Abstract
Spinal implant infection is a rare but significant complication of spinal fusion surgery, and the most common pathogen is Staphylococcus aureus. It is difficult to treat due to this pathogen's biofilm-forming ability and antibiotic resistance. We evaluated the therapeutic outcome of treatments for S aureus spinal implant infections. We retrospectively reviewed all patients with S aureus spinal implant infections at 11 tertiary-care hospitals over a 9-year period. Parameters predictive of treatment failure and recurrence were analyzed by Cox regression. Of the 102 patients with infections, 76 (75%) were caused by methicillin-resistant S aureus (MRSA) and 51 (50%) were late-onset infections. In all, 83 (81%) patients were managed by debridement, antibiotics, and implant retention (DAIR) and 19 (19%) had their implants removed. The median duration of all antibiotic therapies was 52 days. During a median follow-up period of 32 months, treatment failure occurred in 37 (36%) cases. The median time to treatment failure was 113 days, being <1 year in 30 (81%) patients. DAIR (adjusted hazard ratio [aHR], 6.27; P = .01) and MRSA infection (aHR, 4.07; P = .009) were independently associated with treatment failure. Rifampin-based combination treatments exhibited independent protective effects on recurrence (aHR, 0.23; P = .02). In conclusion, among patients with S aureus spinal implant infections, MRSA and DAIR were independent risk factors for treatment failure, and these risk factors were present in the majority of patients. In this difficult-to-treat population, the overall treatment failure rate was 36%; rifampin may improve the outcomes of patients with S aureus spinal implant infections.
Keywords: instrumentation, outcome, rifampin, spondylitis, treatment, vertebral osteomyelitis
1. Introduction
The number of spinal fusion surgeries has increased rapidly over recent decades. Such procedures have multiple indications, including spinal stenosis, degenerative changes, and herniated discs.[1] Such infections after instrumented surgery are uncommon, with rates ranging from 1% to 4%, but these are serious complications in terms of morbidity and healthcare costs.[2–6]
Staphylococcus aureus is the most common etiological agent of spinal implant infections, accounting for 33% to 75% of microbiologically diagnosed cases.[7–9] It is difficult to treat these infections due to the pathogen's formation of a biofilm on the surface of the osteosynthetic device, antimicrobial resistance, and slow-growing variants.[10,11] Hence, implant removal is crucial for successful treatment, but is occasionally not possible because of the potential risk of spinal instability.[11]
Effective antibiotics are important for managing spinal implant infections, particularly if the implant cannot be removed. Rifampin may be a good option because of its excellent bone- and biofilm-penetration abilities and high efficacy against adherent and stationary-phase staphylococci.[12,13] Rifampin-based combination treatments are recommended for patients with orthopedic implant-related infections, particularly if the implant cannot be removed.[14–16] These recommendations are mainly based on studies of S aureus prosthetic joint infections,[17–19] but data on rifampin therapy for S aureus spinal implant infections are limited. We evaluated the therapeutic outcomes of treatments for S aureus spinal implant infections, in particular the efficacy of rifampin-based combinations.
2. Material and methods
2.1. Study design
This observational cohort study was undertaken at 11 tertiary-care hospitals in the Republic of Korea. The study included all adult patients with a S aureus spinal implant infection from January 2006 to December 2014. This study was approved by the Institutional Review Board of Gyeongsang National University Changwon Hospital. Informed consent was waived because of the retrospective nature of the study. We report it following the format recommended by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.[20]
2.2. Inclusion and exclusion criteria
Adult patients (≥16 years of age) who presented with S aureus spinal implant infections were included. Infection was defined as the presence of clinical signs of deep surgical site infection with positive culture results of tissue surrounding the implant from the deep fascia or at least 2 sets of blood cultures.[7] The implant devices were titanium cages, plates, screws, rods, and hooks. Bone grafting without implanted devices was not categorized as spinal implant surgery.[21] Preoperative superficial wound culture results were not taken into account for the diagnosis of infection. Exclusion criteria were a preexisting spinal infection before instrumentation, transfer to another hospital before completing antibiotic therapy, incomplete medical records, and polymicrobial infections.
2.3. Data collection
Medical records were reviewed retrospectively for demographic information, underlying illnesses/conditions, clinical presentation, laboratory and radiological data, medical and surgical treatments, and clinical outcomes.
2.4. Definitions
Patients who developed signs or symptoms of spinal infection (fever, increasing pain, wound drainage, and wound erythema) within 30 days of implant placement were considered to have early onset infection, and all others were considered to have late-onset infection.[7] Cases were classified as treatment failure when infection-related death, primary failure, recurrence, or a new infection at the surgical site occurred.[9] Infection-related death was defined as in-hospital death related to the infection or to its treatment. Primary failure was defined as the need for new surgical debridement after 2 weeks of directed antibiotic therapy but before the end of antibiotic treatment because of signs of uncontrolled infection. Recurrence was defined as recurrent symptoms and signs after completion of antibiotic therapy. Patients were considered to have microbiological recurrence if a needle or open biopsy or blood culture revealed the same organism that caused the initial infection. Patients were considered to have clinical recurrence if biopsy and blood cultures did not reveal the causative organism(s). A new infection was defined as recurrent symptoms and signs after completion of antibiotic therapy caused by a pathogen different from that responsible for the initial infection.
2.5. Surgical and medical therapy
During the study period, surgical therapies were given at the discretion of the treating physicians. The antibiotic regimen and duration of therapy were usually determined by infectious disease specialists, based on culture results. Rifampin together with another antibiotic to which the causative pathogen was susceptible was used for rifampin-susceptible isolates, and rifampin was administered orally at 600 mg daily.
2.6. Statistical analysis
Data were analyzed using SPSS for Windows, version 18.0 (SPSS, Inc., Chicago, IL). Categorical variables were compared using chi-square or Fisher's exact tests, and continuous variables were compared using the Mann–Whitney U-test. Univariate and multivariate analyses of the parameters predictive of overall treatment failure and recurrence were performed using Cox regression. To identify independent predictors of treatment failure and recurrence, all significant variables identified in univariate analyses were included in multivariate analyses. The influence of antimicrobial therapy on outcome was analyzed only for recurrence, because primary failure and infection-related death occurred prior to completion of antibiotic therapy. The rates of treatment-failure-free survival and recurrence–free survival were estimated using the Kaplan–Meier method. The survival curves of the 2 groups were compared using the log-rank test. All analyses were 2-tailed, and a P value ≤0.05 was considered to indicate statistical significance.
3. Results
In all, 118 patients with S aureus spinal implant infections were identified during the study period. Of these, 16 were excluded because of a previous history of spinal infection (n = 8), transfer to other hospitals before completing antibiotic treatment (n = 5), incomplete medical records (n = 2), or polymicrobial infection (n = 1). Therefore, 102 patients were included in the analyses (Fig. 1).
Figure 1.
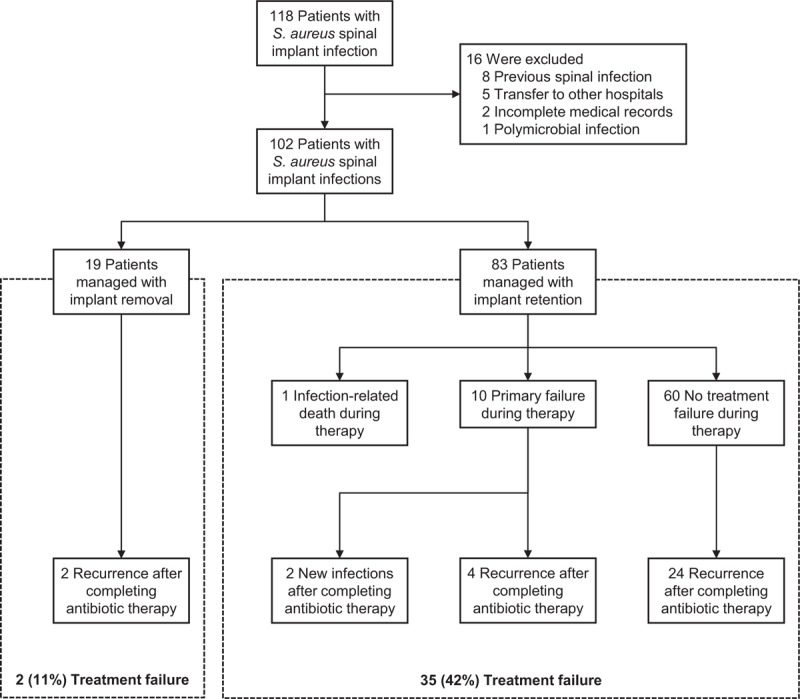
Flow chart of the patient inclusion process and outcomes.
3.1. Patients’ characteristics
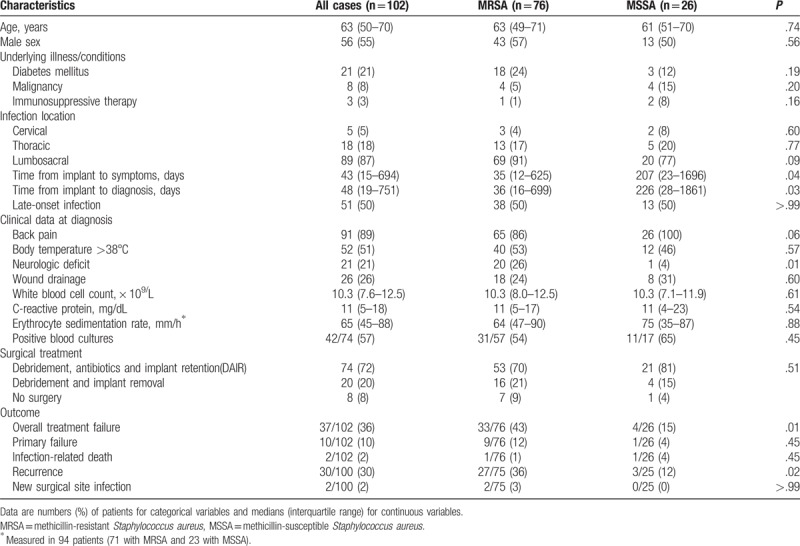
The clinical characteristics of the 102 patients with S aureus spinal implant infection are shown in Table 1. The median age of the cohort was 63 years (interquartile range [IQR], 50–70 years), and 56 (55%) patients were males. Overall, 21 (21%) patients had diabetes mellitus, 8 (8%) had underlying malignancies, and 3 (3%) were undergoing immunosuppressive therapy. In all, 5 (5%) infections were cervical, 18 (18%) were thoracic, and 89 (87%) were lumbosacral. Fifty-one (50%) patients each had early onset infection and late-onset infection. Seventy-six (75%) cases were caused by methicillin-resistant S aureus (MRSA) and 26 (25%) were caused by methicillin-susceptible S aureus (MSSA). The time from implantation to diagnosis was longer in patients with MSSA infections than in those with MRSA infections (median 226 vs 36 days; P = .03). Neurological deficit at diagnosis was more frequent in MRSA than MSSA cases (20% vs 4%; P = .01). There were no other differences in baseline characteristics between the MRSA and MSSA groups.
Table 1.
Clinical characteristics, treatment, and outcomes of patients with spinal implant infections caused by methicillin-resistant Staphylococcus aureus and methicillin-susceptible S aureus.
3.2. Management
Of the 102 patients with infections, 19 (19%) were managed by implant removal and 83 (81%) were managed by debridement, antibiotics, and implant retention (DAIR) (Fig. 1). Implants were removed initially from 6% (3/51) of patients with early onset infection and 31% (16/51) of those with late-onset infection (P = .001).
Table 2 shows the type, route, and duration of antimicrobial therapy received. All patients received intravenous antibiotics as a component of their initial treatment. The median duration of intravenous antibiotic therapy was 41 days (IQR, 22–57 days). Among the 76 patients with MRSA infections, primary parenteral therapy consisted of vancomycin in 59 (78%) patients and teicoplanin in 17 (22%). Among 26 patients with MSSA infections, primary parenteral therapy consisted of cefazolin in 17 (65%) patients, nafcillin in 7 (27%), and vancomycin in 2 (8%) patients. Oral antibiotics were prescribed after completion of intravenous antibiotic therapy in 42 (42%) patients. The median duration of total antibiotic therapy was 52 days (IQR, 34–88 days).
Table 2.
Antimicrobial treatments for spinal implant infections caused by methicillin-resistant Staphylococcus aureus and methicillin-susceptible S aureus.

Thirty (29%) patients received rifampin-based combination therapy for at least 2 weeks. Rifampin was used concurrently with intravenous antibiotics in 14 patients, with oral antibiotics in 6, and with both in 10. Intravenous companion drugs for rifampin consisted of a glycopeptide in 21 patients, cefazolin in 2, and nafcillin in 1. The median interval between initiation of glycopeptide and addition of rifampin to the glycopeptide was 5 days (IQR, 3–12 days). Oral companion drugs for rifampin consisted of a fluoroquinolone in 11 patients, trimethoprim–sulfamethoxazole in 3, and fusidic acid in 2 patients. Of the 30 patients who received rifampin, treatment was stopped before the end of antibiotic therapy in 6: in 2 due to an allergic exanthema and in 4 due to nausea. The median duration of rifampin use was 52 days (IQR, 28–123 days).
3.3. Therapeutic outcomes
The median follow-up duration after treatment initiation was 32 months (IQR, 13–59 months). Eleven patients experienced treatment failure during the initial treatment period: 1 infection-related death and 10 primary failure cases. Of the latter 10, 4 subsequently experienced recurrence and 2 subsequently experienced a new infection after completing antibiotic therapy. Of the 79 patients whose treatment did not fail, 26 experienced recurrence after completing the initial treatment. Ultimately, 36% (37/102) of all patients experienced treatment failure (Fig. 1). The median interval between treatment initiation and treatment failure was 113 days (IQR, 41–257 days; range, 7–2,669 days) and <1 year in 30 (81%) patients.
Analyses of parameters predictive of overall treatment failure are shown in Table 3. Univariate analyses indicated that DAIR, MRSA infection, and a C-reactive protein level ≥ 10 mg/dL were associated with overall treatment failure. Of the 51 patients with early onset infection, 3 were managed by implant removal. In the remaining 48 patients with early onset infection managed by DAIR, the estimated rate of 2-year survival free of treatment failure was 62% (95% confidence interval [CI], 46–74%; Fig. 2A). Among the 51 patients with late-onset infection, the estimated rate of 2-year survival free of treatment failure for the implant removal group was 93% (95% CI, 61–99%), compared to 56% (95% CI, 37–71%) for the DAIR group (log-rank test; P = .009; Fig. 2A). Kaplan–Meier analyses showed that the treatment-failure-free survival rate was lower among MRSA cases than MSSA cases (log-rank test, P = .01; Fig. 2B). Multivariate analyses indicated that DAIR (adjusted hazard ratio [aHR], 6.27; P = .01) and MRSA infection (aHR, 4.07; P = .009) were independently associated with overall treatment failure.
Table 3.
Univariate and multivariate analyses of parameters predicting overall treatment failure, primary failure, and recurrence among 102 patients with Staphylococcus aureus spinal implant infections.
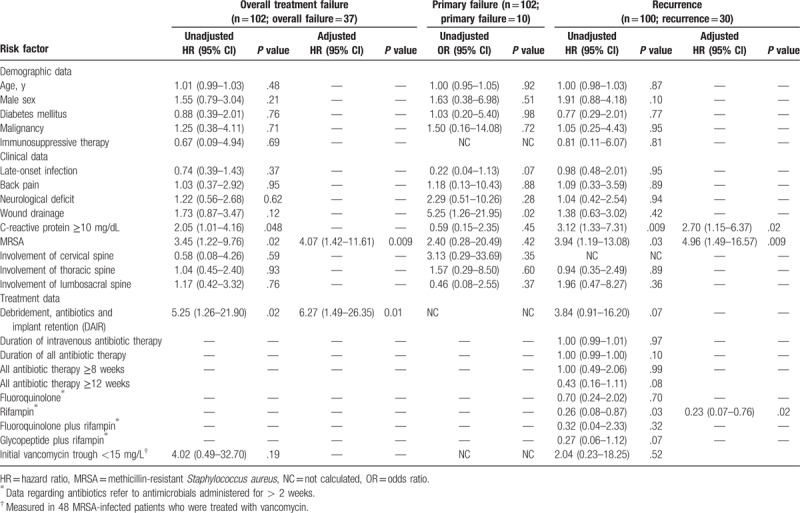
Figure 2.

Kaplan–Meier plots showing the cumulative probabilities of treatment-failure-free survival for early and late-onset infections (A) and methicillin-susceptible (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) infections (B). Three cases of early onset infection managed by implant removal were excluded. MRSA = methicillin-resistant Staphylococcus aureus, MSSA = methicillin-susceptible Staphylococcus aureus.
Of the 102 patients, 2 died before completing antimicrobial therapy for their infection. The remaining 100 patients who completed initial antibiotic therapy were evaluable for recurrence. Of these 100, 30 experienced recurrence after completing antibiotic therapy (24 had microbiological recurrence and 6 had clinical recurrence). The median interval between completion of antibiotic therapy and recurrence was 133 days (IQR, 31–371 days; range, 19–2594 days), and was <1 year in 26 (87%) patients. Recurrence was less common in patients given a rifampin-based combination treatment than in those who received other antimicrobials (10.0% [3/30] vs 39% [27/70]; P = .004). Of the 3 recurrences following rifampin-based combination therapy, 2 were caused by rifampin-susceptible MRSA and one was a clinical recurrence. Kaplan–Meier analyses showed that the recurrence-free survival rate was higher in patients treated with a rifampin-based combination regimen than in those who received other antimicrobials (log-rank test, P = .02; Fig. 3). The protective efficacy of rifampin-based combinations was evident in patients managed by DAIR (P = .004) and those infected with MRSA (P = .001), but not in patients without these risk factors (Fig. 4). Multivariate analyses indicated that MRSA (aHR, 4.96; P = .009) and C-reactive protein levels ≥ 10 mg/dL (aHR, 2.70; P = .02) were independently associated with recurrence (Table 3). Rifampin-based combinations exhibited an independent protective effect against recurrence (aHR, 0.23; P = .02).
Figure 3.
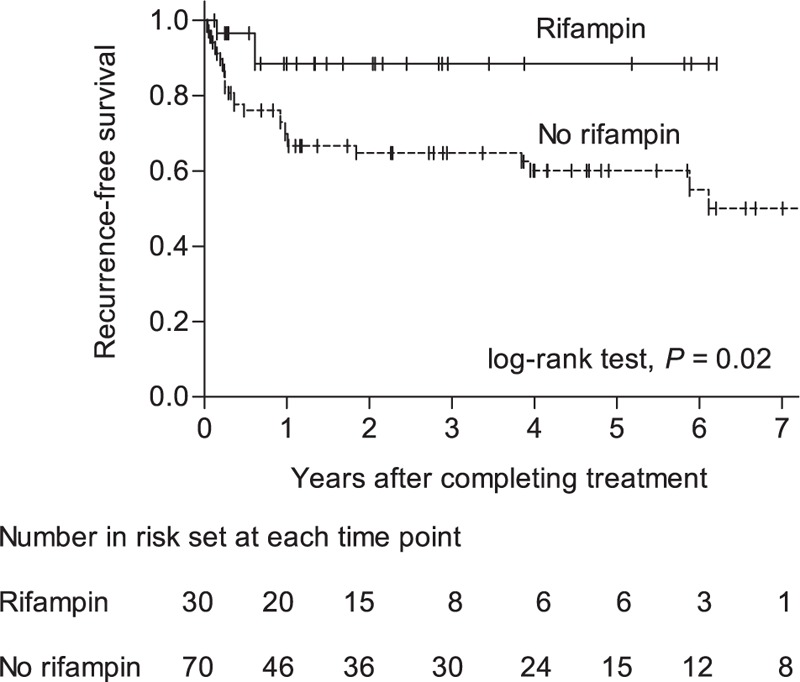
Kaplan–Meier plots showing the cumulative probability of recurrence-free survival according to receipt of concurrent rifampin therapy.
Figure 4.
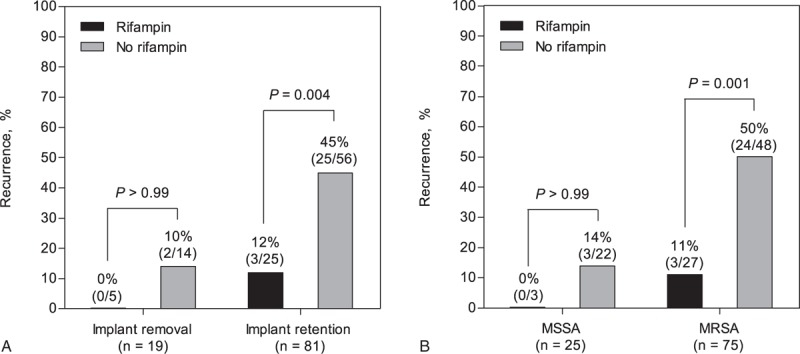
Protective effects of a rifampin-based combination treatment on spinal implant management (A) and methicillin resistance (B).
4. Discussion
To the best of our knowledge, this is one of the largest studies on spinal implant infections and the first to specifically evaluate the therapeutic outcome of treatments for S aureus spinal implant infections. DAIR and MRSA infection were independently associated with treatment failure, and most of our patients had these risk factors. In this difficult-to-treat population, the overall treatment failure rate was 36%, and rifampin-based combination therapy improved patient outcomes.
Our results demonstrate that implant removal is important for successful treatment of S aureus spinal implant infections. This finding is in line with previous studies, including those of such infections caused by various pathogens.[7,8,22–24] The rate of recurrence in our patients was 30%, much higher than the 9.6% in our previous study on native S aureus vertebral osteomyelitis infections (unrelated to spinal instruments).[25] The higher rate of treatment failure among S aureus spinal implant infections may be related to the difficulty eradicating bacteria on the surfaces of spinal implants.[11] The presence of biofilm in a chronic infection can make eradication difficult without foreign body removal. In this context, DAIR has been found to be a significant risk factor for treatment failure, particularly in late-onset infections.[7,23,24] Kowalski et al[7] reported that patients with late-onset spinal implant infection managed by DAIR had a considerably lower 2-year rate of treatment-failure-free survival than those who underwent implant removal (36% vs 84%). Recently, Chen et al[24] reported that delayed treatment for infection > 3 months was significantly associated with spinal implant removal. Consistent with these previous results, our patients with late-onset S aureus implant infection had a lower 2-year rate of treatment-failure-free survival than those who underwent implant removal (56% vs 93%).
We found that antibiotic resistance and the choice of antibiotic were associated with the therapeutic outcome. MRSA infections were independently associated with a higher risk of treatment failure than MSSA infections. Possible explanations for these findings include the lower activity of glycopeptides against staphylococci compared to antistaphylococcal β-lactams,[26–28] limited bone penetration,[12] and impaired activity against biofilm-embedded bacteria.[29] Indeed, vancomycin therapy is reportedly associated with higher recurrence rates than β-lactam antibiotics among patients with MSSA osteomyelitis.[30]
Rifampin-based combination treatments prevented recurrence in patients with S aureus spinal implant infections. It should be noted that, of our patients treated with a rifampin-based combination regimen, 70% received glycopeptide–rifampin therapy. Despite the potential benefit of such combinations, concerns remain regarding the safety of glycopeptide–rifampin combinations for MRSA infections. For MSSA osteoarticular infections, fluoroquinolone–rifampin is the most extensively studied combination and the one for which the available level of evidence is highest.[13,31] In contrast, disappointing results have been obtained in previous studies of vancomycin–rifampin treatment in patients with chronic osteomyelitis [32] and prosthetic joint infection due to MRSA.[19] In previous clinical studies, use of glycopeptide–rifampin treatment for MRSA infections has a risk of failure due to the emergence of rifampin-resistant mutants.[33,34] John et al[35] showed in an animal study that the combination of vancomycin plus rifampin does not completely prevent the emergence of rifampin resistance in cases of infection by high-density MRSA. Therefore, to prevent the emergence of rifampin-resistant strains, it is recommended to combine rifampin with a glycopeptide following surgical debridement,[13] clearance of bacteremia,[36] and ≥2 days of glycopeptide therapy.[14,15] Diffusion of glycopeptide into bone tissue is poor during the first few days but improves thereafter.[12] In our study, all of the patients were treated by surgical debridement, and the median interval from initiation of treatment to addition of rifampin to the glycopeptide was 5 days. In the 21 patients who received glycopeptide–rifampin treatment, the emergence of rifampin resistance was not detected. Our data suggest that glycopeptide–rifampin may be safe for treating S aureus spinal implant infections if rifampin is added to the glycopeptide several days after effective surgical and medical treatments.
Another important consideration for managing spinal implant infections is the duration of antibiotic therapy. Kowalski et al[7] showed that prolonged oral antibiotic suppression prevented treatment failure in patients with early onset infections. In a recent study by Dubee et al, the best outcome (2-year treatment-failure-free survival rate of 88%) was obtained in patients with early onset infections, most of whom were treated with regimens containing fluoroquinolone and/or rifampin for 3 months following DAIR.[9] In contrast to earlier reports, a recent study that included 55 patients with spinal implant infections did not find an association between suppressive antibiotic therapy ≥ 3 months and improved outcomes.[37] We observed a non-significant (P = .08) trend toward a lower recurrence rate among patients treated with any antibiotic for ≥12 weeks. Therefore, we could not firmly conclude whether a prolonged duration of antibiotic therapy was associated with an improved outcome in patients with S aureus spinal implant infections. Nevertheless, based on previous results[7,9] and ours, we suggest that prolonged antibiotic therapy in high-risk patients with such infections, like those treated by DAIR, may improve outcomes. Further studies should evaluate this issue.
Our study had several limitations. First, as in all retrospective studies, the data were incomplete and some patients were lost to follow-up, which may have introduced unrecognized bias into the results. Second, our results were obtained in the setting of antibiotic resistance and frequent attempts to retain implants, and thus caution should be used in extrapolating our results to a different epidemiologic context. Third, this study had limited power to confirm the benefit of rifampin-based therapy among low-risk patients (MSSA infection and implant removal cases).
5. Conclusion
S aureus spinal implant infection is a serious threat to patients who undergo spinal instrumentation surgery and continues to pose challenges to clinicians in terms of antibiotic resistance and difficulty removing prostheses. Our data suggest that not only effective surgical management but also selection of the optimal antibiotic (herein, rifampin) are crucial for improving the outcome of S aureus spinal implant infections.
Author contributions
Conceptualization: Oh-Hyun Cho, Ki-Ho Park.
Data curation: In-Gyu Bae, Song Mi Moon, Seong Yeon Park, Yee Gyung Kwak, Baek-Nam Kim, Shi Nae Yu, Min Hyok Jeon, Tark Kim, Eun Ju Choo, Eun Jung Lee, Tae Hyong Kim, Seong-Ho Choi, Jin-Won Chung, Kyung-Chung Kang, Jung Hee Lee, Yu-Mi Lee, Mi Suk Lee, Ki-Ho Park.
Formal analysis: Ki-Ho Park.
Investigation: Ki-Ho Park.
Supervision: Baek-Nam Kim, Ki-Ho Park.
Writing – original draft: Oh-Hyun Cho, Ki-Ho Park.
Writing – review & editing: Oh-Hyun Cho, Ki-Ho Park.
Footnotes
Abbreviations: aHR = adjusted hazard ratio, DAIR = debridement, antibiotics and implant retention, IQR = interquartile range, MRSA = methicillin-resistant Staphylococcus aureus, MSSA = methicillin-susceptible Staphylococcus aureus.
This work was supported by a grant from Kyung Hee University in 2015 (KHU-20150818).
The authors declare no conflicts of interest and no transparency issues.
References
- [1].Rajaee SS, Bae HW, Kanim LE, et al. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37:67–76. [DOI] [PubMed] [Google Scholar]
- [2].Veeravagu A, Patil CG, Lad SP, et al. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine (Phila Pa 1976) 2009;34:1869–72. [DOI] [PubMed] [Google Scholar]
- [3].Smith JS, Shaffrey CI, Sansur CA, et al. Rates of infection after spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976) 2011;36:556–63. [DOI] [PubMed] [Google Scholar]
- [4].Rao SB, Vasquez G, Harrop J, et al. Risk factors for surgical site infections following spinal fusion procedures: a case-control study. Clin Infect Dis 2011;53:686–92. [DOI] [PubMed] [Google Scholar]
- [5].Liu JT, Liao WJ, Chang CS, et al. Management of deep infection after instrumentation on lumbar spinal surgery in a single institution. Biomed Res Int 2015;2015:842010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ishii M, Iwasaki M, Ohwada T, et al. Postoperative deep surgical-site infection after instrumented spinal surgery: a multicenter study. Global Spine J 2013;3:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kowalski TJ, Berbari EF, Huddleston PM, et al. The management and outcome of spinal implant infections: contemporary retrospective cohort study. Clin Infect Dis 2007;44:913–20. [DOI] [PubMed] [Google Scholar]
- [8].Muschik M, Luck W, Schlenzka D. Implant removal for late-developing infection after instrumented posterior spinal fusion for scoliosis: reinstrumentation reduces loss of correction. A retrospective analysis of 45 cases. Eur Spine J 2004;13:645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dubee V, Lenoir T, Leflon-Guibout V, et al. Three-month antibiotic therapy for early-onset postoperative spinal implant infections. Clin Infect Dis 2012;55:1481–7. [DOI] [PubMed] [Google Scholar]
- [10].Sendi P, Rohrbach M, Graber P, et al. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin Infect Dis 2006;43:961–7. [DOI] [PubMed] [Google Scholar]
- [11].Kasliwal MK, Tan LA, Traynelis VC. Infection with spinal instrumentation: review of pathogenesis, diagnosis, prevention, and management. Surg Neurol Int 2013;4:S392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Landersdorfer CB, Bulitta JB, Kinzig M, et al. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin Pharmacokinet 2009;48:89–124. [DOI] [PubMed] [Google Scholar]
- [13].Coiffier G, Albert JD, Arvieux C, et al. Optimizing combination rifampin therapy for staphylococcal osteoarticular infections. Joint Bone Spine 2013;80:11–7. [DOI] [PubMed] [Google Scholar]
- [14].Societe de Pathologie Infectieuse de Langue Francaise (SPILF). Recommendations for clinical practice. Osteo-articular infection therapy according to materials used (prosthesis, implants, osteosynthesis). Med Mal Infect 2009;39:745–74. [DOI] [PubMed] [Google Scholar]
- [15].Lazennec JY, Fourniols E, Lenoir T, et al. Infections in the operated spine: update on risk management and therapeutic strategies. Orthop Traumatol Surg Res 2011;97:S107–16. [DOI] [PubMed] [Google Scholar]
- [16].Zimmerli W, Sendi P. Orthopaedic biofilm infections. APMIS 2017;125:353–64. [DOI] [PubMed] [Google Scholar]
- [17].Zimmerli W, Widmer AF, Blatter M, et al. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 1998;279:1537–41. [DOI] [PubMed] [Google Scholar]
- [18].Senneville E, Joulie D, Legout L, et al. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus. Clin Infect Dis 2011;53:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lora-Tamayo J, Murillo O, Iribarren JA, et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis 2013;56:182–94. [DOI] [PubMed] [Google Scholar]
- [20].von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- [21].Park KH, Cho OH, Lee YM, et al. Therapeutic outcomes of hematogenous vertebral osteomyelitis with instrumented surgery. Clin Infect Dis 2015;60:1330–8. [DOI] [PubMed] [Google Scholar]
- [22].Collins I, Wilson-MacDonald J, Chami G, et al. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J 2008;17:445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hedequist D, Haugen A, Hresko T, et al. Failure of attempted implant retention in spinal deformity delayed surgical site infections. Spine (Phila Pa 1976) 2009;34:60–4. [DOI] [PubMed] [Google Scholar]
- [24].Chen SH, Lee CH, Huang KC, et al. Postoperative wound infection after posterior spinal instrumentation: analysis of long-term treatment outcomes. Eur Spine J 2015;24:561–70. [DOI] [PubMed] [Google Scholar]
- [25].Park KH, Chong YP, Kim SH, et al. Clinical characteristics and therapeutic outcomes of hematogenous vertebral osteomyelitis caused by methicillin-resistant Staphylococcus aureus. J Infect 2013;67:556–64. [DOI] [PubMed] [Google Scholar]
- [26].Stryjewski ME, Szczech LA, Benjamin DK, Jr, et al. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis 2007;44:190–6. [DOI] [PubMed] [Google Scholar]
- [27].Chang FY, Peacock JE, Jr, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003;82:333–9. [DOI] [PubMed] [Google Scholar]
- [28].Kim SH, Kim KH, Kim HB, et al. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2008;52:192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rose WE, Poppens PT. Impact of biofilm on the in vitro activity of vancomycin alone and in combination with tigecycline and rifampicin against Staphylococcus aureus. J Antimicrob Chemother 2009;63:485–8. [DOI] [PubMed] [Google Scholar]
- [30].Tice AD, Hoaglund PA, Shoultz DA. Risk factors and treatment outcomes in osteomyelitis. J Antimicrob Chemother 2003;51:1261–8. [DOI] [PubMed] [Google Scholar]
- [31].Kim BN, Kim ES, Oh MD. Oral antibiotic treatment of staphylococcal bone and joint infections in adults. J Antimicrob Chemother 2014;69:309–22. [DOI] [PubMed] [Google Scholar]
- [32].Daver NG, Shelburne SA, Atmar RL, et al. Oral step-down therapy is comparable to intravenous therapy for Staphylococcus aureus osteomyelitis. J Infect 2007;54:539–44. [DOI] [PubMed] [Google Scholar]
- [33].Ju O, Woolley M, Gordon D. Emergence and spread of rifampicin-resistant, methicillin-resistant Staphylococcus aureus during vancomycin-rifampicin combination therapy in an intensive care unit. Eur J Clin Microbiol Infect Dis 2006;25:61–2. [DOI] [PubMed] [Google Scholar]
- [34].Lai CC, Tan CK, Lin SH, et al. Emergence of rifampicin resistance during rifampicin-containing treatment in elderly patients with persistent methicillin-resistant Staphylococcus aureus bacteremia. J Am Geriatr Soc 2010;58:1001–3. [DOI] [PubMed] [Google Scholar]
- [35].John AK, Baldoni D, Haschke M, et al. Efficacy of daptomycin in implant-associated infection due to methicillin-resistant Staphylococcus aureus: importance of combination with rifampin. Antimicrob Agents Chemother 2009;53:2719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011;52:e18–55. [DOI] [PubMed] [Google Scholar]
- [37].Keller SC, Cosgrove SE, Higgins Y, et al. Role of suppressive oral antibiotics in orthopedic hardware infections for those not undergoing two-stage replacement surgery. Open Forum Infect Dis 2016;3:ofw176. [DOI] [PMC free article] [PubMed] [Google Scholar]


