Abstract
There is a high incidence of adenovirus (AdV) infection in humans due to the presence of more than 60 types of human adenoviruses (HAdVs). The majority of individuals are exposed to one or more HAdV types early in their lives, leading to the development of AdV type-specific neutralizing antibodies. Similarly, immunization or gene therapy with AdV vectors leads to immune responses to the AdV vector. This ‘vector immunity’ is a concern for AdV vector-based applications for vaccines or gene therapy, especially when the repeated administration of a vector is required. The objective of this investigation was to establish whether AdV neutralizing antibody titers decline sufficiently in a year to permit annual vaccination with the same AdV vector. Naïve or human adenoviral vector group C, serotype 5 (HAdV-C5)-primed mice were mock-inoculated (with PBS) or inoculated i.m. with 108 PFU of either HAd-GFP [HAdV-C5 vector expressing the green fluorescent protein (GFP)] to mimic the conditions for the first inoculation with an AdV vector-based vaccine. At 1, 3, 6, and 10 months post-HAd-GFP inoculation, naïve- or HAdV-primed animals were vaccinated i.m. with 108 PFU of HAd-H5HA [HAdV-C5 vector expressing hemagglutinin (HA) of H5N1 influenza virus]. There was a significant continual decrease in vector immunity titers with time, thereby leading to significant continual increases in the levels of HA-specific humoral and cell-mediated immune responses. In addition, significant improvement in protection efficacy against challenge with an antigenically heterologous H5N1 virus was observed in HAdV-primed animals at 6 months and onwards. These results indicate that the annual immunization with the same AdV vector may be effective due to a significant decline in vector immunity.
Keywords: Adenoviral vectors, vector immunity, longevity of adenoviral vector immunity, prevalence of vector immunity, human adenoviral vector, avian influenza
INTRODUCTION
Adenovirus (AdV) vector-based vaccines induce excellent humoral and cell-mediated immune (CMI) responses[1–5] due to the adjuvant-like effect of Ad vectors in stimulating the innate immune system through both Toll-like receptor (TLR)-dependent and TLR-independent pathways [6, 7]. Ad vector-based influenza vaccines have shown excellent potential in both animal models [8–10] and clinical trials in humans [11–14]. Our immunogenicity and protective efficacy studies in mice demonstrate that Ad vector-based vaccines provide complete protection against challenge with both homologous and antigenically distinct strains of influenza viruses [9, 15].
There is a high incidence of AdV infections in the general population due to the circulation of more than 60 human AdV (HAdV) serotypes. The development of Ad-specific neutralizing antibodies, popularly known as ‘pre-existing vector immunity’ in the majority of individuals [16–18] is a potential concern for Ad vector-based vaccine efficacy. HAdV neutralizing antibody titers in humans in the U.S. was found to be in the range of 256–512 in 16% of the samples [16]. In Sub-Saharan children, a median HAdV-C5 neutralizing antibody titer of 512 was observed [19]. However, it is unclear what levels of vector immunity may have a significant negative impact on the development of effective immune responses. As well, since the use of AdV vectors as vaccines would often require repeated immunization, each immunization might at least temporarily induce or boost vector immunity, making it important to understand the rate of decline of vector immunity with time.
We have evaluated the role of HAd-C5-neutralizing antibodies or vector immunity in impacting the immunogenicity and protection efficacy of a HAdV-C5 vector (HAd-HA-NP) expressing the HA and NP genes of A/Vietnam/1203/04 (H5N1) influenza virus [20]. The mouse groups were primed either intranasally (i.n.) or intramuscularly (i.m.) with varying doses of HAdV-C5, and following the development of vector immunity, the animal groups were immunized with HAd-HA-NP via the i.n. or i.m. route. The immunogenicity and protection results suggested that moderate levels of vector immunity [520 virus-neutralization (VN) titer] did not adversely impact the protective efficacy of the vaccine. Further increases in vector immunity (up to 2240 VN titers) were overcome by either increasing the vaccine dose by 5× or using an alternate route of vaccination. In the presence of exceptionally high levels of vector immunity (~3040 VN titers), immunization with a 5× vaccine dose still resulted in approximately 3.3–3.7 logs reduction in lung titers of the challenge virus.
Canarypox virus-based vaccines are routinely used in pet animals on an annual basis suggesting that the development of immunity against a canarypox vector due to yearly exposure does not negatively impact the vaccine efficacy [21, 22]. In this manuscript, using a mouse model, we addressed whether anti-AdV immunity declines sufficiently in a year to permit annual vaccination with AdV vector-based vaccines. We found that there was a continual decline in the vector immunity with time, leading to a significant increase in humoral and cell-mediated immune responses against the target immunogen. In addition, effective immunogenicity and protection was observed in HAdV-C5-primed animal groups immunized with a HAd vector (HAd-H5HA) expressing the HA gene of A/Hong Kong/156/97(H5N1) (HK/156)] at 6-month and onwards. This study should be valuable for determining the practical utility of AdV vector-based vaccines for human use.
MATERIAL AND METHODS
Cell lines and viruses
All cell lines were grown in minimum essential medium (MEM) (Life Technologies, Gaithersburg, MD) with 10% fetal calf serum (Hyclone, Logan, UT) and 50 μg/ml gentamycin. 293 (human embryonic kidney cells expressing HAdV-C5 E1 proteins) [23] and BHH2C (bovine-human hybrid clone 2C) [24] were used to grow and titrate human adenovirus vectors (HAdVs). MDCK (Madin-Darby canine kidney) cell line was used to titrate influenza virus titers in the lungs for protection studies.
The construction of HAdV-C5 empty vector (HAd-ΔE1E3) [25], HAdV-C5 vector expressing the green fluorescent protein (HAd-GFP) [16], and HAdV-C5 vector expressing HA of A/Hong Kong/156/H5N1 (HAd-H5HA)] [9] have been described previously. Vectors were purified using cesium chloride density gradient ultracentrifugation as described [20].
The influenza virus A/Vietnam/1203/2004(H5N1)-PR8/CDC-RG [VN/1203/RG] which was created by reverse genetics, was grown in embryonated chicken eggs and quantified as tissue culture infectious dose 50 (TCID50) in MDCK cells. VN/1203/RG was used for challenge studies in mice as described in the experimental design. The HA gene in HAd-H5HA vector is from HK/156 influenza virus, which is antigenically distinct from the HA of VN/1203/RG (the challenge virus).
Animal inoculation and experimental design
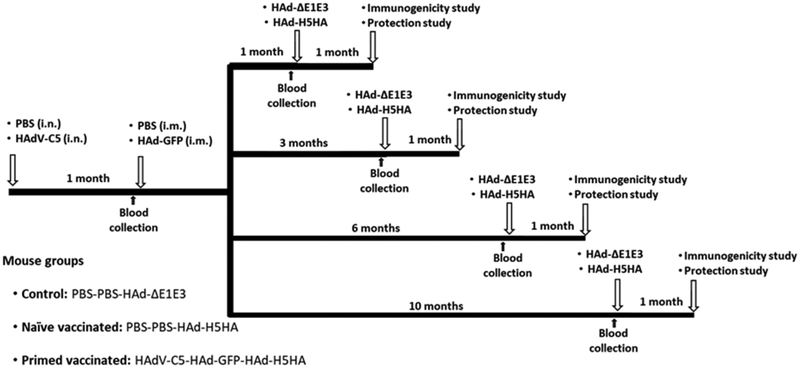
The animal experiments were operated in BSL-2+ lab at Purdue University approved by USDA. The Institutional Animal Care and Use Committee (IACUC), and the Institutional Biosafety Committee (IBC) have approved the protocols for animal inoculations and protection studies. Five to 6-week-old female BALB/c mice (purchased from Harlan Sprague Dawley Inc., Indianapolis, IN) were used to examine the longevity of HAdV-C5 neutralizing antibodies and its impact on the immune responses and protection of the HAd-H5HA vaccine. A diagrammatic representation of the experimental plan is depicted (Fig. 1).
Fig. 1. Diagrammatic representation of animal immunization and challenge studies.
To mimic preexisting vector immunity and the development of vector-specific immune responses following first-time vaccination with an Ad vector-based vaccine, 5 to 6-week-old BALB/c mice were mock-inoculated (with PBS) or inoculated with 107 PFU of HAdV-C5 via the i.n. route (the natural route of HAdV-C5 infection in humans) to develop high levels (>200 virus-neutralizing antibody titers) of pre-existing vector immunity. At 1-month post-inoculation, the primed animals were inoculated i.m. with 108 PFU of HAd-GFP to mimic the conditions for a first inoculation with the AdV vector-based vaccine. Subsequently, at 1, 3, 6, and 10 months post-HAd-GFP inoculation, naïve- or HAdV-primed animals (10 mice/group) were vaccinated i.m. with 108 PFU of HAd-ΔE1E3 or HAd-H5HA. Before each immunization, blood samples were collected from the cheek vein. For immunogenicity studies, five animals from each group were euthanized under anesthesia at 4 weeks after immunization, and the blood and spleen were collected to monitor humoral and cell-mediated immune responses. For protection studies, the remaining five immunized animals were challenged i.n. with 100 mouse infectious dose 50 (MID50) of VN/1203/RG, euthanized at 3 days post-challenge, and the lungs were collected to determine the lung virus titers. PBS, phosphate buffer saline; i.n., intranasal; i.m., intramuscular; HAdV-C5, Human adenovirus type 5; HAd-ΔE1E3, HAdV-C5 empty vector with deletions in E1 and E3 regions; HAd-GFP, HAd-ΔE1E3 vector with the GFP gene inserted in the E1 region; HAd-H5HA, HAd-ΔE1E3 vector with the HA gene from A/HK/156/H5N1 influenza virus inserted in the E1 region.
Control groups.
At Day 0, all control groups were inoculated intranasally (i.n.) with 20 μl/animal of phosphate buffer saline (PBS) under anesthesia (intraperitoneal injection of 90 mg/kg ketamine and 10 mg/kg xylazine in PBS). One month later all control groups were inoculated intramuscularly (i.m.) with 50 μl of PBS. Subsequently, at 1, 3, 6 and 10 months, post-second inoculation with PBS, 10 animals per group were inoculated i.m. with 1 × 108 PFU of HAd-ΔE1E3.
Naïve vaccinated groups.
At Day 0, all naïve-vaccinated groups were inoculated i.n. with 20 μl of PBS under anesthesia and one month later, all animals received 50 μl of PBS i.m. Subsequently, at 1, 3, 6, and 10 months post-second PBS inoculation, 10 animals were inoculated i.m. with 1 × 108 PFU of HAd-H5HA.
Primed vaccinated groups.
At Day 0, all primed-vaccinated groups were inoculated i.n. with 1 × 107 PFU of HAdV-C5 and one month later, all animals were inoculated i.m. with 1 × 108 PFU of HAd-GFP. Subsequently, at 1, 3, 6, and 10 months post-inoculation with HAd-GFP, 10 animals were inoculated with 1 × 108 PFU of HAd-H5HA.
Blood samples were collected from the cheek vein from all mice 3 days before the second and third inoculations for the evaluation of HAdV-C5 neutralizing antibodies and GFP antibody levels using the plaque assay and ELISA, respectively. Blood samples and spleens were collected under anesthesia from 5 mice in each group (control, naïve-vaccinated or primed-vaccinated) at 4 weeks post-third inoculation either with HAd-ΔE1E3, HAd-H5HA or HAd-H5HA, respectively. Serum samples were used to determine the humoral immune responses by ELISA, and splenocytes were used to determine the cell-mediated immune responses by ELISpot. The remaining 5 mice in each group (control, naïve-vaccinated or primed-vaccinated) were challenged at 4 weeks post-third inoculation i.n. with 100 mouse infectious dose 50 (MID50) of A/VN/1203-RG under anesthesia. Three days after the challenge mice were euthanized, and the lungs were collected for evaluating the protection efficacy by determining the lung virus titers.
Vector-neutralizing antibody titration
Serum samples collected from the mouse study (Fig. 1) before the second and the third inoculations, were used to monitor HAdV-C5-neutralization antibody titers by a virus-neutralization assay as described previously [24]. Briefly, serum samples were diluted in PBS 1:10 and inactivated at 56°C for 30 minutes. The inactivated sera were 2-fold serially diluted and incubated with 100 PFU HAdV-C5 at 37°C for 1 h. Each serum/virus mixture was added to BHH-2C cells at 90% confluency in 60-mm tissue culture plates in triplicate. After 30 minutes at 37°C, the agarose overlay (MEM containing 0.5% agarose, 5% fetal calf serum and 1% yeast extract) was added into each plate and incubated at 37°C in 5 % CO2 incubator for 7 days. The number of AdV plaques were counted to determine virus-neutralization titers. The highest serum dilution that reduced the number of virus plaques by 50% compared to the control virus-infected cells was considered as the HAdV-C5-neutralization antibody titer.
Enzyme-linked immunosorbent assay (ELISA)
For measuring the humoral immune responses to GFP or HA, ELISA was used as described previously [26, 27]. Briefly, 96-well ELISA plates (Thermo Fisher Scientific Clear Flat-Bottom Immuno Nonsterile 96-Well Plates) were coated with 0.5 μg/ml of purified GFP protein (Upstate, Temecula, CA) or HA protein of HK/156 (MyBioSource, Inc., San Diego, CA), incubated overnight at 4°C, and blocked with 1% bovine serum albumin (BSA) in PBS. Various serial dilutions of a few mouse serum samples were used to determine the best dilution for all serum samples. Finally, all serum samples were diluted to 1:200, added to the wells containing GFP or HA and incubated for 2 h at room temperature. The plates were washed 4 times with PBST (PBS + Tween 0.5%) and horseradish peroxidase-conjugated goat anti-mouse IgG (anti-mouse IgG-HRP) [Invitrogen|Thermo Fisher Scientific) at a dilution of 1:5000 in PBS containing 0.5% BSA was added into each well and incubated at room temperature for 2 h. The plates were washed 4 times with PBST, and the color development was achieved with BD OptEIA™ ELISA set TMB substrate (Thermo Fisher Scientific) following the manufacturer recommendations. An equal volume of 2N sulfuric acid solution was used to stop the reaction. The optical density readings were measured at 450 nm using a SpectraMax® i3x microplate reader (Molecular Devices, Sunnyvale, CA).
ELISpot Assays
The assays were performed as described previously [9]. The splenocytes were extracted from the spleen tissues and used for mouse anti-interferon-gamma (anti-INFγ) ELISpot assays. Different dilutions of the splenocytes in triplicates were stimulated with HA518 (IYSTVASSL) peptide (H-2Kd−restricted CTL epitope for HA) at a concentration of 0.25 μg/ml. The number of the spot-forming units (SFU) were counted using AID ELiSpot reader 8.0 (Autoimmun Diagnostika GmbH, Germany).
Statistical analyses
One and two-way ANOVA with Bonferroni post-test were conducted using GraphPad Prism 6.0 to determine the statistical significance between groups. The statistical significance was set at p<0.05.
RESULTS
The decline in HAdV-C5-neutralizing antibodies with time in a mouse model
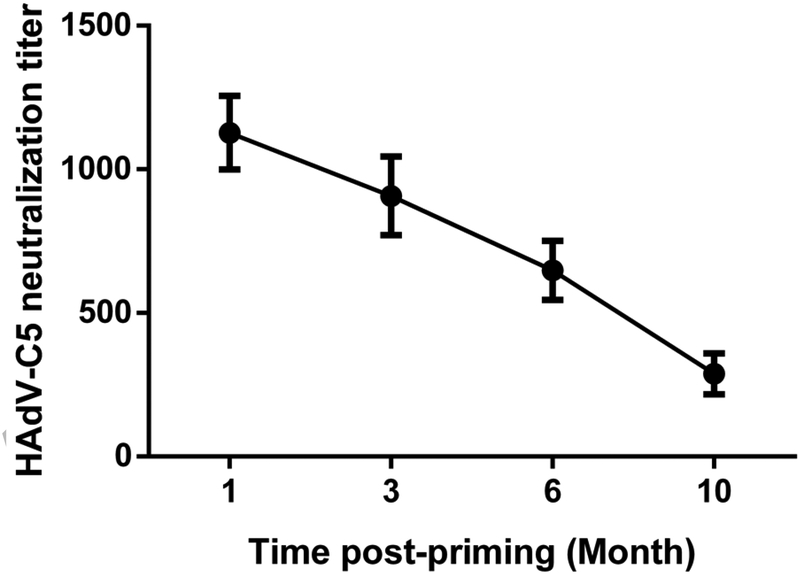
To mimic the pre-existing AdV vector immunity status of humans in the mouse model, we first inoculated mice i.n. with 1 × 107 PFU of HAdV-C5 to develop reasonable levels of HAdV-C5-neutralizing antibodies. A single inoculation of HAdV-C5 resulted in AdV-neutralization titers in the range of 333±67 at 4 weeks post-inoculation (data not shown). Since the AdV vector immunity titer above 200 is considered to be on a higher side in the human population [28], HAdV-C5-neutralization titers in our mouse model closely mimic the situation in humans. Mouse groups having AdV-neutralizing antibodies were inoculated with 1 × 108 PFU of HAd-GFP to mimic the normal human conditions where individuals having pre-existing AdV vector immunity were immunized for the first time with an AdV vectored vaccine. HAdV-C5-neutralizing antibody titers in mice reached 1127±128 at 4 weeks post-inoculation with HAd-GFP (Fig. 2). To monitor the decline in vector immunity with time, HAdV-C5-neutralizing antibody titers in mice were determined at 3, 6 and 10 months post- HAd-GFP inoculation. The HAdV-C5-neutralizing antibody titer started to decline with time and reached 907±137, 648±102, and 288±71.5 after 3, 6 and 10 months, respectively post-inoculation with HAd-GFP (Fig. 2). The decline curve shows that the half-life of HAdV-C5-neutralizing antibodies was approximately 6 months.
Fig. 2. The decline in vector immunity with time in the mouse model.
Six to 8-week-old BALB/c mice were inoculated with 107 PFU of HAdV-C5 via the i.n. route and at 1-month post-inoculation, the primed animals were inoculated i.m. with 108 PFU of HAd-GFP. Following 1, 3, 6, and 10 months post-HAd-GFP inoculation, blood samples were collected from the cheek vein to monitor the development of HAdV-C5-neutralizing titers by virus neutralization assay. The data are depicted as mean ± SD from 10 mice. HAdV-C5, Human adenovirus type 5.
Inhibition in GFP-specific antibody response in HAdV-C5-primed mice compared to naïve mice following inoculation with HAd-GFP
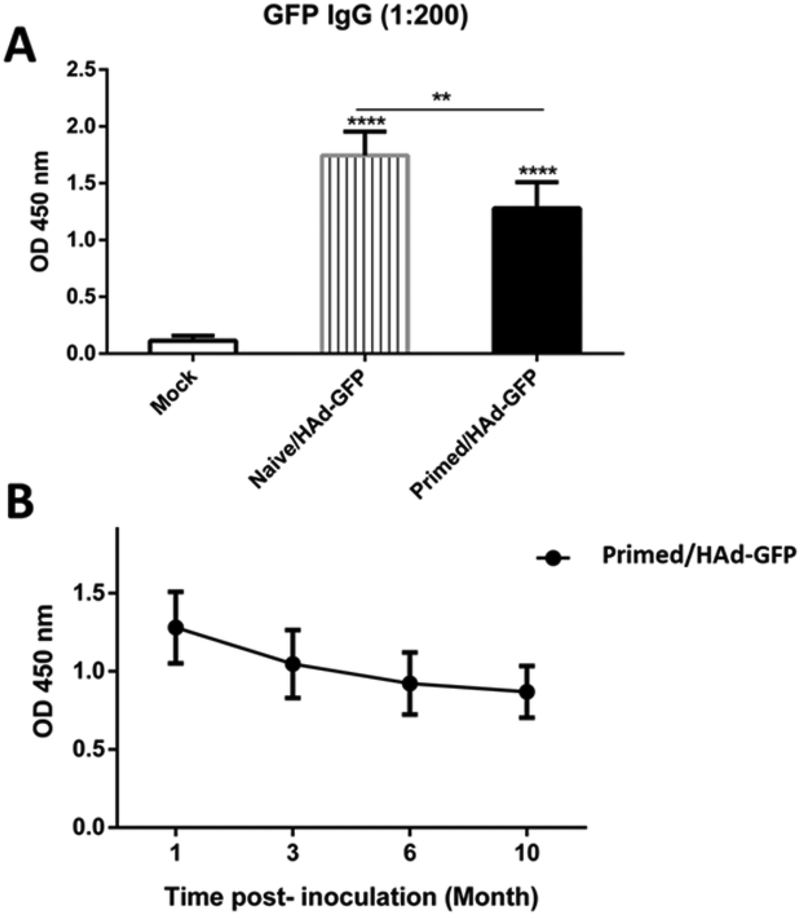
To determine the impact of vector immunity on the resultant humoral immune response, HAdVC5-primed group having vector neutralizing antibody titers of 333±67, or the naïve group, were inoculated with HAd-GFP. There was approximately 26.6% inhibition in GFP-specific ELISA antibody levels in the HAdV-C5-primed group compared to the naïve group (Fig. 3A) suggesting that significant levels of reporter-specific humoral immune response occurred even in the presence of high levels of vector immunity. Furthermore, to monitor the decline in reporter-specific antibody levels with time, GFP-specific ELISA antibody levels in the HAdV-C5-primed group were determined at 3, 6 and 10 months post-HAd-GFP inoculation. The GFP-specific antibody levels declined with time and decreased to 81.8, 72, and 67.8% after 3, 6 and 10 months, respectively, compared to the one month level post-HAd-GFP inoculation (Fig. 3B) indicating that AdV vector immunization provides long-lasting humoral immune responses.
Fig. 3. Inhibition of GFP-specific antibody response in HAdV-C5-primed mice compared to naïve mice following inoculation with HAd-GFP.
A) Naïve or HAdV-C5-primed mice were immunized with HAd-GFP, and blood samples were collected at 1 month post-immunization. B) HAdV-C5-primed mice were immunized with HAd-GFP, and blood samples were collected at 1, 3, 6 and 10 months post-immunization. GFP-specific ELISA antibody levels were determined in all serum samples. **, significant at p<0.01 and ****, significant at p<0.0001. The statistical analysis was done by Bonferroni post-test and one-way ANOVA using GraphPad Prism 6.
Improvement in HA-specific humoral and cell-mediated immune responses following decline of HAdV-C5 neutralizing antibody titers with time
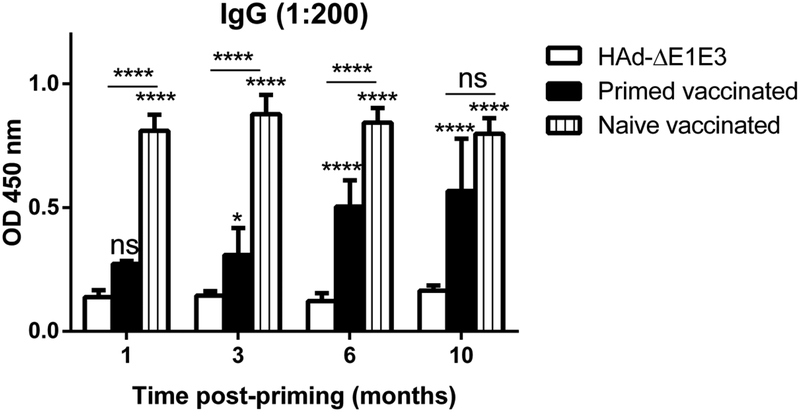
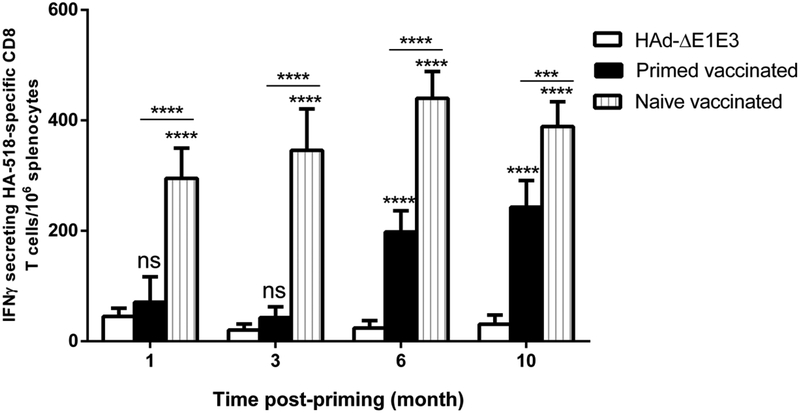
To mimic the situation in humans after the first vector inoculation, HAdV-C5-primed mouse groups were inoculated with HAd-GFP. This led to the development of very high titers of vector neutralizing antibodies in the range of 1127±128 at 4 weeks post-inoculation with HAd-GFP. These levels of HAdV-C5-neutralizing antibodies allowed us to monitor the improvement in HA-specific humoral and cell-mediated immune responses following the decline of HAdV-C5 neutralizing antibody titers with time. Age-matched naïve or HAdV-C5-primed mouse groups were immunized i.m. once with 1 × 108 PFU of HAd-H5HA at 1, 3, 6, and 10 months post-inoculation with HAd-GFP. Detectable levels of hemagglutination inhibition (HI) or virus neutralization (VN) titers against antigenically distinct challenge virus, VN/1203/RG, were not observed (data not shown). However, earlier we have demonstrated that immunization of mice with HAd-H5HA led to significantly higher levels of HI and VN titers against the homologous influenza virus[29]. Therefore, here we used purified HA protein of a homologous H5N1 virus for measuring HA-specific antibody titers by ELISA. HA-specific ELISA antibody levels in naïve vaccinated groups at 1, 3, 6 and 10 months were similar, showing that mouse age did not significantly affect this response (Fig. 4). However, the HA-specific ELISA antibody responses in primed vaccinated groups correlated inversely with the HAdV-C5 neutralizing antibody titers, continually increasing with time since previous HAdV-C5 exposure (Fig. 4). As expected, the maximum inhibition of HA-specific antibody levels was observed in the primed vaccinated group that was immunized 1-month post-inoculation with HAd-GFP. At 10 months post-inoculation with HAd-GFP, vector neutralizing antibodies titers were declined to 288±71.5 and the HA-specific ELISA antibody levels in the primed vaccinated group were not significantly different from those in naïve mice (Fig. 4). The HA-specific ELISA antibody levels at 10 months were significantly better than those in the primed vaccinated groups at 1, 3 or 6 months (Fig. 4). AdV vectors are known to induce significant levels of CMI responses to the antigenic protein, and the CMI responses to influenza antigens are capable of conferring heterologous as well as heterosubtypic protection against influenza viruses [30–33]. Because of the importance of CMI responses, the HA518 epitope-specific CD8+ immune response was also monitored to determine the improvement in HA-specific CMI responses following decline of HAdV-C5 neutralizing antibody titers with time. As with antibody responses, there was a continual increase in the number of IFNγ-secreting CD8+ T cells in the spleens of the primed vaccinated groups as the vector immunity declined with time (Fig. 5). The maximum inhibition in the number of IFNγ-secreting CD8+ T cells in the spleens were observed in the primed vaccinated groups that were immunized 1 or 3 months post-inoculation with HAd-GFP (Fig. 5). At 10 months post-inoculation with HAd-GFP, the numbers of IFNγ-secreting CD8+ T cells in the spleens of the primed vaccinated group at 10 months were significantly higher than in the primed vaccinated groups at 1, 3 or 6 months. In contrast to the antibody response, the CD8+ T cell response remained somewhat lower than that of the naïve vaccinated group even at 10 months (Fig. 5). As with antibody responses, the number of IFNγ-secreting CD8+ T cells in the spleens of naïve vaccinated groups at 1, 3, 6 and 10 months did not change significantly as the mice aged (Fig. 5).
Fig. 4. Increase in HA-specific antibody levels following immunization with HAd-H5HA with a decline in vector immunity with time.
At 1, 3, 6, and 10 months post-HAd-GFP inoculation, naïve or HAdV-primed BALB/c mice were immunized i.m. with 108 PFU of HAd-H5HA, and at 4 weeks after the immunization with HAd-H5HA, the blood samples were collected to monitoring the development of HA-specific antibody levels by ELISA. ns, non-significant at p<0.05; *, significant at p<0.05; and ****, significant at p<0.0001. The statistical analysis was done by Bonferroni post-test and two-way ANOVA using GraphPad Prism 6.
Fig. 5. Increase in the number of HA-specific IFNγ secreting CD8 T cells following immunization with HAd-H5HA with a decline in vector immunity.
At 1, 3, 6, and 10 months post-HAd-GFP inoculation, naïve or HAdV-primed BALB/c mice were immunized i.m. with 108 PFU of HAd-H5HA, and at 4 weeks after the immunization with HAd-H5HA and the spleens were collected to monitoring the number of HA-specific IFNγsecreting CD8 T cells by ELISpot. The data are represented as the mean ± standard deviation (SD) of spot-forming units (SFU). ns, non-significant at p<0.05; *, significant at p<0.05; **, significant at p<0.01; ***, significant at p<0.001; and ****, significant at p<0.000; The statistical analysis was done by Bonferroni post-test and two-way ANOVA using GraphPad Prism 6.
Enhancement in heterologous H5N1 influenza protection efficacy and improvement in HA-specific humoral and cell-mediated immune responses following the decline of HAdV-C5 neutralizing antibody titers with time
The impact of vector immunity on the vaccine efficacy can be best assessed by determining the protective effectiveness following the challenge. To evaluate the differences in protective efficacy between the primed vaccinated groups and the naïve vaccinated groups following the decline of HAdV-C5 neutralizing antibody titers with time, we used an antigenically distinct H5N1 virus (VN/1203/RG) for challenge studies. Since immunization with HK/156 HA will not confer complete protection against challenge with VN/1203/RG even in the naïve vaccinated groups, this allowed us a better comparison between naïve vaccinated and primed vaccinated groups. For evaluating influenza virus protection efficacy, the lung viral titers in the primed vaccinated groups were monitored and compared with the lung viral titers of the naïve vaccinated or control groups. Significant reduction in lung viral titers is an essential parameter of vaccine protective efficacy, and it is also a strong indicator of the inhibition of virus transmission.
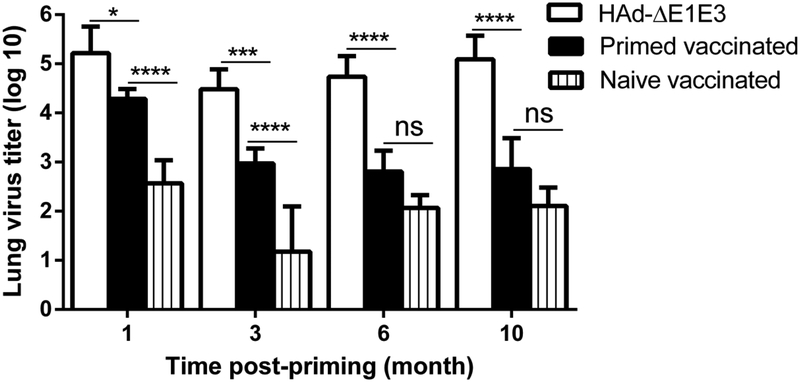
To determine enhancement in heterologous H5N1 influenza protection efficacy and improvement in HA-specific humoral and cell-mediated immune responses following the decline of HAdV-C5 neutralizing antibody titers with time. The control, naïve vaccinated and primed vaccinated mouse groups were inoculated with HAd-ΔE1E3, HAd-H5HA, or HAd-H5HA respectively at 1, 3, 6, and 10 months post-inoculation with HAd-GFP. The control, naïve vaccinated, and primed vaccinated groups were challenged with 100 MID50 of VN/1203/RG one-month post-vaccination. In the control groups, the lung virus titers at 1, 3, 6 and 10 months were 5.22, 4.48, 4.74 and 5.09 logs, respectively, and in the naïve vaccinated groups, the lung virus titers at 1, 3, 6 and 10 months were 2.57, 1.18, 2.07 and 2.11 logs, respectively (Fig. 6). In the primed vaccinated groups, the lung virus titers at 1, 3, 6 and 10 months were 4.29, 2.97, 2.81 and 2.86 logs, respectively (Fig. 6). Significant and continual reductions in the lung virus titers in the primed vaccinated groups were observed as the vector immunity declined with time (Fig. 6). The lung virus titers in the naïve vaccinated group were significantly lower than those in the control or the primed vaccinated groups at 1 and 3 months, but at 6 and 10 months, the differences in the lung virus titers between the primed and naïve vaccinated groups were statistically similar (Fig. 6). The protection results, therefore, correlate with the improvement of both humoral and CMI responses after 6 months post-inoculation with HAd-GFP.
Fig. 6. Enhancement in heterologous H5N1 influenza protection efficacy following the decline of HAdV-C5 neutralizing antibody titers with time.
At 1, 3, 6, and 10 months post-HAd-GFP inoculation, naïve or HAdV-primed BALB/c mice were immunized i.m. with 108 PFU of HAd-H5HA or HAd-ΔE1E3, and at 4 weeks after the immunization with HAd-H5HA and the animals were challenged i.n. with 100 mouse infectious dose 50 (MID50) of VN/1203/RG, euthanized at 3 days post-challenge, and the lungs were collected and processed for lung virus titers to monitor protection efficacy. The data are shown as mean Log10 TCID50±SD, and the detection limit was 0.5 Log10 TCID50/ml. ns, non-significant at p<0.05; *, significant at p<0.05; **, significant at p<0.01; ***, significant at p<0.001; and ****, significant at p<0.000. The statistical analysis was done by Bonferroni post-test and two-way ANOVA using GraphPad Prism 6.
DISCUSSION
Pre-existing AdV vector immunity is an important factor that could significantly inhibit the efficacy of an AdV vector by compromising vector uptake by susceptible cells due to the presence of vector-neutralizing or vector cross-neutralizing antibodies. AdV is a common human pathogen, and there are more than 60 different types of HAdVs that are known to infect humans, so that the majority of individuals are infected with one or more AdV types early in their lives leading to a high occurrence of pre-existing AdV vector immunity [17, 18, 34]. Therefore, several less prevalent HAdV types, as well as nonhuman AdVs including ChAdV, BAdV, and others, have developed as gene delivery systems to circumvent pre-existing vector immunity [5, 35–39].
Earlier we demonstrated that moderate levels of vector immunity [520 virus-neutralization (VN) titer] did not adversely impact the protective efficacy of a two-dose HAdV-C5-based influenza vaccine [20]. Further increases in vector immunity (up to 2240 VN titers) were overcome either by increasing the vaccine dose by 5-fold or by using an alternate route of vaccination. Furthermore, in the presence of exceptionally high levels of vector immunity (~3040 VN titers), immunization with a 5-fold vaccine dose still resulted in approximately 3.3–3.7 log reductions in lung virus titers. Similarly, in a separate study, the impact of vector immunity on a HAdV-C5-based HIV vaccine in phase I clinical trial was shown to be overcome by increasing the vaccine dose [40]. Therefore, moderate to high levels of vector immunity do not present an insurmountable obstacle to using AdV vectors as vaccines.
In addition to pre-existing AdV vector immunity, the development of AdV vector-specific neutralizing antibodies could play an important role in inhibiting the vaccine efficacy of the same vector carrying the same or a different insert on repeated immunizations. This is a particular concern for vaccines, such as for influenza, which is typically given annually. Here we examined whether AdV neutralizing antibody titers decline enough to permit annual vaccination with the same AdV vector. To address this critical need, we mimicked the pre-existing AdV vector immunity in the mouse model by immunizing the first time with a HAd vector containing the GFP gene, followed by immunization with the same vector expressing HA of an H5N1 influenza virus 1, 3, 6, and 10 months post-HAd-GFP inoculation. By using a single dose of vaccine vector and challenging with an antigenically heterologous H5N1 influenza virus, we tested the ability of the vaccine to induce a robust immune response despite the presence of pre-existing vector immunity.
HAdV-C5-neutralizing antibody titers declined with a half-life of approximately 6 months. Preexisting vector titers of 333±67 only inhibited the development of GFP-specific antibody levels to approximately 26.6% compared to naïve animals inoculated with HAd-GFP. By10-month post-HAd-GFP inoculation, HAdV-C5-neutralizing antibody titers dropped below 300, suggesting that immunization with the same vector will be effective. As expected, there was a continual decline in GFP-specific antibody levels with time, but significant levels persisted at least for 10 months signifying that immunogen-specific immune responses with AdV vectors are usually long-lasting. Earlier in naïve mice immunized twice with HAdV vector expressing HA of a H5N1 virus, significant levels of humoral and CMI responses conferring complete protection from morbidity and mortality following challenge with a lethal H5N1 influenza virus were demonstrated even after one year [10].
Immunization of mice shortly after initial inoculation with HAdV vectors, in the presence of high levels of vector immunity, did lead to a significant reduction in both CMI and humoral immune responses to the vaccine antigen. However, as vector immunity declined over time, humoral as well as CMI immune responses following immunization with HAd-H5HA were easily detectable, and by 10 months post-HAd-GFP inoculation were more mildly affected by vector immunity. Moreover, these immune responses, particularly at 6 and 10 months, resulted in significant protection as measured by reduction in the lung virus titers following challenge with an antigenically heterologous H5N1 influenza virus. Again, by 10 months post-HAd-GFP inoculation, protection was very similar to that seen in mice that had no vector immunity, further supporting the notion that annual vaccination with an AdV vector-based vaccine will be practical.
One of the major limitations of the mouse model is the lifespan since one-year-old mouse is an old mouse with somewhat impaired immune system. Also, mice are not a natural host for HAdV-C5, and hence there is no possibility of recurrent infection in the periods between vaccinations. Therefore, the decay of vector immunity with time in humans may vary significantly in the mouse model. Since it may not be easy to conduct a vector immunity decline study for an AdV that is a common human pathogen due to the likelihood of natural exposure during the study period. It will be necessary to conduct a study in humans with a less prevalent HAdV or nonhuman AdV vector to determine the decay kinetics of vector immunity. Serum samples from the human subjects immunized with an AdV-based vaccine, e.g., Ebola vaccine, over time may serve as an excellent source to determine the decay of vector immunity with time in humans.
In summary, it is clear from this study that both pre-existing AdV vector immunity and the development of vector-neutralizing antibodies following the first inoculation with any AdV vector are important in determining a successful strategy for an AdV vector-based gene delivery. Development of some less prevalent HAdV types, as well as nonhuman AdVs as gene delivery vehicles, is essential to have optimum options for various applications ranging from the vaccine to gene therapy. Our results suggest that it may be better to use a different AdV vector for the second inoculation if the period between the two inoculations is less than 6 months. However, the same vector may be used again successfully if the period between the two inoculations is more than 10 months at least for the vaccine applications.
Acknowledgments:
This work was supported by the Public Health Service grants - AI059374 from the National Institute of Allergy and Infectious Diseases, and the Hatch and Animal Health Funds. We thank Donna Schrader, and Sara Penka for their assistance. Ekramy Sayedahmed was supported by an Egyptian Government Scholarship for PhD studies at Purdue University and was on study leave from the College of Veterinary Medicine, Benha University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None of the authors has any conflict of interest.
Ethics statement: All animal experiments were conducted following the approvals and guidelines of the Institutional Animal Care and Use Committee (IACUC), and the Institutional Biosafety Committee (IBC).
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, U.S. Department of Health and Human Services.
REFERENCES
- [1].Sharma PK, Dmitriev IP, Kashentseva EA, Raes G, Li L, Kim SW, et al. Development of an adenovirus vector vaccine platform for targeting dendritic cells. Cancer Gene Ther. 2018;25:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang C, Zhou D. Adenoviral vector-based strategies against infectious disease and cancer. Hum Vaccin Immunother. 2016;12:2064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wold WS, Toth K. Adenovirus Vectors for Gene Therapy, Vaccination and Cancer Gene Therapy. Curr Gene Ther. 2013;13:421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vemula SV, Mittal SK. Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin Biol Ther. 2010;10:1469–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bangari DS, Mittal SK. Development of nonhuman adenoviruses as vaccine vectors. Vaccine. 2006;24:849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81:3170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sharma A, Tandon M, Ahi YS, Bangari DS, Vemulapalli R, Mittal SK. Evaluation of Cross-Reactive Cell-Mediated Immune Responses among Human, Bovine and Porcine Adenoviruses. Gene Ther. 2010;17:634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gao W, Soloff AC, Lu X, Montecalvo A, Nguyen DC, Matsuoka Y, et al. Protection of Mice and Poultry from Lethal H5N1 Avian Influenza Virus through Adenovirus-Based Immunization. J Virol. 2006;80:1959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hoelscher MA, Garg S, Bangari DS, Belser JA, Lu X, Stephenson I, et al. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet. 2006;367:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hoelscher MA, Jayashankar L, Garg S, Veguilla V, Lu X, Singh N, et al. New pre-pandemic influenza vaccines: an egg- and adjuvant-independent human adenoviral vector strategy induces long-lasting protective immune responses in mice. Clin Pharmacol Ther. 2007;82:665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Van Kampen KR, Shi Z, Gao P, Zhang J, Foster KW, Chen DT, et al. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine. 2005;23:1029–36. [DOI] [PubMed] [Google Scholar]
- [12].Gurwith M, Lock M, Taylor EM, Ishioka G, Alexander J, Mayall T, et al. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect Dis. 2013;13:238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liebowitz D, Lindbloom JD, Brandl JR, Garg SJ, Tucker SN. High titre neutralising antibodies to influenza after oral tablet immunisation: a phase 1, randomised, placebo-controlled trial. Lancet Infect Dis. 2015;15:1041–8. [DOI] [PubMed] [Google Scholar]
- [14].Kim L, Liebowitz D, Lin K, Kasparek K, Pasetti MF, Garg SJ, et al. Safety and immunogenicity of an oral tablet norovirus vaccine, a phase I randomized, placebo-controlled trial. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hoelscher MA, Singh N, Garg S, Jayashankar L, Veguilla V, Pandey A, et al. A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J Infect Dis. 2008;197:1185–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bangari DS, Mittal SK. Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 2004;105:127–36. [DOI] [PubMed] [Google Scholar]
- [17].Kostense S, Koudstaal W, Sprangers M, Weverling GJ, Penders G, Helmus N, et al. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. Aids. 2004;18:1213–6. [DOI] [PubMed] [Google Scholar]
- [18].Nwanegbo E, Vardas E, Gao W, Whittle H, Sun H, Rowe D, et al. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin Diagn Lab Immunol. 2004;11:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thorner AR, Vogels R, Kaspers J, Weverling GJ, Holterman L, Lemckert AA, et al. Age dependence of adenovirus-specific neutralizing antibody titers in individuals from sub-Saharan Africa. J Clin Microbiol. 2006;44:3781–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pandey A, Singh N, Vemula SV, Couetil L, Katz JM, Donis R, et al. Impact of preexisting adenovirus vector immunity on immunogenicity and protection conferred with an adenovirus-based H5N1 influenza vaccine. PLoS One. 2012;7:e33428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Taylor J, Meignier B, Tartaglia J, Languet B, VanderHoeven J, Franchini G, et al. Biological and immunogenic properties of a canarypox-rabies recombinant, ALVAC-RG (vCP65) in non-avian species. Vaccine. 1995;13:539–49. [DOI] [PubMed] [Google Scholar]
- [22].Paoletti E Applications of pox virus vectors to vaccination: an update. Proc Natl Acad Sci U S A. 1996;93:11349–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. [DOI] [PubMed] [Google Scholar]
- [24].van Olphen AL, Mittal SK. Development and characterization of bovine × human hybrid cell lines that efficiently support the replication of both wild-type bovine and human adenoviruses and those with E1 deleted. J Virol. 2002;76:5882–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Noblitt LW, Bangari DS, Shukla S, Knapp DW, Mohammed S, Kinch MS, et al. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. Cancer Gene Ther. 2004;11:757–66. [DOI] [PubMed] [Google Scholar]
- [26].Mittal SK, Middleton DM, Tikoo SK, Babiuk LA. Pathogenesis and immunogenicity of bovine adenovirus type 3 in cotton rats (Sigmodon hispidus). Virology. 1995;213:131–9. [DOI] [PubMed] [Google Scholar]
- [27].Mittal SK, Aggarwal N, Sailaja G, van Olphen A, HogenEsch H, North A, et al. Immunization with DNA, adenovirus or both in biodegradable alginate microspheres: effect of route of inoculation on immune response. Vaccine. 2000;19:253–63. [DOI] [PubMed] [Google Scholar]
- [28].Tomita K, Sakurai F, Tachibana M, Mizuguchi H. Correlation between adenovirus-neutralizing antibody titer and adenovirus vector-mediated transduction efficiency following intratumoral injection. Anticancer Res. 2012;32:1145–52. [PubMed] [Google Scholar]
- [29].Singh N, Pandey A, Jayashankar L, Mittal SK. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against human adenovirus. Mol Ther. 2008;16:965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vemula SV, Ahi YS, Swaim AM, Katz JM, Donis R, Sambhara S, et al. Broadly protective adenovirus-based multivalent vaccines against highly pathogenic avian influenza viruses for pandemic preparedness. PLoS One. 2013;8:e62496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006;12:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sambhara S, Kurichh A, Miranda R, Tumpey T, Rowe T, Renshaw M, et al. Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLUISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage function. Cell Immunol. 2001;211:143–53. [DOI] [PubMed] [Google Scholar]
- [33].Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol. 2008;82:1350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mittal SK, Ahi YS, Vemula SV. 19 - Xenogenic Adenoviral Vectors A2 - Curiel, David T Adenoviral Vectors for Gene Therapy (Second Edition). San Diego: Academic Press; 2016. p. 495–528. [Google Scholar]
- [36].Moffatt S, Hays J, HogenEsch H, Mittal SK. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology. 2000;272:159–67. [DOI] [PubMed] [Google Scholar]
- [37].Lopez-Gordo E, Podgorski II, Downes N, Alemany R. Circumventing antivector immunity: potential use of nonhuman adenoviral vectors. Hum Gene Ther. 2014;25:285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Barouch DH, Tomaka FL, Wegmann F, Stieh DJ, Alter G, Robb ML, et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13–19). Lancet. 2018;392:232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vujadinovic M, Khan S, Oosterhuis K, Uil TG, Wunderlich K, Damman S, et al. Adenovirus based HPV L2 vaccine induces broad cross-reactive humoral immune responses. Vaccine. 2018;36:4462–70. [DOI] [PubMed] [Google Scholar]
- [40].Catanzaro AT, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–49. [DOI] [PMC free article] [PubMed] [Google Scholar]