Abstract
Neutrophils are an important source of IL-1β secretion in bacterial infections, where they infiltrate affected tissues in log-fold higher numbers than macrophages. Neutrophils also have functional NLRP3 and NLRC4 inflammasomes that can process pro-IL-1β to the bioactive 17kD form. In the current study, we examined the role of IL-1β in response to corneal infection with the filamentous fungus A. fumigatus, and found that neutrophils were the predominant source of bioactive IL-1β in the cornea. We also found that caspase-11−/− mice exhibited the same susceptibility phenotype as IL-1β−/−, ASC−/−, NLRP3−/− and caspase-1−/− mice, with impaired neutrophil recruitment to infected corneas and increased hyphal growth. We further demonstrate that caspase-11 is required for caspase-1 activation and IL-1β processing during infection. In vitro, we show that caspase-11 is regulated by the common type I IFN receptor (IFNAR1) through JAK-STAT signaling, and that caspase-11 is required for speck formation and caspase-1 activity. Aspergillus spores (conidia) stimulate IL-1β processing and secretion by neutrophils by activation of Dectin-1 and signaling through the Raf1 kinase/MEKK rather than the spleen tyrosine kinase (Syk) pathway. Collectively, these findings reveal unexpected regulation of IL-1β production by neutrophils in response to pathogenic fungi.
Keywords: Aspergillus, neutrophil, IL-1β, NLRP3, inflammasome, Dectin-1, Caspase-11, fungal keratitis
Introduction
IL-1β serves as a central cytokine in infection and immunity (1). Neutrophils are generally not considered to be an important source of IL-1β compared with macrophages; however, we, and others reported that neutrophils not only produce the pro-form of IL-1β in response to bacterial infections, but also have active NLRP3 and NLRC4 inflammasomes that generate active caspase-1, resulting in cleavage of the 31kD pro-form into the bioactive 17kD cleaved cytokine (2–4). Further, those reports demonstrate that in marked contrast to macrophages that undergo pyroptosis, neutrophils remain viable in response to Streptococcus pneumoniae - induced NLRP3 activation, Salmonella induced NLRC4 activation and LPS/ATP induced NLRP3 activation.
In the current study, we examined the role of IL-1β in a well-established model of fungal keratitis in which neutrophils are the predominant cells that infiltrate infected corneas (5–9). This model reflects early stage blinding human fungal keratitis, which is characterized by a predominant neutrophil infiltration and IL-1β gene expression (10). We found that in addition to requiring IL-1β, NLRP3 and caspase-1 to regulate A. fumigatus corneal infection, caspase-11 has an essential role in the development of corneal opacity and neutrophil infiltration in addition to regulating fungal growth, which is regulated by IFNAR. Together, results from this study identify caspase-11 as an important player in fungal keratitis that is based on its unexpected role mediating IL-1β secretion by neutrophils in the absence of pyroptosis.
Materials and Methods
Source of Mice
C57BL/6, caspase-1/11−/−, caspase-11−/− deficient and IFNAR1−/− mice were purchased from The Jackson Laboratory. Dectin-1 (clec7a−/−) and IL-1β deficient mice (6–8 weeks old) were a kind gift from Dr. Yoichiro Iwakura (University of Tokyo, Tokyo, Japan). Caspase-1/11−/−, were generated by R. Flavell (Yale University, New Haven, CT), and NLRP3−/− and ASC−/− mice were generated by Millenium Phamaceuticals. All C57BL/6 and gene knockout mice were bred in the ALARC approved animal facility at Case Western Reserve University; therefore, all mice were exposed to the same environment. We used 6–8 week old male and female mice in all experiments.
Aspergillus strain and growth conditions
The RFP-expressing strain of A. fumigatus (Af293) constitutively expresses monomeric dsRed protein, and was generated by Dr. Michelle Momany at the University of Georgia (Athens, GA) (5). Af293 were harvested from Sabouraud dextrose Agar (SDA) plate culture, and conidia were obtained through sterile gauze, then conidia were cultured in Sabouraud dextrose Broth (SDB) for 6 hours to allow germination and expression of β-glucan (5).
Antibodies and Reagents
All commercial reagents and antibodies are listed in Supplemental Table 1.
Murine model of fungal keratitis
Conidia were harvested from SDA and suspended in PBS. Mice were anaesthetized by intraperitoneal injection of 1.2% tribromoethanol, and a 30-gauge needle was used to make a pocket in the corneal stroma, after which a 33-gauge Hamilton syringe was inserted, and 2 μl of 1 × 105 conidia in PBS was injected into the corneal stroma. Corneal opacity and corneal fungal grown were photographed using a high-resolution stereo fluorescence MZFLIII microscope (Leica Micro- systems) and Spot RT Slider KE camera (Diagnostics Instruments). All images were captured using SpotCam software (RT Slider KE; Diagnostics Instruments). Corneal opacity and corneal red-fluorescence were quantified by Metamorph software as described (6).
A. fumigatus growth in infected corneas (CFU)
Infected whole eyes were homogenized in 1ml of sterile PBS using the Retsch Mixer Mill MM300 (Qiagen, Valencia, CA) at 33Hz for 4 minutes. Log dilutions were made and samples were plated on SDA plates at 37oC for 48 hours. The number of colony forming units (CFU) was determined by direct counting.
Western blot analysis
Corneas were dissected and homogenized using the Retsch Mixer Mill MM300 as described above. Protein content was quantified using the BCA kit (Thermofisher), and 10–20 μg protein extracts were subjected to standard western blot techniques as we reported (3, 11).
Bone marrow neutrophils and BM derived macrophages
Neutrophils were isolated from single cell suspensions of bone marrow using the negative selection EasySep™ mouse neutrophil enrichment kit (STEMCELL, Vancouver, Canada), which yields >95% neutrophils as confirmed by flow cytometry. To derive bone marrow macrophages, total bone marrow cells were incubated with MCSF for 10 days as described. Cells were >95% macrophages as determined by flow cytometry using F/480 antibody.
Cell culture
Purified conidia were cultured in in Sabouraud dextrose Broth (SDB) for 6 hours to allow germination and expression of β-glucan (5). Bone marrow neutrophils (95% purity) were pre-treated with the caspase-11 inhibitor Wedelolactone, the caspase-1 inhibitor AC-YVAD-CMK, STAT1/3 inhibitor Ruxolitinib, Raf1 inhibitor GW5074, Syk inhibitor R406, MEKK inhibitor U0126 or JNK inhibitor SP600125 at concentrations that we previously found were effective (3, 7, 12), or as recommended by the manufacturers. The source and catalogue numbers of all inhibitors are listed in Supplemental Table 1). Neutrophils were then incubated at 370C in 5% CO2 with conidia (10:1 conidia: neutrophils) for 3h to examine caspase-1 activity (FLICA), or for 6h to measure IL-1β. Cells were lysed, and pro-and cleaved IL-1β were detected by western blot. Total IL-1β in cell free supernatants was quantified by ELISA.
Flow Cytometric analysis
Infected corneas were dissected, the vascularized iris was removed, and corneas were incubated in 500 μl collagenase type I (Sigma-Aldrich) at 82 U/cornea for 1–2h at 37oC. For intracellular IL-1β, total cells were collected by centrifugation, and incubated in fixation buffer and in permeabilization buffer according to the manufacturer’s directions (eBioscience, San Diego, CA). Cells were resuspended in 200 μl FACS buffer containing 4μg/cornea Fc blocking Ab (anti-mouse CD16/32; eBioscience) on ice for 10 minutes, followed by FITC conjugated anti-rat NIMP-R14 Ab for 1h, washed and incubated in fixation buffer and in permeabilization buffer according to the manufacturer’s directions (eBioscience, San Diego, CA) and stained with APC conjugated anti-mouse IL-1β Ab. Cells were analyzed using an Accuri C6 flow cytometer (BD Bio. CA) and gated based on the Fluorescence minus one (FMO). Cells were also analyzed using the Amnis Imagestream.
FAM-FLICA Caspase-1 Assay
Neutrophils (1×106 cells/ml) were incubated with 1×106 heat-killed, swollen conidia for 3h, or with 100 ng/ml LPS for 3h and ATP for 45 min. were then incubated with the FAM-YVAD-FMK (ImmunoChemistry Tech., MN), which binds active caspase-1, and cells were counterstained with DAPI (Invitrogen). Active caspase-1 was detected by fluorescence microscopy and flow cytometry as we described (3).
Cytokine quantification
IL-1β, IFN-β CXCL1 and CXCL2 in digested corneas or in supernatants of neutrophil cultures were quantified by 2-site ELISA according to manufacturer’s directions (R&D Systems, MN). Bioactive IL-1β was quantified using the HEK-blue IL-1R expressing cells according to manufacturer’s directions (InVivoGen). Although these cells respond to IL-1α and IL-1β, bioactivity of IL-1β was examined by adding neutralizing antibodies.
Statistical Analysis
Data were analyzed by unpaired Student t test (comparing 2 samples) or by ANOVA with Tukey post hoc analysis for >2 samples using GraphPad Prism (San Diego, CA). Statistical significance was defined as p < 0.05.
RESULTS
Aspergillus fumigatus corneal infection is regulated by IL-1β, NLRP3 and ASC
To examine the role of IL-1β and the NLRP3/ASC inflammasome in fungal keratitis, we utilized a well-established murine model in which A. fumigatus conidia (spores) are injected directly into the corneal stroma (5). C57BL/6 and IL-1β−/− mice were infected intrastromally with 2 μl saline containing 1 × 105 conidia of A. fumigatus strain Af293 expressing dsRed. After 24h, hyphae had invaded the corneal stroma (Figure 1A), and total hyphal mass was significantly higher in infected IL-1β−/− compared with C57BL/6 mice (Figure 1A, B). Conversely, corneas of IL-1β−/− mice exhibit significantly less corneal opacification compared with C57BL/6 mice (Figure 1C, D).
Figure 1. Impaired fungal killing in A. fumigatus infected IL-1β−/−, NLRP3−/− and ASC−/− mice.
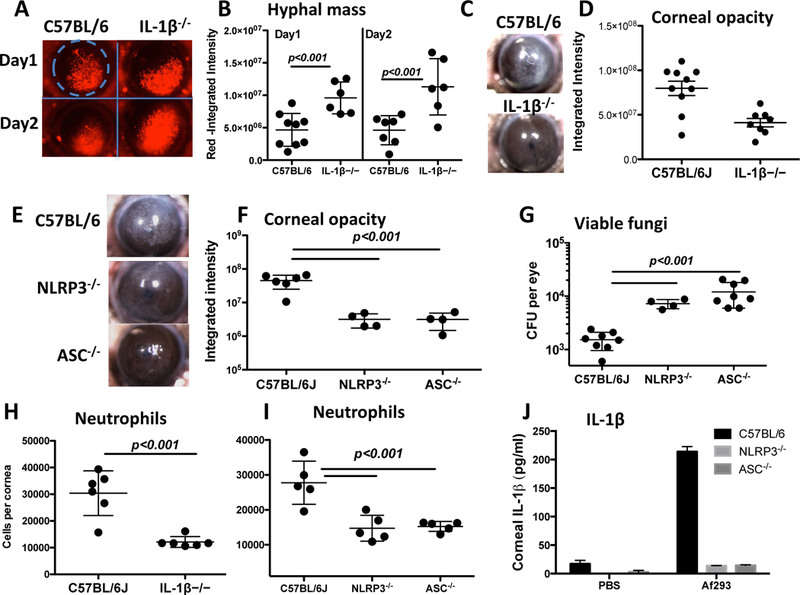
A-D: Corneas of IL-1β−/− mice infected with A. fumigatus conidia and examined for hyphal growth (A, B), and for corneal opacification (C, D) as shown by representative images (A, C) (original magnification is x20). Hyphal mass and corneal opacity were quantified by image analysis (B, D). Data points represent individual corneas. E-G: Corneal opacification (E, F) and colony forming units (CFU, G) in infected NLRP3−/− and ASC−/− corneas. H, I: Neutrophil numbers in infected corneas following collagenase digestion, and total Ly6G+ cells per cornea was quantified by flow cytometry. J: Total IL-1β in homogenized corneas of NLRP3−/− and ASC−/− mice measured by ELISA. P values were calculated by 2 way ANOVA with Tukey post analysis (3 or more data sets) or by unpaired students t test (2 data sets). Data are representative of three repeat experiments.
To determine the role of the NLRP3 inflammasome in regulating fungal growth, NLRP3−/− and ASC−/− mice were infected with Af293 (not expressing dsRed). As shown in Figure 1E-G, NLRP3−/− and ASC−/− mice had significantly higher CFU, but less corneal opacification than C57BL/6 mice. Our prior studies showed that rapid infiltration of neutrophils is essential to control fungal growth in the cornea, and that neutrophil infiltration results in corneal opacification (5). Longer-term infections in IL-1β−/−, NLRP3−/− and ASC−/− mice results in corneal perforation due to unregulated fungal growth (not shown). Finally, total cells in infected corneas were incubated with the anti-Ly6G antibody NIMP-R14 following collagen digestion, and examined by flow cytometry. As shown in Figure 1H-I, the number of Ly6G+ neutrophils was significantly lower in infected corneas from NLRP3−/− and ASC−/− compared with C57BL/6 mice, which was consistent with increased fungal growth and less corneal opacification (Figure 1E-G).
Finally, IL-1β was measured in corneas from infected and control mice. As shown in Figure 1J, the IL-1β response in NLRP3−/− and ASC−/− mice was undetectable compared to the robust response observed in infected C57BL/6 mice.
Together, these findings demonstrate that IL-1β, NLRP3 and ASC are required to regulate A. fumigatus hyphal growth in the cornea, and that IL-1β production in the cornea is dependent on NLRP3 and ASC.
Neutrophils are the predominant source of IL-1β in A. fumigatus infection
As the decreased IL-1β in infected NLRP3−/− and ASC−/− corneas correlates with impaired neutrophil infiltration, and we reported that neutrophils utilize the NLRP3 inflammasome to cleave IL-1β in Streptococcus pneumoniae infected corneas (3), we examined if neutrophils are the source of IL-1β in Aspergillus infected C57BL/6 corneas.
Infected C57BL/6 corneas were dissected 24h post – infection, incubated with collagenase, and Ly6G+ neutrophils with intracellular IL-1β were quantified by flow cytometry. As shown in Figure 2A, 47.7% total cells were IL-1β positive, and 44% were Ly6G+, indicating that the majority (92.2%) of IL-1β positive cells in A. fumigatus infected corneas are neutrophils. Intracellular IL-1β is also shown in representative Ly6G+, CD11β+ neutrophils (Figure 2B). However, as the antibody to IL-1β does not differentiate between the 31kD pro-form and the 17kD cleaved, bioactive form of IL-1β, we also processed infected corneas for western blot analysis. As shown in Figure 2C, the cleaved 17kD IL-1β was detected in infected corneas.
Figure 2. Neutrophils produce IL-1β in A. fumigatus corneal infection.
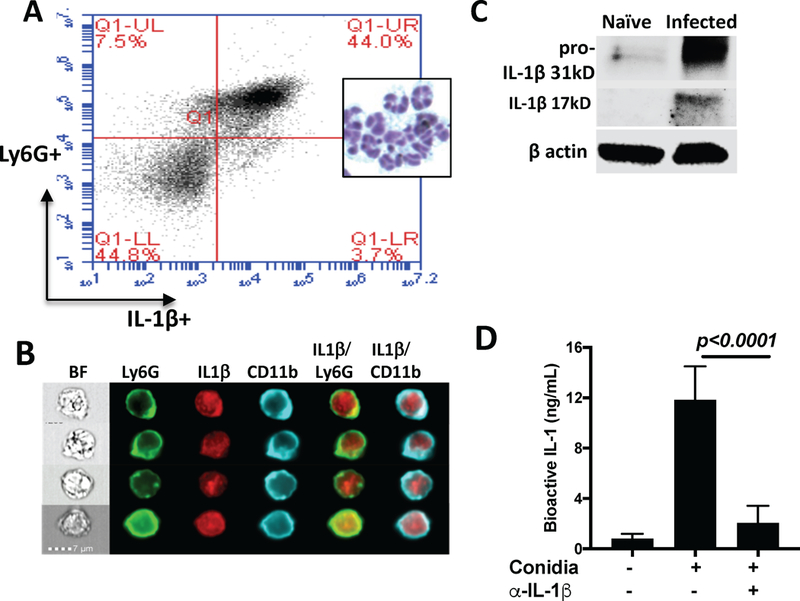
A: A. fumigatus infected corneas of C57BL/6 mice were collagenase digested 24h post infection, and Ly6G+ and intracellular IL-1β were quantified by flow cytometry. Inset shows polymorphonuclear cells from infected corneas after staining with Wrights-Giemsa. B: Representative neutrophils from infected corneas immunostained with Ly6G (NIMPR-14) and intracellular IL-1β examined by Imagestream analysis. C.Infected corneas were homogenized, run on SDS-PAGE, and IL-1β was detected by western blot. D. Homogenates of infected corneas were incubated with IL-1R reporter HEK cells, and total bioactive IL-1 was quantified. Data in A and C are from 5 corneas pooled together; Data in panel D are mean+/− SD from corneas from individual mice. All these experiments were repeated twice with similar results.
Finally, we used IL-1R reporter cells to quantify bioactive IL-1β. Infected corneas were homogenized, and bioactive IL-1 was quantified using IL-1R reporter cells as described in Methods. This assay is more sensitive than ELISA, and infected corneas were found to have ~10–15 ng/ml bioactive IL-1, which was reduced >90% in the presence of neutralizing IL-1β antbody (Figure 2D), indicating that IL-1β in infected corneas is bioactive.
Collectively, these findings demonstrate that neutrophils are not only the predominant source of IL-1β in Aspergillus infected corneas, but also that IL-1β is cleaved and has bioactivity.
Caspase-11 is required for Caspase-1 and IL-1β cleavage in vivo
To examine the role of caspase-1 in IL-1β production and A. fumigatus infection, we used caspase-1−/− mice that are also deficient in caspase-11 (caspase-1/11−/− mice), and mice that are deficient only in caspase-11 (13). We found that infected caspase-1/11−/− and caspase 11−/− mice had significantly elevated hyphal mass and viable A. fumigatus compared with infected C57BL/6 corneas (Figure 3A-C). These mice also had 3–5 fold fewer neutrophils compared with C57BL/6 corneas (Figure 3D), most likely due to impaired CXC chemokine production by resident macrophages as we reported in earlier studies (5). Together, these findings demonstrate an unexpected role for caspase 11 in the host response to a fungal infection.
Figure 3. Impaired fungal killing and IL-1β cleavage in infected Caspase-11−/− mice.
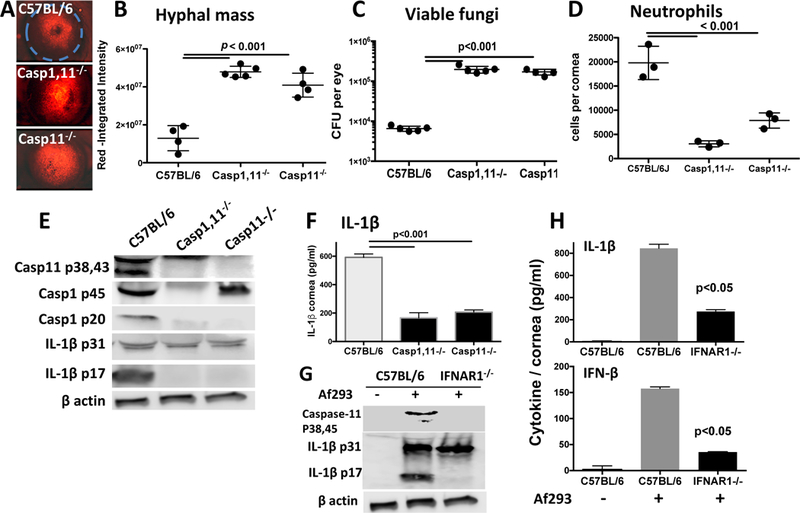
A-D: Corneas of caspase-1−/− and caspase-1/11−/− mice 24h post-infection with A. fumigatus conidia. Representative RFP hyphae in infected corneas (A), and hyphal mass for each cornea was quantified by image analysis (B). Fungal viability was measured by CFU (C), and total Ly6G+ neutrophils per cornea were quantified by flow cytometry (D). Experiments were performed as described in the legend to Figure 1; data points represent individual corneas. E-H: Corneas from infected C57BL/6, caspase-1−/− and caspase-1/11−/− mice were homogenized and examined for pro- and cleaved forms of IL-1β (E), and secreted IL-1β (F). G,H. Corneas from C57BL/6 and IFNAR−/− mice were examined for pro- and cleaved IL-1β (G), and secreted IL-1β and IFN-β (H). production in infected corneas from control and IFNAR−/− mice (I). A: original magnification was x20; B-D: data points represent individual mice; E,G: Pooled corneas from three mice; F, H: mean+/−SD from 3–5 mice. P values were derived by ANOVA with a Tukey post-test.
To examine the effect of caspase 11 on IL-1β cleavage, infected C57BL/6, caspase 1/11−/− and caspase 11−/− corneas were homogenized, and examined by western blot analysis. As shown in Figure 3E, in contrast to C57BL/6 mice, caspase-1 p20 and IL-1β p17 were not detected in caspase 11−/− corneas, indicating that caspase-11 is required for caspase-1 and subsequent IL-1β cleavage. Secreted IL-1β (pro- and cleaved) was also significantly lower in infected Caspase-11−/− corneas (Figure 3F).
As the type 1 IFN receptor (IFNAR) has been shown to induce caspase-11 expression (14), we next examined the effect of IFNAR on IL-1β production and cleavage in Aspergillus infected corneas. Figure 3G, H shows significantly reduced total IL-1β and the absence of IL-1β cleavage in IFNAR−/− compared with C57BL/6 corneas, indicating a requirement for IFNAR in IL-1β production in vivo. IFNAR1−/− mice also showed reduced IFN-β production compared with infected C57BL/6 corneas (Figure 3I).
Caspase-11 and IFNAR1 are required for caspase-1 and IL-1β cleavage by bone marrow neutrophils
We took two complementary approaches to determine the role of neutrophil caspase-11 on IL-1β production and on caspase-1 and IL-1β cleavage; using neutrophils from gene knockout mice; and using well-characterized inhibitors to block caspase-11 production by neutrophils from C57BL/6 mice. Neutrophils were isolated by negative selection from total bone marrow cells from C57Bl/6 and caspase-11−/− mice, and incubated with A. fumigatus swollen conidia. Secreted IL-1β was quantified by ELISA, and processed caspase-1 p20 and IL-1β p17 were detected by Western blot.
Figure 4A shows significantly less secreted IL-1β in caspase-11−/− compared with C57BL/6 neutrophils, whereas there was no difference in CXCL1 production (Figure 4B), indicating that there is no intrinsic deficiency in the ability of caspase-11−/− neutrophils to respond to Aspergillus. Also, conidia alone were sufficient to induce IL-1β production by neutrophils, and pre-incubation with LPS as signal 1 was not required (not shown).
Figure 4. Impaired IL-1β cleavage in neutrophils from Caspase-1/11−/−, Caspase-11−/− and IFNAR−/− mice.
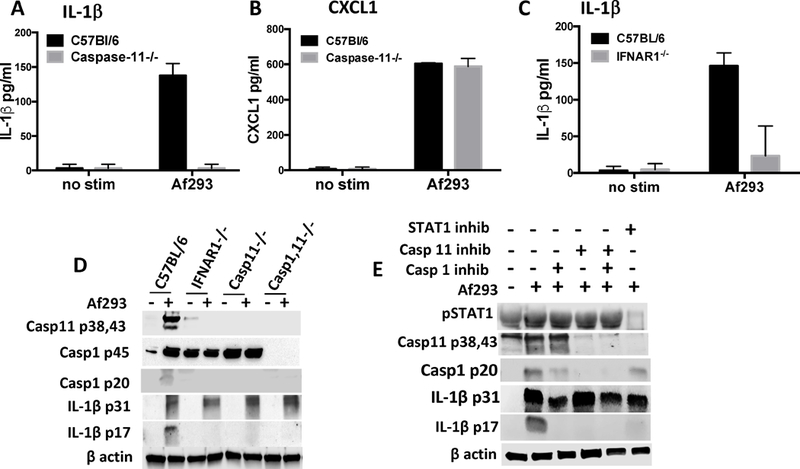
A,B: IL-1β and CXCL1 production by bone marrow neutrophils from C57BL/6 and caspase-11−/− mice incubated 6h with live Aspergillus swollen conidia (Asp), and quantified by ELISA. C: IL-1β induced by LPS/nigericin; and IL-1β produced by neutrophils from IFNAR−/− mice (D). E: Western blot of cleaved IL-1β in Aspergillus – stimulated bone marrow neutrophils from caspase-1/11−/−, caspase-11−/−, and IFNAR−/− mice. F: Bone marrow neutrophils from C57BL/6 mice incubated with Aspergillus in the presence of either the JAK/STAT inhibitor Ruxolitinib, the caspase-11 inhibitor Wedelolactone, or the caspase-1 inhibitor YVAD. A-C: p values were determined by students t test. Experiments were repeated 3 times with similar results.
As IFNAR signaling induces caspase-11 expression (14), we also examined the role of IFNAR in IL-1β production and IL-1β cleavage. As shown in Figure 4C, total IL-1β secretion was significantly lower in neutrophils from IFNAR−/− compared with C57BL/6 mice. Similarly, cleaved caspase-1 p20 and IL-1β p17 were detected in C57BL/6 neutrophils incubated with swollen conidia, but not in neutrophils from either IFNAR−/− or Caspase 11−/− mice (Figure 4D), indicating a requirement for IFNAR and caspase-11 in caspase-1 and IL-1β cleavage.
As a second approach, bone marrow neutrophils from C57BL/6 mice were incubated with A. fumigatus swollen conidia in the presence of the caspase 11 inhibitor Wedelolactone, which blocks caspase-11 gene expression by inhibiting p65 activation (15, 16). Neutrophils were also incubated with the caspase 1 inhibitor YVAD, or with the JAK/STAT inhibitor ruxolitinib.
We found that these inhibitors blocked the predicted targets, as ruxolitinib inhibited STAT1 phosphorylation, that Wedelolactone blocked caspase-11 production, and that YVAD inhibited caspase-1 activation, resulting in less p20 (Figure 4E). (Note that although the p20 fraction was recently shown not to be enzymatically active (17), the presence of p20 indicates that caspase-1 has undergone auto-cleavage that generates the bioactive p33/p10 products.) Ruxolitinib inhibition also reflected the results with IFNAR−/− neutrophils showing inhibition of caspase-11, caspase-1 and IL-1β p17 (Figure 4E). (Although inhibition of caspase-1 p20 was not complete, it was sufficient to block IL-1β cleavage and inhibit secretion.) Additionally, inhibition of caspase-1 or caspase-11 also blocked the cleavage of bioactive IL-1β (Figure 4E), supporting the data seen with knockout neutrophils.
As with caspase-1−/− neutrophils, YVAD inhibited IL-1β processing by neutrophils, and similarly, Wedelolactone inhibition of caspase 11 resulted in impaired caspase-1 and IL-1β processing neutrophils, which supports the findings with caspase-11−/− neutrophils (Figure 4D). Together, these observations indicate that caspase-11 regulates caspase-1 activity. Ruxolitinib inhibition also reflected the results with IFNAR−/− neutrophils showing inhibition of caspase-11, caspase-1 and IL-1β p17 (Figure 4E). (Although inhibition of caspase-1 p20 was not complete, it was sufficient to block IL-1β cleavage and inhibit secretion.)
Collectively, these findings support the concept that IL-1β processing by neutrophils in response to Aspergillus occurs by IFNAR/JAK-STAT - induced production of caspase-11, which functions upstream of caspase-1.
Selective role for caspase-11 in regulating FLICA speck formation in response to A. fumigatus conidia
As a third approach to examine the role of caspase-11 in caspase-1 activation in neutrophils, bone marrow neutrophils from C57BL/6 and caspase-11−/− mice were incubated with swollen conidia, and caspase-1 activation in the context of visible NLRP3/ASC/caspase-1 inflammasomes (specks) was examined using FITC-YVAD (FLICA) as an indicator of caspase-1 activity, and the number of FLICA+ cells was quantified using the flow cytometry function of the Amnis Imagestream.
As shown in Figure 5A, >50% of total neutrophils from C57BL/6 mice were FLICA+ following incubation with swollen (β-glucan expressing), heat killed conidia. In contrast, <5% of caspase-11−/− neutrophils were FLICA+. There was no intrinsic defect in caspase-11 neutrophils as LPS/ATP stimulation induced >50% FLICA+ cells in neutrophils from C57BL/6 and caspase-11−/− neutrophils (Figure 5A,B). Consistent with the selective role for caspase-11 in conidia – induced speck formation, there was no difference in IL-1β production between caspase-11−/− and C57BL/6 neutrophils following stimulation with LPS/ATP (Figure 5C).
Figure 5. FLICA speck formation in response to A. fumigatus conidia.
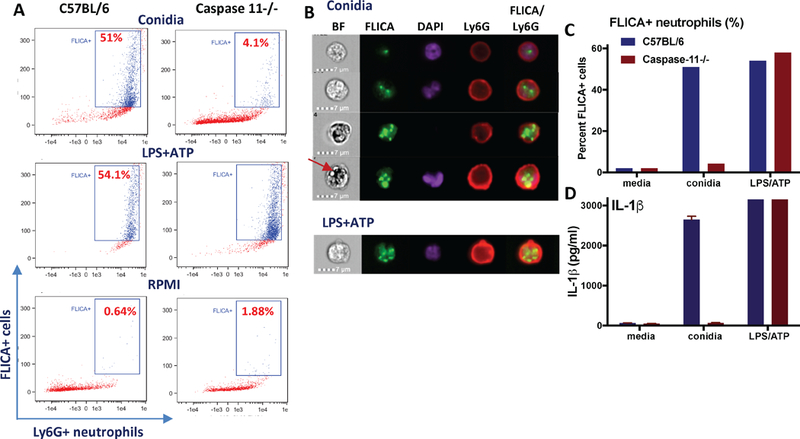
A. Bone marrow neutrophils from C57BL/6 and caspase-11−/− mice were incubated with either swollen conidia or with LPS/ATP in the presence of FITC-YVAD (FLICA) as an indicator of caspase-1 activity. FLICA positive cells were quantified using the flow cytometry function of the Amnis Imagestream. B. Quantification of FLICA+ neutrophils following stimulation with A. fumigatus conidia or LPS/ATP. C. IL-1β production by the same neutrophils. D. Representative photomicrographs of neutrophils with one or more FLICA+ specks (arrow indicates intracellular conidia that do not overlap with FLICA specks). Data shown are representative of two repeat experiments.
Representative images show neutrophils with single or multiple specks in response to conidia or LPS/ATP (Figure 5D), which we also found by Imagestream and confocal microscopy in neutrophils stimulated with Streptococcus pneumoniae (3).
Neutrophil IL-1β cleavage is dependent on Dectin-1 and the Raf1 pathway
Dectin-1 is a c-type lectin that recognizes β-glucan on the fungal cell wall, and mediates phagocytosis (18–20). We reported that Dectin-1 is required to regulate hyphal growth and corneal disease caused by A. fumigatus (5, 7), and that Dectin-1 is expressed on bone marrow neutrophils (6).
To examine if Dectin-1 is required for neutrophil IL-1β production, bone marrow neutrophils from Dectin-1−/− mice were incubated with swollen A. fumigatus conidia, and secreted and cleaved IL-1β were detected by ELISA and western blot analyses. As shown in Figure 6A, IL-1β secretion by Dectin-1−/− neutrophils stimulated with Aspergillus conidia was significantly less than that of neutrophils from C57BL/6 mice. Further, there was less pro- and cleaved IL-1β in Dectin-1−/− compared with C57BL/6 neutrophils (Figure 6B).
Figure 6. Dectin-1 signaling pathways in IL-1β production.
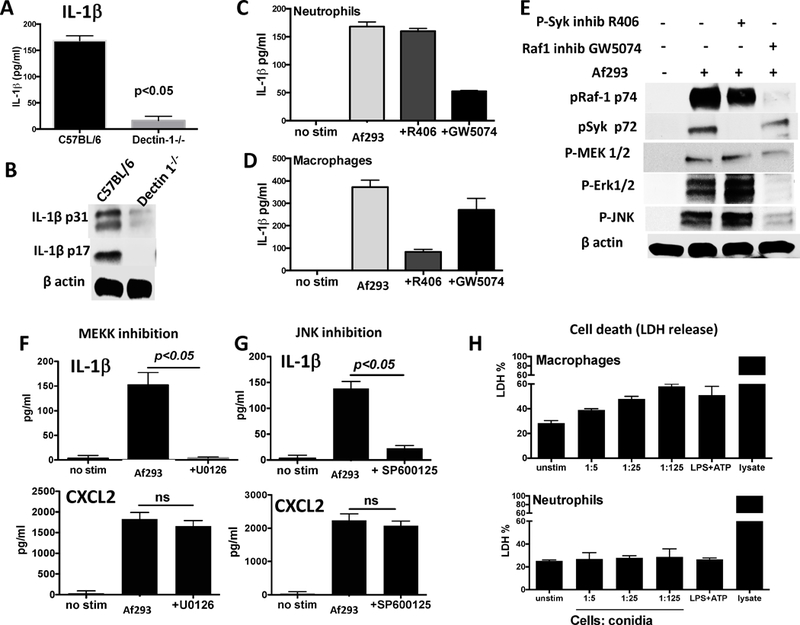
A,B: IL-1β production by bone marrow neutrophils from C57BL/6 and Dectin-1−/− mice stimulated with Aspergillus swollen conidia and examined by ELISA (A) and Western blot analysis (B). C,D. IL-1β production by bone marrow neutrophils (C) or bone marrow derived macrophages (D) from C57BL/6 mice following incubation with Aspergillus swollen conidia in the presence of p-Syk inhibitor R406 or the Raf-1 kinase inhibitor GW5074. E. IL-1β cleavage and cell signaling in C57BL/6 bone marrow neutrophils from mice incubated with Aspergillus and either R406 or GW5074. F,G. IL-1β and CXCL2 production by neutrophils from C57BL/6 mice and incubated with MEKK inhibitor U0126 or JNK inhibitor SP600125. H. LDH release from bone marrow neutrophils compared with bone marrow derived macrophages incubated with Aspergillus swollen conidia. Experiments were repeated twice with similar results.
As Dectin-1 can signal through either the Syk or the Raf1 pathway (21, 22), bone marrow neutrophils from C57BL/6 mice were incubated 6h with swollen A. fumigatus conidia in the presence of either the p-Syk inhibitor R406 or with the p-Raf1 inhibitor GW5074, and IL-1β in the supernatant was quantified by ELISA. As shown in Figure 6C, IL-1β was inhibited by GW5074, but not R406, indicating that Dectin-1 signals through p-Raf1. Conversely, IL-1β production by bone marrow derived macrophages was selectively inhibited by R406 (Figure 6D). GW5074, but not R406, inhibited neutrophil Raf1, Erk1/2, JNK, and MEK1/2 phosphorylation (Figure 6E), which is characteristic of RAF1 activation of the MEKK / MAPK pathway (21, 22). Consistent with the neutrophil data, secreted IL-1β was significantly lower in the presence of either the MEK inhibitor U1026 or the JNK inhibitor SP600125, neither of which inhibited CXCL2 production (Figure 6F, G), which is more dependent on NFκB activation. Although pharmacological inhibitors can have off-target effects, we found that Syk inhibitor F506 and Raf1 inhibitor GW5074 are selective in blocking Raf1 and Syk phosphorylation, respectively. Similarly, these inhibitors selectively blocked IL-1β production by neutrophils and macrophages.
Finally, we examined the effect of conidia on inducing pyroptosis, defined as caspase-1 dependent cell death. Neutrophils and macrophages were incubated with increasing numbers of swollen conidia, and pyroptosis was measured by uptake of LDH as described (3). Whereas macrophage pyroptosis was dose-dependent and reached ~60%, neutrophil cell death did not increase over unstimulated cells, indicating that conidia do not induce neutrophil pyroptosis (Figure 6H). This is consistent with the absence of pyroptosis in neutrophils incubated with Streptococcus pneumoniae or Salmonella typhimurium (2, 3).
Together, these findings demonstrate that in contrast to macrophages, A. fumigatus activation of the Raf1 pathway is essential for Dectin-1 – mediated secretion of IL-1β by neutrophils, and that neutrophils do not undergo pyroptosis.
DISCUSSION
Aspergillus and Fusarium molds are the most common infectious agents leading to corneal blindness in developing countries, often occurring as a result of ocular trauma (23). We reported that neutrophils comprise >90% of total cells in patients with early stage corneal ulcers, which also have highly elevated gene expression of NLRP3 and ASC, and of pro-inflammatory cytokines, including IL-1β (10).
We also reported that neutrophils are the predominant cells in murine models of fungal keratitis, and that IL-1β is elevated during infection (5, 24); in the current study, we demonstrate that neutrophils are the major source of IL-1β in fungal infected corneas, that neutrophil IL-1β is processed to the 17kD product by the NLRP3/ASC inflammasome and caspase-1, and that neutrophil derived IL-1β in infected corneas has pronounced bioactivity. Further, genetic deletion of any of these proteins results in impaired fungal killing. Surprisingly, we also found that infected caspase-11−/− mice exhibited the same phenotype as caspase-1/11−/− mice, and that caspase-11 acts upstream of caspase-1 in infected mice and in bone marrow neutrophils stimulated in vitro with A. fumigatus conidia.
Caspase-11 gene expression and protein production can be induced by type I and type II IFNs (14); hence IFN-β and IFN-γ induce caspase-11, and dendritic cells from IFNAR−/− mice exhibit impaired IL-1β production in response to bacterial stimulation (14). We also found impaired IL-1β processing and secretion in Aspergillus infected IFNAR−/− mice, and caspase-11 was not detected in bone marrow neutrophils from IFNAR−/− mice or from C57BL/6 mice treated with the JAK/STAT inhibitor ruxolitinib. These observations indicate that IFNAR regulates expression of caspase-11 in Aspergillus- stimulated neutrophils in a manner similar to that described for macrophages (14).
Finally, we show that A. fumigatus conidia activate Dectin-1 on neutrophils; however, in contrast to macrophages that primarily signal through spleen tyrosine kinase, Dectin-1 preferentially activates the Raf1 pathway, leading to MAPK signaling. We also showed that Dectin-1 is required for production of the pro-form of IL-1β and that the Raf1 pathway mediates IL-1β secretion by neutrophils. A recent study shows the Dectin-1 (and Dectin-2) activation by pathogenic Histoplasma yeast induces assembly of the NLRP3 inflammasome in dendritic cells (25); therefore a similar mechanism may be in place that activates the NLRP3 inflammasome in neutrophils incubated with A. fumigatus conidia.
IFN-β is also produced during corneal infection, and we found that the type I IFN receptor (IFNAR1) is required for production of caspase-11 in vivo, and in bone marrow neutrophils in vitro. This role for type I IFN in promoting IL-1β production is distinct from earlier reports that type I IFN used in treatment of autoimmune disease is due at least in part to suppression of IL-1β production (26, 27). In those studies, type I IFN signaling inhibited NLRP3 inflammasome inhibition, whereas in our study and that of Rathinam and Fitzgerald (14), IFNAR - induced caspase-11 was required for caspase-1 activation.
We also found that in the absence of caspase-11, neutrophils did not form caspase-1 specks in response to swollen conidia, whereas there was no effect of caspase-11 deficiency on speck formation by LPS/ATP - induced neutrophils. Together with the western blot data in Figure 4 where cleaved caspase-1 was not detected in the absence of, or following inhibition of caspase-11, these observations strongly indicate that caspase-11 functions upstream of the NLRP3 inflammasome and caspase-1 activation. Broz and Monack reported that caspase-11 (activated by intracellular LPS) is required for NLRP3 inflammasome assembly and therefore acts upstream of the inflammasome (13), whereas Rathinam and Fitzgerald reported that caspase-11 mediates caspase-1 activation in the absence of NLRP3 (14). Rathinam also indicates that caspase-11 undergoes auto-activation, whereas Broz suggests a scaffold is required (reviewed in (28)). Given that these macromolecular specks can be detected using probes for ASC, NLRP3 or activated caspase-1, we propose that in neutrophils, caspase-11 functions upstream of the inflammasome.
Caspase-1 and caspase-11 cleave the pore forming protein Gasdermin-D (GSDMD) in the cytosol from the auto-inhibitory form to release the N-terminal product, which inserts into the inner leaflet of the plasma membrane. Oligomerization in the membrane leads to pore formation in macrophages and dendritic cells that results in pyroptotic cell lysis and release of bioactive IL-1β (29, 30). We found that neutrophils stimulated with A. fumigatus conidia secrete IL-1β in the absence of cell lysis, which is consistent with prior reports by us and by Schroder et. al. showing that neutrophil activation of the NLRP3 or NLRC4 inflammasome generates caspase-1 – dependent processing of IL-1β, but the neutrophils do not undergo pyroptosis (2, 3, 11). Although we have yet to determine the mechanism of lysis - independent IL-1β release by neutrophils, limited Gasdermin-D cleavage can result in pore formation and release of cleaved IL-1β in the absence of pyroptosis (31, 32). Further, neutrophil elastase can cleave GSDMD in aging neutrophils, leading to lytic cell death (33).
In addition to pyroptosis, neutrophils undergo cell death by release of neutrophil extracellular traps (NETosis), which is characterized by early nuclear decondensation and swelling, followed by DNA together with histones and antimicrobial peptides that are extruded from the cells (34). Two recent reports demonstrated that GSDMD regulates cell death by NETosis: Zychlinsky and co-workers used a novel GSDMD inhibitor to block NETosis (35), whereas Schroder et al showed that not only are caspase-11 (activated by intracellular LPS) and GSDMD required for extrusion of NETs, but are also required for nuclear decondensation (36). We recently reported that NETs are induced in A. fumigatus infected corneas; however, NETosis is not required for hyphal killing either in vitro or in vivo (37).
Our findings on the role of caspase-11 differ from those of Kanneganti and colleagues using a murine model of pulmonary aspergillosis that requires systemic immunosuppression with cyclophosphamide and cortisone that reflects the immune suppressed state of patients that occurs as a result of leukemia, neutropenia or allogeneic cell transplantation (38, 39) (In contrast, patients with fungal keratitis are not immune compromised (23)). We found a requirement for caspase-11 in caspase-1 and IL-1β cleavage by neutrophils, whereas these investigators reported that bone marrow derived dendritic cells from caspase-1−/− or caspase-1/11−/− mice did not produce IL-1β whereas caspase-11−/− dendritic cells produced IL-1β at the same level as control cells from C57BL/6 mice (40).
We recently reported a difference in the role of calprotectin in A. fumigatus infection of corneas compared with lungs (9), which may also relate to the differences between our studies and those of Kanneganti. This group also reported that A. fumigatus activates the NLRP3 and the AIM2 inflammasome, and that both are required for infected mice to survive and to clear the pathogens (41). Although we did not examine AIM2, we found impaired IL-1β production, neutrophil infiltration and fungal killing in infected corneas of mice that are deficient only in NLRP3. The underlying differences between these reports and our current data are also likely based on the major source of IL-1β being dendritic cells rather than neutrophils.
In conclusion, these studies add to our understanding of the role of caspase-11 in IL-1β production by neutrophils in a clinically relevant model of fungal corneal infections. Given that neutrophils are recruited to bacterial and fungal infected tissues in large numbers, it is highly likely has these findings will be relevant to multiple other infections.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the VSRC Core Facility, especially Scott Howell, Cathy Doller, and Dawn Smith for outstanding technical assistance. We also thank George Dubyak for helpful discussions.
The research was funded by NIH grant EY18612 (EP) and by Department of Veterans Affairs Merit Award I01BX002607 (AGH). Other funding sources include the P30 Core Grant for Vision Research P30EY011373 and The Research to Prevent Blindness Foundation.
REFERENCES
- 1.van den Berg LM, Gringhuis SI, and Geijtenbeek TB, 2012. An evolutionary perspective on C-type lectins in infection and immunity. Ann N Y Acad Sci 1253: 149–158. [DOI] [PubMed] [Google Scholar]
- 2.Chen KW, Gross CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ, and Schroder K, 2014. The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell Rep 8: 570–582. [DOI] [PubMed] [Google Scholar]
- 3.Karmakar M, Katsnelson M, Malak HA, Greene NG, Howell SJ, Hise AG, Camilli A, Kadioglu A, Dubyak GR, and Pearlman E, 2015. Neutrophil IL-1beta processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J Immunol 194: 1763–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karmakar M, Sun Y, Hise AG, Rietsch A, and Pearlman E, 2012. Cutting edge: IL-1beta processing during Pseudomonas aeruginosa infection is mediated by neutrophil serine proteases and is independent of NLRC4 and caspase-1. J Immunol 189: 4231–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leal SM Jr., Cowden S, Hsia YC, Ghannoum MA, Momany M, and Pearlman E, 2010. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog 6: e1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leal SM Jr., Vareechon C, Cowden S, Cobb BA, Latge JP, Momany M, and Pearlman E, 2012. Fungal antioxidant pathways promote survival against neutrophils during infection. J Clin Invest 122: 2482–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrion Sde J, Leal SM Jr., Ghannoum MA, Aimanianda V, Latge JP, and Pearlman E, 2013. The RodA Hydrophobin on Aspergillus fumigatus Spores Masks Dectin-1- and Dectin-2-Dependent Responses and Enhances Fungal Survival In Vivo. J Immunol 191: 2581–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leal SM Jr., Roy S, Vareechon C, Carrion SD, Clark H, Lopez-Berges MS, Dipietro A, Schrettl M, Beckmann N, Redl B, Haas H, and Pearlman E, 2013. Targeting Iron Acquisition Blocks Infection with the Fungal Pathogens Aspergillus fumigatus and Fusarium oxysporum. PLoS Pathog 9: e1003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark HL, Jhingran A, Sun Y, Vareechon C, de Jesus Carrion S, Skaar EP, Chazin WJ, Calera JA, Hohl TM, and Pearlman E, 2016. Zinc and Manganese Chelation by Neutrophil S100A8/A9 (Calprotectin) Limits Extracellular Aspergillus fumigatus Hyphal Growth and Corneal Infection. J Immunol 196: 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karthikeyan RS, Leal SM Jr., Prajna NV, Dharmalingam K, Geiser DM, Pearlman E, and Lalitha P, 2011. Expression of innate and adaptive immune mediators in human corneal tissue infected with Aspergillus or fusarium. J Infect Dis 204: 942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karmakar M, A. KM, Dubyak GR, and Pearlman E, 2016. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nature Communications 15;7:10555. doi: 10.1038/ncomms10555: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor PR, Roy S, Meszaros EC, Sun Y, Howell SJ, Malemud CJ, and Pearlman E, 2016. JAK/STAT regulation of Aspergillus fumigatus corneal infections and IL-6/23-stimulated neutrophil, IL-17, elastase, and MMP9 activity. J Leukoc Biol 100: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, and Monack DM, 2012. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490: 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, and Fitzgerald KA, 2012. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150: 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobori M, Yang Z, Gong D, Heissmeyer V, Zhu H, Jung YK, Gakidis MA, Rao A, Sekine T, Ikegami F, Yuan C, and Yuan J, 2004. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death Differ 11: 123–130. [DOI] [PubMed] [Google Scholar]
- 16.Yuan F, Chen J, Sun PP, Guan S, and Xu J, 2013. Wedelolactone inhibits LPS-induced pro-inflammation via NF-kappaB pathway in RAW 264.7 cells. J Biomed Sci 20: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boucher D, Monteleone M, Coll RC, Chen KW, Ross CM, Teo JL, Gomez GA, Holley CL, Bierschenk D, Stacey KJ, Yap AS, Bezbradica JS, and Schroder K, 2018. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J Exp Med 215: 827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown GD, and Gordon S, 2001. Immune recognition. A new receptor for beta-glucans. Nature 413: 36–37. [DOI] [PubMed] [Google Scholar]
- 19.Dambuza IM, and Brown GD, 2015. C-type lectins in immunity: recent developments. Curr Opin Immunol 32: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Underhill DM, and Pearlman E, 2015. Immune Interactions with Pathogenic and Commensal Fungi: A Two-Way Street. Immunity 43: 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geijtenbeek TB, and Gringhuis SI, 2016. C-type lectin receptors in the control of T helper cell differentiation. Nat Rev Immunol 16: 433–448. [DOI] [PubMed] [Google Scholar]
- 22.Geijtenbeek TB, and Gringhuis SI, 2009. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol 9: 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas PA, and Kaliamurthy J, 2013. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect 19: 210–220. [DOI] [PubMed] [Google Scholar]
- 24.Tarabishy AB, Aldabagh B, Sun Y, Imamura Y, Mukherjee PK, Lass JH, Ghannoum MA, and Pearlman E, 2008. MyD88 regulation of Fusarium keratitis is dependent on TLR4 and IL-1R1 but not TLR2. J Immunol 181: 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang TH, Huang JH, Lin HC, Chen WY, Lee YH, Hsu LC, Netea MG, Ting JP, and Wu-Hsieh BA, 2017. Dectin-2 is a primary receptor for NLRP3 inflammasome activation in dendritic cell response to Histoplasma capsulatum. PLoS Pathog 13: e1006485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, and Tschopp J, 2011. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34: 213–223. [DOI] [PubMed] [Google Scholar]
- 27.Inoue M, Williams KL, Oliver T, Vandenabeele P, Rajan JV, Miao EA, and Shinohara ML, 2012. Interferon-beta therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Sci Signal 5: ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broz P, and Monack DM, 2013. Noncanonical inflammasomes: caspase-11 activation and effector mechanisms. PLoS Pathog 9: e1003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, and Dueber EC, 2016. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A 113: 7858–7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, and Dixit VM, 2015. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526: 666–671. [DOI] [PubMed] [Google Scholar]
- 31.Evavold CL, Ruan J, Tan Y, Xia S, Wu H, and Kagan JC, 2018. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 48: 35–44 e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heilig R, Dick MS, Sborgi L, Meunier E, Hiller S, and Broz P, 2018. The Gasdermin-D pore acts as a conduit for IL-1beta secretion in mice. Eur J Immunol 48: 584–592. [DOI] [PubMed] [Google Scholar]
- 33.Kambara H, Liu F, Zhang X, Liu P, Bajrami B, Teng Y, Zhao L, Zhou S, Yu H, Zhou W, Silberstein LE, Cheng T, Han M, Xu Y, and Luo HR, 2018. Gasdermin D Exerts Anti-inflammatory Effects by Promoting Neutrophil Death. Cell Rep 22: 2924–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, and Zychlinsky A, 2004. Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535. [DOI] [PubMed] [Google Scholar]
- 35.Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B, Kruger R, Herzig A, and Zychlinsky A, 2018. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol 3 6689. [DOI] [PubMed] [Google Scholar]
- 36.Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, and Schroder K, 2018. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol 3 6676. [DOI] [PubMed] [Google Scholar]
- 37.Clark HL, Abbondante S, Minns MS, Greenberg EN, Sun Y, and Pearlman E, 2018. Protein Deiminase 4 and CR3 Regulate Aspergillus fumigatus and beta-Glucan-Induced Neutrophil Extracellular Trap Formation, but Hyphal Killing Is Dependent Only on CR3. Front Immunol 9: 1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nucci F, Nouer SA, Capone D, Anaissie E, and Nucci M, 2015. Fusariosis. Semin Respir Crit Care Med 36: 706–714. [DOI] [PubMed] [Google Scholar]
- 39.Kosmidis C, and Denning DW, 2015. The clinical spectrum of pulmonary aspergillosis. Thorax 70: 270–277. [DOI] [PubMed] [Google Scholar]
- 40.Man SM, Karki R, Briard B, Burton A, Gingras S, Pelletier S, and Kanneganti TD, 2017. Differential roles of caspase-1 and caspase-11 in infection and inflammation. Sci Rep 7: 45126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karki R, Man SM, Malireddi RK, Gurung P, Vogel P, Lamkanfi M, and Kanneganti TD, 2015. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe 17: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


