Abstract
Pentraxin-3 (PTX3) is a glycoprotein involved in inflammation and immune regulation of cancer. The aim of this study was to evaluate the serum PTX3 level in patients with colorectal cancer (CRC) and analyze its prognostic significance.
A total of 263 consecutive patients underwent radical resection for primary CRC and 126 healthy controls were enrolled in this study. Serum PTX3 level was measured within the day before surgery though enzyme-linked immunosorbent assays, comparing with the level of healthy control. Baseline demographic and clinical characteristics were recorded. The association between serum PTX3 level and survival outcome was analyzed by the Kaplan–Meier with Log-Rank test and Cox regression methods.
Mean serum PTX3 level in CRC patients was higher than that of healthy control (13.8 ± 3.2ng/mL versus 3.3 ± 1.2ng/mL, P < .001). Finally, 55 (20.9%) patients out of all 263 patients studied had died during following-up period. All patients were divided into 2 groups using the optimal cutoff value (12.6 ng/mL) of PTX3 level using a sensitivity of 68.0% and a specificity of 71.7% as optimal conditions from receiver operating curve analysis. Patients with a PTX3≥12.6ng/mL had poorer 5 years overall survival rate (76.6% versus 67.8%, P = .025) patients with a PTX3 < 12.6ng/mL in univariate analysis and serum PTX3 level also been confirmed as an independent predictor for survival for CRC in multivariate analysis (Hazard ratio, 1.468; 95% [confidence interval] CI, 1.081–1.976; P < .001).
Serum PTX3 level can serve as an independent prognostic biomarker for CRC patients after curative resection.
Keywords: colorectal cancer, pentraxin-3, survival
1. Introduction
Colorectal cancer (CRC) is the third most common gastrointestinal cancer and the fifth leading cause of cancer-related mortality in China.[1,2] Despite improvements of surgical techniques and adjuvant therapies and implementation of comprehensive standard treatments, the overall prognosis of CRC patients still poor.[3–5] High morbidity of metastasis and relapse were considered as main contributors to unsatisfactory long-term prognosis for CRC patients underwent standard treatments.[6,7] It has been revealed both inflammation and immunity dysregulation was closely associated with proliferation and migration of tumor cells.[8–10] However, there was no sufficient data focusing on the significance of biomarker involved both inflammation and immunity in CRC patients.
Pentraxin-3 (PTX3), as a glycoprotein secreting by immunocytes in response to stimulating, plays an important role in inflammation, apoptosis and vascular remodeling, meanwhile, acting as essential components of innate immunity.[11–13] Moreover, PTX3 sustains cellular proliferation, angiogenesis, insensitivity to apoptosis, thus prompting cancer cell invasion and proliferation though protein kinase B (AKT) and nuclear factor-k-gene binding (NF-kB) signaling pathways, as well as facilitate tumor escape from immunosurveillance.[14,15] Furthermore, emerging evidences showed that serum PTX3 level served as a novel diagnostic and prognostic biomarker of various types of malignancies, such as prostate, lung and pancreatic cancer.[16–18] These findings furtherly revealed the potential vital role of PTX3 in the development of cancers. In this study, we try to evaluate the expression level of PTX3 in peripheral blood of patients with operable CRC and analyzed its prognostic significance.
2. Materials and methods
2.1. Study population
This retrospective study was performed in accordance with the relevant guidelines and regulations and approved by the institutional review boards of the Second Hospital of Shandong University. Written informed consent was obtained from all study participants. The data of 263 primary CRC patients undergone curative resection at the Department of Gastroenterology in the Second Hospital of Shandong University between July 1, 2012, and July 1, 2017, was collected. Inclusion criteria included histologically confirmed tumor, node, and metastases (TNM) stage I, II and III CRC, more than 18 years of age and life expectancy more than 24 weeks. Patients with preoperative acute and severe comorbidity and adjuvant treatments, such as acute systemic infection, other malignancies, autoimmune diseases or inflammation and preoperative adjuvant chemotherapy, were excluded. All patients underwent R0 resection and postoperative radiotherapy and/or chemotherapy. A control group consisted of 126 age- and sex-matched healthy volunteers were enrolled at the corresponding duration of this study.
Clinical and histopathological characteristics of all patients were collected from the clinical records by 1 surgeon and check by another surgeon, including gender, age, tumor site, tumor size, tumor invasion depth, lymph node involvement, TNM stage, pathological differentiation and operation records. Clinical staging was evaluated by postoperative tumor tissue histopathological examination and clinical assessment according to the American Joint Committee on Cancer (AJCC) TNM classification. All Patients were regularly followed up by clinical visiting or telephone every 3 months for the first 2 years, every 6 months for the next 3 years, and once annually thereafter. Enhanced abdominal computed tomography (CT) or magnetic resonance imaging (MRI) scans were performed generally every 12 months. Clinical follow-up period lasted from the date of surgery to either the time of death or January 2018. Outcome was assessed as 5-years overall survival (OS) rate. OS was accurately defined as the duration from date of surgery to death.
2.2. Measurement of PTX3 serum levels
Sterile peripheral blood (5 mL) samples were obtained from each patient on the day before surgery, as well as from the healthy controls at the corresponding duration of study. Then blood samples were immediately centrifuged at 3000 rpm for 10 minutes to obtain serum, then transferred to tubes and immediately stored at −80 °C for further process. Serum levels of PTX3 were evaluated by commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (R&D systems, Minneapolis, MN).
2.3. Statistical Analysis
Statistical analysis was conducted using the SPSS 20.0 (IBM) in this study. P < .05 (2- sided) was considered statistically significant. Continuous variable was represented by mean ± standard deviation (SD), and categorical variable was represented by frequency. The χ2 test or Fisher exact test was used to compare different of categorical variable between 2 groups while continuous variable values were analyzed by independent student's t test. Receiver operating characteristic curve (ROC) analysis was performed to calculate optimal cutoff values for plasma PTX3 levels predicting survival. The 5 years OS rate and survival curve was studied in Kaplan–Meier survival analysis by using the log-rank test. The Cox regression model was used to assess the hazard ratio and multivariate analysis.
3. Results
3.1. Patient characteristics
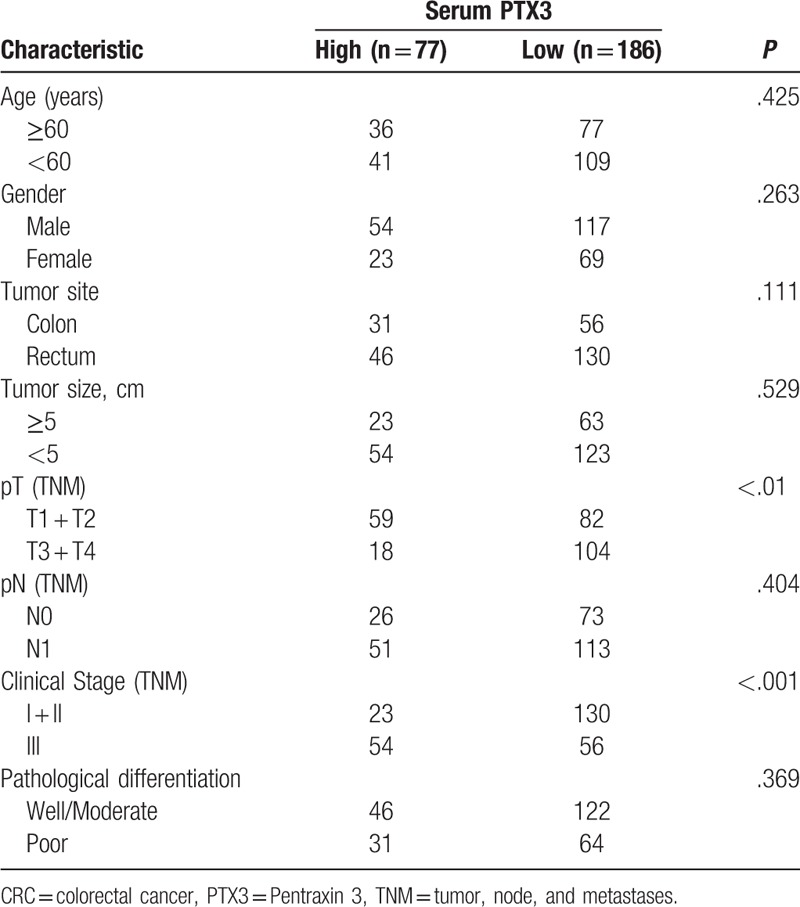
Table 1 briefly summarizes the clinicopathologic characteristics of 263 CRC patients. No significant statistical difference was found in age and gender of patients, tumor size and location, histological differentiation type, tumor invasion depth and lymph node involvement between patients with a high PTX3 level (≥12.6ng/mL) and those with a low PTX3 (<12.6 ng/mL). However, we found that there was significant relationship between preoperative serum PTX3 level and clinical stage, patients with a high PTX3 level may have an advanced cancer. In details, in group of 77 patients with a higher PTX3 level, 23 patients had TNM I or II tumor and 54 with TNM III lesion (P <.001, Table 1).
Table 1.
Correlation between serum PTX3 level and clinicpathologic characteristics of CRC patients.
3.2. Serum PTX3 level in CRC patients
The mean serum PTX3 level of 263 primary CRC patients was 13.8 ± 3.2ng/mL, which was significantly higher than that of healthy control (n = 126; 3.3 ± 1.2ng/mL; P < .001, Fig. 1). In receiver operating characteristic curve analysis, the optimal cutoff levels for the PTX3 predicting OS of CRC patients was 12.6ng/mL using a sensitivity of 68.0% and a specificity of 71.7% as optimal conditions, respectively. The area under the curve was 0.732 with a 95% confidence interval between 0.658 and 0.806, P < .001. (Fig. 2).
Figure 1.
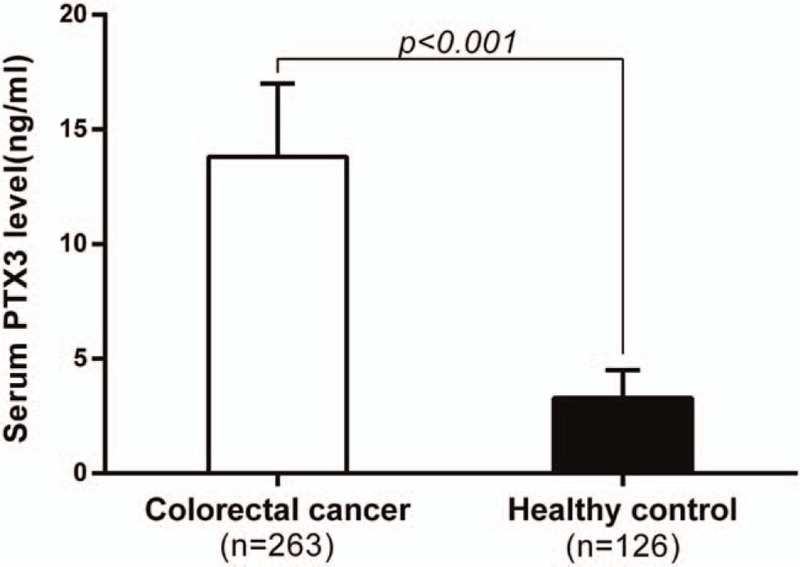
Serum pentraxin-3 level in colorectal patients and healthy controls. Colorectal cancer patients had significant higher mean serum PTX3 level than that of healthy control (13.8 ± 3.2 ng/mL versus 3.3 ± 1.2 ng/mL, P < .001). PTX3 = pentraxin 3.
Figure 2.
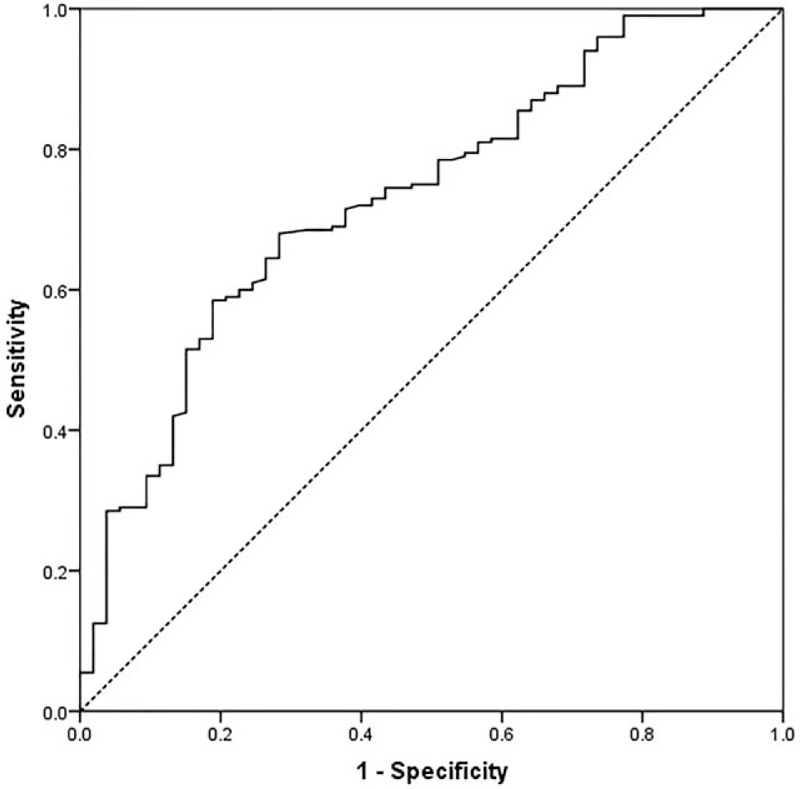
Receiver-operator characteristic curve for pentraxin-3. The areas under the curve were 0.732 with a 95% confidence interval (95% CI) for the area between 0.658 and 0.806. CI = confidence interval.
3.3. Prognostic significance of serum PTX3 level for CRC patients
The median follow-up time was 33.5 months (6.1–85.1months). During follow-up period of this study, there were 55 (20.9%) tumor-special death patients and 208 survival patients, respectively. All 263 CRC patients were divided into high PTX3 group (≥12.6ng/mL) and low PTX3 group (≥12.6ng/mL) according to the optimal cutoff value obtained from ROC curve analysis. In univariate survival analysis, we found that patients in low PTX3 group had a statistically better postoperative prognosis than those with high PTX3 in term of 5-years OS rate (76.6% versus 67.8%, P = .025), (Table 2, Fig. 3). In addition, histological differentiation type (P = .012), tumor invasion depth (P = .021) and lymph node involvement (P = .001) were also significantly associated with postoperative OS of CRC patients (Table 2).
Table 2.
The prognostic characteristics of CRC patients.
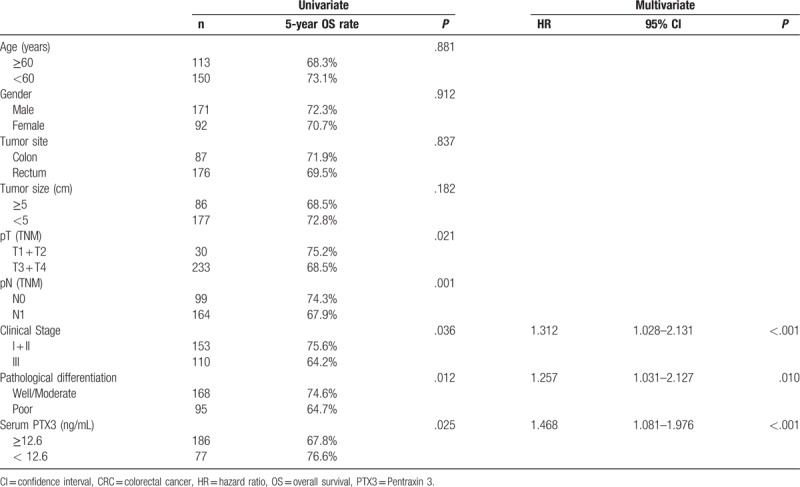
Figure 3.
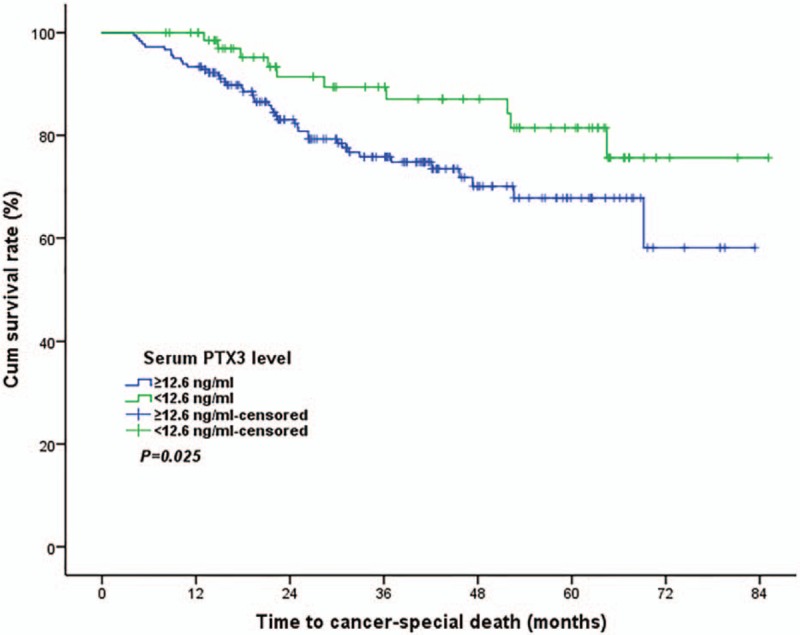
Prognostic significance of preoperative serum pentraxin-3 level for colorectal cancer. Panel showed patients with a high serum PTX3 level had a significantly lower 5 years overall survival rate than those with low serum PTX3 (76.6% versus 67.8%, P = .025). PTX3 = pentraxin 3.
A multivariate analysis enrolled sex and gender of patients, tumor size and location, histological differentiation type, clinical stage (TNM stage), serum PTX3 level into the COX regression model to identify independent predictive factors for OS of operable CRC patients. We found that serum PTX3 level (Hazard ratio, 1.468; 95% CI, 1.081–1.976; P < .001) and clinical Stage (Hazard ratio, 1.312; 95% CI, 1.028–2.131; P < .001) and pathological differentiation (Hazard ratio, 1.257; 95% CI, 1.031–2.127; P = .01) were the independent prognostic factors for CRC patients (Table 2).
4. Discussion
In present study, we have investigated serum PTX3 level in CRC patients and found that significantly higher serum PTX3 levels in CRC patients compared with that in healthy controls. Furthermore, we also analyzed a potential role of serum PTX3 obtained before surgical resection as a candidate biomarker to predict postoperative prognosis of CRC and showed serum PTX3 level was significantly associated with survival of CRC patients in both univariate and multivariate survival analysis. According to these findings, we concluded that serum PTX3 level can be evaluated for an optimal risk stratification of individual CRC patients, and which also can serve as a potential biomarker to predict postoperative survival of CRC patients.
PTX3, as a member of the long pentraxin superfamily, is produced by innate immunity cells, which plays an important role in tumor-related inflammation.[19,20] Moreover, PTX3 is overexpressed in various malignancies and promotes the severity of tumors, such as lung cancer, glioma and breast cancer.[21–23] In this study, we found that elevated serum PTX3 level in CRC comparing to healthy controls is significantly associated to TNM stage, which is consistent with prior similar studies.[24] However, underlying mechanisms of serum PTX3 elevation in patients with cancer are not fully understood. PTX3 is an inflammatory factor, which has similar function with C-reactive protein.[25] In tumor microenvironment, tumor endothelial cells and macrophages overexpress PTX3 in response to inflammatory signals, in turn, PTX3 promotes development of tumor by stimulating angiogenesis and inhibiting apoptosis.[26]
Furthermore, we furtherly confirmed the prognostic significance of serum PTX3 in multivariate COX analysis enrolled classical prognostic predictor including TNM stage and histological differentiation. In addition, patients with a high serum PTX3 had poorer 5-years survival rate than those with a low PTX3. Therefore, the risk of patients a high serum PTX3 should be aware, and receive more proactive treatments. Otherwise, PTX3 may be considered as a novel therapy target. Ying et al used lentivirus-mediated small hairpin RNA (shRNA) to knock down PTX3 expression in cervical cancer cell lines, which inhibited cell viability, diminished colony forming ability, and induced cell cycle arrest.[27] Choi et al found that PTX3 silencing using siRNA-specific siRNA prevented breast cancer cell migration.[28] However, there was no sufficient information concerning the potential role and mechanism of PTX3 in CRC in vivo and vitro setting.
In present, we calculated the cut-off value for the PTX3 though ROC analysis, which is different from that of previous studies. Thus, although PTX3 serves as a useful prognostic biomarker for CRC, the optimum cut-off value for the PTX3 may differ in various studies enrolled patients with different stage, or different endpoint and statistics mothed.[29]
There were several limitations in our study. One problem was the retrospective, relative small scale and single center design of study. A large-scale multi-center prospective study would allow further confirmation of the prognostic significance of PTX3 for CRC patients. Furthermore, the optimum cut-off value for the preoperative PTX3 predicting survival of CRC remains unknown, although 12.6 was set as the cut-off value by ROC analysis. A meta-analysis including various PTX3 validation studies may be required to confirm more definite cutoff values for the PTX in CRC patients.
In conclusion, our study confirmed that CRC patients have higher serum PTX3 level, which is correlated to TNM stage. More important, we found that preoperative serum PTX3 level can serve as an independent prognostic predictor for operable CRC.
Author contributions
Conceptualization: Lianrong Guo.
Data curation: Yangying Zhao, Bin Liu.
Formal analysis: Lianrong Guo, Yangying Zhao.
Funding acquisition: Lianrong Guo.
Investigation: Lianrong Guo, Yangying Zhao.
Methodology: Lianrong Guo.
Project administration: Lianrong Guo.
Resources: Bin Liu.
Software: Yangying Zhao, Bin Liu.
Supervision: Lianrong Guo.
Writing – original draft: Yangying Zhao, Bin Liu.
Writing – review & editing: Lianrong Guo, Bin Liu.
Footnotes
Abbreviations: CRC = colorectal cancer, OS = overall survival, PTX3 = pentraxin-3.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Chen W. Cancer statistics: updated cancer burden in China. Chin J Cancer Res 2015;27:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fang YJ, Wu XJ, Zhao Q, et al. Hospital-based colorectal cancer survival trend of different tumor locations from 1960s to 2000s. PloS One 2013;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nakajima J, Iida T, Okumura S, et al. Recent improvement of survival prognosis after pulmonary metastasectomy and advanced chemotherapy for patients with colorectal cancer. Eur J Cardiothorac Surg 2017;51:869–73. [DOI] [PubMed] [Google Scholar]
- [5].Quidde J, Denne L, Kutscheidt A, et al. Baseline and on-treatment markers determining prognosis of first-line chemotherapy in combination with bevacizumab in patients with metastatic colorectal cancer. Oncol Res Treat 2017;40:21–6. [DOI] [PubMed] [Google Scholar]
- [6].Karakas Y, Dizdar O. Tumor sidedness and prognosis in colorectal cancer: is microbiome the missing link. JAMA Oncol 2017;3:1000. [DOI] [PubMed] [Google Scholar]
- [7].Gui L, Wang Z, Han J, et al. High expression of orai1 enhances cell proliferation and is associated with poor prognosis in human colorectal cancer. Clin Lab 2016;62:1689–98. [DOI] [PubMed] [Google Scholar]
- [8].de la Cruz-Merino L, Henao Carrasco F, Vicente Baz D, et al. Immune microenvironment in colorectal cancer: a new hallmark to change old paradigms. Clin Dev Immunol 2011;2011:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Qu D, Shen L, Liu S, et al. Chronic inflammation confers to the metabolic reprogramming associated with tumorigenesis of colorectal cancer. Cancer Biol Ther 2017;1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McConnell BB, Yang VW. The role of inflammation in the pathogenesis of colorectal cancer. Curr Colorectal Cancer Rep 2009;5:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kotooka N, Inoue T, Fujimatsu D, et al. Pentraxin3 is a novel marker for stent-induced inflammation and neointimal thickening. Atherosclerosis 2008;197:368–74. [DOI] [PubMed] [Google Scholar]
- [12].Naito A, Tanabe N, Jujo T, et al. Pentraxin3 in chronic thromboembolic pulmonary hypertension: a new biomarker for screening from remitted pulmonary thromboembolism. PloS One 2014;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Inforzato A, Doni A, Barajon I, et al. PTX3 as a paradigm for the interaction of pentraxins with the complement system. Semin Immunol 2013;25:79–85. [DOI] [PubMed] [Google Scholar]
- [14].Hu FQ, Qiao T, Xie X, et al. Knockdown of the inflammatory factor pentraxin-3 suppresses growth and invasion of lung adenocarcinoma through the AKT and NF-kappa B pathways. J Biol Regul Homeost Agents 2014;28:649–57. [PubMed] [Google Scholar]
- [15].Fornai F, Carrizzo A, Ferrucci M, et al. Brain diseases and tumorigenesis: the good and bad cops of pentraxin3. Int J Biochem Cell Biol 2015;69:70–4. [DOI] [PubMed] [Google Scholar]
- [16].Stallone G, Cormio L, Netti GS, et al. Pentraxin 3: a novel biomarker for predicting progression from prostatic inflammation to prostate cancer. Cancer Res 2014;74:4230–8. [DOI] [PubMed] [Google Scholar]
- [17].Diamandis EP, Goodglick L, Planque C, et al. Pentraxin-3 is a novel biomarker of lung carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res 2011;17:2395–9. [DOI] [PubMed] [Google Scholar]
- [18].Kondo S, Ueno H, Hosoi H, et al. Clinical impact of pentraxin family expression on prognosis of pancreatic carcinoma. Br J Cancer 2013;109:739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Garlanda C, Bottazzi B, Bastone A, et al. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Ann Rev Immunol 2005;23:337–66. [DOI] [PubMed] [Google Scholar]
- [20].Schenk JM, Kristal AR, Neuhouser ML, et al. Biomarkers of systemic inflammation and risk of incident, symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol 2010;171:571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang D, Ren WH, Gao Y, et al. Clinical significance and prognostic value of pentraxin-3 as serologic biomarker for lung cancer. Asian Pac J Cancer Prev 2013;14:4215–21. [DOI] [PubMed] [Google Scholar]
- [22].Locatelli M, Ferrero S, Martinelli Boneschi F, et al. The long pentraxin PTX3 as a correlate of cancer-related inflammation and prognosis of malignancy in gliomas. Journal Neuroimmunol 2013;260:99–106. [DOI] [PubMed] [Google Scholar]
- [23].Basu S, Harris H, Wolk A, et al. Inflammatory F2-isoprostane, prostaglandin F2alpha, pentraxin 3 levels and breast cancer risk: the Swedish mammography cohort. Prostaglandins Leukot Essent Fatty Acids 2016;113:28–32. [DOI] [PubMed] [Google Scholar]
- [24].Choi B, Lee EJ, Shin MK, et al. Upregulation of brain-derived neurotrophic factor in advanced gastric cancer contributes to bone metastatic osteolysis by inducing long pentraxin 3. Oncotarget 2016;7:55506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Okutani D. The role of long pentraxin 3, a new inflammatory mediator in inflammatory responses. Nihon Rinsho Men’eki Gakkai kaishi Jpn J Clin Immunol 2006;29:107–13. [DOI] [PubMed] [Google Scholar]
- [26].Abderrahim-Ferkoune A, Bezy O, Chiellini C, et al. Characterization of the long pentraxin PTX3 as a TNFalpha-induced secreted protein of adipose cells. J Lipid Res 2003;44:994–1000. [DOI] [PubMed] [Google Scholar]
- [27].Ying TH, Lee CH, Chiou HL, et al. Knockdown of pentraxin 3 suppresses tumorigenicity and metastasis of human cervical cancer cells. Sci Rep 2016;6:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Choi B, Lee EJ, Song DH, et al. Elevated Pentraxin 3 in bone metastatic breast cancer is correlated with osteolytic function. Oncotarget 2014;5:481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang J, Wang TY, Niu XC. Increased plasma levels of pentraxin 3 are associated with poor prognosis of colorectal carcinoma patients. Tohoku J Exp Med 2016;240:39–46. [DOI] [PubMed] [Google Scholar]


