Abstract
Background:
Cocaine use disorder is characterized by compulsive drug-seeking that persists long into abstinence. Work using rodent models of cocaine addiction has found evidence for reversal learning deficits 21 days after non-contingent cocaine administration and 60 days after self-administration. Here we sought to determine if a deficit in reversal learning is present 3–4 weeks after cessation of cocaine self-administration, when relapse to cocaine-seeking is robust. Conversely, we hypothesized that reversal learning training would protect against relapse, similar to other forms of environmental enrichment.
Methods:
Male rats underwent short access (ShA, 2h/10d) or long access (LgA, 1h/7d then 6h/10d) cocaine self-administration, followed by 21–29 days of abstinence. During abstinence, a subset of rats underwent training in a plus-maze that required an egocentric strategy to earn a sucrose reward. Following response acquisition and retention, the ability to reverse the spatial navigation strategy was tested.
Results:
Total trials to criteria and total errors made did not differ between the groups during response acquisition, retention, or reversal. On the first reversal test, ShA rats performed better than LgA and control rats. ShA rats’ performance worsened over time. There were no effects of cognitive training or length of cocaine access on context-primed relapse of cocaine-seeking.
Conclusions:
The present data indicate that perhaps LgA cocaine self-administration does not produce adaptations to regions mediating context-primed relapse as it does for cocaine and cocaine-associated cue-induced reinstatement of drug-seeking. A time-dependent deficit in reversal learning was found only in ShA rats. Reversal learning training did not protect against cocaine relapse.
Keywords: context, relapse, reinstatement, extended access, reversal learning
1. Introduction
Cocaine addiction is a chronic relapsing disease that is characterized by drug craving and loss of inhibitory control (Jentsch and Taylor, 1999). A major challenge in the successful treatment of cocaine addiction is reducing the risk of relapse, which remains high after months or even years of abstinence (Dackis and O’Brien, 2001; Mendelson and Mello, 1996). Concomitant with a high risk of relapse, cocaine addicts also display cognitive deficits in the domain of reversal learning (Ersche et al., 2008). Reversal learning is an orbito-frontal-cortex (OFC)- dependent task (McAlonan and Brown, 2003) where a cue predictive of reward becomes predictive for non-reward (or punishment) and vice versa. Chronic cocaine administration has been reported to impair reversal learning (Calu et al., 2007; McCracken and Grace, 2013). However, these studies either utilized non-contingent cocaine administration or assessed deficits during late withdrawal from cocaine self-administration (60+ days post-cocaine). As such, one goal of the present work was to assess reversal learning in early withdrawal (less than 30 days post-cocaine), at a time when a large body of literature examines cocaine relapse in laboratory rodents.
The OFC lies at the intersection of cognitive deficits and motivation to seek cocaine. Human cocaine users exhibit OFC abnormalities (Volkow and Fowler, 2000), such as hypoactivity during baseline conditions and enhanced activation in response to cocaine- associated cues (Bolla et al., 2003; Bonson et al., 2002; Matochik et al., 2003). OFC and basolateral amygdala (BLA) functional inactivation inhibits drug context-induced cocaine- seeking behavior (Lasseter et al., 2009, 2010, 2011). Thus, it is possible that deficits in the OFC- dependent task of reversal learning are related to an increased propensity to relapse.
Previous work from our laboratory found that rats that experience 2–3 weeks of instrumental extinction of the operant response made to obtain cocaine display different neuroadaptations in the NA compared to rats that experience only abstinence from cocaine without instrumental extinction (Knackstedt et al., 2010; LaCrosse et al., 2016). Furthermore, the beta-lactam antibiotic, ceftriaxone, attenuates cocaine-seeking and associated NA glutamate efflux following operant extinction (Trantham-Davidson et al., 2012) but not abstinence (LaCrosse et al., 2016). Thus, it seems that extinction training confers benefits that may be protective against cocaine relapse (Knackstedt et al., 2010). Human cocaine users rarely, if ever, experience instrumental extinction in the drug-taking environment; however, another learning strategy that engages cortical processes may be protective against cocaine relapse. While a history of cocaine self-administration has the potential to impact reversal learning, it is also possible that training rats on such a task will impact cocaine-seeking. Several studies suggest that learning tasks confer similar benefits as enriched environments (EE), including increased synaptogenesis and neurogenesis (Black et al., 1990; Döbrössy et al., 2003; Leuner et al., 2004). Typical EE housing offers increased novelty, complexity, and opportunities for the performance of naturalistic behaviors, such as social interactions and exercise. Such EE housing prevents relapse to cocaine induced both by contextual and discrete drug-associated cues (e.g., Chauvet et al., 2009; Thiel et al., 2009, 2010). Although little is known about the mechanisms underlying the attenuation of relapse by EE, differences in neuronal morphology and glutamate and dopamine transmission in areas relevant to drug addiction, such as the prefrontal cortex (PFC) and nucleus accumbens (NA), have been hypothesized (Thiel et al., 2012, 2010).
Thus, the second goal of the present work was to assess whether training in a reversal learning task outside the drug-associated context during early withdrawal from cocaine self- administration would protect against relapse to cocaine-seeking. We asked these questions using two different lengths of access to cocaine self-administration - short access (ShA; 2 hours/day for 10 days) and long access (LgA; 1 hour/day for 7 days, 6 hours/day for 10 days). We hypothesized that greater cocaine intake would produce greater deficits in reversal learning and that reversal learning would attenuate context-primed relapse to cocaine seeking.
2. Methods
2.1. Drugs
Cocaine hydrochloride was obtained from the NIDA Controlled Substances Program (Research Triangle Institute, NC, USA). Cocaine (4 mg/mL cocaine concentration at a dose of 1 mg/infusion in 0.1mL, i.v.) was prepared in 0.9% sodium chloride.
2.2. Animals
43 adult male Sprague-Dawley rats (250–300 g upon arrival, Charles River Laboratories, Raleigh, NC, USA) were housed individually in a temperature- and humidity-controlled vivarium on a 12 hour reverse light cycle and rats were allotted approximately 15–20g chow/day such that they were maintained at 90% of free-feeding weight. All experiments were conducted during the rats’ dark, active phase. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Florida and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
2.2.1. Catheter surgery.
Animals were anesthetized with a mixture of ketamine (87.5 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.) and a catheter (SILASTIC silicone tubing, ID 0.51 mm, OD 0.94 mm, Dow Corning, Midland, MI, USA) inserted into the right jugular vein. The other end of the catheter was inserted subcutaneously through the shoulder blades and exited the skin through a small dermal hole. The catheter was then attached to a guide cannula that was secured with a backpack (Instech, Plymouth Meeting, PA, USA). Rats received ketorolac (3 mg/kg, i.p.) for three days and cefazolin (100 mg/mL, i.v.) for seven days following surgery and received 0.2 ml of heparinized saline (100 U/mL, i.v.) before and after each self-administration session to maintain the patency of the catheter. Catheter patency was verified periodically via methohexital sodium (10 mg/ml; Eli Lilly, Indianapolis, IN, USA). Rats were allowed five days of recovery before starting self-administration of cocaine.
2.3. Cocaine self-administration, abstinence, and reinstatement
Rats were placed in operant self-administration chambers (30×24×30 cm; Med Associates, St. Albans, VT, USA) each equipped with two retractable levers. Pressing of the active lever resulted in an intravenous infusion of cocaine (1 mg/mL/infusion) paired with a discrete five second auditory tone (2900 Hz) and light cue, while pressing the inactive lever resulted in no delivery of drug or associated cues. Infusions of cocaine were followed by a 20- second timeout period where further active lever presses resulted in no drug delivery or paired cues. Both active and inactive lever presses as well as total infusions were recorded using Med PC IV software (Med Associates). Long access (LgA) rats experienced seven days of one-hour sessions followed by 10 days of six-hour sessions. Short access (ShA) rats experienced daily two-hour cocaine self-administration sessions until reaching 10 days of 10 or more cocaine infusions/session. Yoked-saline rats (n=7) received intravenous saline (0.9% physiological saline, 0.1 mL/infusion) when their yoked counterpart received a cocaine infusion. Five rats experienced catheter failure during cocaine self-administration and were excluded from the experiment. Two additional rats died from illness during self-administration, leaving 29 rats that successfully completed cocaine self-administration, and seven yoked-saline rats.
Following self-administration, a subset of rats (ShA n=7; LgA n=9) were trained in a reversal learning task described in detail below. To control for variables inherent to the maze task, such as running the maze, handling, and sugar consumption, “abstinence only” (ABS) rats (ShA n=5; LgA n=8) were allowed to explore the maze while consuming an equal number of sugar pellets, but were not trained in the learning task. After completion of the maze task, or an equivalent number of days of ABS (see timeline in Fig. 1A), animals were placed into operant chambers and tested for context-primed relapse of cocaine-seeking behaviors. During the relapse test session, lever pressing did not produce either drug infusion or the presentation of cues. Presses on the previously associated active and inactive levers were recorded for two hours.
Figure 1.
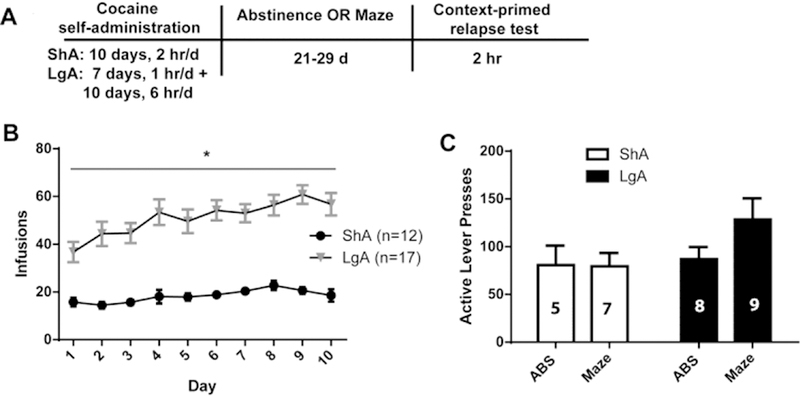
Extended access to cocaine self-administration resulted in greater cocaine intake but not greater context-primed relapse to cocaine seeking. A). A timeline of the self-administration and relapse procedure. B) LgA rats that had access to cocaine for 6 h/session displayed significantly greater intake than ShA rats that had access to cocaine for 2 h/session. C) During a 2 h context-primed relapse test, presses on the previously active lever were unaffected by cocaine intake or exposure to the maze task. Data are depicted as mean ± SEM.
2.4. Reversal learning task
Maze description:
A black Plexiglass plus-maze was used for this task, with four arms extending out from a central area (13 × 13 cm). Each of the four arms was 48 cm long, 13 cm wide, and 30 cm tall. A removable piece of black Plexiglass was used to block one arm of the maze to form a “T” configuration. The start arm for each trial was pseudorandom to discourage use of an allocentric spatial strategy to locate the reward. Three maze arms (South, East, and West) were used as the start arm for each trial on a rotating basis. The North arm was only used for probe trials (described below). The maze room was approximately 2.1 × 2.1 m and the maze itself was positioned at a height of 75 cm from the floor. Visual cues in the room included a 13 × 13 cm black ‘+’ symbol hanging on the west wall, a 13 × 13 cm black ‘X’ hanging on the south wall, a wire bookshelf with a red lamp attached was positioned along on the north wall, and the door to the room was located on the east wall. All trials were run under dim red light. The maze was cleaned with 70% ethanol between each rat.
Maze Task:
The maze task was adopted from McCracken and Grace (2013).
Habituation, Days 1–3: rats were given 20 sucrose pellets in the home cage.
Habituation, Days 4–7: rats learned to consume sucrose pellets from the wells at the end of each arm by baiting arms and the food well. Habituation criterion was defined as consuming all pellets in the maze within 15 minutes.
Turn Bias, Day 8: Turn bias was determined by the direction (right or left) that a rat turned four or more times out of seven trials.
Response Discrimination, Day 9: Rats were trained in the Response Discrimination task and learned to turn in the opposite direction of their turn bias in order to gain a sucrose reward. For example, if the rat preferred to turn Left on Day 8, he was trained to turn Right on Day 9. After successful completion of a trial, rats were placed in a holding cage for an inter-trial interval of 15 seconds while the maze was reset for the next trial. Training continued until a criterion of ten consecutive, correct choices was met. If an incorrect decision was made during a trial, the trial continued until the correct decision was made. After nine correct choices were made in succession, the North arm was used for a probe trial that confirmed task acquisition via an egocentric strategy. During probe trials, if a rat made a correct response, Response Discrimination would conclude. If a rat made an incorrect response during this trial, training would continue until the rat made four consecutive correct choices followed by another probe trial. This continued until a rat made a correct choice on the probe trial. The maximum trials per day allowed was 30; the maximum number of days rats underwent Response Discrimination was three.
Response Retention, Day 10: The day after ten consecutive successful trials in the Response Discrimination task, the ability to retain this training was assessed. The procedure and criterion previously described was also used on this day of retention testing.
Reversal Learning Test, Day 11: Animals were required to turn in the opposite direction from that in the Response Discrimination and Retention tasks in order to receive the sucrose. Training/testing proceeded in an identical way as for Response Discrimination (after nine consecutive correct choices, rats underwent a probe trial).
Reversal Retention, Day 12: The day after successful reversal, a Reversal Retention test confirmed the previous day’s learning. Once an animal completed the first Reversal Learning and Reversal Retention tests successfully, the reward arm was changed again, for another reversal learning test. This continued until a total of three reversals were completed.
2.5. Statistical analysis
Graph Pad Prism (version 6.0) was used to analyze the behavioral data. For all statistical analyses used, the alpha level was set at p = 0.05. Self-administration and reversal learning data were analyzed with Repeated Measures ANOVA with time as a within-subjects factor and “Learning” (Maze or Abstinence) as a between-subjects variable. Relapse data were analyzed with a two-factor ANOVA, with Learning and Access as between-subjects factors. Maze response acquisition was analyzed with one-way ANOVAs. Dependent variables included the percent of total correct trials, the number of errors, and number of trials, calculated as 1-(total errors/total trials run), the sum of all incorrect trials, and sum of all trials, respectively. Significant main effects and/or interactions were followed by post-hoc Tukey analyses to further examine differences. Pearson’s correlations were used to explore the relationship between active lever presses during relapse and reversal learning performance. Finally, upon detection of group differences in the percent of correct trials in the absence of changes in total number of errors and trials, we explored the potential that the timing of an error influenced the percent correct metric. To do so, the total number of times a probe trial error was made was compared to the total number of times either no probe trial error was made and/or no probe trial was attempted; these numbers were compared between groups using a Chi-square test. We performed this analysis for both Reversal 1 and Reversal 3 data separately.
3. Results
In the present study, rats self-administered cocaine on a ShA or LgA schedule. LgA rats self-administered significantly more cocaine than ShA rats across the ten sessions (Figure 1B), evidenced by a significant main effect of Access (F(1, 27) = 71.52, p < 0.0001) but no Access x Time interaction. The mean total (mg/kg) cocaine intake for ShA rats was 211.8 ± 14.2 and for LgA was 645.8 ±41.9. Context-primed relapse (Figure 1C) was not affected by length of cocaine Access (F(1, 25) = 1.144, p = 0.295), Learning (F(1, 25) = 2.192, p = 0.1512), or their interaction (F(1,25) = 1.306, p = 0.2639). Inactive lever presses during context-primed relapse did not differ between groups (F(3, 25) = 0.2649, p = 0.85).
Figure 2A depicts the maze training schedule, where rats underwent a series of response discrimination, retention, and reversal learning tests involving sucrose seeking in a T-shaped maze. During response discrimination, total errors did not differ by cocaine Access (Figure 2B, F(2, 20) = 0.2583, p = 0.7749), nor did the total number of trials to reach criterion (Figure 2C, F(2,20) = 0.3487, p = 0.7098). Similarly, total errors (Figure 2D, F(2, 20) = 0.8321, p = 0.4496) and total number of trials to reach criterion (Figure 2E, F(2, 20) = 0.6629, p = 0.5263) did not differ significantly by cocaine Access.
Figure 2.
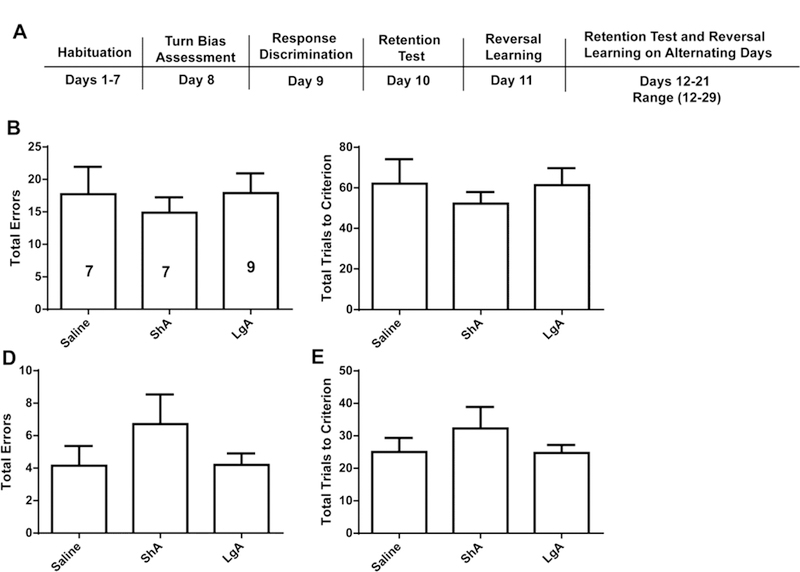
Acquisition and retention of the correct response in a 4-arm maze was not affected by either short- or long-access cocaine self-administration. A) A timeline of the maze procedure. During response discrimination, (B) total errors and (C) total trials to criterion did not differ by group. During the retention trial, (D) total errors also did not differ by group, nor did (E) the total trials to criterion. Data are depicted as the mean ± SEM.
For percent correct trials during Reversal Learning (Figure 3A), a significant Access x Trial interaction was detected (F(4, 40) = 3.081, p = 0.0265), as well as a significant main effect of Access (F(2, 20) = 3.391, p = 0.05). ShA rats performed significantly better relative to saline rats on the first reversal (p<0.05), and significantly worsening across time (Day 1 vs. Day 3 p < 0.05). On Day 3 ShA performed significantly worse than LgA (p < 0.05). No significant effects of Access, Trial, or Access × Trial interaction were observed in the total errors made during reversal (Figure 3B) and the total number of reversal learning trials (Figure 3C). Thus, the timing of the error was altered by a history of ShA cocaine, and not total errors made, or trials required. Further exploration of the data revealed that the total number of errors made during a probe trial on Reversal 1 was significantly different across groups (χ2(2, N = 65) = 7.02, p = 0.029). Whereas this type of error was not exhibited by the ShA group, rats in the LgA and Saline groups exhibited this type of error seven and five times, respectively. The number of errors made during a probe trial on Reversal 3 did not significantly differ across groups (χ2(2, N = 40) = 3.78, p = 0.226). The number of active lever presses during relapse did not correlate with the percent correct on reversal trials for any of the three reversal learning days.
Figure 3.
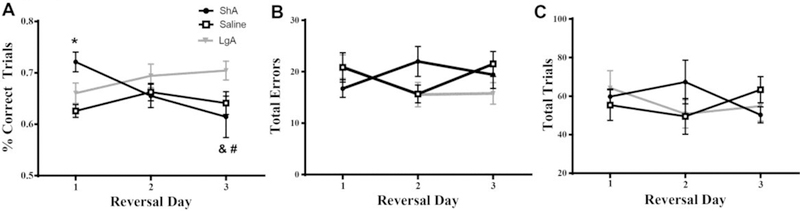
Reversal of the response requirement in the maze was affected by a history of cocaine self-administration. A) The number of correct trials (expressed as a percentage of total trials completed) was affected by cocaine access, with the ShA cocaine group displaying better performance than saline rats on the first reversal and worse performance by the third reversal. (B) Total number of errors and (C) total number of trials to meet criteria did not differ by group. * = p<0.05 compared to Saline; # = p<0.05 compared to LgA cocaine; & = p<0.05 compared to day 1. Data are depicted as the mean ± SEM.
4. Discussion
We used a rat model of cocaine self-administration followed by abstinence and tested the ability of a potentially translational learning intervention to prevent relapse. We found no differences in cocaine-seeking in a context induced test of relapse between rats that learned a maze task and control rats that were placed in the maze but were not trained in the task. This suggests our learning task was not an effective intervention to prevent relapse. We tried to isolate the experience-dependent learning component of EE, and did not provide opportunities for subjects to engage in natural behaviors, including social interactions and exercise, or exposure to complexity and novelty, which all contribute to the beneficial effects of the EE paradigm (van Praag et al., 2000). Potentially, for cognitive training to have EE-like effects on mechanisms mediating cocaine-seeking, more training expanding into other cognitive domains, or in conjunction with exercise, is necessary. However, other isolated components of EE, such as exercise, have been reported to reduce the rewarding-properties of cocaine. In a rat model of cocaine self-administration, female rats were weaned into individual cages with or without a running wheel (Smith et al., 2008). At nine weeks of age, rats were implanted with jugular catheters and trained to self-administer cocaine using a fixed-ratio schedule of reinforcement, and then tested for breakpoints using a progressive ratio schedule and responses maintained using two doses of cocaine (0.3 or 1.0 mg/kg/infusion). Exercising rats acquired the task at the same rate of sedentary rats; however, they had lower breakpoints at the higher dose of cocaine (Smith et al., 2008). Studies using a multicomponent (i.e., novelty, complexity, and social groups) EE paradigm and that rear subjects within such conditions, have largely been successful at using EE as a treatment to reduce cocaine-seeking (e.g., acquisition of self-administration, escalation of intake), especially at low doses of cocaine self-administration (Chauvet et al., 2012; Gipson et al., 2011; Ranaldi et al., 2011; Solinas et al., 2008, Thiel et al., 2012, 2011, 2010, 2009). Interestingly, although the EE paradigm reduces cocaine-seeking, effects are transient such that once EE is removed, the benefits are lost (Chauvet et al., 2012; Thiel et al., 2011).
We are the first to compare context-primed relapse after abstinence between groups undergoing ShA vs. LgA cocaine self-administration. We found that, although the LgA rats self- administered more cocaine than ShA rats, there were no differences in cocaine-seeking during context-primed relapse. This finding is in contrast to our previous work that showed that following extinction training, LgA rats displayed greater lever presses than ShA rats during a cocaine-primed reinstatement test. This effect was observed only when reinstatement was primed with a 30 mg/kg priming dose of cocaine, but not a 3 or 10 mg/kg dose (Knackstedt and Kalivas, 2007). In a model similar to the one used here, when abstinence was employed instead of extinction training, LgA rats displayed greater lever presses than did ShA rats during a cueprimed relapse test (Ferrario et al., 2005). Thus, contrasting our data with the literature indicates that perhaps LgA to cocaine self-administration produces adaptations to regions mediating cocaine and cocaine-associated cue-induced reinstatement of drug seeking, but not those mediating context-primed relapse. Future exploration of such differences is warranted, given that context-primed relapse following abstinence is more likely to resemble the experience of human cocaine addicts, who do not typically undergo instrumental extinction as used in the classic extinction-reinstatement animal model of relapse.
Regardless of length of access to self-administration, cocaine did not significantly impair reversal learning on the first reversal test day, relative to cocaine-naïve rats, as was found previously after non-contingent cocaine injections (McCracken and Grace, 2013). The length of cocaine access did affect the percent of correct trials across reversals. However, unexpectedly, the ShA rats performed better than saline rats on Reversal Day 1, worsened across reversals, and performed worse compared to LgA rats on Reversal Day 3. This pattern did not manifest in the number of errors or total trials. This may have occurred because the total percent trials correct, unlike number of errors, was influenced by the timing of the error. For example, if the rat made an error on the probe (i.e., 10th) trial, the protocol dictated he had to run a minimum of five more trials, thereby adding to the total number of trials completed. Our data support this potential effect, as the number of times an error was made on a probe trial was indeed different across groups, with the LgA and Saline control rats having more of such errors during Reversal 1; however, the same was not true for Reversal 3. There may still be some potential that after ShA cocaine, rats were able to perform the first reversal faster due to a prior experience with cocaine- reinforced learning in the operant chamber (as opposed to yoked-saline rats that did not receive cocaine reinforcement). For example, Lucantonio and colleagues (2014) trained rats to self- administer cocaine or oral sucrose for three hours a day across 14 days. After three weeks of abstinence, rats were trained to respond in a Pavlovian conditioning over-expectation task that consisted of three phases: conditioning, compound training, and extinction. After cocaine, rats were faster at extinguishing in this OFC-dependent extinction phase compared to vehicle rats (Lucantonio et al., 2014). Although it is unlikely that prior operant training was responsible for the increased performance in the ShA rats in Reversal 1, as LgA rats experienced an even greater number of cocaine-reinforced operant responses without demonstrating benefit in this reversal, the LgA rats did outperform the other groups by Reversal 3. Future studies will need to confirm the effect of prior operant training on reversal learning.
A potential reason that more cocaine-induced impairments in reversal performance were not observed is the working memory load at 15 seconds inter-trial intervals was low. Other studies using OFC-dependent tasks to study cognitive deficits due to cocaine also do not detect differences at such short delays, but did using longer delays of 30 and 60 seconds (Radley et al., 2015), and 70 and 130 seconds (George et al., 2008). Moreover, we believe we are the first to assess performance in this OFC-dependent maze task in animals following self-administration of cocaine. We followed the methods of McCracken and Grace (2013), who previously reported cocaine-induced deficits in reversal learning following 28 days withdrawal from 14 days of intraperitoneal injections of cocaine (30 mg/kg), for a total of 420 mg/kg administered. Despite attaining greater cocaine intake (the mean intake for LgA rats was 645.8 mg/kg), rats in the present study failed to display deficits in reversal learning following cocaine self-administration. Thus, it may be possible that bolus administration of cocaine produces more significant changes to the circuitry mediating reversal learning.
Many preclinical studies using the reinstatement model assess relapse at 21–30 days of withdrawal, at a time when relapse to cocaine-seeking is robust. To our knowledge, we are the first to assess OFC-dependent reversal learning at this time of withdrawal. Calu et al. (2007) reported deficits in reversal learning using a go, no-go operant task more than two months after cessation of intravenous cocaine self-administration. Potentially, had we tested the LgA group later in withdrawal (~60 days), we may have seen results in our LgA rats similar to those of ShA rats and to those previously reported by Calu et al. (2007). The effects of ShA cocaine self- administration on reversal learning observed here were modest. Moreover, although statistically significant, this effect may not be biologically relevant and thus future work and replication is now needed.
For our intent to test the effects of learning during abstinence on cocaine relapse, we believe the maze task was appropriate. With the failure of this task to attenuate context-induced cocaine seeking, an effective cognitive intervention that prevents compulsive relapse to cocaine seeking is still needed.
Highlights.
6h vs. 2h of cocaine access did not affect context-primed cocaine-seeking
Reversal learning task performance declined for rats that had 2h of cocaine access
Training in the maze task did not reduce context-primed relapse of cocaine-seeking
Acknowledgements
The authors would like to thank Lizhen Wu for her excellent technical assistance on this project.
Role of the Funding Source
This work was funded by National Institutes of Health grants DA033436 and DA037270 awarded to L.A.K. The authors declare no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
Conflict of Interest
The authors declare no competing interests. This article is original and is not being considered for publication elsewhere.
References
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT, 1990. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc. Natl. Acad. Sci. USA 87, 5568–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M, 2003. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision- making task. Neuroimage 19, 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED, 2002. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology 26, 376–386. [DOI] [PubMed] [Google Scholar]
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G, 2007. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn. Mem 14, 325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Goldberg SR, Jaber M, Solinas M, 2012. Effects of environmental enrichment on the incubation of cocaine craving. Neuropharmacology 63, 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M, 2009. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology 34, 2767–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, O’Brien CP, 2001. Cocaine dependence: a disease of the brain’s reward centers. J. Subst. Abuse Treat 21, 111–117. [DOI] [PubMed] [Google Scholar]
- Döbrössy MD, Drapeau E, Aurousseau C, Le Moal M, Piazza PV, Abrous DN, 2003. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol. Psychiatry 8, 974–982. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ, 2008. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology 197, 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE, 2005. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol. Psychiatry 58, 751–759. [DOI] [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF, 2008. Extended access to cocaine self- administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology 33, 2474–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT, 2011. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology 214, 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW, 2007. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J. Pharmacol. Exp. Ther 322, 1103–1109. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW, 2010. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J. Neurosci 30, 7984–7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCrosse AL, Hill K, Knackstedt LA, 2016. Ceftriaxone attenuates cocaine relapse after abstinence through modulation of nucleus accumbens AMPA subunit expression. Eur. Neuropsychopharmacology 26, 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA, 2009. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur. J. Neurosci 30, 1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Wells AM, Xie X, Fuchs RA, 2011. Interaction of the basolateral amygdala and orbitofrontal cortex is critical for drug context-induced reinstatement of cocaine- seeking behavior in rats. Neuropsychopharmacology 36, 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA, 2010. Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr. Top. Behav. Neurosci 3, 101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ, 2004. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J. Neurosci 24, 7477–7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantonio F, Takahashi YK, Hoffman AF, Chang CY, Bali-Chaudhary S, Shaham Y, Lupica CR, Schoenbaum G, 2014. Orbitofrontal activation restores insight lost after cocaine use. Nat. Neurosci 17, 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet J-L, Bolla KI, 2003. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage 19, 1095–1102. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ, 2003. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav. Brain Res 146, 97–103. [DOI] [PubMed] [Google Scholar]
- McCracken CB, Grace AA, 2013. Persistent cocaine-induced reversal learning deficits are associated with altered limbic cortico-striatal local field potential synchronization. J. Neurosci 33, 17469–17482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, 1996. Management of cocaine abuse and dependence. N. Engl. J. Med 334, 965–972. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Anderson RM, Cosme CV, Glanz RM, Miller MC, Romig-Martin SA, LaLumiere RT, 2015. The contingency of cocaine administration accounts for structural and functional medial prefrontal deficits and increased adrenocortical activation. J. Neurosci 35, 11897–11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaldi R, Kest K, Zellner M, Hachimine-Semprebom P, 2011. Environmental enrichment, administered after establishment of cocaine self-administration, reduces lever pressing in extinction and during a cocaine context renewal test. Behav. Pharmacol 22, 347–353. [DOI] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML, 2008. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend 98, 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M, 2008. Reversal of cocaine addiction by environmental enrichment. Proc. Natl. Acad. Sci. USA 105, 17145–17150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Chauvet C, Jaber M, 2010. Prevention and treatment of drug addiction by environmental enrichment. Prog. Neurobiol 92, 572–592. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT, 2009. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol. Biochem. Behav 92, 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Engelhardt B, Hood LE, Peartree NA, Neisewander JL, 2011. The interactive effects of environmental enrichment and extinction interventions in attenuating cue- elicited cocaine-seeking behavior in rats. Pharmacol. Biochem. Behav 97, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Painter MR, Pentkowski NS, Mitroi D, Crawford CA, Neisewander JL, 2012. Environmental enrichment counters cocaine abstinence-induced stress and brain reactivity to cocaine cues but fails to prevent the incubation effect. Addict. Biol 17, 365– 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Pentkowski NS, Peartree NA, Painter MR, Neisewander JL, 2010. Environmental living conditions introduced during forced abstinence alter cocaine- seeking behavior and Fos protein expression. Neuroscience 171, 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL, 2009. Anti-craving effects of environmental enrichment. Int. J. Neuropsychopharmacol 12, 1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA, 2012. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J. Neurosci 32, 12406–12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Praag H, Kemperman G, Gage FH 2000. Neural consequences of environmental enrichment. Nat. Rev. Neurosci 1:191–8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, 2000. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cereb. Cortex 10, 318–325. [DOI] [PubMed] [Google Scholar]


