Supplemental Digital Content is available in the text
Keywords: epilepsy, pediatrics, seizure, vitamin D
Abstract
Background
: Vitamin D deficiency is highly prevalent among children with epilepsy. Lack of high-quality evidence led to variability among scientific societies recommendations. Therefore, we aim to determine the efficacy of different common doses used in the pediatric practice to maintain optimal 25-hydroxy vitamin D (25 [OH] vitamin D) level in children with epilepsy and normal baseline 25 (OH) vitamin D level over 6 months of supplementation.
Methods
: This is a protocol for phase IV pragmatic randomized superiority controlled open-label trial at King Saud University Medical City in Riyadh. Children with epilepsy and receiving chronic antiepliptic medication and normal baseline 25 (OH) vitamin D level will be randomly assigned to receive Cholecalciferol 400 IU/day versus 1000 IU/day for 6 months. Our primary outcome is the proportion of children with vitamin D insufficiency (25 (OH) vitamin D level < 75nmol/L) at 6 months. Secondary outcomes include seizure treatment failure, seizure frequency, parathyroid hormone (PTH) levels, bone mineral density, and safety.
Discussion: Our trial is set out to evaluate the efficacy of common different vitamin D maintenance doses on 25 (OH) vitamin D level, seizure control, and bone health for children with epilepsy. The results of our study will possibly help in shaping current vitamin D guidelines for vitamin D supplementation in children with epilepsy and provide a link between 25 (OH) vitamin D level and seizure control.
1. Introduction
Vitamin D (vitD) deficiency is a global health concern affecting all age groups. In Saudi Arabia, it affects around 95.3% of children aged 6 to 15 years.[1,2] VitD plays a crucial rule in maintaining bone health, muscle strength, immune function, neurotransmission in the central nervous system in healthy children.[3–5] While in children with epilepsy, low vitD level tends to increase seizure frequency. Large epidemiological studies reported significant seasonal variation in seizure frequency, with the least seizures occurring during summer and most during winter.[6] The increased seizure frequency during winter was attributed to low vitD level.
A large body of evidence—since 1960s—indicates that antiepileptic drugs (AEDs) impact bone metabolism negatively leading to impaired bone quality and increased risk of fractures. This observation led to extensive research on the interaction between AEDs and vitD metabolism. Cohort studies concluded that enzyme-inducer AEDs lowers 25 (OH) vitamin D level compared to none enzyme inducers making epilepsy patient at much higher risk for vitD deficiency.[7–19] Although, there are inconsistencies regarding the vitD lowering effect of the same AEDs in different studies, this may be due to differences in the study design, geographic location, or dietary habits between study populations.
VitD treatment for adults with epilepsy and vitD deficiency was carried out in 2 pilot studies. They found a reduction of seizure frequency by 30% in the treatment group compared to the control group.[20,21] Up to date, only one randomized controlled trial (RCT) assessed the impact of different vitD maintenance doses for children with epilepsy. The study included 78 children aged 10 to 18 years who are on long-term AED therapy. Children were randomized to receive 400 IU/day versus 2000 IU/day over 1 year regardless of the presence of vitD deficiency at baseline.[22] They reported comparable bone mineral density (BMD) level between both groups irrespective of their baseline vitD level, as well similar mean 25 (OH) vitamin D levels at 1 year. However, they did not report seizure frequency.
Many scientific societies had recommended vitD maintenance supplementations with 400 IU daily for all children. Yet, because of insufficient evidence to recommend a specific maintenance dose for children with epilepsy, the American Academy of Pediatrics had recommended 400 IU daily similar to healthy children, and 400 and 1000 IU for children who were treated recently for vitD deficiency, while the Endocrine Society recommended a higher dose ranging from 600 to 1000 IU daily for children at risk of developing vitD deficiency.[23,24] Therefore, we aim to determine the efficacy of standard dose of Cholecalciferol 400 IU/day that is used for healthy children versus 1000 IU/day that is recommended to be used for children at increased risk for developing vitD deficiency would be sufficient to maintain optimal 25 (OH) vitamin D level in children with epilepsy and normal baseline 25 (OH) vitamin D level over 6 months of supplementation, provide clinically significant reduction in seizure treatment failure, and improve bone health. Additionally, we aim to assess the presence of differential effect based on body mass index (BMI), use of enzyme-inducer AED, and number of AEDs used.
2. Methods
2.1. Trial design
This is phase IV pragmatic randomized superiority controlled open-label trial. The study protocol follows the SPIRIT guidelines for reporting protocol items for RCTs.[25] The trial was approved by the institutional review board at King Saud University (IRB no. E-17-2425). Informed consent was given to the patients and parents. The study population is recruited from the pediatric neurology clinic at King Saud University Medical City (KSUMC) starting December 2017 for 2 years. The KSUMC is a tertiary academic center that provides care to > 250 children with epilepsy yearly. The clinical trials.gov registration number is NCT03536845.
2.2. Eligibility criteria
Children with epilepsy are eligible to the study if they are aged 2 to 16 years and being treated with AEDs. Children will be excluded from the study if they had pre-existing vitD metabolism problems such as vitD dependent rickets, malabsorption syndromes, kidney disease, or liver disease. In addition to children with hypercalcemia at baseline total corrected calcium > 2.5 mg/dL, 25 (OH) vitamin D level > 250 nmol/L, or urine calcium: creatinine ration > 1.2 mol/mol, or > 0.41 g/g.
2.3. Treatment and randomization
Eligible patients will be approached during their routine neurology clinic visit by the research team for recruitment. Upon their approval, the research team will obtain informed consent and assent from the family and the child. At baseline, we will measure the child height, weight, vitD deficiency risk factors, seizure type, seizure control, current AEDs, concomitant medications. Additionally, we will perform laboratory workup at baseline, 3 months, and 6 months: 25 (OH) vitD level, AED level, alkaline phosphatase, corrected total calcium, parathyroid hormone (PTH), urine calcium:creatinine ratio, liver enzymes, renal function test (see Supplemental Digital Content which contain data collection form of the study with detailed laboratory requirement in each clinic visit).
All children with 25 (OH) vitaminD level < 75 nmol/L at baseline will be treated with Cholecalciferol 5000 IU/day for 8 weeks, and 30 to 75 mg/kg/day of elemental calcium in 3 divided doses for 4 weeks. Patients will be given the option of taking 35,000 IU once/week during the treatment phase according to the patient preference. After completion of 8 weeks course on vitD, 25 (OH) vitamin D level will be measured (Fig. 1).[23,24] A treatment course is necessary before starting maintenance dose because it is not ethical to randomize children to maintenance therapy when they are deficient. The treatment course of 8 weeks will be repeated if 25 (OH) vitamin D level is still < 75 nmol/L. Upon normalization of vitD level, the patient will be randomized into 2 groups. Group “A” will receive 400 IU\day of Cholecalciferol, and group “B” will receive 1000 IU\day of Cholecalciferol for 6 months. While children with normal 25 (OH) vitamin D level > 75 nmol/L will be randomized immediately. Cholecalciferol is provided in the form of drops manufactured by Novartis Vi-De 3 10 mL drops, 1 mL = 45 drops = 4500 IU; each drop provides 100 IU. Concomitant multivitamins that contain vitD, or other vitD preparations will not be allowed during the trial.
Figure 1.
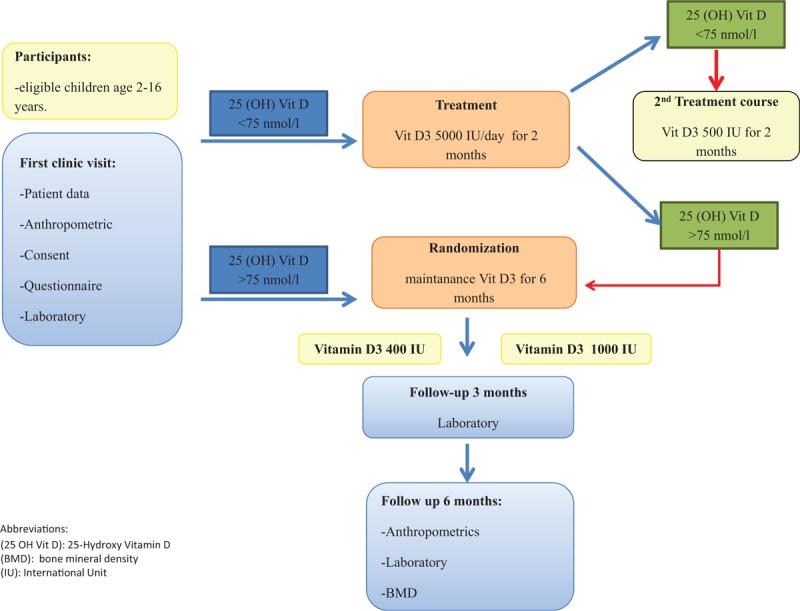
Study flow diagram.
The randomization schedule for patients using monotherapy AED will be stratified blocked randomization based on the type of AEDs (P450 enzyme-inducer vs P450 nonenzyme-inducer) and BMI (normal vs overweight and obese). BMI categories are defined according to the Centers for Diseases Control and Prevention (CDC) growth charts. While, for children on polytherapy AED (≥2 AEDs), the randomization schedule will be stratified based on BMI status only. The randomization schedule will be produced by the primary investigator using online software Sealed Envelope Ltd. 2017 https://www.sealedenvelope.com/simple-randomiser (Accessed December 2, 2017), and concealed through opaque sealed envelopes drawn sequentially. Owing to the nature of pragmatic trial, patients and study team will not be blinded to treatment after treatment allocation to simulate real-life clinical setting, and we will not introduce any strategy to improve compliance. All study procedures will comply with the Good Clinical Practice and Declaration of Helsinki.
2.4. Trial safety
Although vitD toxicity, hypercalcemia, and hypercalciuria are not commonly seen among children receiving standard vitD supplementation dose, we will monitor children for such complications at 3 and 6 months. This is because of the lack of RCT evidence for children with epilepsy, and because some children with epilepsy are bedridden which increases their risk of hypercalcemia and hypercalciuria.
Hypercalcemia will be defined as total corrected calcium > 2.5 mg/dL, hypercalciuria as urine calcium:creatinine ratio > 1.2 mol/mol, and vitD toxicity as 25 (OH) vitamin D level > 250 nmol/L. Any patient who develops those adverse events at any stage of the study will discontinue vitD and receive appropriate treatment if hypercalcemia developed.
2.5. Outcome measurement
Our primary outcome is the proportion of children with vitD insufficiency. Serum 25 (OH) vitamin D level will be measured using Electrochemiluminescence binding assay (ECLIA) from Roche diagnostics. VitD insufficiency will be defined as a 25 (OH) vitamin D level < 75 nmol/L according to the Institute of Medicine.[26] Although, the American Academy of Pediatrics (AAP) suggests using a 25 (OH) vitamin D level < 50 nmol/L in children as a definition for insufficiency, because it is sufficient to prevent rickets,[24] the Canadian Paediatric Society suggests maintaining 25 (OH) vitamin D level for children > 75 nmol/L, because adult data suggest that maintaining serum 25 (OH) vitamin D level > 75 to 80 nmol/L is required to minimize bone calcium resorption, and maximize intestinal calcium absorption.[27]
The secondary outcomes include proportion of children with AED treatment failure, mean seizure frequency, mean serum PTH, and bone mineral density (BMD). Treatment failure will be defined as a composite outcome of clinically significant increase in seizure frequency, or need to add new AED to control breakthrough seizure, or increase in the AED dose to control seizure that is not secondary to poor compliance, or decreased AED level, or intercurrent illness. This composite outcome provides better sensitivity in capturing possible treatment failure that is not captured as increased seizure frequency. Seizure frequency will be measured using parental report of seizure frequency at baseline and at 6 months. Any change in seizure frequency by 50% from baseline will be considered clinically significant.
3. Statistical analyses
3.1. Sample size estimation
Our primary outcome is 25 (OH) vitamin D level < 75 nmol/L between 2 independent groups. Prior data indicated that vitD deficiency prevalence among children receiving monotherapy AED and 400 IU of vitD is 0.45. If the true deficiency prevalence for children receiving 1000 IU is 0.1,[28,29] we will need to study 28 patients in each study arm to be able to reject the null hypothesis that the deficiency prevalence for both groups is equal with probability B = 0.85, and α=0.05. Furthermore, to be able to test our subgroup hypothesis of the impact of AED type and BMI status on vitD level we will need at least 56 patients in each arm. With an expected drop-out rate of 20%, we will need a total of 135 patients on monotherapy.
Prior data indicated that patients using polytherapy AED tend to have lower vitD level by −16 to −18 ± 13.6 compared to monotherapy.[30,31] Although, no prior data indicated a difference in vitD level among those using polytherapy according to their BMI. However, such data exists for monotherapy patients. Therefore we used estimates from monotherapy to inform our sample size estimation. We will need to study 28 patients in each arm (total of 68 patients with expected drop out rate of 20%) to test the hypothesis of the impact of monotherapy versus polytherapy AED with B = 0.85, alpha 0.05.
The current sample size will enable us to test difference in seizure treatment failure among the 2 intervention groups. Prior data indicate that the estimates of treatment failure among children receiving placebo are 0.6. If the true failure rate for children receiving vitD supplementation is 0.9,[20,21] we will need to study 36 patients in each arm with probability B = 0.85, and α=0.05.
4. Data analysis
We will analyze continuous outcomes using student t-test for independent samples for normally distributed data and Wilcoxon sign test for non-parametric data using the intention to treat analysis. We will present adjusted and unadjusted estimates for within groups proportion and rate comparisons. VitD treatment response and seizure treatment failure will be adjusted for AED type, number of used AEDs, and BMI using logistic regression model. Missing data will be handled using the last observation carried forward.
5. Discussion
VitD deficiency among children with epilepsy is an overlooked issue despite that evidence suggests its negative impact on seizure control and overall bone health. VitD plays an important role in brain development and behavior as well.[32–34] VitD has shown to have a neuroactive property in the brain that influences brain development.[3] Animal studies suggest an anticonvulsant effect of vitD. Siegel et al. showed that vitD3 delivered to the hippocampus of rodents elevated the threshold for chemically induced seizures and that vitD receptor knockout mice had increased seizure susceptibility.[35]
Our trial is set out to evaluate the efficacy of common different vitD maintenance doses on 25 (OH) vitamin D level, seizure control, and bone health for children using monotherapy and polytherapy to control epilepsy. In addition to estimating the health care burden of vitD deficiency among children with epilepsy, the results of our study will possibly help in shaping current vitD guidelines for vitD supplementation for children with epilepsy, provide an evidence link between 25 (OH) vitamin D level and seizure control.
The key strengths of our protocol include the pragmatic trial design to simulate real clinical practice setting, use of the currently recommended vitD doses by AAP and endocrine society, the outcome definition, and proposed analysis plan to confirm the previously reported interaction between vitamin D dose and AED cytochrome P450 inducers and BMI. We anticipate that the short duration of the study to possibly impact the observed link between 25 (OH) vitamin D level and our assessed outcomes.
Acknowledgment
This RCT is funded by Dallah Healthcare, Kingdom of Saudi Arabia, and Grant number (CMRC-DHG-1/006). Dallah healthcare has no role in planning, collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
Author contributions
Conceptualization: Reem Abdullah Al Khalifah, Abrar Hudairi, Doua Al Homyani, Muddathir H Hamad, Fahad A Bashiri.
Data curation: Reem Abdullah Al Khalifah, Doua Al Homyani, Muddathir H Hamad.
Formal analysis: Reem Abdullah Al Khalifah.
Funding acquisition: Reem Abdullah Al Khalifah, Fahad A Bashiri.
Investigation: Fahad A Bashiri.
Methodology: Reem Abdullah Al Khalifah, Abrar Hudairi.
Project administration: Reem Abdullah Al Khalifah, Abrar Hudairi, Doua Al Homyani, Muddathir H Hamad, Fahad A Bashiri.
Resources: Reem Abdullah Al Khalifah, Muddathir H Hamad, Fahad A Bashiri.
Visualization: Reem Abdullah Al Khalifah, Muddathir H Hamad.
Writing – original draft: Abrar Hudairi.
Writing – review & editing: Reem Abdullah Al Khalifah, Doua Al Homyani, Muddathir H Hamad, Fahad A Bashiri.
Reem Abdullah Al Khalifah orcid: 0000-0002-5304-3528
Supplementary Material
Footnotes
Abbreviations: 25 OH vitamin D = 25-hydroxy vitamin D, AAP = American Academy of Pediatrics, AEDs = antiepileptic drugs, BMD = bone mineral density, BMI = body mass index, CDC = Centers for Diseases Control and Prevention, ECLIA = electrochemiluminescence binding assay, IU = International Unit, PTH = parathyroid hormone, RCT = randomized controlled trial.
Trial registration: NCT03536845
Protocol version 2
Access to data: All investigators will have access to the final dataset.
Dissemination policy: We plan to disseminate the trial results in peer-reviewed journal, and scientific meetings.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Al Shaikh AM, Abaalkhail B, Soliman A, et al. Prevalence of vitamin D deficiency and calcium homeostasis in Saudi children. J Clin Res Pediatr Endocrinol 2016;8:461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Muthayya S, Rah JH, Sugimoto JD, et al. The global hidden hunger indices and maps: an advocacy tool for action. PLoS One 2013;8:e67860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc 2013;88:720–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cebeci AN, Ekici B. Epilepsy treatment by sacrificing vitamin D. Expert Rev Neurother 2014;14:481–91. [DOI] [PubMed] [Google Scholar]
- [5].Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- [6].Holló A, Clemens Z, Lakatos P, et al. Int J Neurosci 2014;124:387–93. [DOI] [PubMed] [Google Scholar]
- [7].Nettekoven S, Strohle A, Trunz B, et al. Effects of antiepileptic drug therapy on vitamin D status and biochemical markers of bone turnover in children with epilepsy. Eur J Pediatr 2008;167:1369–77. [DOI] [PubMed] [Google Scholar]
- [8].Shellhaas RA, Barks AK, Joshi SM. Prevalence and risk factors for vitamin D insufficiency among children with epilepsy. Pediatr Neurol 2010;42:422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Misra A, Aggarwal A, Singh O, et al. Effect of carbamazepine therapy on vitamin D and parathormone in epileptic children. Pediatr Neurol 2010;43:320–4. [DOI] [PubMed] [Google Scholar]
- [10].Aksoy A, Sonmez FM, Deger O, et al. The effects of antiepileptic drugs on the relationships between leptin levels and bone turnover in prepubertal children with epilepsy. J Pediatr Endocrinol Metab 2011;24:703–8. [DOI] [PubMed] [Google Scholar]
- [11].Borusiak P, Langer T, Heruth M, et al. Antiepileptic drugs and bone metabolism in children: data from 128 patients. J Child Neurol 2013;28:176–83. [DOI] [PubMed] [Google Scholar]
- [12].Rieger-Wettengl G, Tutlewski B, Stabrey A, et al. Analysis of the musculoskeletal system in children and adolescents receiving anticonvulsant monotherapy with valproic acid or carbamazepine. Pediatrics 2001;108:E107. [DOI] [PubMed] [Google Scholar]
- [13].Verrotti A, Greco R, Latini G, et al. Increased bone turnover in prepubertal, pubertal, and postpubertal patients receiving carbamazepine. Epilepsia 2002;43:1488–92. [DOI] [PubMed] [Google Scholar]
- [14].El-Hajj Fuleihan G, Dib L, Yamout B, et al. Predictors of bone density in ambulatory patients on antiepileptic drugs. Bone 2008;43:149–55. [DOI] [PubMed] [Google Scholar]
- [15].Fong CY, Riney CJ. Vitamin D deficiency among children with epilepsy in South Queensland. J Child Neurol 2014;29:368–73. [DOI] [PubMed] [Google Scholar]
- [16].Nicolaidou P, Georgouli H, Kotsalis H, et al. Effects of anticonvulsant therapy on vitamin D status in children: prospective monitoring study. J Child Neurol 2006;21:205–9. [DOI] [PubMed] [Google Scholar]
- [17].Guo CY, Ronen GM, Atkinson SA. Long-term valproate and lamotrigine treatment may be a marker for reduced growth and bone mass in children with epilepsy. Epilepsia 2001;42:1141–7. [DOI] [PubMed] [Google Scholar]
- [18].Cansu A, Yesilkaya E, Serdaroglu A, et al. Evaluation of bone turnover in epileptic children using oxcarbazepine. Pediatr Neurol 2008;39:266–71. [DOI] [PubMed] [Google Scholar]
- [19].Rauchenzauner M, Griesmacher A, Tatarczyk T, et al. Chronic antiepileptic monotherapy, bone metabolism, and body composition in non-institutionalized children. Dev Med Child Neurol 2010;52:283–8. [DOI] [PubMed] [Google Scholar]
- [20].Christiansen C, Rodbro P, Sjo O. Anticonvulsant action” of vitamin D in epileptic patients? A controlled pilot study. Brit Med J 1974;2:258–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hollo A, Clemens Z, Kamondi A, et al. Correction of vitamin D deficiency improves seizure control in epilepsy: a pilot study. Epilepsy Behav 2012;24:131–3. [DOI] [PubMed] [Google Scholar]
- [22].Mikati MA, Dib L, Yamout B, et al. Two randomized vitamin D trials in ambulatory patients on anticonvulsants: impact on bone. Neurology 2006;12:2005–14. [DOI] [PubMed] [Google Scholar]
- [23].Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- [24].Misra M, Pacaud D, Petryk A, et al. D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 2008;122:398–417. [DOI] [PubMed] [Google Scholar]
- [25].Chan AW, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ (Clinical research ed ) 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health 2007;12:583–98. [PMC free article] [PubMed] [Google Scholar]
- [28].Gallo S, Comeau K, Vanstone C, et al. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA 2013;309:1785–92. [DOI] [PubMed] [Google Scholar]
- [29].Rajakumar K, Moore CG, Yabes J, et al. Effect of vitamin D3 supplementation in black and in white children: a randomized, placebo-controlled trial. J Clin Endocrinol Metab 2015;100:3183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lee YJ, Park KM, Kim YM, et al. Longitudinal change of vitamin D status in children with epilepsy on antiepileptic drugs: prevalence and risk factors. Pediatr Neurol 2015;52:153–9. [DOI] [PubMed] [Google Scholar]
- [31].Lee SH, Yu J. Risk factors of vitamin D deficiency in children with epilepsy taking anticonvulsants at initial and during follow-up. Ann Pediatr Endocrinol Metab 2015;20:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Eilander A, Gera T, Sachdev HS, et al. Multiple micronutrient supplementation for improving cognitive performance in children: systematic review of randomized controlled trials. Am J Clin Nutr 2010;91:115–30. [DOI] [PubMed] [Google Scholar]
- [33].Lam LF, Lawlis TR. Feeding the brain - The effects of micronutrient interventions on cognitive performance among school-aged children: A systematic review of randomized controlled trials. Clin Nutr (Edinburgh, Scotland) 2017;36:1007–14. [DOI] [PubMed] [Google Scholar]
- [34].Lips P. Vitamin D physiology. Progr Biophys Mol Biol 2006;92:4–8. [DOI] [PubMed] [Google Scholar]
- [35].Kalueff AV, Minasyan A, Tuohimaa P. Anticonvulsant effects of 1,25-dihydroxyvitamin D in chemically induced seizures in mice. Brain Res Bull 2005;67:156–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


