatory conditions such as pelvic infection, endometriosis, pancreatitis, or tuberculous peritonitis.[4]
Ultrasound has limitations as a screening tool for ovarian cancer because of differences in ovarian size and blood flow according to the location of the ovaries and the menstrual cycle. To overcome these limitations, a scoring system has been developed using various indicators such as the internal structure of the tumor, wall thickness, tumor size, and mediastinal structure.[10] However, this system has poor positive prediction rates for the early detection of ovarian cancer. Moreover, malignant tumors resemble benign conditions such as endometriosis and hemorrhagic teratoma cysts, and ovarian cancer may be confused with ovarian cysts.[11,12]
Abdomen-pelvic CT aids in the identification of peritoneal disease. However, the CT findings of tuberculous peritonitis and peritoneal carcinomatosis are similar, and it is difficult to correctly differentiate these diseases.[13] Particularly when the ovary is normal-sized with peritoneal fat infiltration, differentiating between benign and malignant peritonitis may be difficult. In particular, the CT findings of ovarian cancer and tuberculous peritonitis are comparable, although ovarian capsular change and ovarian parenchymal attenuation are distinctive distinguishing features.[14]
This case highlights the limitations of ovarian cancer predictors. Our patient had normal CA-125 levels and no definitive clinical symptoms suspicious for ovarian cancer. The likelihood of developing ovarian cancer is higher in patients with a significantly enlarged ovary,[11] but ovarian cancer is rarely suspected in cases with a normal ovary volume as seen in our patient. van Nagell and DePriest[15] recommended ultrasound and monitoring of CA-125 levels in postmenopausal women with a complex mass <5 cm and normal CA-125 levels and surgical intervention in cases with increasing morphologic complexity and elevated CA-125 values. Thus, ovarian cancer seemed improbable in our patient since she had a small complex ovary mass (3 cm) with a solid portion on ultrasound findings and normal CA-125 levels. Although abdomen-pelvis CT scanning was performed, ovarian cancer was not detected, and she was misdiagnosed with tuberculosis peritonitis based on the small volume of ascites, omentum inflammation, peritoneal thickening, and ascending colon wall thickening. Because of her persistent abdominal discomfort and inconclusive CT findings, we performed a biopsy for a more definitive diagnosis and thus detected early ovarian cancer.
Our case has few limitations. First, we did not evaluate enough tests before surgery to differentiate between ovarian cancer and tuberculosis. According to previous studies, human epididymis protein 4 (HE4) with CA-125 in differential diagnosis between epithelial ovarian cancer and peritoneal tuberculosis.[16] However, because there was no suspicion of ovarian cancer before surgery, there was a limit to more accurate clinical tests. Second, we did not perform frozen biopsy after oophorectomy of the left ovary during first surgery. Because the size of the left ovary was normal, the possibility of malignancy was not taken into consideration.
Our findings suggest the importance of surgical biopsy and histopathology in postmenopausal women with nonspecific symptoms that are similar to tuberculosis peritonitis to evaluate for ovarian cancer even if this diagnosis appears unlikely. An early accurate diagnosis is important for prompt, appropriate treatment and to improve prognosis.
Author contributions
Conceptualization: Aeli Ryu
Supervision: Aeli Ryu
Writing – original draft: Seong Taek Mun, Aeli Ryu, Si-Hyong Jang
Footnotes
Abbreviations: CA 19–9 = carbohydrate antigen 19–9, CA-125 = cancer antigen 125, CBC = complete blood count, CEA = carcinoembryonic antigen, CT = computed tomography, HE4 = human epididymis protein 4.
This research was supported by Soonchunhyang University Research Funds.
The authors have no conflicts of interest relevant to this article.
References
- [1].Bray F, Ferlay J, Laversanne M, et al. Cancer incidence in five continents: inclusion criteria, highlights from Volume X and the global status of cancer registration. Int J Cancer 2015;137:2060–71. [DOI] [PubMed] [Google Scholar]
- [2].Andersen ES, Knudsen A, Rix P, et al. Risk of malignancy index in the preoperative evaluation of patients with adnexal masses. Gynecol Oncol 2003;90:109–12. [DOI] [PubMed] [Google Scholar]
- [3].Sant M, Chirlaque Lopez MD, Agresti R, et al. Survival of women with cancers of breast and genital organs in Europe 1999-2007: results of the EUROCARE-5 study. Eur J Cancer 2015;51:2191–205. [DOI] [PubMed] [Google Scholar]
- [4].Paik ES, Kim JH, Kim TJ, et al. Prognostic significance of normal-sized ovary in advanced serous epithelial ovarian cancer. J Gynecol Oncol 2018;29:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gosein MA, Narinesingh D, Narayansingh GV, et al. Peritoneal tuberculosis mimicking advanced ovarian carcinoma: an important differential diagnosis to consider. BMC Res Notes 2013;6:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang YC, Lu JJ, Chen CH, et al. Peritoneal tuberculosis mimicking ovarian cancer can be diagnosed by polymerase chain reaction: a case report. Gynecol Oncol 2005;97:961–3. [DOI] [PubMed] [Google Scholar]
- [7].Lawrie TA, Winter-Roach BA, Heus P, et al. Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer. Cochrane Database Syst Rev 2015;CD004706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].FIGO Committee on Gynecologic Oncology. Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia. Int J Gynaecol Obstet 2009;105:3–4. [DOI] [PubMed] [Google Scholar]
- [9].Fung MF, Bryson P, Johnston M, et al. Cancer Care Ontario Practice Guidelines Initiative Gynecology Cancer Disease Site Group. Screening postmenopausal women for ovarian cancer: a systematic review. J Obstet Gynaecol Can 2004;26:717–28. [DOI] [PubMed] [Google Scholar]
- [10].Niemi RJ, Saarelainen SK, Luukkaala TH, et al. Reliability of preoperative evaluation of postmenopausal ovarian tumors. J Ovarian Res 2017;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. Gynecologists’ Committee on Practice, Practice Bulletin No. 174: evaluation and management of adnexal masses. Obstet Gynecol 2016;128:e210–26. [DOI] [PubMed] [Google Scholar]
- [12].Alcazar JL. Ultrasound-based IOTA simple rules allow accurate malignancy risk estimation for adnexal masses. Evid Based Med 2016;21:197. [DOI] [PubMed] [Google Scholar]
- [13].Patel SM, Lahamge KK, Desai AD, et al. Ovarian carcinoma or abdominal tuberculosis? A diagnostic dilemma: study of fifteen cases. J Obstet Gynaecol India 2012;62:176–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shim SW, Shin SH, Kwon WJ, et al. CT differentiation of female peritoneal tuberculosis and peritoneal carcinomatosis from normal-sized ovarian cancer. J Comput Assist Tomogr 2017;41:32–8. [DOI] [PubMed] [Google Scholar]
- [15].van Nagell JR, DePriest PD. Management of adnexal masses in postmenopausal women. Am J Obstet Gynecol 2005;193:30–5. [DOI] [PubMed] [Google Scholar]
- [16].Zhang L, Chen Y, Liu W, et al. Evaluating the clinical significances of serum HE4 with CA125 in peritoneal tuberculosis and epithelial ovarian cancer. Biomarkers 2016;21:168–72. [DOI] [PubMed] [Google Scholar]


