Abstract
Rationale
The endocannabinoid system is under active investigation as a pharmacological target for obesity management due to its role in appetite regulation and metabolism. Exogenous cannabinoids such as tetrahydrocannabinol (THC) stimulate appetite and food intake. However, there are no controlled observations directly linking THC to changes of most of the appetite hormones.
Objectives
We took the opportunity afforded by a placebo-controlled trial of smoked medicinal cannabis for HIV-associated neuropathic pain to evaluate the effects of THC on the appetite hormones ghrelin, leptin and PYY, as well as on insulin.
Methods
In this double-blind cross-over study, each subject was exposed to both active cannabis (THC) and placebo.
Results
Compared to placebo, cannabis administration was associated with significant increases in plasma levels of ghrelin and leptin, and decreases in PYY, but did not significantly influence insulin levels.
Conclusion
These findings are consistent with modulation of appetite hormones mediated through endogenous cannabinoid receptors, independent of glucose metabolism.
Keywords: THC, Cannabinoid, Ghrelin, Leptin, Peptide YY, PYY, Neuroendocrine, Delta-9-tetrahydrocannabinol, Appetite
1. Introduction
Delta-9-Tetrahydrocannabinol (THC), a cannabinoid (CB) receptor partial agonist, is widely recognized to affect appetite and food intake (Williams et al., 1998). This orexigenic action is believed to occur at CB1 receptors in the hypothalamus. While previous studies have demonstrated functional relationships between endogenous endocannabinoid systems and individual appetite hormones in animals (Di Marzo et al., 2001; Tucci et al., 2004), direct, controlled observations in humans have not been reported. In this study, we sought to evaluate the potential effects of THC on appetite mediators in humans.
Insulin, ghrelin, peptide YY (PYY) and leptin are individually modulated in response to food intake and energy homeostasis. The primary role of insulin is to permit cellular uptake of circulating glucose after a meal. Thus, the pancreas releases insulin into the blood after ingestion of carbohydrates, regardless of changes in appetite. Ghrelin, PYY and leptin, are appetite-mediating hormones with differing stimuli for release. Rising ghrelin levels are associated with increased appetite; conversely, ghrelin release from the stomach fundus is downregulated after eating (Castaneda et al., 2010; Cummings et al., 2004). In contrast, PYY release from the gastrointestinal mucosa appears to mediate satiety, with blood levels increasing after food intake (Valassi et al., 2008). Leptin, a hormone secreted by fat cells, acts via hypothalamic receptors to inhibit feeding and increase thermogenesis (Chin-Chance et al., 2000; Kennedy et al., 1997; Valassi et al., 2008). Leptin has been shown to be a key factor in maintaining energy homoeostasis and appears to have dual regulatory functions in this role. In humans maintaining a relatively stable weight, the circadian pattern of leptin secretion is highly regular, showing a large diurnal variation independent of food intake that has a peak around midnight and a trough in the morning, upon which smaller, superimposed drops occur after meals (Cummings et al., 2001). However, leptin responds more dynamically in states of acute energy imbalances (e.g. weight-loss or weight-gain programs) to restore energy homoeostasis (Arora and Anubhuti, 2006; Dardeno et al., 2010).
Few animal studies have examined direct stimulation of CB1 receptors and measured its effects on appetite hormones. Zbucki et al. (2008) demonstrated that a single ip injection of cannabinoid agonist induced increased plasma ghrelin. Animal work has also demonstrated that ghrelin stimulation of appetite depends upon the presence of a functional CB1 receptor (Kola et al., 2008; Tucci et al., 2004).
Oral administration of a CB1 antagonist reduces plasma leptin levels in obese subhuman primates (Wagner et al., 2010), although this decrease could have been due to a coincident weight loss. A single intravenous leptin injection into rats reduces hypothalamic levels of the endocannabinoids, anandamide and 2-arachidonyl-glycerol (2-AG), in the hypothalamus. Conversely, defective leptin signaling is associated with elevated hypothalamic, but not cerebellar, levels of endocannabinoids. Taken together, these experiments show that activation of the cannabinoid system drives the release of leptin and that endocannabinoids in brain areas specifically associated with feeding are under partial negative control of leptin (Di Marzo et al., 2001).
To the best of our knowledge, no studies have been published linking PYY with the cannabinoid system; however, circulating levels of the appetite hormones are interrelated. PYY is thought to inhibit ghrelin release (Cone et al., 2001) while leptin appears to negatively regulate ghrelin (Barazzoni et al., 2003).
To assess the effects of exogenous cannabinoids on appetite hormones, we measured plasma levels of ghrelin, leptin, and PYY in HIV-infected subjects before and after receiving smoked cannabis or placebo in a clinical trial. Because food intake was not controlled in this study, we measured insulin levels to evaluate whether the observed changes in appetite hormones were confounded by food intake.
1. Results
Of the 28 subjects enrolled in the parent clinical trial, 7 elected to participate in this substudy. All were men with documented HIV infection; the mean age was 43.3 (SD 3.3) and the mean years of education was 13 (SD 2.9). Median Body Mass Index (BMI) was 25.24 [IQR 22.59, 31.56]. Five subjects were Caucasian and 2 were African-American. The median current CD4 count was 304 [IQR 262, 515]. Five subjects had undetectable plasma HIV RNA and the remaining two had plasma HIV RNA levels of 4.88 and 4.82 log10 copies per mL. Of the 7 participants, 6 took combination antiretroviral therapy (CART) and the remaining one took no antiretroviral medications. The 6 CART regimens were: lamivudine/stavudine/indinavir, lamivudine/abacavir/tenofovir/boosted lopinavir, lamivudine/abacavir/tenofovir/didanosine/efavirenz, lamivudine/stavudine/zidovudine/efavirenz/boosted fos-amprenavir, stavudine/nevirapine/nelfinavir/saqinavir, and lamivudine/abacavir/tenofovir/fos-amprenavir.
Fig. 1 shows that during the cannabis treatment week THC was readily detected at clinically relevant levels in afternoon samples, but was never detected in the morning samples, consistent with complete wash out of THC in blood from the previous day’s treatment. During the placebo week, THC levels were undetectable at both timepoints.
Fig. 1.
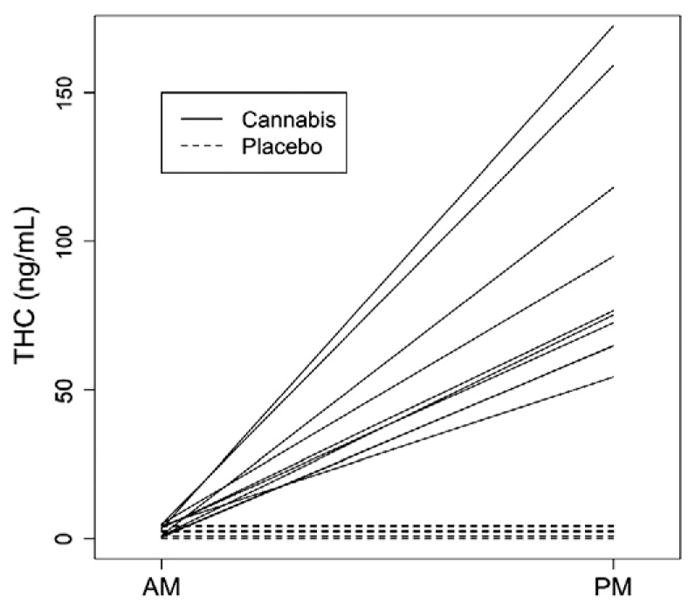
Unadjusted delta-9-tetrahydrocannabinol (THC) levels before and after administration of cannabis (solid lines) or placebo (dotted lines). Each line represents the change in an individual subject’s THC level from the morning (before treatment) and afternoon (after treatment).
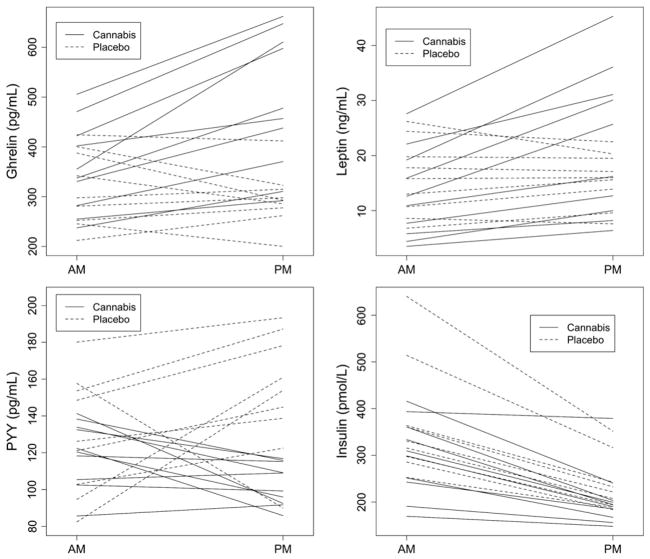
Table 1 displays median hormone levels according to treatment week and sampling time. Morning hormone levels did not significantly differ between the two treatment periods. Fig. 2 shows unadjusted changes in hormone levels from morning to afternoon according to treatment period. On average, both ghrelin and leptin levels increased with cannabis treatment and slightly decreased or remained unchanged with placebo. PYY levels decreased or remained the same with cannabis, whereas with placebo all but one of the participants’ PYY levels increased. Insulin levels decreased from morning to afternoon regardless of treatment.
Table 1.
Blood hormone levels [median (IQR)] according to measurement time.
| Group | Sample time (AM/PM) | Ghrelin (pg/ml) | Leptin (ng/ml) | PYY (pg/ml) | Insulin (pmol/l) |
|---|---|---|---|---|---|
| Placebo | AM | 298 (253, 388) |
15.8 (10.7, 19.8) |
126 (103, 154) |
331 (298, 364) |
| PM | 293 (278, 315) |
16.0 (13.9, 19.5) |
154 (139, 178) |
222 (194, 243) |
|
| Cannabis | AM | 346 (294, 417) |
11.8 (6.3, 18.4) |
121 (109, 134) |
304 (245, 354) |
| PM | 467 (387, 608) |
21.0 (10.7, 30.9) |
104 (93, 114) |
192 (172, 203) |
Fig. 2.
Unadjusted plasma hormone levels before (morning, post-breakfast, pretreatment) and after (afternoon, between meals, post-treatment) administration of cannabis (solid lines) or placebo (dotted lines). Each line represents an individual subject’s data during one treatment week.
In the mixed effects analysis, after adjusting for intra-subject correlation, cannabis administration was associated with an increase in ghrelin levels from morning-to-afternoon by 42.4% (95% CI [27.7%, 58.7%]) compared to a decrease of 12.0% with placebo (95% CI [−21.5%, −1.4%]; p<0.001). Leptin levels also increased significantly more with cannabis (+67.1%, 95%CI [+44.6%, +93.1%]) compared to placebo (+11.7%, 95%CI [−4.4%, +30.4%]), p<0.001). PYY levels decreased with cannabis (−14.2%, 95%CI [−24.9%, −2.0%]), but increased significantly with placebo administration (+23.2%, 95%CI [+7.2%, +41.6%], p<0.001). Insulin levels dropped to a similar extent both with cannabis (−30.4%, 95%CI [−37.1%, −23.0%]) and placebo (−32.3%, 95%CI [−39.2%, −24.7%], difference in change, p=0.69). However, the variability of the insulin decrease differed for the two treatments: cannabis SD=13.8% vs placebo SD=5.2% (p=0.011, Bartlett’s test of homogeneity). There was a trend toward a higher proportion of subjects with 25% or more reduction in insulin levels in the placebo group compared to the THC group: 9/9 (100%) vs 6/10 (60%), p=0.087 (Fisher’s exact test). Thus, for some subjects, insulin levels did not drop as much after cannabis administration as they did after placebo administration.
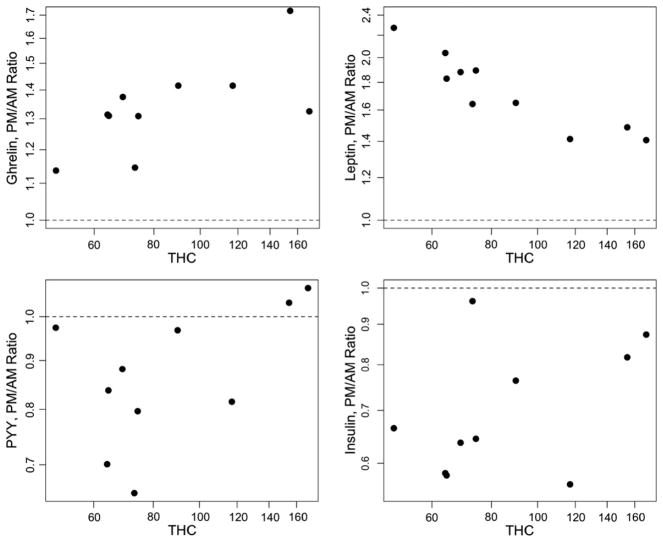
We also included a dose–response analysis on THC levels. During the active cannabis week, we explored whether the change in hormone levels between morning measurements (prior to smoking cannabis) and afternoon levels (post-smoking) were dependant on the measured afternoon levels of THC. The statistical model is a regression of change in hormone levels (on log scale) between morning and afternoon, as a function of log (THC), controlling for morning levels of the hormone. The plots in Fig. 3 show a THC dose relationship for ghrelin and leptin. For ghrelin, higher THC is associated with a greater increase in ghrelin (p=0.032). For leptin, however, higher THC is associated with a smaller increase in the hormone (p<0.001). Change in PYY and insulin failed to show any significant correlation with THC levels (p=0.11 and 0.62, respectively).
Fig. 3.
THC dose–response plots for ghrelin, leptin, PYY and insulin. For each hormone, plasma THC levels are plotted against log transformed PM/AM ratios of the hormone level.
2. Discussion
This study demonstrated significant alterations of the appetite hormones ghrelin, PYY and leptin in blood after smoking cannabis at doses that yielded substantial blood THC levels and produced therapeutic analgesia in patients with pain related to HIV sensory neuropathy. Ghrelin levels increased and PYY levels decreased as hypothesized after smoking cannabis, but not placebo. Cannabis-related changes in these hormones had a magnitude similar to what has been observed with food intake over the course of a day in normal volunteers (7) (14), suggesting physiological relevance.
Ghrelin is an orexigenic protein released by the stomach fundus and acting on receptors in the hypothalamus in a manner correlated with increased appetite. Changes in THC and ghrelin in this study demonstrated a modest dose–response correlation such that individuals obtaining higher levels of THC showed higher baseline-adjusted ghrelin levels after smoking. These findings are consistent with data from previous animal studies showing that administration of cannabinoid agonists yielded elevated plasma ghrelin (9) whereas administration of cannabinoid antagonists attenuated a fasting induced increase in ghrelin (15). PYY is a satiety hormone released by the gastrointestinal mucosa, also acting on the hypothalamus, that has been shown to have an inverse relationship with ghrelin (12).
Leptin is a hormone secreted by fat cells that inhibits feeding behavior via hypothalamic receptors. Thus in prior studies, increased appetite has been associated with decreased leptin, and we expected cannabis to result in reduced leptin levels (8). Instead, we observed significantly increased leptin levels with cannabis. One possible explanation is that high levels of exogenous cannabinoid stimulation might feedback negatively on endogenous cannabinoid production, leading to increases in leptin.
It is possible that caloric intake, rather than THC, influenced appetite hormone levels. We did not control for or carefully measure caloric intake in this study. However, we did measure insulin levels, which predictably increase following caloric intake (specifically carbohydrates) and decrease with fasting. In our study, insulin levels fell with both treatments in a manner predicted by the sampling times (morning/post-breakfast and afternoon/between meals).
We also included a dose–response analysis to determine if the plasma THC levels correlated with the degree of change in hormone levels seen during the active cannabis week, controlling for morning hormone levels. THC dose was significantly correlated with change in ghrelin and leptin levels. The THC dose correlation with change in ghrelin levels was in the expected direction. Specifically, higher THC levels were associated with greater increases in ghrelin between morning (pre-smoking) and afternoon (post-smoking). The changes in leptin levels were also correlated with plasma THC levels. However, this was a strong negative correlation, which was unexpected. Thus, higher THC levels were correlated with a smaller increase in leptin levels. So, although smoking THC increases both ghrelin and leptin levels, higher THC concentrations led to a greater increase in ghrelin but the increase in leptin levels was actually greatest at lower levels of THC. We considered several possible reasons for this apparent contradiction but were unable to provide a satisfactory explanation. We considered the possibility that the individuals with the highest THC levels may have smoked most recently and the negative dose correlation was actually a reflection of a lag between the initial rise in ghrelin and a secondary increase in leptin (which would have provided further support for the hypothesis that leptin levels rose after cannabis administration in order to downregulate endocannabinoid levels). This was not the case. It is worth noting that self-titrated dose was significantly correlated with measured THC levels. BMI did not predict self-titrated cannabis dose or change in leptin levels. There was no THC dose effect on change in PYY levels. As expected, there was also no correlation between THC and insulin levels. Further investigation of the dose effect of THC on appetite hormone levels (leptin and PYY, in particular) is needed to replicate and offer insight into the findings presented here.
This study has several limitations. Treatment and sampling schedules for our subjects were not balanced, introducing the possibility of bias. Thus, while five subjects contributed data from the placebo and cannabis treatment periods, two subjects contributed data only for one treatment period. Also, for some subjects the morning and afternoon samples were drawn on different days, rather than the same day. The random effects statistical modeling was designed to minimize biases resulting from these factors. For example, this method allowed the morning and afternoon samples from different days to be included, while recognizing that they would display a weaker correlation than samples drawn on the same day. Additionally, this method allowed multiple observations from the same subject to be used, recognizing that they would be more correlated than observations from different subjects. This careful accounting for the different sources of variance and correlation in the data makes it possible to use all available data in an efficient way, without sacrificing the integrity of the analysis. However, to further evaluate the potential influence of unbalanced sampling on our results, we assessed a subset of the data in which the morning and afternoon samples were collected during the same day. The analysis of this subset confirmed the results of the random effects model analysis.
Because all of our subjects were HIV seropositive and had distal sensory polyneuropathy, these results may not be generalizable to HIV negative individuals without neuropathy. However, the observed hormone levels and fluctuations under placebo conditions in our subjects were similar to those reported previously for healthy, HIV negative volunteers (4). Although some HIV-infected individuals experience wasting, our volunteers had normal to somewhat elevated body mass indexes. Our analysis focused on cannabinoid effects on appetite hormones, rather than potential disease effects, since each subject served as his own control in this cross-over study design. Nevertheless it is possible that cannabinoid effects would be different in HIV negative individuals, and our findings should be replicated in uninfected subjects. All participants in this study were men. As significant differences between the sexes in both the circulating levels of appetite mediators and the level of response to biological cues have been documented (5), further studies are needed to investigate whether cannabinoids affect the endocannabinoid system in women in the same way as in men.
An additional limitation of this study is the relatively sparse sampling schedule, which could have missed important alterations in appetite hormones over shorter or longer time scales. Future studies should use more frequent and systematic sampling to better delineate the time-effect relationship between cannabinoid receptor activation and changes in appetite mediators. As our subjects were screened to have negative urine toxicology at entry, and exposure was relatively brief (one week), our data may not generalize to longer cannabis use.
In conclusion, this study demonstrated significant alterations in the hormones ghrelin and PYY in humans, consistent with modulation of appetite hormones mediated by cannabis through endogenous cannabinoid receptors. Increases in leptin may reflect its role as an inhibitory regulator of the endocannabinoid system. These findings support further evaluation of interventions directed at manipulating the endocannabinoid system for the treatment of eating disorders and obesity.
3. Experimental Procedure
3.1. Study Design
This was a prospective subgroup analysis of data from a randomized clinical trial (Ellis et al., 2009). Subjects were enrolled in a phase II, single group, double-blind, placebo-controlled, cross-over trial of smoked cannabis for the short-term treatment of neuropathic pain associated with HIV infection. Each subject participated in five study phases over 7 weeks: (1) a 1-week wash-in phase to obtain baseline pain measurements; (2) 5 days of smoked active or placebo cannabis; (3) 2 week wash-out to allow for drug clearance; (4) 5 days smoked active or placebo cannabis; and (5) 2 week final wash-out. Samples collected for this study were from the active cannabis and placebo treatment weeks according to the schedule described below.
3.2. Subjects
To be eligible for the parent clinical trial, subjects had documented HIV infection and painful distal sensory polyneuropathy determined by clinical neurological examination and electrophysiological studies. Neuropathic pain was defined as distal, symmetric pain affecting the toes, feet and lower legs. Abnormal clinical exam findings had to include at least 2 signs from the following list: distal, bilateral reduction in vibratory sensation, or sharp-dull discrimination, or reduced or absent ankle reflexes compared to knees. Exclusion criteria were (1) current DSM-IV substance use disorders (including cannabis); (2) lifetime history of cannabis dependence; (3) previous psychosis with or intolerance to cannabinoids; (4) concurrent use of approved cannabinoid medications (i.e. Marinol); (5) positive urine toxicology screen for cannabinoids during the wash-in week before initiating study treatment; and (6) serious concomitant medical conditions such as congestive heart failure that might affect participant safety or the conduct of the trial. Participation in the appetite hormone substudy was optional according to the subject’s preference.
3.3. Procedures
The clinical trial was performed as an outpatient study at the General Clinical Research Center at the University of California, San Diego (UCSD) Medical Center. This study was approved and monitored by the UCSD Institutional Review Board, the Research Advisory Panel of California, the US Food and Drug Administration, the US Drug Enforcement Administration, the US Department of Health and Human Services, and the University of California Center for Medicinal Cannabis Research. All participants gave written informed consent to participate.
Cannabis and placebo cigarettes were provided by the National Institute on Drug Abuse and were constructed of the same base material. Active strengths ranged from 1% to 8% delta-9-tetrahydrocannabinol (THC) concentration by weight. Placebo cigarettes were made from whole plant material with cannabinoids removed and were identical in appearance to active cigarettes. Previous work has demonstrated substantial variability between individuals in sensitivity to the therapeutic (analgesic) and adverse (sedation, tachycardia) effects of THC. The size of the therapeutic window, measured as the difference between the lowest therapeutic and highest tolerated doses, also demonstrates considerable inter-individual variability. To accommodate these features, we used a dose-titration strategy that permitted selection of an optimized dosing schedule for each individual. On Day 1 of each treatment week (active vs placebo cannabis), the first smoking session used a 2% THC cigarette, which was then titrated upwards (to 4% and then 8%) or downwards (to 1%) to maximize pain relief while maintaining tolerable side effects. This dose-finding strategy identified a target dose on Day 1 that was carried through the remainder of the treatment week. During treatment weeks, subjects smoked cannabis or placebo under direct observation in the Clinical Research Center during 4 sessions distributed over the course of an 8-hour day (Ellis et al., 2009).
3.4. Blood Sampling Schedule
Blood samples were collected in the morning (pre-smoking) and afternoon (after last smoking session) on days 2, 3, 4 or 5. The time since last meal and the time since last smoking session were also recorded. Seven participants were enrolled in this sub-study and each contributed 1–2 pairs of samples (AM and PM) for the treatment weeks completed. All seven completed the active THC week, but two of these subjects did not complete the placebo week. For 3 pairs of samples during the placebo week and 3 pairs of samples during the THC week, the morning sample was from a different day (one day earlier, in all cases) than the afternoon sample. The statistical analysis accounted for the complexity of this design as described below.
3.5. Laboratory Assays
Ghrelin levels were measured by enzyme immunoassay (EIA) with a detection range of 130–10,000 pg/ml (Phoenix Pharmaceuticals Human Ghrelin EIA Kit). Leptin was also measured by an EIA with a range of 1–1000 ng/ml (R&D Systems Human Leptin Quantikine ELISA Kit). PYY levels were determined by an EIA kit with a range of 60–10,000 pg/ml (Phoenix Pharmaceuticals PYY (3–36) (Human) EIA Kit). Insulin levels were measured with the ALPCO Insulin ELISA Kit with a range of 7–1400 pmol/l. THC concentrations were quantified in plasma samples using electron impact gas chromatography/mass spectrometry with a lower limit of quantification of 0.5 μg/l (Huang et al., 2001).
Before administering study treatments, all subjects underwent comprehensive clinical and laboratory evaluations. Plasma HIV RNA was quantified by reverse transcriptase–polymerase chain reaction (Amplicor, Roche Diagnostic Systems, Indianapolis, IN) using the ultrasensitive assay (lower limit of quantitation, 50 copies per ml). Blood CD4+lymphocyte counts were measured by flow cytometry. Standard blood chemistry and hematology panels were performed.
3.6. Statistical Methods
The results of primary and confirmatory analysis strategies were compared. In the primary analysis, random effects linear models were applied to evaluate changes in ghrelin, insulin, leptin, and PYY levels between morning (pre-smoking) and afternoon (post-smoking) sessions, comparing the placebo and active THC periods, as follows. A variance components (random effects) model was fitted with the logarithm of the ghrelin, insulin, leptin, and PYY blood levels as a response, and a model term for the afternoon session for each of the THC and placebo periods. The error term consisted of several independent components, for (i) subject (ii) visit day for each subject, and (iii) blood draw. The error terms for subject and for visit within subject were treated as random effects. This model accounts for the correlation of levels for the same subject at different time points, and for the correlation of levels taken in the morning and afternoon of the same day, for a given subject. This modeling strategy was necessary because not all morning-afternoon pairs of observations came from the same day. In addition, this model accounts for the within-subject correlation of the placebo week and THC week draws. Restricted Maximum Likelihood (REML) models were fit using the lme4 package within the R statistical software (R Development Core Team, 2008).
The second, confirmatory analysis strategy considered the data subset including 13 pairs of observations where the morning and afternoon draws were taken on the same day, and analyzed the change in the ghrelin, insulin, leptin, and PYY levels between morning and afternoon. Since this analysis yielded consistent results with the previous analysis, but with fewer data points, only the results of the REML analysis are reported here. The difference in standard deviations of the reduction in insulin levels in the two groups was tested using Bartlett’s test of homogeneity.
Acknowledgments
This project was supported by Grant C00-SD-104 from the University of California, Center for Medicinal Cannabis Research.
The HIV Neurobehavioral Research Center (HNRC) is supported by Center award MH 62512 from NIMH.
*The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Igor Grant, M.D.; Co-Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and J. Allen McCutchan, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Scott Letendre, M.D., Edmund Capparelli, Pharm. D., RachelSchrier, Ph.D., Terry Alexander, R.N., Debra Rosario, M.P.H., Shannon LeBlanc; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), Steven Paul Woods, Psy.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D.; Matthew Dawson; Neuroimaging Component: Terry Jernigan, Ph.D. (P.I.), Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John Hesselink, M.D., Jacopo Annese, Ph.D., Michael J. Taylor, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Ian Everall, FRCPsych., FRCPath., Ph.D., Cristian Achim, M.D., Ph.D.; Neurovirology Component: Douglas Richman, M.D., (P.I.), David M. Smith, M.D.; International Component: J. Allen McCutchan, M.D.,(P.I.); Developmental Component: Ian Everall, FRCPsych., FRCPath., Ph.D. (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Rodney von Jaeger, M.P.H.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman (Data Systems Manager); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Tanya Wolfson, M.A.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Footnotes
Disclosure
At the time of this study, Ben Gouaux was an employee of the Center for Medicinal Cannabis Research at the University of California, San Diego, the study sponsor. Mr. Gouaux was a Research Associate with the CMCR and assisted the investigator with regulatory issues, oversight/monitoring, data preparation and analysis, and preparation of the article.
References
- Arora S, Anubhuti Role of neuropeptides in appetite regulation and obesity — a review. Neuropeptides. 2006;40:375–401. doi: 10.1016/j.npep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Barazzoni R, Zanetti M, Stebel M, Biolo G, Cattin L, Guarnieri G. Hyperleptinemia prevents increased plasma ghrelin concentration during short-term moderate caloric restriction in rats. Gastroenterology. 2003;124:1188–1192. doi: 10.1016/s0016-5085(03)00281-6. [DOI] [PubMed] [Google Scholar]
- Castaneda TR, Tong J, Datta R, Culler M, Tschop MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31:44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Chin-Chance C, Polonsky KS, Schoeller DA. Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J Clin Endocrinol Metab. 2000;85:2685–2691. doi: 10.1210/jcem.85.8.6755. [DOI] [PubMed] [Google Scholar]
- Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab. 2004;287:E297–E304. doi: 10.1152/ajpendo.00582.2003. [DOI] [PubMed] [Google Scholar]
- Dardeno TA, Chou SH, Moon HS, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Front Neuroendocrinol. 2010;31:377–393. doi: 10.1016/j.yfrne.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672–680. doi: 10.1038/npp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Moody DE, Andrenyak DM, Smith EK, Foltz RL, et al. Simultaneous determination of delta9-tetrahydrocannabinol and 11-nor-9-carboxy-delta9-tetrahydrocannabinol in human plasma by solid-phase extraction and gas chromatography-negative ion chemical ionization-mass spectrometry. J Anal Toxicol. 2001;25:531–537. doi: 10.1093/jat/25.7.531. [DOI] [PubMed] [Google Scholar]
- Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, et al. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82:1293–1300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
- Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. [Google Scholar]
- Tucci SA, Rogers EK, Korbonits M, Kirkham TC. The cannabinoid CB1 receptor antagonist SR141716 blocks the orexigenic effects of intrahypothalamic ghrelin. Br J Pharmacol. 2004;143:520–523. doi: 10.1038/sj.bjp.0705968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18:158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Wagner JD, Zhang L, Kavanagh K, Ward GM, Chin JE, et al. A selective cannabinoid-1 receptor antagonist, PF-95453, reduces body weight and body fat to a greater extent than pair-fed controls in obese monkeys. J Pharmacol Exp Ther. 2010;335:103–113. doi: 10.1124/jpet.110.168187. [DOI] [PubMed] [Google Scholar]
- Williams CM, Rogers PJ, Kirkham TC. Hyperphagia in pre-fed rats following oral delta9-THC. Physiol Behav. 1998;65:343–346. doi: 10.1016/s0031-9384(98)00170-x. [DOI] [PubMed] [Google Scholar]
- Zbucki RL, Sawicki B, Hryniewicz A, Winnicka MM. Cannabinoids enhance gastric X/A-like cells activity. Folia Histochem Cytobiol. 2008;46:219–224. doi: 10.2478/v10042-008-0033-4. [DOI] [PubMed] [Google Scholar]