Abstract
The use of fibular graft for the reconstruction of bone defects has been demonstrated to be a reliable method. The aim of this study was to assess the clinical outcome of graft union, functional outcome (hypertrophy of the graft bones) and complications of both non-vascularized and vascularized grafts.
From 1981 to 2015, 10 patients were treated using non-vascularized fibular graft or free vascularized fibular graft. The outcomes were bony union time, graft hypertrophy and complications based on radiograph and functional outcomes according to the Musculoskeletal Tumor Society (MSTS) score. Mobility of the ankle at the donor site was evaluated using the Kofoed ankle score system.
This study included 10 patients with an average follow-up of 6.8 years. The union rate for all patients was 100%. The mean union time was 21.3 weeks for vascularized fibular grafts and 30.5 weeks for non-vascularized fibular grafts (P = .310). There was a significant difference between the upper limbs and the lower limbs regarding hypertrophy of the grafts in 5 patients (P = .003). The mean MSTS score in 10 patients was 84% (range 53%–97%). Stress fracture of the graft occurred in 1 patient. Donor site complications, including valgus deformity and length discrepancy, between 2 legs occurred in 2 patients who were under 18 years of age at the time of operation (P = .114). The mean Kofoed score was 96.8 (range 88–100).
A greater increase in hypertrophy of grafts was observed with reconstruction in the lower limbs. There was no difference in MSTS score between these 2 types of grafts. Children were more likely to experience the valgus deformity at the donor site after harvesting the fibula. Keeping at least the distal 1/4 of the fibula intact during the surgery is a valid means of ensuring ankle stability at the donor site, and children should be considered for prophylactic distal tibiofibular synostosis creation to prevent the valgus deformity of the ankle at the donor site.
Keywords: bone defect reconstruction, donor site complication, fibular graft, functional evaluation
1. Introduction
Bone recalcitrant nonunion and bone defects usually follow trauma, resection of a malignant tumor of the musculoskeletal system and osteomyelitis. Instead of amputation, which was used in the past, limb salvage surgeries, including non-vascularized or vascularized autografts and allografts,[1–3] bone transport and replacement with prostheses,[4,5] are performed. Among the above methods, non-vascularized autogenous bone graft use is an important treatment strategy that has been performed for over a hundred years since 1911.[6] In 1975, Taylor GI successfully used the free vascularized fibular graft for the reconstruction of skeletal defects.[7] The fibula is a tubular bone that has a suitable length, geometrical shape and mechanical strength and is considered to be the best donor bone for large bone defects.[8] Free vascularized fibular grafts achieve higher union rate than non-vascularized fibular grafts in the reconstruction of long bone defects.[9–25]
Currently, however, most studies have focused on the bony union rate or the functional outcome of the recipient site after the fibular graft, but few studies have reported on donor site complications and the ankle functional outcome of the donor site. Additionally, no studies have reported changes in hypertrophy of the grafts more than 10 years after the fibular graft surgery.
In this study, 10 patients with bone defects treated by non-vascularized fibular graft or free vascularized fibular graft were included. We intended to assess the clinical outcomes of graft union, functional outcome, hypertrophy of the graft bones and complications, which may be influenced by various factors from both non-vascularized and vascularized grafts.
2. Patients and methods
2.1. Patients
This study was a retrospective review and analysis approved by the Wuhan University institutional review boards. Between 1981 and 2015, 10 patients (mean age at operation 24.8 years, range 6 to 56 years, female/male = 4/6) had undergone surgery using a non-vascularized fibular graft or free vascularized fibular graft (vascularized/non-vascularized = 4/6) for reconstructions, and we excluded those with a follow-up period of less than 1 year; the average follow-up period was 6.8 years (range 1.0–26.0 years). The indication for surgery was a bone defect caused by a fracture (n = 2), osteomyelitis with a bony defect (n = 2) (Figs 1A and 3A), resection of fibrous dysplasia of bone (n = 3), or resection of a tumor (n = 3) (Fig. 2A). The recipient sites for the grafts included the femur (n = 3), radius (n = 2), tibia (n = 3), humerus (n = 1), and ilium (n = 1). Table 1 shows the patients’ data.
Figure 1.
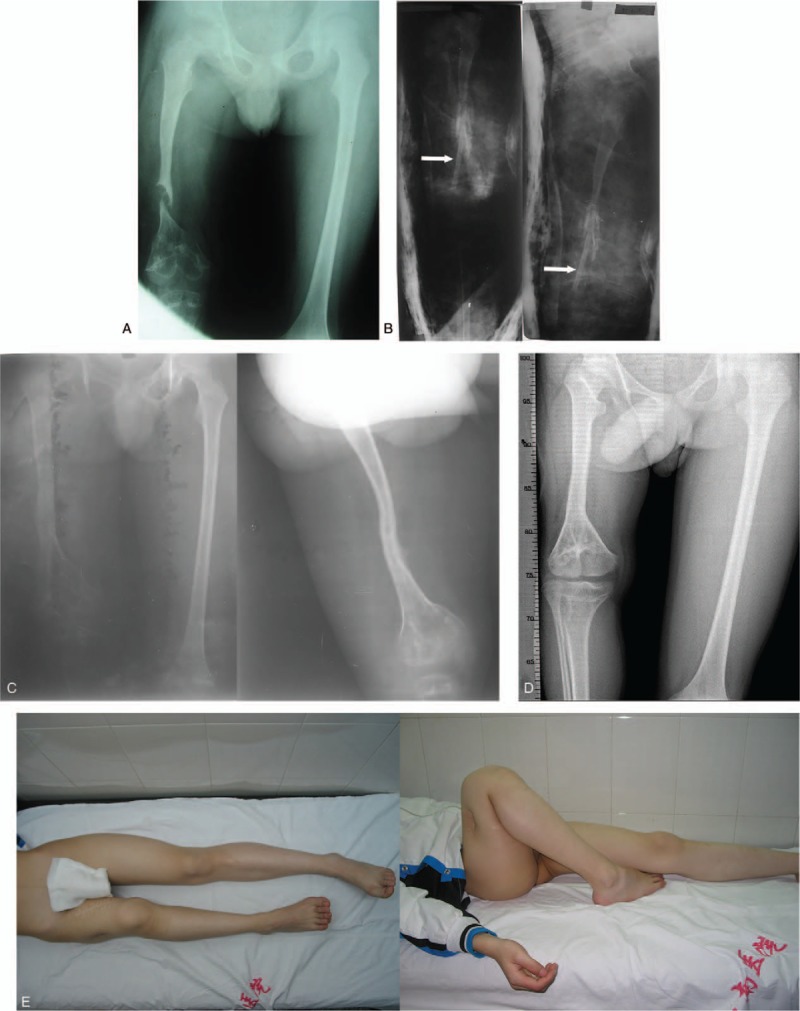
A: A 6-year-old boy was diagnosed with a massive bone defect in the right femur caused by osteomyelitis. B: Non-vascularized fibular graft (white arrows) harvested for construction. C: At 59 weeks after the surgery, the graft was consolidated. D, E: At 144 months after the surgery, significant hypertrophy of graft was achieved with excellent functional results, but there was a 22 cm discrepancy between his left and right lower extremities.
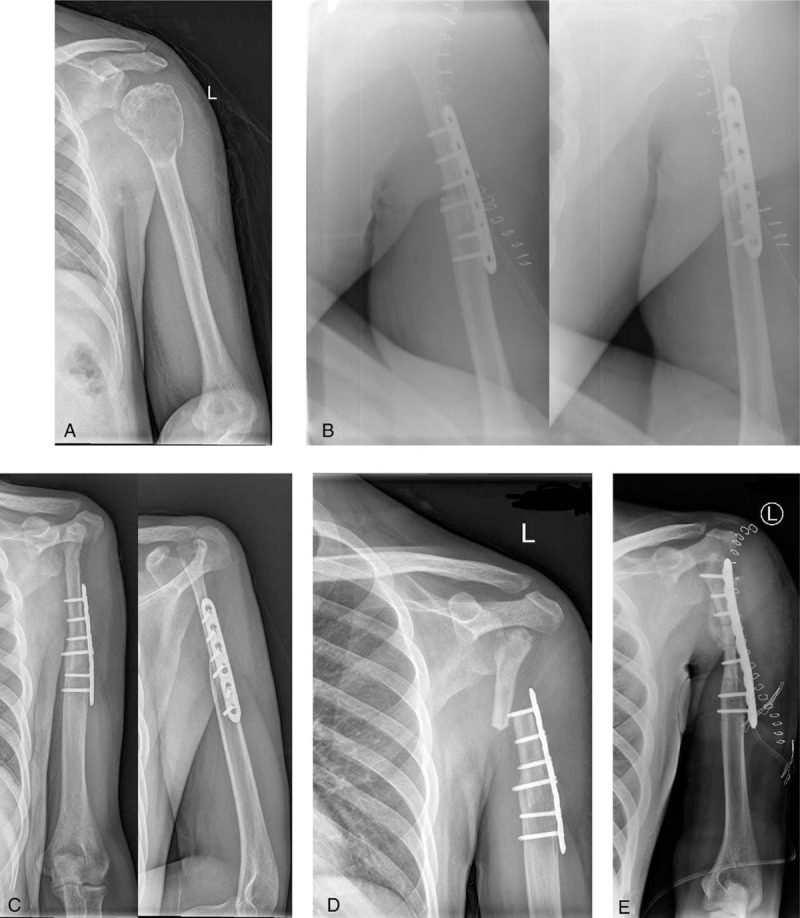
Figure 3.
A: An 8-year-old boy was diagnosed with a bone defect in the right radius caused by osteomyelitis. B: Free vascularized fibular graft (white arrows) harvested for construction. C: At 20 weeks after the surgery, the graft was consolidated. D, E: Radiographs taken 216 months postoperatively show excellent bony healing and functional results. F, G: A significant valgus deformity at the donor site and a length discrepancy between the 2 legs occurred at the last follow-up.
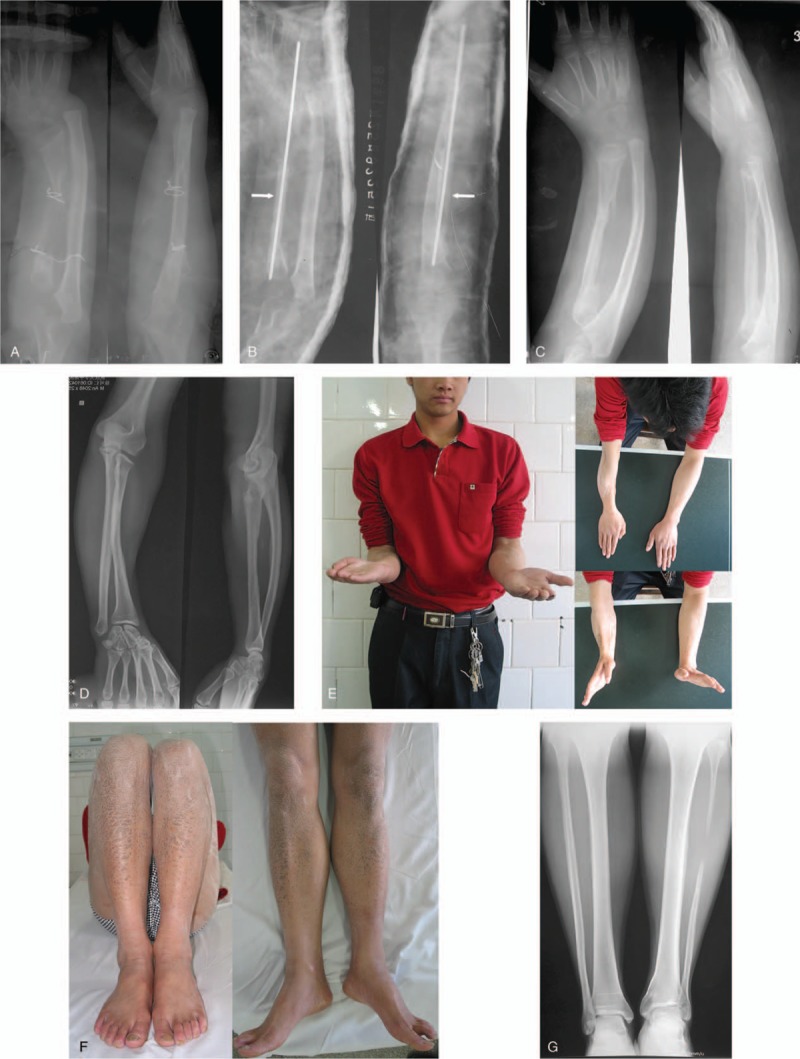
Figure 2.
A: A 28-year-old man was diagnosed with a left proximal humeral giant cell tumor. B: After resection of the tumor, the defect in the left humerus was reconstructed with a free vascularized fibular head graft. C: At 21 weeks after the surgery, bony healing was obtained without significant hypertrophy. D: At 7 months after the operation, a stress fracture occurred at the level of the proximal extreme of the plate. E: The fracture was treated with plate removal, iliac bone graft and fixation with a new plate.
Table 1.
Patient characteristics.
2.2. Surgical techniques
The fibulas were obtained using a posterior lateral approach, and at least the distal 1/4 length of the fibulas in all patients were retained to ensure the stability of the ankle. With the exception of 4 patients who previously had bone fractures with internal/external fixation or debridement of osteomyelitis, all patients had tumor resection or debridement and reconstruction at the same time. The graft used no fixation devices for stabilization of the ilium after resection of the tumor in 1 case. In 3 cases, the fibula grafts were wedged into the bone as an intercalary segment for reconstruction or only fixed with Kirschner wire. The reconstruction was stabilized with a plate in 6 cases. Overall, 10 patients underwent a reconstruction with a single fibular graft. Allograft or iliac bone grafts were used as a supplement in 6 patients. In 1 case, the medial formal condylus osteoperiosteal flap was pedicled with the descending genicular vessels at the same time that the fibular graft was placed to cover the bone defect and the graft[26] (Fig. 1). None of the patients had undergone treatment with chemotherapy or radiotherapy. The mean length of the fibula was 9.9 cm (range 6–16).
2.3. Methods of follow-up and statistical analysis
The patients were followed systematically until bone healing was accomplished. Bone healing, hypertrophic changes of the graft and surgical complications (such as stress fracture and nonunion) were confirmed by plain radiographs or tomograms if necessary. Bony union of the graft was evaluated by the occurrence of bony trabeculae bridging on at least plain radiography at 1- to 3-month intervals. The hypertrophy of the graft was evaluated using diameters of recipient bone and the graft determined from the plain radiographs both immediately post-surgery and at the final follow-up according to the hypertrophy index of De Boer and Wood.[27] The patients were evaluated functionally using the Musculoskeletal Tumor Society (MSTS) score[28] at the final follow-up. The system of MSTS assigns numerical values (0–5) for each of 6 categories: pain, function and emotional acceptance in upper and lower extremities; supports, walking and gait in the lower extremity; hand positioning, dexterity and lifting ability in the upper extremity. Demographic information and a patient satisfaction component are included. Ankle mobility of the donor site was also assessed using the Kofoed ankle score system.[29] The system includes 3 categories: pain, function and mobility, with a total points of 100. A score greater than 85 means excellent, between 75 and 85 means good and less than 75 means unacceptable.
The differences were determined using 1-way analysis of variance (ANOVA) and the chi-square test. Statistical analysis was performed using SPSS software (version 19.0; SPSS, Chicago, IL). A P value < .05 indicated statistical significance.
3. Results
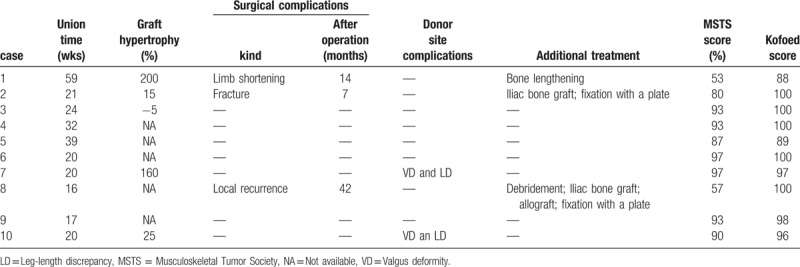
Detailed data are presented in Table 2.
Table 2.
Results.
3.1. Bony union and hypertrophy
Primary bony union was achieved in all 10 patients (100%) at both the proximal and distal ends of the graft segment. The average time to consolidation for bony healing was 26.8 weeks (range 16–59 weeks). There was no significant difference between vascularized grafts and non-vascularized grafts (P = .310), and the extra treatment with allograft or iliac bone graft did not influence bony union (P = .618). Moreover, the method of stabilization between the plate and other type of fixation had no significant effect on bony healing (P = .599). Graft hypertrophy was detected in 5 cases. In people who underwent reconstruction of the pelvis or for whom the graft was inlayed in the bone, accurate detection of graft hypertrophy was difficult to achieve by plain radiographs. Significant hypertrophy of more than 20% was detected in 3 cases, and the other 2 cases did not demonstrate significant changes (less than 20%). The type of the graft (P = .170) and the method of stabilization (P = .171) also had no influence on graft hypertrophy. Two patients had reconstructions of the lower extremity, and 3 patients had reconstructions of the upper limb, and there was a significant difference between them (P = .003).
3.2. Functional outcome
The average overall MSTS score at the last follow-up was 84% (range 53%–97%). Two patients had low MSTS scores: a boy (case 1) with a score of 53% who had his right femoral shaft replaced and who had a right lower extremity that was 22 cm shorter than the left lower extremity (Fig. 1E), and a girl (case 8) with a low score of 57% who underwent reconstruction of her femur and who later suffered a recurrence of fibrous dysplasia with fracture. The MSTS score of patients with non-vascularized fibular grafts (n = 6) was 80% (range 53% to 97%), whereas the MSTS score of patients with vascularized fibular grafts (n = 4) was 90% (range 80%–97%). Among the 10 patients, we found no significant difference in the MSTS scores between the vascularized fibular graft and non-vascularized fibular graft groups (P = .366).
3.3. Complications
No significant complications occurred in the intraoperative or immediate postoperative period. There was no problem with regard to the blood supply of the vascularized fibular graft shortly after the operation. Delayed postoperative complications, including fracture, occurred in 2 patients (20%). One patient had a stress fracture (Fig. 2D), and the other patient was diagnosed with a recurrence of fibrous dysplasia with fracture (case 8). Both fractures occurred after the graft union was achieved. Both cases were initially treated by internal fixation. Case 2 underwent a plate removal, iliac bone graft and fixation with a new plate (Fig. 2E). Case 8 underwent debridement, iliac bone graft, allograft and fixation with a plate. Both cases achieved bone healing after the surgery. A severe limb shortening occurred in 1 case (case 1) with a 22-cm discrepancy between his left and right lower extremities (Fig. 1D). This patient underwent a right femur lengthening with an external fixator 11 years after the reconstruction surgery.
Complications occurred at the donor site in 2 patients (case 7 and 10; 20%), as observed on radiographs of the donor site. Both patients had a mild valgus deformity of the ankle without ankle pain. The valgus deformity was 4.0 degrees in case 7 and 10.3 degrees in case 10. The distal fibula was 4.5 mm upward in case 7 and 3.2 mm in case 10. The 2 patients also had a length discrepancy between the 2 legs (Fig. 3F and 3G). At the time of operation, 5 patients were aged greater than or equal to 18 years, and 5 patients were aged less than 18 years. There was no significant difference between the 2 groups (P = .114). There were no painful neuromata, vascular injuries, long-lasting ankle pain or nerve injuries identified in any of the 10 patients. The mean Kofoed score was 96.8 (range 88–100), and overall, the 10 patients had excellent scores (>85 points) and function of the ankle at the donor site.
4. Discussion
Reconstruction of bone defects caused by trauma, osteomyelitis, or tumor resection has always been a problem for orthopedic surgeons. There have been many recent reports of the successful application of vascularized fibular grafts,[7,9–15,24] and non-vascularized grafts are rarely mentioned at present.
In a series of bone defects treated by vascularized fibular grafts, bone consolidation was reported to be obtained in 86% to 95% of cases at a mean of 3.6 to 12 months.[8,12,13,17,30] Enneking et al[20] found a primary union rate in 63% of non-vascularized fibular grafts within the first 12 months through clinical trials involving long bone reconstructions. In our study, the overall bony union rate was 100%, and the average time to consolidation for bony union was 26.8 weeks (range 16–59 weeks). There was no significant difference between the mean union time for vascularized fibular grafts (21.3 weeks) and non-vascularized fibular grafts (30.5 weeks) (P = .310). Han et al[31] reported that reconstruction of a skeletal defect due to osteomyelitis using a vascularized bone graft might not have a satisfactory result. In our study, however, both patients with osteomyelitis achieved primary bony union, which suggested that a thorough debridement is necessary before a fibular graft is placed in patients with osteomyelitis.
Krieg et al found a significant difference in hypertrophy between vascularized and non-vascularized fibular grafts.[18] It was mentioned in the literature[12,13,17,27] that hypertrophy of vascularized fibular grafts varies between 37% and 90%; by comparison, hypertrophy occurs in an average of 32% of non-vascularized grafts. In clinical research on vascularized fibular grafts, only 43% of vascularized fibular grafts achieved hypertrophy after 12 months,[27] and extreme hypertrophy occurred between 2 and 3 years based on the literature.[17,27] Enneking et al[20] found that 32% of non-vascularized fibular grafts demonstrated biological activity and hypertrophy. If there was a small diameter in contact at the recipient site, such as in the bones of the upper extremities, the diameter of the graft was unlikely to increase notably, which these authors did not take into consideration. In our study (Figs 1D, 2C and 3D), among the 5 grafts that could be detected, 4 of them increased in diameter. In the present study, we found a significant difference in hypertrophy (P = .003) when the host site was changed from the upper limbs to the lower limbs and it was reported earlier.[12] Studies have suggested that hypertrophy was achieved earlier in grafts without internal fixation, such as screws or plates, and there was a greater degree of hypertrophy due to the higher mechanical load on the junctions.[27] We also found that grafts fixed with a nail or a plate had a mean 128.3% change in hypertrophy compared to 5% for the grafts without nail or plate (P = .171). According to Soldado et al,[32–34] an extra vascularized periosteal placement might accelerate the bone union due to a unique osteo- and angiogenic potential. In our study, case 1 underwent a non-vascularized fibular graft and a transplant of the medial formal condylus osteoperiosteal flap pedicled with the descending genicular vessels,[26] which achieved a 200% hypertrophy, which is in keeping with these reports.
Among the 10 patients, we found no significant difference between the vascularized fibular graft and non-vascularized fibular graft groups in the MSTS scores (P =.366). However, using the MSTS score might have limitations, such as how the reconstructions were assessed. As with the population, the evaluation of function may be influenced by the ages of patients; therefore, the true function might not be reflected by the MSTS scores.
Non-vascularized fibular graft is a simpler, less expensive and a shorter procedure than the use of vascularized graft and allows remodeling of the fibula at the donor site.[18] However, vascularized graft is a better choice when there is not a good cover with soft tissue and good blood supply or stabilization of the graft is difficult. Vascularized graft should be also used primarily in the lower leg and the forearm.
Stress fracture was 1 of the most prevalent delayed postoperative complications of the graft, occurring in between 15% and 40% of cases according to previous studies.[13,27,30] In our study, stress fractures occurred in 10% of cases, and the patient who had his graft fractured underwent proximal humerus reconstruction after giant cell tumor resection using a free vascularized fibular head graft. The fracture occurred after the union of the graft junctions had been consolidated.
Donor site complications include painful neuromata, vascular injury, long-lasting ankle pain, nerve injury, and ankle instability. Complication rates for vascularized fibular graft are between 7% and 35%, as reported in the literature,[13,30,35–37] whereas the rate for non-vascularized fibular grafts vary between 4% and 16%.[18,20,38,39] A common complication is valgus deformity of the ankle at the donor site in children, and it is reported to occur in 10% to 42% of cases according to the literature.[13,40,41] In our study, this complication occurred in 2 of our patients (20%). Although we did not find a significant difference in age, the complication seems to occur more frequently in people aged less than 18 years old (40%) compared to those 18 years and older (P = .114). Nathan et al[37] found that children were more likely to develop ankle instability than adults, and our study confirmed this finding. Excellent ankle functional outcomes were obtained at the donor site according to the Kofoed ankle score system, which supported that keeping at least the distal 1/4 of the fibula intact during the surgery is a reliable option to ensure ankle stability at the donor site. Although has a potential danger of arthrosis of the ankle joint, a primary synostosis of the distal tibia and fibula it might be advisable for children to prevent the valgus deformity of the ankle at the donor site.
An advantage of our study was the time of follow-up; 3 patients were followed for more than 10 years. Another advantage was the evaluation of the ankle at the donor site, which presented excellent results in all patients. A limitation in the present study was that the MSTS scores may not assess the exact function of the limbs; a score defined by quality of life could probably be used to increase the accuracy of functional evaluation. The most serious weakness and limitation was the number of patients, and the results may be more relevant as we increase the number of cases in the future.
5. Conclusions
The application of fibular grafts in the reconstruction of bone defects caused by trauma, osteomyelitis or tumor resection represents an effective treatment option. Compared to vascularized fibular grafts, non-vascularized fibular grafts may need a longer time until union and should not be used when there is not a good cover with soft tissue and good blood supply or stabilization of the graft is difficult, but non-vascularized fibular graft is a simpler, less expensive and a shorter procedure than the use of vascularized graft. In terms of graft hypertrophy, similar hypertrophy can be obtained by both the abovementioned fibular grafts, but a greater increase in graft diameter might be achieved if the reconstruction is in the lower limbs. An extra transplant of vascularized periosteum might facilitate graft union and hypertrophy. In regards to functional outcome, patients had an average MSTS score of 84%, and there was no significant difference between non-vascularized and vascularized grafts. For donor site complications, a potential valgus deformity of the ankle should be considered before performing a fibular graft surgery on a child regardless of whether a vascularized or non-vascularized fibular graft is employed. Additionally, keeping at least the distal 1/4 of the fibula intact is a valid way to ensure ankle stability at the donor site, and children should be considered for prophylactic distal tibiofibular synostosis creation to prevent the valgus deformity of the ankle at the donor site.
Acknowledgments
The authors would like to acknowledge and express gratitude for the generous permission and consent provided by the patients.
Author contributions
Conceptualization: Siyi Liu, Jinhai Tan.
Data curation: Siyi Liu.
Formal analysis: Siyi Liu.
Funding acquisition: Siyi Liu, Jinhai Tan, Shengxiang Tao.
Investigation: Siyi Liu, Huiyi Liu, Zonghuan Li.
Methodology: Siyi Liu.
Project administration: Siyi Liu, Shengxiang Tao, Jinhai Tan.
Resources: Siyi Liu, Shengxiang Tao, Jinhai Tan.
Software: Siyi Liu.
Supervision: Xiang Hu.
Writing – original draft: Siyi Liu.
Writing – review & editing: Siyi Liu, Jinhai Tan, Xiang Hu, Zonghuan Li.
Siyi Liu orcid: 0000-0002-6733-3497
Footnotes
Abbreviation: MSTS = Musculoskeletal Tumor Society.
This study was supported by the Hubei Province Health and Family Planning Scientific Research Project (WJ2018H0001) and the Innovation and Cultivation Program funded by Zhongnan Hospital of Wuhan University (znpy2017030).
The authors have no conflicts of interest to disclose.
References
- [1].Brigman BE, Hornicek FJ, Gebhardt MC, et al. Allografts about the knee in young patients with high-grade sarcoma. Clin Orthop Relat Res 2004;421:232–9. [DOI] [PubMed] [Google Scholar]
- [2].Dick HM, Malinin TI, Mnaymneh WA. Massive allograft implantation following radical resection of high-grade tumors requiring adjuvant chemotherapy treatment. Clin Orthop Relat Res 1985;197:88–95. [PubMed] [Google Scholar]
- [3].Mankin HJ, Gebhardt MC, Jennings LC, et al. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res 1996;324:86–97. [DOI] [PubMed] [Google Scholar]
- [4].Upton J, Kocher MS, Wolfort FG. Reconstruction following resection of malignancies of the upper extremity. Surg Oncol Clin N Am 1996;5:847–92. [PubMed] [Google Scholar]
- [5].Wang J, Shen J, Dickinson IC. Functional outcome of arthrodesis with a vascularized fibular graft and a rotational latissimus dorsi flap after proximal humerus sarcoma resection. Ann Surg Oncol 2011;18:1852–9. [DOI] [PubMed] [Google Scholar]
- [6].Walter M. Resection de l’extremite inferieure du radius pour osteosarcome: greffe de l’extremité supériuie du péroné. Bull Et Mem Soc de Chir de Par 1911;37:739–47. [Google Scholar]
- [7].Taylor GI, Miller GD, Ham FJ. The free vascularized bone graft: a clinical extension of microvascular techniques. Plast Reconstr Surg 1975;55:533–44. [DOI] [PubMed] [Google Scholar]
- [8].Keiichi Muramatsu, Koichiro Ihara, Kazuteru Doi, et al. Reconstruction of massive femur defect with free vascularized fibula graft following tumor resection. Anticancer Res 2006;26:3679–84. [PubMed] [Google Scholar]
- [9].Hariri A, Mascard E, Atlan F, et al. Free vascularised fibular graft for reconstruction of defects of the lower limb after resection of tumour. J Bone Joint Surg Br 2010;92:1574–9. [DOI] [PubMed] [Google Scholar]
- [10].Amr SM, El-Mofty AO, Amin SN, et al. Reconstruction after resection of tumors around the knee: role of the free vascularized fibular graft. Microsurgery 2000;20:233–51. [DOI] [PubMed] [Google Scholar]
- [11].Kryger Zol B, Dumanian Gregory A. Reconstruction of extremity long bone defects after sarcoma resection with vascularized fibula flaps: a 10-year review. Plast Reconstr Surg 2007;119:915–26. [DOI] [PubMed] [Google Scholar]
- [12].Hsu RWW, Wood MB, Sim FH, et al. Free vascularised fibular grafting for reconstruction after tumour resection. J Bone Joint Surg Br 1997;79:36–42. [DOI] [PubMed] [Google Scholar]
- [13].Shea KG, Coleman DA, Scott SM, et al. Microvascularized free fibular grafts for reconstruction of skeletal defects after tumor resection. J Pediatr Orthop 1997;17:424–32. [PubMed] [Google Scholar]
- [14].Minami A, Usui M, Ogino T, et al. Simultaneous reconstruction of bone and skin defects by free fibular graft with a skin flap. Microsurgery 1986;7:38–45. [DOI] [PubMed] [Google Scholar]
- [15].Eward WC, Kontogeorgakos V, Levin LS, et al. Free vascularized fibular graft reconstruction of large skeletal defects after tumor resection. Clin Orthop Relat Res 2010;468:590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Başarir K, Selek H, Yildiz Y, et al. Nonvascularized fibular grafts in the reconstruction of bone defects in orthopedic oncology. Acta Orthop Traumatol Turc 2005;39:300–6. [PubMed] [Google Scholar]
- [17].El Gammal TA, El-Sayed A, Kotb MM. Hypertrophy after free vascularized fibular transfer to the lower limb. Microsurgery 2002;22:367–70. [DOI] [PubMed] [Google Scholar]
- [18].Krieg AH, Hefti F. Reconstruction with non-vascularised fibular grafts after resection of bone tumours. J Bone Joint Surg Br 2007;89:215–21. [DOI] [PubMed] [Google Scholar]
- [19].Krieg AH, Lenze U, Gaston MS, et al. The outcome of pelvic reconstruction with non-vascularised fibular grafts after resection of bone tumours. J Bone Joint Surg Br 2010;92:1568–73. [DOI] [PubMed] [Google Scholar]
- [20].Enneking WF, Eady JL, Burchardt H. Autogenous cortical bone grafts in the reconstruction of segmental skeletal defects. J Bone Joint Surg Am 1980;62:1039–58. [PubMed] [Google Scholar]
- [21].Lawal YZ, Garba ES, Ogirima MO, et al. Use of non-vascularized autologous fibula strut graft in the treatment of segmental bone loss. Ann Afr Med 2011;10:25–8. [DOI] [PubMed] [Google Scholar]
- [22].Chadha M, Arora SS, Singh AP, et al. Autogenous nonvascularized fibula for treatment of giant cell tumor of distal end radius. Arch Orthop Trauma Surg 2010;130:1467–73. [DOI] [PubMed] [Google Scholar]
- [23].Abuhassan FO, Shannak A. Non-vascularized fibular graft reconstruction after resection of giant aneurysmal bone cyst (ABC). Strateg Trauma Limb Reconstr 2010;5:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Donati D, Di Liddo M, Zavatta M, et al. Massive bone allograft reconstruction in high-grade osteosarcoma. Clin Orthop Relat Res 2000;377:186–94. [DOI] [PubMed] [Google Scholar]
- [25].Estrella EP, Wang EH. A comparison of vascularized free fibular flaps and nonvascularized fibular grafts for reconstruction of long bone defects after tumor resection. J Reconstr Microsurg 2017;33:194–205. [DOI] [PubMed] [Google Scholar]
- [26].Chen ZG, Chen XQ, Yu GR, et al. Transposition of medial femoral condylus osteoperiosteal flap pedicled with the descending genicular vessels. Chin J Microsurg 1998;21:174–6. [Google Scholar]
- [27].De Boer HH, Wood MB. Bone changes in the vascularized fibular graft. J Bone Joint Surg Br 1989;71:374–8. [DOI] [PubMed] [Google Scholar]
- [28].Enneking WF, Dunham W, Gebhardt MC, et al. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 1993;286:241–6. [PubMed] [Google Scholar]
- [29].Kofoed H, Stürup J. Comparison of ankle arthroplasty and arthrodesis. A prospective series with long-term follow-up. Foot 1994;4:6–9. [Google Scholar]
- [30].Minami A, Kasashima T, Iwasaki N, et al. Vascularised fibular grafts. An experience of 102 patients. J Bone Joint Surg Br 2000;82:1022–5. [DOI] [PubMed] [Google Scholar]
- [31].Han C-S, Wood MB, Bishop AT, et al. Vascularised bone transfer. J Bone Joint Surg Am 1992;74:1441–9. [PubMed] [Google Scholar]
- [32].Soldado F, Fontecha CG, Barber I, et al. Vascularized fibular periosteal graft: a new technique to enhance bone union in children. J Pediatr Orthop 2012;32:308–13. [DOI] [PubMed] [Google Scholar]
- [33].Soldado F, Garcia FC, Haddad S. Treatment of congenital pseudarthrosis of the tibia with vascularized fibular periosteal transplant. Microsurgery 2012;32:397–400. [DOI] [PubMed] [Google Scholar]
- [34].Soldado F, Diaz-Gallardo P, Sena-Cabo L. Vascularized fibular grafts extended with vascularized periosteum in children. Microsurgery 2017;37:410–5. [DOI] [PubMed] [Google Scholar]
- [35].Weiland A. Current concepts review: vascularized free bone transplants. J Bone Joint Surg Am 1981;63:166–9. [PubMed] [Google Scholar]
- [36].Arai K, Toh S, Tsubo K, et al. Complications of vascularized fibula graft for reconstruction of long bones. Complications of vascularized fibula graft for reconstruction of long bones. Plast Reconstr Surg 2002;109:2301–6. [DOI] [PubMed] [Google Scholar]
- [37].Nathan SS, Hung-Yi L, Disa JJ. Ankle instability after vascularized fibular harvest for tumor reconstruction. Ann Surg Oncol 2005;12:57–64. [DOI] [PubMed] [Google Scholar]
- [38].Yadav SS. Dual-fibular grafting for massive bone gaps in the lower extremity. J Bone Joint Surg Am 1990;72:486–94. [PubMed] [Google Scholar]
- [39].Al-Zharani S, Harding MG, Kremli M, et al. Free fibular graft still has a place in treatment of bone defects. Injury 1993;24:551–4. [DOI] [PubMed] [Google Scholar]
- [40].Pacelli L, Gillard J, McLoughlin SW, et al. A biomechanical analysis of donor-site ankle instability following free fibular graft harvest. J Bone Joint Surg Am 2003;85:597–603. [DOI] [PubMed] [Google Scholar]
- [41].Tang CL, Mahoney JL, Mckee MD, et al. Donor site morbidity following vascularized fibular grafting. Microsurgery 1998;18:383–6. [DOI] [PubMed] [Google Scholar]


