Supplemental Digital Content is available in the text
Keywords: clinical stage T2N0, esophageal carcinoma, induction therapy, surgery
Abstract
Objective:
It is still controversial whether patients with clinical T2N0M0 (cT2N0M0) esophageal cancer are treated with induction therapy. The aim of this study was to determine the effect of induction therapy on cT2N0M0 esophageal cancer.
Methods and materials:
We searched PubMed, Embase, the Cochrane Library, and Medline databases from inception up to May 1, 2017. This meta-analysis was performed to compare odds ratios (OR) for 5-year overall survival (OS), pathologically understaged and overstaged after esophagectomy.
Results:
Eight retrospective studies of 2646 patients were included in the meta-analysis. Data showed that no statistically significant difference in 5-year over survival was observed between induction therapy group and direct operation group. The pooled OR and 95% confidence interval (CI) for 5-year OS were 0.92 (95% CI = 0.72–1.18; P = .52). Whereas, compared with induction therapy group, direct operation group had more pathologically understaged and less overstaged after esophagectomy.
Conclusions:
Currentclinical staging for T2N0M0 esophageal carcinoma remains inaccurate. In this study, we found that direct operation group had more pathologically understaged and less overstaged after esophagectomy compared with induction therapy group. Induction therapy could degrade the tumor staging but not improve the patient's survival.
1. Introduction
Locally advanced stages of esophageal carcinoma are generally treated with induction chemoradiotherapy (CRT), followed by resection.[1] In contrast, the appropriate treatment of early carcinomas is generally treated with direct resection. A randomized phase III trial reported neoadjuvant CRT did not improve R0 resection rate or survival but enhanced postoperative mortality in patients with stage I or II esophageal cancer, compared with direct operation.[2] The effect of neoadjuvant CRT on patients with the clinical stage of T2N0M0 esophageal cancer remains uncertain.
The optimal treatment of esophageal cancer patients with clinical T2N0M0 (cT2N0M0) remains controversial. Killinger et al reported patients with pT2N0M0 disease had a survival rate on par with patients with pT1N0M0,[3] and Song et al reported adjuvant therapy did not improve the survival of pT2N0M0 patients.[4] In fact, the accuracy rate of cT2N0M0 staging is poor, with the majority of esophageal cancers presenting with more advanced disease.[5,6] Some studies had found that induction therapy did not improve survival of patients with cT2N0 esophageal cancer and recommended surgery alone as the primary treatment approach.[7–9] Other studies had also found that induction therapy did not improve survival, but due to the significant understaging of T2N0M0 patients, they recommended induction therapy.[10,11] In addition, there was a study found that induction therapy was harmful to survival. They believed that surgery should be performed directly and those patients with understaging should receive postoperative adjuvant therapy.[12] Therefore, the aim of this study is to determine the effect of induction therapy on cT2N0M0 esophageal cancer.
2. Methods and materials
All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
2.1. Literature search
We searched PubMed, Embase, the Cochrane Library, and Medline databases from inception up to May 1, 2017. We used combinations of the following search terms: “esophageal carcinoma,” “esophageal, carcinoma,” “esophageal cancer,” “esophageal, cancer,” “T2N0,” “T2,” “surgery,” “operation,” “induction therapy” “neoadjuvant CRT ” and “neoadjuvant therapy.” The database search was restricted to human research articles written in English.
2.2. Selection criteria
The following eligibility criteria were applied:
all the patients were diagnosed with esophageal carcinoma;
survival data were reported or could be extrapolated based on published data results;
Studies with Patients were treated with neoadjuvant chemotherapy, radiotherapy or neoadjuvant CRT.
Exclusion criteria:
reviews, case reports, editorials, commentaries, and letters;
duplicate publications;
absence of critical information for the calculation date.
2.3. Data extraction
Data from eligible studies were extracted independently by 2 reviewers. The following data were collected: title, the first author, year of the publication, country, study period, study design, mean age, number of patients, induction therapy, postoperative pathology stage, and survival outcome. The primary endpoint of this meta-analysis was the 5-year OS. The secondary endpoints were pathologically understaged and overstaged after esophagectomy. Survival outcome data were extracted directly or calculated from the Kaplan–Meier survival curves.
2.4. Statistical analysis
The meta-analysis was carried out using Revman 5.3 software (The Nordic Cochrane Centre, Copenhagen, Denmark). We analyzed survival outcomes using odds ratio (OR) with 95% CI. We extracted data from the primary studies first. For studies reporting only available in the figures, we calculated data using Engauge Digitizer Version 4.1 (Free Software Foundation). We first extracted several specific points from the survival curves using Engauge Digitizer version 4.1 to obtain 2 lists of survival rates at specific time points from the 2 survival curves. We then input the extracted survival rates at specific time points into the spreadsheet developed to calculate the odds ratios (OR) and 95% CI. OR > 1 and the 95% CI did not overlap 1 with P < .05 was considered statistically significant difference. We assessed heterogeneity using the X2 test with significance defined as P < .10 and using I2 with a maximum value of 50% for low heterogeneity. The Mantel–Haenszel method for fixed effects was used to pool data for P ≥ .10 or I2 ≤ 50%. Otherwise, a random effect model was used. Software Stata 12 (Stata Corporation, College Station, TX) was used to calculate publication bias, and then the symmetry of the funnel plot was confirmed by Egger test with a P < .05.
2.5. Quality assessment
Study quality assessment was guided by the Newcastle–Ottawa Scale according to the following 3 items: the selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure or outcome of interest for case-control or cohort studies respectively.
3. Results
3.1. Study characteristics
The literature search of databases identified 457 records. After scanning titles and abstracts, 72 duplicate and 357 unrelated studies were excluded. As shown in Figure 1, additional 20 studies were further excluded for reviews (n = 16), and insufficient data (n = 4). Finally, 2646 patients in a total of 8 studies were included in the present met-analysis, among which 961 patients with cT2N0 esophageal carcinoma received induction therapy and 1685 patients did not.
Figure 1.
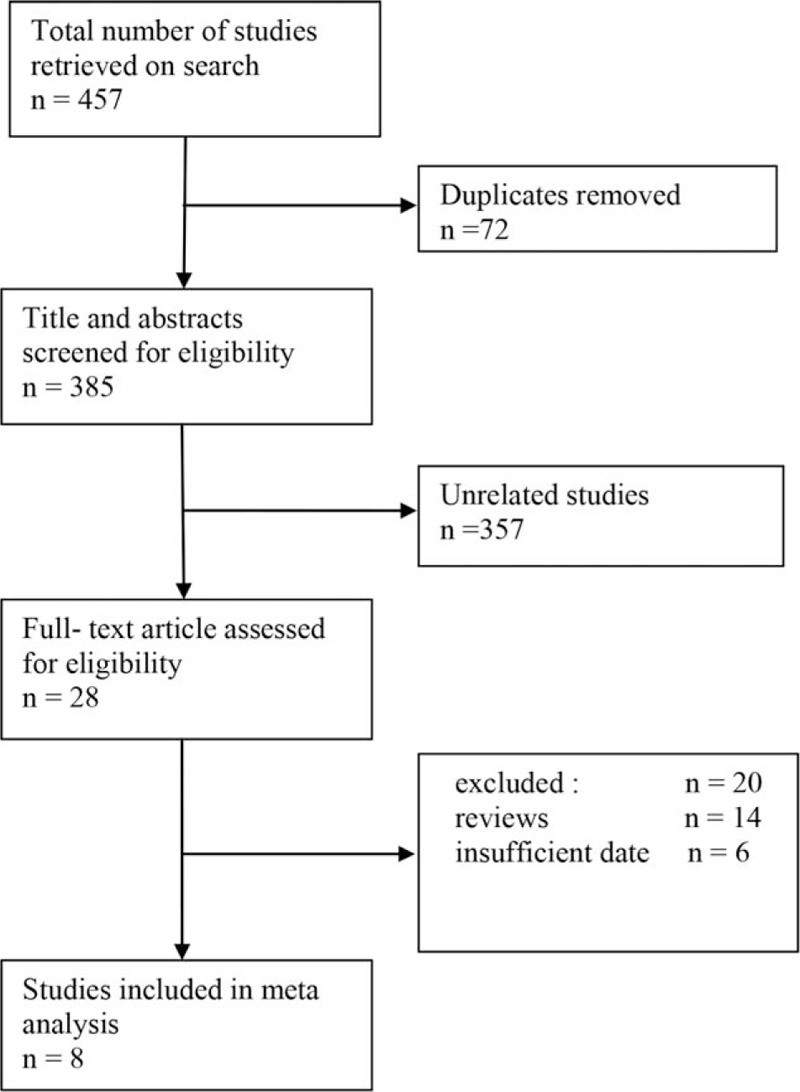
Flowchart of literature search and selection.
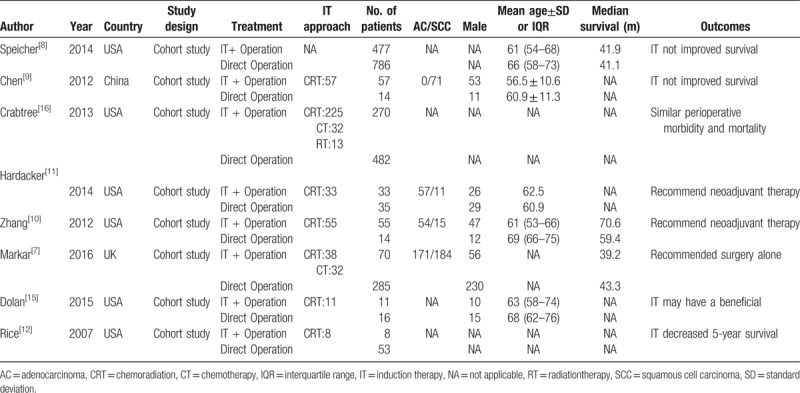
All the studies were retrospective cohort studies. The detailed characteristics of the included studies are showed in Table 1. All the patients in the 8 studies were treated with CRT. The mean age in induction therapy group was lower than that in direct operation group.
Table 1.
Main characteristics of all the included studies.
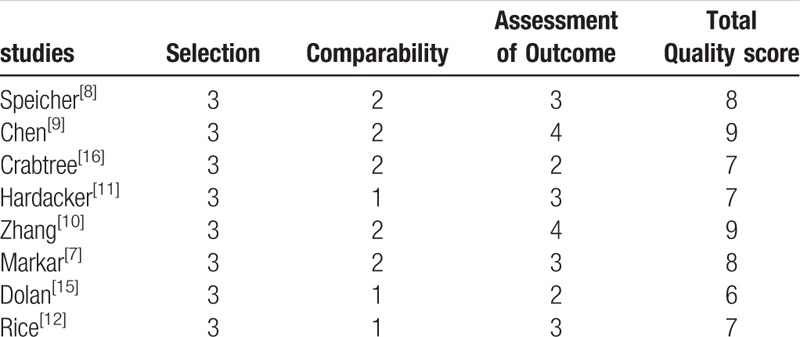
Assessing the Quality of Nonrandomized Studies with the Newcastle–Ottawa Scale is given in Table 2. The final scores varied from 5 to 7.
Table 2.
The Newcastle–Ottawa Scale for assessing the quality of studies.
3.2. 5-year overall survival (OS)
Six studies representing 832 patients with direct operation and 388 patients with induction therapy reported 5-year OS. The pooled OR and 95% CI by comparing the direct operation group on 5-year OS was 0.92 (95% CI = 0.72–1.18; P = .52) (Fig. 2). There was no significant difference in 5-year OS for the 2 groups.
Figure 2.
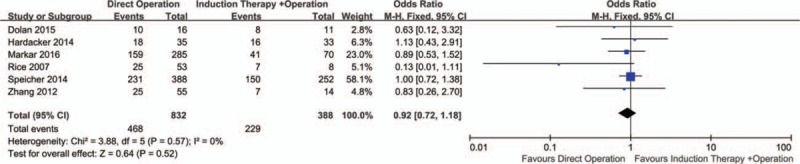
Forest plot of the comparison between induction therapy and direct operation group for 5-year OS. OS = overall survival.
3.3. Pathological stage understaged
Seven studies including 2585 patients reported pathological T stage and N stage understaged. Six studies including 2230 patients reported pathological stage understaged. The pooled analysis showed that compared with induction therapy group, T stage (OR = 1.23; 95% CI = 1.02–1.48; P = .03), N stage (OR = 1.47; 95% CI = 1.22–1.76; P < .0001) and pathological stage (OR = 1.38; 95% CI = 1.15–1.64; P = .0004) were significant understaged in the direct operation group (Fig. 3). However, the weight of Speicher[8] is too large, and after removing this study, it was found that there was no significant difference with T stage (OR = 1.06; 95% CI = 0.81–1.38; P = .68). In contrast, N stage (OR = 1.70; 95% CI = 1.12–2.59; P = .01) and pathologic stage (OR = 1.38; 95% CI = 1.05–1.81; P = .02) still had significant differences. In the direct operation group, the degree of N stage underestimation was higher than T stage (35.1% versus 30.1%).
Figure 3.
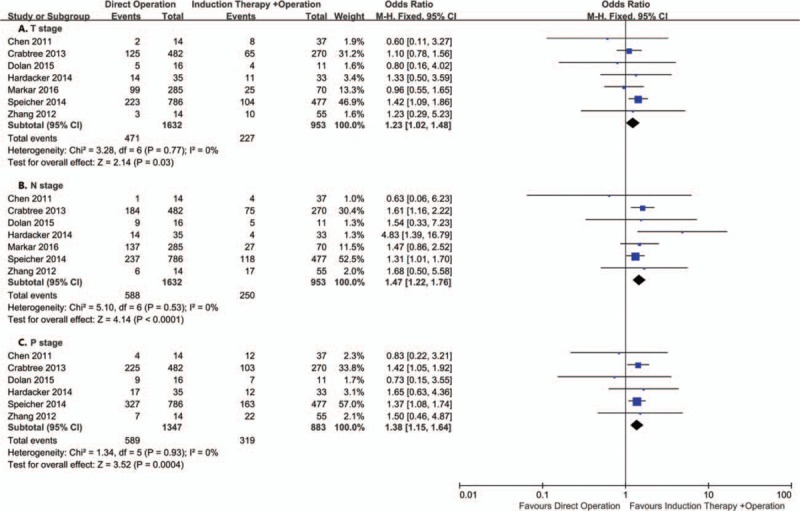
Forest plot of the comparison between induction therapy and direct operation group for understaged.
3.4. Pathological stage overstaged
Same as pathological stage understaged, 7 studies including 2585 patients reported pathological T stage and N stage overstaged and 6 studies including 2230 patients reported pathological stage overstaged. The pooled analysis showed that compared with direct operation group, T stage (OR = 0.73; 95% CI = 0.62–0.87; P = .0003), N stage (OR = 0.60; 95% CI = 0.50–0.72; P < .00001) and pathological stage (OR = 0.70; 95% CI = 0.58–1.69; P = .007) were significant overstaged in the induction therapy group (OR = 0.67; 95% CI = 0.54–0.84; P = .0001) (Fig. 4). Similarly, after removing the study of Speicher,[8] there were no significant difference with T (OR = 0.82; 95% CI = 0.65–1.05; P = .12) and pathologic stage (OR = 0.78; 95% CI = 0.58–1.04; P = .09).
Figure 4.
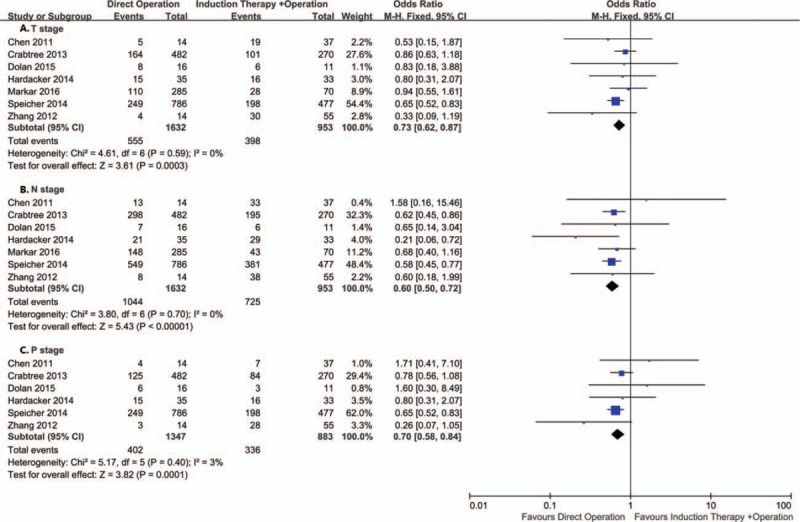
Forest plot of the comparison between induction therapy and direct operation group for overstaged.
3.5. Heterogeneity and publication bias
No heterogeneity were found among the studies, therefore a fixed-effect model was applied. No publication bias was detected by the Egger test when the data of pathological T stage was used as the outcome (P = .20, 95% CI = −1.87–0.51, Supplementary Fig. 1).
4. Discussion
In this study, the results showed that neoadjuvant therapy for cT2N0M0 esophageal cancer patients did not improve survival. Speicher[8] reported that induction therapy was not independently associated with risk of death. Zhang[10] reported induction therapy did not translate into prognostic benefits in survival, but they recommend neoadjuvant therapy to all cT2N0 patients because of understaged cT2N0 patients. However, Markar[7] reported that surgery alone treatment approach could achieve similar short- and long-term outcomes compared with neoadjuvant therapy. So they recommended surgery alone treatment as the primary treatment. Furthermore, Rice reported induction therapy decreased 5-year OS compared with direct operation.[12]
Induction therapy for cT2 N0M0 did not translate into an significant improvement in survival. Some previous studies confirmed that induction therapy could improve the survival compared to direct operation in patients with locally advanced stages of esophageal cancer.[13,14] Actually, understaged cT2N0M0 is locally advanced stages esophageal cancer. These patients should be recommended for induction therapy. Dolan[15] also reported that understaged cT2N0M0 patients with postoperative adjuvant therapy had better survival than surgery alone. However, overstaged cT2N0M0 is T1 stage esophageal cancer. Surgical resection of primary tumor and regional lymph nodes may be enough to control locoregional disease. We hypothesized that induction therapy for understaged cT2N0M0 patients could have resulted in superior survival, but it may increased the risk of treatment-related death for all other staged cT2N0M0 patients. We need to improve the stage methods for patients with esophageal cancer to avoid unnecessary preoperative treatment.
There was a distinct difference between the 2 groups in postoperative pathology staging. Compared with induction therapy group, there were more understaged and less overstaged in the direct operation group. The results indicated that induction therapy may make the clinical stage lower. Although CT, EUS, and PET-CT were used to accurately determine the correct pathologic stage of cT2N0 patients, they were still unreliable.[5,6,16] Induction therapy may improve survival outcomes in understaged patients,[1] but it did not translate into better survival in all cT2N0 patients. In fact, induction therapy can down stage tumor and improve survival outcome for advanced esophageal carcinoma.[17,18] However, a large group of patients were clinically overstaged, the efficacy of neoadjuvant therapy may not be significant for all cT2N0 patients. Rice reported that overstaged patients treated by surgery alone had a 5-year OS similar to that of patients with pathological TNM (pTNM), and understaged patients had a better 5-year survival than patients with pTNM if they had postoperative adjuvant therapy. But induction therapy decreased 5-year survival compared with undergoing direct surgery.[12]
There are several limitations in this study. First, only retrospective studies on induction therapy for cT2N0M0 esophageal cancer have been published. In this study, the mean age of induction therapy group was lower than direct operation group. There was a physician selection bias. Smaller tumors, older patients, more complications might be recommended direct operation. Second, the response to neoadjuvant CRT is different according to different histology of the tumor, however, most of the studies included in adenocarcinoma and squamous cell carcinoma patients. The squamous cell carcinoma patients showed a better survival outcome than those with adenocarcinoma.[19] In addition, the radiation dose, fractionation and chemotherapy agents were different.
5. Conclusions
In summary, current clinical staging T2N0 esophageal carcinoma remains inaccurate. In this study, we found that direct operation group had more pathologically understaged and less overstaged after esophagectomy compared with induction therapy group. Induction therapy couid degrade the tumor staging but not improve the patient's survival.
Author contributions
Data curation: HongWei Lv, Si-Ning Shen, Ji-Wei Cheng.
Formal analysis: Wen-Qun Xing.
Investigation: HongWei Lv.
Methodology: HongWei Lv, Wen-Qun Xing, Si-Ning Shen, Ji-Wei Cheng.
Software: HongWei Lv, Ji-Wei Cheng.
Writing – original draft: HongWei Lv, Ji-Wei Cheng.
Writing – review & editing: HongWei Lv, Si-Ning Shen.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, cT2N0M0 = clinical T2N0M0, CRT = chemoradiotherapy, OR = odds ratios, OS = overall survival, pTNM = pathological TNM.
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
References
- [1].Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226–34. [DOI] [PubMed] [Google Scholar]
- [2].Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416–22. [DOI] [PubMed] [Google Scholar]
- [3].Killinger WA, Jr, Rice TW, Adelstein DJ, et al. Stage II esophageal carcinoma: the significance of T and N. J Thorac Cardiovasc Surg 1996;111:935–40. [DOI] [PubMed] [Google Scholar]
- [4].Song Y, Tao G, Guo Q, et al. Survival benefit of surgery with radiotherapy vs surgery alone to patients with T2-3N0M0 stage esophageal adenocarcinoma. Oncotarget 2016;7:21347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shin S, Kim HK, Choi YS, et al. Clinical stage T1-T2N0M0 oesophageal cancer: accuracy of clinical staging and predictive factors for lymph node metastasis. Eur J Cardiothorac Surg 2014;46:274–9. [DOI] [PubMed] [Google Scholar]
- [6].Crabtree TD, Yacoub WN, Puri V, et al. Endoscopic ultrasound for early stage esophageal adenocarcinoma: implications for staging and survival. Ann Thorac Surg 2011;91:1509–15. [DOI] [PubMed] [Google Scholar]
- [7].Markar SR, Gronnier C, Pasquer A, et al. Role of neoadjuvant treatment in clinical T2N0M0 oesophageal cancer: results from a retrospective multi-center European study. Eur J Cancer 2016;56:59–68. [DOI] [PubMed] [Google Scholar]
- [8].Speicher PJ, Ganapathi AM, Englum BR, et al. Induction therapy does not improve survival for clinical stage T2N0 esophageal cancer. J Thorac Oncol 2014;9:1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen WH, Chao YK, Chang HK, et al. Long-term outcomes following neoadjuvant chemoradiotherapy in patients with clinical T2N0 esophageal squamous cell carcinoma. Dis Esophagus 2012;25:250–5. [DOI] [PubMed] [Google Scholar]
- [10].Zhang JQ, Hooker CM, Brock MV, et al. Neoadjuvant chemoradiation therapy is beneficial for clinical stage T2 N0 esophageal cancer patients due to inaccurate preoperative staging. Ann Thorac Surg 2012;93:429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hardacker TJ, Ceppa D, Okereke I, et al. Treatment of clinical T2N0M0 esophageal cancer. Ann Surg Oncol 2014;21:3739–43. [DOI] [PubMed] [Google Scholar]
- [12].Rice TW, Mason DP, Murthy SC, et al. T2N0M0 esophageal cancer. J Thorac Cardiovasc Surg 2007;133:317–24. [DOI] [PubMed] [Google Scholar]
- [13].Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681–92. [DOI] [PubMed] [Google Scholar]
- [14].van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–84. [DOI] [PubMed] [Google Scholar]
- [15].Dolan JP, Kaur T, Diggs BS, et al. Significant understaging is seen in clinically staged T2N0 esophageal cancer patients undergoing esophagectomy. Dis Esophagus 2016;29:320–5. [DOI] [PubMed] [Google Scholar]
- [16].Crabtree TD, Kosinski AS, Puri V, et al. Evaluation of the reliability of clinical staging of T2 N0 esophageal cancer: a review of the society of thoracic surgeons database. Ann Thorac Surg 2013;96:382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sanford NN, Catalano PJ, Enzinger PC, et al. A retrospective comparison of neoadjuvant chemoradiotherapy regimens for locally advanced esophageal cancer. Dis Esophagus 2017;30:1–8. [DOI] [PubMed] [Google Scholar]
- [18].Samson P, Puri V, Robinson C, et al. Clinical T2N0 esophageal cancer: identifying pretreatment characteristics associated with pathologic upstaging and the potential role for induction therapy. Ann Thorac Surg 2016;101:2102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].De Vita F, Di Martino N, Orditura M, et al. Preoperative chemoradiotherapy for squamous cell carcinoma and adenocarcinoma of the esophagus: a phase II study. Chest 2002;122:1302–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


