Abstract
Background:
Many studies have investigated how cognitive control may be compromised in cocaine addiction. Here, we extend this literature by employing spatial Independent Component Analysis (ICA) to describe circuit dysfunction in relation to impairment in response inhibition in cocaine addiction.
Methods:
Fifty-five cocaine-dependent (CD) and 55 age- and sex-matched non-drug-using healthy control individuals (HC) participated in the study. Task-relatedness of 40 independent components (ICs) was assessed using multiple regression analyses of component time courses with the modeled time courses of hemodynamic activity convolved with go success (GS), stop success (SS) and stop error (S ). This procedure produced beta-weights that represented the degree to which each IC was temporally associated with, or ‘engaged’, by each task event.
Results:
Behaviorally, CD participants showed prolonged stop signal reaction times (SSRTs) as compared to HC participants (p<0.01). ICA identified two networks that showed differences in engagement related to SS between CD and HC (p<0.05, FDR-corrected). The activity of the fronto-striatal-thalamic network was negatively correlated with SSRTs in HC but not in CD, suggesting a specific role of this network in mediating deficits of response inhibition in CD individuals. In contrast, the engagement of the fronto-parietal-temporal network did not relate to SSRTs, was similarly less engaged for both SS and SE trials, and may reflect attentional dysfunction in cocaine addiction.
Conclusions:
This study highlights the utility of ICA in identifying neural circuitry engagement related to SST performance and suggests that specific networks may represent important targets in remedying executive-control impairment in cocaine addiction.
Keywords: Cocaine Abuse, fMRI, ICA, Stop Signal Task, SSRT
1. Introduction
Cocaine addiction is a debilitating and relapsing disorder (McLellan et al., 2000; Yuferov et al., 2005). Previous work has suggested deficits in cognitive control as an etiological process of habitual drug use in cocaine addiction (Ersche et al., 2011; Garavan and Hester, 2007; Goldstein and Volkow, 2011). ortical and subcortical dysfunction has been suggested in brain imaging studies of addictions to cocaine or other stimulants (Aron and Paulus, 2007; Goldstein and Volkow, 2011; Hanlon et al., 2009; Hanlon et al., 2011; Hester and Garavan, 2004; Kaufman et al., 2003; Moeller et al., 2005; Peters et al., 2016; Wesley et al., 2011; Zhang et al., 2018). The medial and lateral frontal cortical regions, in particular, have been repeatedly implicated in deficits of response inhibition in association with cocaine misuse (Connolly et al., 2012; Hester et al., 2013; Hester and Garavan, 2004; Lundqvist, 2010). For example, attenuated responses in executive-cortical regions during Go-NoGo task performance was observed in cocaine users (Kaufman et al., 2003). Striatal activation during performance of the Stroop color-word interference task was correlated with the drug-free rate of urine screen and longer duration of self-reported abstinence (Brewer et al., 2008). Combining Bayesian model of stop-signal-task (SST) performance and functional magnetic resonance imaging (fMRI), we highlighted a distinct role of the frontal-subcortical structures in Bayesian learning for goal-directed control and deficits in learning in cocaine dependent (CD) individuals (Hu et al., 2015a; Ide et al., 2014). Among individuals without and with addictions including to cocaine, impulsivity has been associated with smaller volumes in the hippocampus, amygdala ,and insula (Yip et al., 2018). Deficits in goal-directed action control, particularly in situations that involve uncertain outcomes (Mirabella, 2014), represent a prominent feature of drug addiction. Together, these studies support structural and functional differences in cortical and subcortical regions in association with deficits in cognitive control and related constructs in cocaine addiction. Along with incentivized salient responses to drug cues (Berridge and O’Doherty, 2014; Berridge and Robinson, 2016; Chow et al., 2016; Hickey and Peelen, 2015; Mirabella et al., 2007), cognitive control dysfunction may perpetuate habitual drug use in cocaine-addicted individuals.
Functional connectivity analyses have identified regional interactions associated with cognitive dysfunction in neuropsychiatric populations. Cocaine dependence was related to altered functional interactions of the insula with the prefrontal cortex, suggesting the influence of interoceptive information on cognitive-control and decision-making processes (Cisler et al., 2013). Compulsive cocaine use was associated with decreased cortico-striatal and increased limbic-striatal resting-state functional connectivity (rsFC) (Hu et al., 2015b). Disrupted connectivity dynamics, as reflected by power spectrum scale invariance (PSSI) of cerebral blood oxygenation-level dependent (BOLD) signal, in fronto-parietal-temporal areas was related to compromised post-signal behavioral adjustment in CD individuals (Ide et al., 2016). Interhemispheric coupling of executive-control networks was weakened during early abstinence of cocaine use (McCarthy et al., 2017). Our recent study of dynamic functional connectivity study described a decrease in temporal flexibility of executive networks in CD as compared to non-drug-using control (HC) participants (Zhang et al., 2018a). These studies suggest the importance of characterizing the functional network organization of cerebral activity in support of cognitive control and how these network activities are disrupted in cocaine addiction.
Independent component analysis (ICA) represents a useful tool to investigate functional network activity. A data-driven method, ICA uncovers hidden factors from a set of measurements such that the sources of the observed data are maximally independent (Calhoun and Adali, 2006; Calhoun et al., 2001a; Calhoun et al., 2002; Lange et al., 1999; McKeown et al., 2003; McKeown et al., 1998). Applied to fMRI data, ICA is capable of identifying functionally integrated brain regions, or functional networks, through a decomposition of the blood oxygenation level-dependent (BOLD) signal into maximally independent systems displaying temporally synchronous activity. In contrast to the general linear modeling of BOLD signals, ICA demonstrates the advantage of uncovering task-related regions with concurrent but opposite changes in response to task events (Xu et al., 2015; Xu et al., 2016). ICA has been widely used to describe the cerebral functional organization. For instance, we recently employed ICA to understand how the thalamus is functionally parcellated according to connectivities with identified components (Zhang and Li, 2017). In previous studies, we applied ICA to fMRI data of the SST and characterized the component networks in response to go success, stop success and stop error trials (Zhang and Li, 2012) and how motor network activities may contribute to individual variation in the stop-signal-reaction time (SSRT) (Zhang et al., 2015b). We have also used ICA to identify networks linked to Stroop performance, cocaine addiction and its treatment (Worhunsky et al., 2013), reward and loss processing in cocaine addiction (Worhunsky et al., 2017), and Go-NoGo performance and drinking behaviors among college students (Worhunsky et al., 2016).
In this study, we used ICA to identify network engagement related to response inhibition during SST performance and aimed to characterize how CD and HC individuals may differ in circuitry recruitment. During SST performance, participants must override a prepotent motor response, monitor errors, and adjust the speed of responding after encountering an error (Duann et al., 2009; Hu et al., 2015a; Ide et al., 2013; Li et al., 2006b; Zhang and Li, 2012). The capacity of response inhibition is quantified by the SSRT, the time it requires for subjects to successfully inhibit a response half of the time, with a longer SSRT reflecting impairment in response inhibition. We examined the temporal profiles of the activity during different trial types and how CD and HC participants differed in engaging these component activities. Following our previous work on regional responses relating to stop-signal inhibition (Duann et al., 2009; Li et al., 2008), we hypothesized that a network involving fronto-parietal-subcortical circuits, including the thalamus and subthalamic nucleus, maybe specifically engaged during stop success (SS) trials. In particular, as compared to HC individuals, CD individuals would display reduced SS-related engagement of this functional network.
2. Methods
2.1. Participants
Fifty-five and 55 HC adults participated (Table 1). CD participants met DSM-IV criteria for cocaine dependence, resided in an inpatient treatment unit and tested positive for cocaine but no other substances in urine toxicology prior to admission. All subjects were required to be physically healthy and without major medical or neurological illnesses. Other exclusion criteria included a past or current diagnosis of psychotic disorder or other substance-use disorders except for nicotine dependence. CD patients were admitted to a locked inpatient research unit, where abstinence is strictly monitored during the entire study period. On average, CD participants were abstinent for 13.5 ± 2.6 (mean ± SD) days, including the time when they were abstinent before admission, prior to MRI. All participants provided written, informed consent in accordance with a protocol approved by the Yale Human Investigation Committee.
Table 1.
Demographics of participants
| CD (n=55) | HC (n=55) | P Value | |
|---|---|---|---|
| Age (years) | 40.3± 7.3 | 39.1 ± 10.4 | 0.460a |
| Gender (M/F) | 42/13 | 35/20 | 0.145b |
| Amount of average monthly cocaine use (Dambacher et al.) in the prior year |
17.7± 29.3 | N/A | N/A |
| Amount per use in grams | 1.0 ± 1.2 | N/A | N/A |
| Days of cocaine use in the prior month | 13.8± 8.5 | N/A | N/A |
| Years of cocaine use | 17.3± 8.4 | N/A | N/A |
Note: values are mean ± S.D.
two-sample t test.
x2 test
2.2. Behavioral Task
Participants performed a SST (Hu and Li, 2012; Li et al., 2009) in which go and stop trials were randomly intermixed in presentation with an inter-trial interval of 2 s. fixation dot appeared on screen to signal the beginning of each trial. After a fore-period varying from 1 to 5 s (uniform distribution), the dot became a circle – the “go” signal – prompting participants to press a button quickly. The circle disappeared at button press or after 1 s if the participant failed to respond. In approximately one-quarter of trials, the circle was followed by a ‘cross’ – the stop signal – prompting participants to withhold button press. The trial terminated at button press or after 1 s if the participant successfully inhibited the response. The time between the go and stop signals, the stop signal delay (SSD), started at 200 ms and varied from one stop trial to the next according to a staircase procedure, increasing and decreasing by 67 ms each after a successful and failed stop trial (Levitt, 1971). With the staircase procedure, we anticipated that participants would succeed in withholding the response half of the time. Participants were trained briefly on the task before imaging to ensure that they understood the task. They were instructed to quickly press the button when they saw the go-signal while keeping in mind that a stop signal might come up in some trials. In the scanner, they completed four 10-minute sessions of the task, with approximately 100-trials in each session. There was a total of approximately 300-go and 100-stop trials.
2.3. Behavioral Data Analysis
A critical SSD was computed for each participant that represented the time delay required for the participant to successfully withhold the response in half of the stop trials, following a maximum likelihood procedure (Wetherill et al., 1966). Briefly, SSDs across trials were grouped into runs, with each run being defined as a monotonically increasing or decreasing series. We derived a mid-run estimate by taking the median SSD of every second run. The critical SSD was computed by taking the mean of all mid-run SSDs. It was reported that, except for experiments with a small number of trials (< 30), the mid-run measure was close to the maximum likelihood estimate of X50 (50% positive response, Wetherill et al., 1966). The SSRT was computed for each participant by subtracting the critical SSD from the median go-trial reaction time (Logan et al., 1984).
2.4. FMRI Procedures and Data Analyses
All imaging data were collected using a 3T Siemens Trio scanner (Siemens AG, Erlangen, Germany) while subjects performed the SST. Tobacco- and caffeine-using subjects were allowed to smoke and drink coffee or other caffeinated beverages until 30 m before the fMRI studies. Functional images were acquired with a single-shot gradient-echo echo-planar imaging (EPI) sequence, with 32 axial slices parallel to the AC-PC line covering the whole brain, using published parameters (Li et al., 2006a; Li et al., 2006c): TR=2000 ms, TE=25 ms, bandwidth=2004 Hz/pixel, flip angle=85°, FOV=220×220 mm2, matrix=64×64, slice thickness=4mm, and no gap. A high-resolution 3D structural image (MPRAGE; 1mm resolution) was also obtained for anatomical co-registration.
Functional MRI data were preprocessed with Statistical Parametric Mapping 12 (SPM12; Wellcome Department of Imaging Neuroscience, University College London, UK) with established routines (Farr et al., 2014; Manza et al., 2016; Wang et al., 2018; Zhang et al., 2012). Images from the first five TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation. Images of each individual subject were first corrected for slice timing and realigned (motion-corrected) (Andersson et al., 2001; Hutton et al., 2002). A mean functional image volume was constructed for each subject for each run from the realigned image volumes. The anatomical images (T1-weighted) were co-registered to the mean functional image and normalized to an MNI (Montreal Neurological Institute) template with affine registration followed by nonlinear transformation using a unified segmentation and registration framework (Ashburner and Friston, 2005). The normalization parameters determined for the anatomical volume were then applied to the corresponding functional image volumes for each subject.
2.5. Independent Component Analysis
ICA was performed on the fMRI time series using the Group ICA of fMRI Toolbox (GIFT v4.0b; http://icatb.sourceforge.net) (Calhoun et al., 2001b) to identify spatially independent and temporally coherent networks (Calhoun et al., 2001b; Calhoun et al., 2008). The data from CD and HC individuals were concatenated into a single group and reduced through principal component analysis. An infomax algorithm (Bell and Sejnowski, 1995) was used to separate the dataset into 40 maximally independent components (ICs) in order to identify large-scale functional brain networks (Abou- lseoud et al., 2010). This analysis was repeated 20 times with ICASSO to assess the repeatability of ICs (Himberg et al., 2004). Finally, component time courses and spatial maps were back-reconstructed for each participant (Calhoun et al., 2001b).
Task-relatedness of 40 components was assessed using multiple regression analyses of component time courses with the modeled time courses of canonical hemodynamic activity convolved with the three task events: go success (GS), stop success (SS) and stop error (SE). This procedure produces beta-weights that represent the degree to which each IC is temporally associated with, or ‘engaged’, by each task event. We compared CD and HC participants with respect to the beta-weights relating to SS with two-sample t-tests, and components of interest were determined to be those exhibiting a significant group difference (P<0.05, FDR-corrected). The ICs that demonstrated a significant difference in SS-task-related beta-weights were selected for further analyses, with the subject-level spatial source maps of the component time courses entered into factorial models in SPM12 to determine their regional patterns at a voxel-level P<0.000001, corrected for family-wise error (FWE) of multiple comparisons, with an extent threshold of 40 voxels (Zhang and Li, 2012).
2.6. Analysis of Group-By-SSRT Interaction and Linear Regression Against SSRT
In an additional analysis, we evaluated whether the differences between CD and HC in network engagement reflected differences in SSRTs primarily (see Results). For both CD and HC groups, participants were divided by a median split according to the SSRT into two sub-groups: low SSRT and high SSRT. Thus, with low SSRT CD (lCD), high SSRT CD (hCD), low SSRT HC (lHC), and high SSRT HC (hHC), we conducted a 2 × 2 repeated-measures ANOVA on the beta-weights of networks of interest and reported SSRT and group main effects as well as the interaction effect. This analysis permits distinguishing whether the activity of the components of interest differ between subject groups, SSRT groups, and/or interact in a group-by-subject manner.
We also performed a regression analysis of IC beta-weights against SSRTs. Considering 2-components of interest (see Results) and 3-sets of beta-weights (GS, SS, and SE), we used a corrected value of 0.05/6=0.0083 to define statistical significance. To examine whether CD and HC differ in the relationship between IC beta-weights and SSRTs, we compared the slopes of linear regressions between the two groups (Zar, 1999) as in earlier studies of alcohol addiction (Hu et al., 2018; Ide et al., 2017).
3. . Results
3.1. Behavioral Findings
Table 2 summarizes the performanceMANUSCRIPTmeasuresfromtheSST.BothCDandHC participants succeeded in stopping approximately half of the time, suggesting the success of SSD staircasing in tracking the performance. CD and HC groups did not differ in SS rates, mean go-trial reaction time (goRT) or SE reaction time (Higley et al.). CD participants showed significantly lower go-response rates (t108=−3.24, p=0.002) and longer SSRTs (t108=2.6, p=0.011) than HC participants. The values of SSRTs were within the range as reported in earlier work (Federico and Mirabella, 2014).
Table 2.
Stop Signal Task performance.
| CD (n=55) | HC (n=55) | T Value | P Value | Cohen’s d | |
|---|---|---|---|---|---|
| GR (%) | 95.0 ± 7.3 | 98.3 ± 2.0 | t108 = −3.24 | 0.002* | 0.617 |
| SS (%) | 52.0 ± 3.1 | 51.8 ± 3.3 | t108 = 0.27 | 0.789 | 0.062 |
| goRT (ms) | 643± 125 | 642± 126 | t108 = 0.07 | 0.942 | 0.008 |
| SSRT (ms) | 240± 49 | 219± 36 | t108 = 2.6 | 0.011* | 0.488 |
| SERT (ms) | 534± 100 | 559±115 | t108 = −1.23 | 0.222 | 0.232 |
Note: GR: go response; SS: stop success; goRT: go trial reaction time; SSRT: stop-signal reaction time; SERT: stop-error reaction time; values are mean ± S.D.
p<0.05, two-sample t test. GR instead of GS rate was presented here, as all go trials with a RT were included in the computation of SSRT.
3.2. Functional Brain Networks Identified By ICA
3.2.1. Fronto-Striatal-Thalamic Network.
A fronto-striatal-thalamic network (Fig. 1) included rostral, dorsal anterior, and middle cingulate cortex and supplementary motor area (SMA), bilateral anterolateral frontal cortex including predominantly the superior frontal gyrus, insula, and striatum, as well as thalamic and subthalamic areas. This network was positively engaged during SE, SS and GS trials in HC participants, and positively engaged with SE in CD participants. Moreover, SS-related engagement in CD participants was lower than those in HC participants (t108=−3.47, p=0.015, FDR-corrected).
Fig. 1.
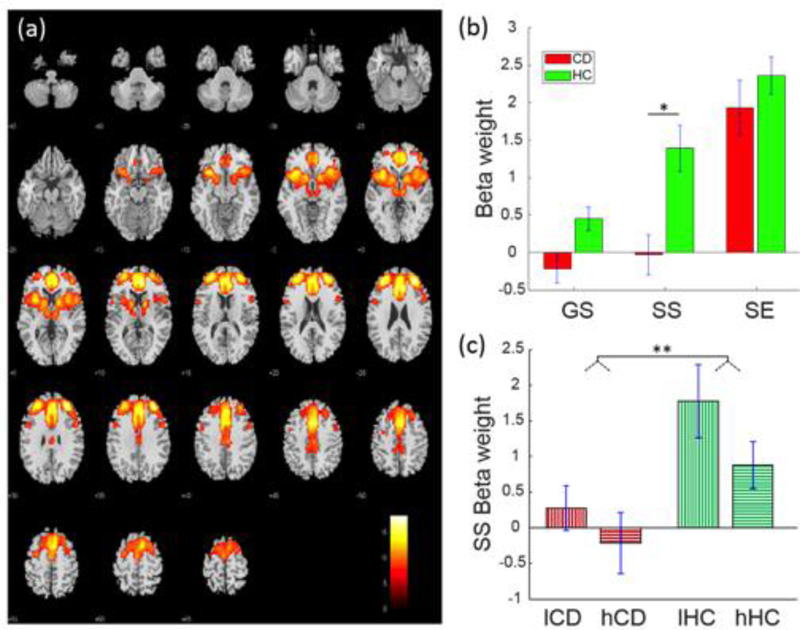
Fronto-striatal-thalamic network displaying a group difference in SS-related engagement (a) Spatial map of the brain regions positively integrated into the functional network displayed at P < 0.000001 (n = 110). (b) Beta-weights of GS, SS and SE trials each in CD and HC participants for the identified fronto-striatal-thalamic network. (c) Beta-weights relating to low and high SSRT subgroups each within the CD and HC groups. *p<0.05, **p<0.01, two-sample t test.
Compared to HC, CD showed prolonged SSRT, suggesting impaired response inhibition, and decreased engagement of the fronto-striatal-thalamic network. We thus examined whether the differences in behavioral performance and network engagement were related. In a two-way ANOVA of group (CD, HC) and SSRT (low, high SSRT; median split in each group), there was a difference between CD and HC (F1,108=10.27, p=0.002), but no SSRT main or group-by-SSRT interaction effect (Table 3). Pearson linear regression showed a negative association between SS-related engagement and SSRTs in HC (r=−0.38, p=0.004) but not in CD (r=−0.08, p=0.558) participants (Fig. 3; Table 4). However, although the Kolmogorov-Smirnov test suggested that the SS beta-weights were normally distributed (p=0.148), the Iglewicz and Hoaglin’s test indicated a potential outlier, under the recommended threshold of 3.5. Thus, we also employed a Spearman regression, which is robust to outliers, to examine the correlation of SS beta-weights and SSRT. SS beta-weights correlated inversely with SSRTs in HC participants at a numerically more robust value (rho=−0.22, p=0.109) than in CD participants (rho=−0.10, p=0.47), but neither correlation was significant.
Table 3.
Two-way ANOVA for interaction of SSRT (low, high) by group (CD, HC)
| SSRT | Group | Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Functional network | F1,108 | p | η2 | F1,108 | p | η2 | F1,108 | p | η2 |
| Fronto-striatal- thalamic |
2.92 | 0.090 | 0.052 | 10.27 | 0.002** | 0.168 | 0.25 | 0.619 | 0.005 |
| Fronto-parietal- temporal |
0.19 | 0.667 | 0.004 | 8.08 | 0.005** | 0.130 | 0.32 | 0.575 | 0.130 |
Note:
p<0.05
p<0.01. η2: eta squared
Fig. 3.
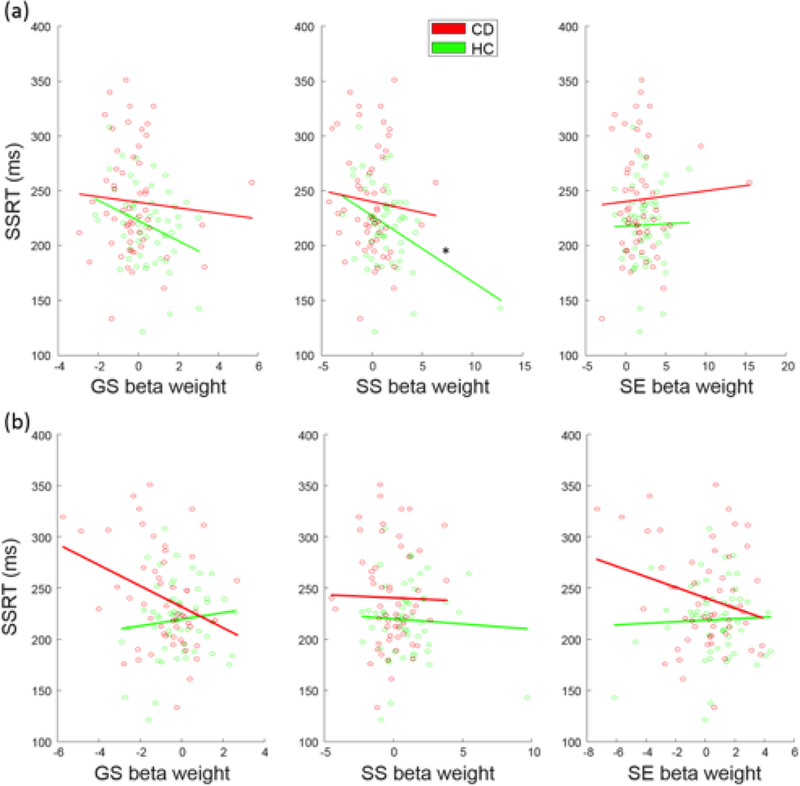
Correlation between SSRT and beta-weights relating to (a) fronto-striatal-thalamic network engagement in CD and HC participants across three task events and (b) fronto- parietal-temporal network engagement in CD and HC participants across three task events. The line indicates the fit according to Pearson regression. *p<0.0083.
Table 4.
Linear regression of beta-weights relating to SSRTs in CD and HC participants
| GS | SS | SE | |||||
|---|---|---|---|---|---|---|---|
|
Functional network |
Group | r | p | r | p | r | p |
| Fronto-striatal-thalamic | CD | −0.07 | 0.611 | −0.08 | 0.558 | 0.05 | 0.703 |
| HC | −0.30 | 0.026 | −0.38 | 0.004* | 0.02 | 0.879 | |
|
Fronto-parietal- temporal |
CD | −0.32 | 0.018 | −0.02 | 0.887 | -0.24 | 0.073 |
| HC | 0.11 | 0.416 | −0.06 | 0.690 | 0.04 | 0.777 | |
Note:
p<0.05/(2×3)=0.0083, corrected for multiple comparisons. GS = Go Success, SS = Stop Success, SE = Stop Error.
3.2.2. Fronto-parietal-temporal network.
A fronto-parietal-temporal network (Fig. 2) included bilateral inferior parietal lobules, angular and supramarginal gyri, and bilateral but predominantly right middle and inferior frontal cortices, the middle and inferior temporal cortex, Parahippocampal gyrus, and cerebellum. This network was positively engaged during SS and SE trials in HC participants and negatively engaged with GS trials in CD participants. Moreover, the engagement was lower in CD than of HC participants in SS trials (t=−3.08, p=0.035, FDR-corrected). two-way group-by-SSRT ANOVA showed a group main effect (F1,108 = 8.08, P = 0.005) but no SSRT main or interaction effect (Table 3). In linear regressions, no beta-weights were significantly correlated with SSRTs at the corrected threshold (Table 4).
Fig. 2.
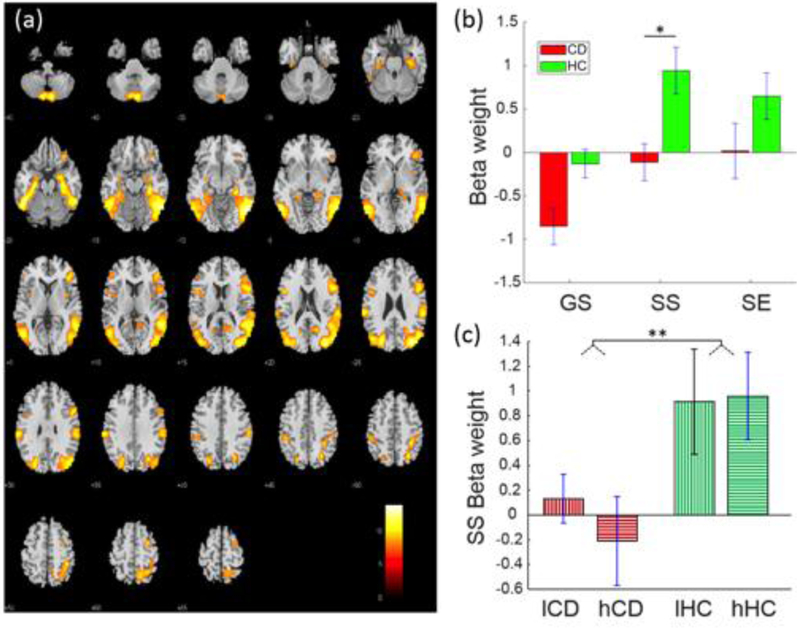
Fronto-parietal-temporal network displaying a group difference in SS-related engagement. (a) Spatial map of the brain regions positively integrated into the functional network displayed at p<0.000001 (n=110). (b) Beta-weights of GS, SS and SE trials each in CD and HC participants for the identified fronto-parietal-temporal network. (c) Beta-weights in low and high SSRT subgroups each in the CD and HC groups. *p<0.05, **p<0.01, two-sample t test. GS = Go Success, SS = Stop Success, SE = Stop Error, lCD = CD participants with low SSRTs, hCD = CD participants with high SSRTs, lHC = HC participants with low SSRTs, hHC = HC participants with high SSRTs.
4. Discussion
Using ICA, we examined differences in functionally integrated brain activations (or networks) in association with response inhibition in CD and HC participants. Largely consistent with our hypotheses, fronto-striatal-thalamic, and fronto-parietal-temporal networks were identified and found to be less engaged during responses to SS trials in CD as compared with HC participants. In particular, a shorter SSRT indicated better inhibitory control in HC than in CD and the SS beta-weights relating to fronto-striatal-thalamic network engagement were negatively correlated with SSRTs in HC but not in CD participants, suggesting that this network may be particularly related to the capacity of response inhibition in HC individuals and not operating in such a manner in CD participants. As CD participants showed prolonged SSRTs as compared to HC participants, we tested whether the difference in fronto-striatal-thalamic network engagement between CD and HC participants simply reflected this difference in SSRTs. The results of group-by-SSRT ANOVA suggested otherwise; there was a significant group main effect but not a SSRT main effect or a group-by-SSRT interaction, suggesting that differences between CD and HC participants in fronto-striatal-thalamic network engagement may involve cognitive and affective processes other than response inhibition.
4.1. Fronto-Striatal-Thalamic Network
Fronto-striatal-thalamic regions and circuitry have been implicated in complex cognitive functions including response inhibition (Morein-Zamir and Robbins, 2015), working memory (Clemensson et al., 2017; Darki and Klingberg, 2015), and reward processing (de Leeuw et al., 2015). Dysfunction of fronto-striatal-thalamic circuits has been postulated to be relevant to drug addictions (Limbrick-Oldfield et al., 2013; Nestor et al., 2017; Worhunsky et al., 2013).
Here, engagement of a fronto-striatal-thalamic network was diminished during response inhibition in as compared to HC participants. In particular, both the striatum and subthalamic nucleus were part of a fronto-striatal-thalamic network and have been implicated in response inhibition in earlier studies (Li et al., 2008; Mirabella et al., 2013; Mirabella et al., 2012). However, it is worth noting that brain regions within fronto-striatal-thalamic circuits may show different patterns of changes during other cognitive processes. For instance, cocaine cues increased activation in CD participants in left dorsolateral and orbitofrontal cortex as well as the ventral striatum as compared with HC participants in response to appetitive vs. control cues (Wilcox et al., 2011). Thus, individual brain regions may reconfigure as distinct networks in partaking in different cognitive and affective challenges. Speculatively, this consideration may also explain a lack of significant SSRT main effect and/or group-by-SSRT interaction effect in the ANOVA. He fronto-striatal-thalamic network activities may differ between CD and HC participants in other processes that were not captured by response inhibition in the SST. Overall, the current findings suggest the utility of ICA in providing new insights into network brain functions and dysfunctions in studies of the neural bases of neuropsychiatric conditions.
The beta-weights relating to engagement of the identified fronto-striatal-thalamic network did not differ between CD and HC participants for SE trials. SE trials are arousing (Critchley, 2002; Zhang et al., 2014; Zhang et al., 2012) and individuals who are more impulsive have demonstrated higher physiological arousal to SE trials during the SST (Zhang et al., 2015a). Along with the earlier discussion that the fronto-striatal-thalamic network may respond to drug cues, it is possible that CD demonstrated higher saliency response to SE than HC, potentially masking group differences (Castelluccio et al., 2014; Connolly et al., 2012; Rose et al., 2014). On the other hand, studies have reported diminished response to prediction errors, which are also salient, in CD as compared to HC (Parvaz et al., 2015), and evidence is mixed in relation to how error-related responses may predict relapse (Luo et al., 2013; Marhe et al., 2013). Examining the engagement of fronto-striatal-thalamic functional networks across multiple behavioral tasks, including those involving response inhibition and cue-elicited craving, should help clarify such considerations.
4.2. Fronto-parietal-temporal network
Previous studies have shown fronto-parietal-temporal regions and circuitry broadly involved in cognitive control across behavioral tasks and participant pools (Barros-Loscertales et al., 2011; Hester et al., 2007; Ide et al., 2016; Wang et al., 2017; Worhunsky et al., 2013; Zhang and Li, 2012). The current results showed significantly weaker engagement during SS trials of the identified fronto-parietal-temporal network in CD as compared with HC participants. On the other hand, fronto-parietal-temporal component engagement did not correlate with SSRTs either in HC or CD participants, suggesting that this network may not be as directly involved in response inhibition in the SST as is the identified fronto-striatal-thalamic network. Previous studies have shown that broader dysfunction of a fronto-parietal circuit in CD may contribute to disrupted connectivity dynamics in association with post-signal behavioral adjustment (Hester et al., 2007; Ide et al., 2016). More recently, we found that dynamic functional connectivity of a fronto-parietal control network was also compromised in CD participants (Zhang et al., 2018). Further studies are needed to understand whether the fronto -parietal-temporal network would be engaged specifically in response to post-signal slowing trials. This would require a larger data set from individual subjects, as the trial numbers tend to be much smaller when post-signal trials are distinguished in terms of their reaction times.
Engagement of the identified fronto-parietal-temporal network was diminished in CD participants for both SS and SE trials, as compared with HC participants. In contrast to the go trials, both SS and SE trials involved the stop signal, which is highly salient. Thus, the finding may suggest a broader attentional dysfunction in CD individuals. The identified fronto-parietal-temporal network includes regions of the attention network, which responds to goal-directed movements to attended stimuli (Kim et al., 2014). The stop signal appeared in only approximately one out of four trials and was highly significant, as it instructed a change in the action plan. Thus, diminished fronto-parietal-temporal network engagement may reflect dysfunction of attentional circuitry in response to the stop signal in CD individuals. The fronto-parietal-temporal network also involved the temporal and occipital cortices and hippocampal regions, which is critical to memory and sensory encoding and retrieval, although data have also suggested a role of higher order occipital cortical neurons in goal selection (Mirabella et al., 2007). Greater engagement of a spatially similar network has been identified during successful response inhibition using a Go/NoGo task. In young adult drinkers, the greater inhibition-related engagement was associated with slower overall reaction times and reduced commission errors (Worhunsky et al., 2016). A similar fronto-parietal-temporal inhibition-related network was also identified across healthy adults and adolescents (Steven et al., 2007). The authors noted differences in this network in effective connectivity influences to and from traditional fronto-parietal and fronto-striatal-thalamic networks associated with age, reaction time, and hit rates. In the current study, the fronto-parietal-temporal network was not associated with SS, suggesting CD may enlist alternative cognitive or neural mechanisms during the performance of the SST. These relationships should be investigated in future work.
4.3. Limitations
Multiple study limitations should be noted. First, a single behavioral task was employed in the current study. Imaging data collected across multiple behavioral tasks may help identify network activities that participate in psychological constructs of inter-related importance to cocaine addiction. Studies are also needed to understand whether the identified fronto-parietal-temporal network may be engaged specifically in response to post-signal slowing trials or relate to other cognitive strategies in SST performance. Second, data from CD and HC participants were included in a single ICA to identify networks related to inhibitory control. This approach may limit the ability to fully characterize all component networks that may contribute to inhibition in individuals with CD; however, this approach allows direct comparison with HC of engagement patterns across shared functional networks. Third, the data were collected from several different studies. Although the imaging parameters were identical, we did not have the same clinical measures for all participants. Thus, clinically relevant measures (for example, of impulsivity, anxiety, and depression) that may influence findings but were not accounted for should be examined in future studies. Fourth, the SSRT reflects the capacity of reactive control, and more studies are needed to examine how proactive control may be compromised in cocaine addiction (Hu et al., 2015a; Wang et al., 2018). Finally, inhibitory control represents one dimension of possible etiological processes relating to drug addiction. The incentive salience hypothesis (Berridge and Robinson, 2016) characterizes robust and mostly unconscious motivations driven by environmental cues as a critical process of habitual drug use. Studies of tasks to address the interaction of inhibitory control and incentive salience may help uncover other neural pathways relevant to cocaine addiction (Hickey and Peelen, 2015; Mirabella et al., 2007).
5. Conclusions
By applying ICA to SST fMRI data, we identified fronto-striatal-thalamic and fronto-parietal-temporal networks showing differences in relation to SS trials between CD and HC participants. The engagement of the fronto-striatal-thalamic network was negatively correlated with SSRTs in HC but not in CD participants, suggesting a direct role of this independent component in mediating deficits of response inhibition in CD individuals. In contrast, engagement of the fronto-parietal-temporal network did not relate to SSRTs and was similarly diminished for both SS and SE trials. Diminished fronto-parietal-temporal network engagement may reflect attentional dysfunction in the CD. This study adds to the literature by characterizing cortico-subcortical network dysfunction in CD and highlights the utility of ICA in distinguishing neural circuit activities in response to complex cognitive processes. More work is needed to examine how deficits in behavioral control may interact with drug cues and other factors in processes underlying cocaine addiction.
Highlights.
Response inhibition is compromised in cocaine dependent people (CD).
CD showed prolonged stop signal reaction time (SSRT), compared to controls (HC).
ICA showed diminished engagement of the fronto-striatal-thalamic network in CD.
The network engagement is associated with response inhibition in HC but not CD.
Acknowledgments
The study was supported by NIH grants DA023248, DA039136, DA040032, DA042998, and DA044749, and the State of Connecticut. The funding agencies were otherwise not involved in data collection or analysis, or in the decision to publish these results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
We have not conflicts of interest with respect to the current work. The author reports no conflicts of interest with respect to the content of this manuscript. Dr. Marc Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for and advised Shire, INSYS, RiverMend Health, Opiant/Lakelight Therapeutics, and Jazz Pharmaceuticals; has received unrestricted research support from Mohegan Sun Casino and grant support from the National Center for Responsible Gaming; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for and/or advised legal and gambling entities on issues related to addictions and impulse control disorders; has provided clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has edited journals and journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
References
- Abou-Elseoud A, Starck T, Remes J, Nikkinen J, Tervonen O, Kiviniemi V, 2010. The Effect of Model Order Selection in Group PICA. Hum. Brain Mapp 31, 1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Hutton C, Ashburner J, Turner R, Friston K, 2001. Modeling geometric deformations in EPI time series. Neuroimage 13,903–919. [DOI] [PubMed] [Google Scholar]
- Aron JL, Paulus MP, 2007. Location, location: using functional magnetic resonance imaging to pinpoint brain differences relevant to stimulant use. Addiction 102, 33–43. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ, 2005. Unified segmentation. Neuroimage 26, 839–851. [DOI] [PubMed] [Google Scholar]
- Barros-Loscertales A, Bustamante JC, Ventura-Campos N, Llopis JJ, Parcet MA, Avila C, 2011. Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine-dependent group. Psychiatry Res 194, 111–118. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ, 1995. An information-maximization approach to blind separation and blind deconvolution. Neural. Comput 7, 1129–1159. [DOI] [PubMed] [Google Scholar]
- Berridge KC, O’Doherty JP, 2014. From experienced utility to decision utility, in: Glimcher PW, Fehr E (Eds.), Neuroeconomics (Second Edition), Decision Making and the Brain Academic Press, Cambridge, MA., pp. 335–351. [Google Scholar]
- Berridge KC, Robinson TE, 2016. Liking, wanting and the incentive-sensitization theory of addiction. Am. Psychol 71, 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN, 2008. Pretreatment brain activation during Stroop Task is associated with outcomes in cocaine-dependent patients. Biol. Psychiat 64, 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, 2006. Unmixing fMRI with independent component analysis - Using ICA to characterize high-dimensional fMRI data in a concise manner. Ieee Eng. Med. Biol 25, 79–90. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, McGinty VB, Pekar JJ, Watson TD, Pearlson GD, 2001a. fMRI activation in a visual-perception task: Network of areas detected using the general linear model and independent components analysis. Neuroimage 14, 1080–1088. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ, 2001b. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp 14, 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD, 2008. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum. Brain Mapp 29, 828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Pekar JJ, McGinty VB, Adali, Watson TD, Pearlson GD, 2002. Different activation dynamics in multiple neural systems during simulated driving. Hum. Brain Mapp 16, 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelluccio BC, Meda SA, Muska CE, Stevens MC, Pearlson GD, 2014. Error processing in current and former cocaine users. a 8, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow JJ, Nickell JR, Darna M, Beckmann JS, 2016. Toward isolating the role of dopamine in the acquisition of incentive salience attribution. Neuropharmacology 109, 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Elton A, Kennedy AP, Young J, Smitherman S, James GA, Kilts CD, 2013. ltered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiat. Res-Neuroim 213, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemensson EK, Clemensson LE, Riess O, Nguyen HP, 2017. The BACHD Rat Model of Huntington Disease shows signs of fronto-striatal dysfunction in two operant conditioning tests of short-term memory. Plos One 12, e0169051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Foxe JJ, Nierenberg J, Shpaner M, Garavan H, 2012. The neurobiology of cognitive control in successful cocaine abstinence. DrugAlcoholDepend 121,45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci 3, 201–215. [DOI] [PubMed] [Google Scholar]
- Critchley HD, 2002. Electrodermal responses: What happens in the brain. Neuroscientist 8, 132–142. [DOI] [PubMed] [Google Scholar]
- Dambacher F, Sack AT, Lobbestael J, Arntz A, Brugman S, Schuhmann T, 2014. A network approach to response inhibition: Dissociating functional connectivity of neural components involved in action restraint and action cancellation. Eur. J. Neurosci 39, 821–831. [DOI] [PubMed] [Google Scholar]
- Darki F, Klingberg T, 2015Therole of fronto-parietal and fronto-striatal networks in the development of working memory: A longitudinal study. Cereb. Cortex 25, 1587–1595. [DOI] [PubMed] [Google Scholar]
- de Leeuw M, Kahn RS, Vink M, 2015. Fronto-striatal dysfunction during reward processing in unaffected siblings of schizophrenia patients. Schizophr. Bull 41, 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann JR, Ide JS, Luo X, Li CS, 2009. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J. Neurosci 29, 10171–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET, 2011. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain 134, 2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr OM, Hu S, Matuskey D, Zhang S, Abdelghany O, Li CSR, 2014. The effects of methylphenidate on cerebral activations to salient stimuli in healthy adults. Exp. Clin. Psychopharm 22, 154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico P, Mirabella G, 2014. Effects of probability bias in response readiness and response inhibition on reaching movements. Exp. Brain Res 232, 1293–1307. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R, 2007. The role of cognitive control in cocaine dependence. Neuropsychol. Rev 17, 337–345. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci 12, 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Porrino LJ, 2009. Loss of functional specificity in the dorsal striatum of chronic cocaine users. Drug Alcohol Depend 102, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Stapleton JR, Laurienti PJ, Porrino LJ, 2011. The association between frontal-striatal connectivity and sensorimotor control in cocaine users. Drug Alcohol Depend 115, 240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Bell RP, Foxe JJ, Garavan H, 2013. The influence of monetary punishment on cognitive control in abstinent cocaine-users. Drug Alcohol Depend 133, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H, 2004. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J. Neurosci 24, 11017–11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Simoes-Franklin C, Garavan H, 2007. Post-error behavior in active cocaine users: poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology 32, 1974–1984. [DOI] [PubMed] [Google Scholar]
- Hickey C, Peelen MV, 2015. Neural mechanisms of incentive salience in naturalistic human vision. Neuron 85, 512–518. [DOI] [PubMed] [Google Scholar]
- Higley J, Hasert M, Suomi S, Linnoila M, 1991. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc. Natl. Acad. Sci. U. S. A 88, 7261–7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himberg J, Hyvarinen A, Esposito F, 2004. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 22, 1214–1222. [DOI] [PubMed] [Google Scholar]
- Hu S, Ide JS, Chao HH, Zhornitsky S, Fischer KA, Wang W, Zhang S, Chiang-shan RL, 2018. Resting state functional connectivity of the amygdala and problem drinking in non-dependent alcohol drinkers. Drug Alcohol Depend 185, 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Ide JS, Zhang S, Li CS, 2015a. Anticipating conflict: Neural correlates of a Bayesian belief and its motor consequence. Neuroimage 119, 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Li CSR, 2012. Neural processes of preparatory control for stop signal inhibition. Hum. Brain Mapp 33, 2785–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YZ, Salmeron BJ, Gu H, Stein EA, Yang YH, 2015b. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. Jama Psychiat 72, 584–592. [DOI] [PubMed] [Google Scholar]
- Hutton C, Bork A, Josephs O, Deichmann R, Ashburner J, Turner R, 2002. Image distortion correction in fMRI: A quantitative evaluation. Neuroimage 16, 217–240. [DOI] [PubMed] [Google Scholar]
- Ide JS, Hu S, Zhang S, Mujica-Parodi LR, Li CSR, 2016. Power spectrum scale invariance as a neural marker of cocaine misuse and altered cognitive control. Neuroimage Clin 11, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Shenoy P, Yu AJ, Li CS, 2013. Bayesian prediction and evaluation in the anterior cingulate cortex. J. Neurosci 33, 2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Zhang S, Hu S, Sinha R, Mazure CM, Li CR, 2014. Cerebral gray matter volumes and low-frequency fluctuation of BOLD signals in cocaine dependence: duration of use and gender difference. Drug Alcohol Depend 134, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Zhornitsky S, Hu S, Zhang S, Krystal JH, Chiang-shan RL, 2017. Sex differences in the interacting roles of impulsivity and positive alcohol expectancy in problem drinking: A structural brain imaging study. Neuroimage Clin 14, 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H, 2003. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J. Neurosci 23, 7839–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, 2014. Involvement of the dorsal and ventral attention networks in oddball stimulus processing: a meta-analysis. Hum. Brain Mapp 35, 2265–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange N, Strother SC, Anderson JR, Nielsen FA, Holmes AP, Kolenda T, Savoy R, Hansen LK, 1999. Plurality and resemblance in fMRI data analysis. Neuroimage 10, 282–303. [DOI] [PubMed] [Google Scholar]
- Levitt H, 1971. Transformed up-down methods in psychoacoustics. J. Acoust. Soc. Am 49, 467–477. [PubMed] [Google Scholar]
- Li CS, Chao HH, Lee TW, 2009. Neural correlates of speeded as compared with delayed responses in a stop signal task: an indirect analog of risk taking and association with an anxiety trait. Cereb. Cortex 19, 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Yan P, Sinha R, Lee TW, 2008. Subcortical processes of motor response inhibition during a stop signal task. Neuroimage 41, 1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CSR, Huang C, Constable RT, Sinha R, 2006a. Gender differences in the neural correlates of response inhibition during a stop signal task. Neuroimage 32, 1918–1929. [DOI] [PubMed] [Google Scholar]
- Li CSR, Huang C, Constable RT, Sinha R, 2006b. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J. Neurosci 26, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CSR, Milivojevic V, Kemp K, Hong K, Sinha R, 2006c. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend 85, 205–212. [DOI] [PubMed] [Google Scholar]
- Limbrick-Oldfield EH, van Holst RJ, Clark L, 2013. Fronto-striatal dysregulation in drug addiction and pathological gambling: Consistent inconsistencies? Neuroimage Clin 2, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA, 1984. On the ability to inhibit simple and choice reaction-time responses - A model and a method. J. Exp. Psychol. Human 10, 276–291. [DOI] [PubMed] [Google Scholar]
- Lundqvist T, 2010. Imaging cognitive deficits in drug abuse. Curr. Top Behav. Neurosci 3, 247–275. [DOI] [PubMed] [Google Scholar]
- Luo X, Zhang S, Hu S, Bednarski SR, Erdman E, Farr OM, Hong K-I, Sinha R, Mazure CM, Li C.-s.R., 2013. Error processing and gender-shared and-specific neural predictors of relapse in cocaine dependence. Brain 136, 1231–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manza P, Hu S, Ide JS, Farr OM, Zhang S, Leung HC, Li CSR, 2016. The effects of methylphenidate on cerebral responses to conflict anticipation and unsigned prediction error in a stop-signal task. J. Psychopharmacol 30, 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhe R, van de Wetering BJ, Franken IH, 2013. Error-related brain activity predicts cocaine use after treatment at 3-month follow-up. Biol. Psychiat 73, 782–788. [DOI] [PubMed] [Google Scholar]
- McCarthy JM, Zuo CS, Shepherd JM, Dias N, Lukas SE, Janes AC, 2017. Reduced interhemispheric executive control network coupling in men during early cocaine abstinence: A pilot study. Drug Alcohol Depend 181, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Hansen LK, Sejnowski TJ, 2003. Independent component analysis of functional MRI: what is signal and what is noise? Curr. Opin. Neurobiol 13, 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ, 1998. Analysis of fMRI data by blind separation into independent spatial components. Hum. Brain Mapp 6, 160–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD, 2000. Drug dependence, a chronic medical illness - Implications for treatment, insurance, and outcomes evaluation. Jama 284, 1689–1695. [DOI] [PubMed] [Google Scholar]
- Mirabella G, 2014. Should I stay or should I go? Conceptual underpinnings of goal-directed actions. Front. Syst. Neurosci 8, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella G, Bertini G, Samengo I, Kilavik BE, Frilli D, Della Libera C, Chelazzi L, 2007. Neurons in area V4 of the macaque translate attended visual features into behaviorally relevant categories. Neuron 54, 303–318. [DOI] [PubMed] [Google Scholar]
- Mirabella G, Iaconelli S, Modugno N, Giannini G, Lena F, Cantore G, 2013. Stimulation of subthalamic nuclei restores a near normal planning strategy in Parkinson’s patients. Plos One 8, e62793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella G, Iaconelli S, Romanelli P, Modugno N, Lena F, Manfredi M, Cantore G, 2012. Deep brain stimulation of subthalamic nuclei affects arm response inhibition in Parkinson’s patients. Cereb. Cortex 22, 1124–1132. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA, 2005. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: Diffusion tensor imaging. Neuropsychopharmacology 30, 610–617. [DOI] [PubMed] [Google Scholar]
- Morein-Zamir S, Robbins TW, 2015. Fronto-striatal circuits in response-inhibition: Relevance to addiction. Brain Res 1628, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor LJ, Murphy A, McGonigle J, Orban C, Reed L, Taylor E, Flechais R, Paterson LM, Smith D, Bullmore ET, Ersche KD, Suckling J, Tait R, Elliott R, Deakin B, Rabiner I, Lingford-Hughes A, Nutt DJ, Sahakian B, Robbins TW, Consortium I, 2017. Acute naltrexone does not remediate fronto-striatal disturbances in alcoholic and alcoholic polysubstance-dependent populations during a monetary incentive delay task. Addict. Biol 22, 1576–1589. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, Konova AB, Proudfit GH, Dunning JP, Malaker P, Moeller SJ, Maloney T, Alia-Klein N, Goldstein RZ, 2015. Impaired neural response to negative prediction errors in cocaine addiction. J. Neurosci 35, 1872–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SK, Dunlop K, Downar J, 2016. Cortico-striatal-thalamic loop circuits of the salience network: A central pathway in psychiatric disease and treatment. Front. Syst. Neurosci 10, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose EJ, Salmeron BJ, Ross TJ, Waltz J, Schweitzer JB, McClure SM, Stein EA, 2014. Temporal difference error prediction signal dysregulation in cocaine dependence. Neuropsychopharmacology 39, 1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven MC, Kiehl KA, Pearlson GD, Calhoun VD, 2007. Functional neural networks underlying response inhibition in adolescents and adults. Behav. Brain. Res 181, 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shen H, Lei Y, Zeng LL, Cao F, Su L, Yang Z, Yao S, Hu D, 2017a. Altered default mode, fronto-parietal and salience networks in adolescents with Internet addiction. Addict. Behav 70, 1–6. [DOI] [PubMed] [Google Scholar]
- Wang W, Hu S, Ide JS, Zhornitsky S, Zhang S, Yu A, Li C-SR, 2018a. Motor preparation disrupts proactive control in the stop signal task. Front. Hum. Neurosc 12, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Hanlon CA, Porrino LJ, 2011. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiat. Res-Neuroim 191, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetheril GB, Chen H, Vasudeva RB, 1966. Sequential estimation of quantal response curves- A new method of estimation. Biometrika 53, 439–454. [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR, 2011. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend 115, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worhunsky PD, Dager AD, Meda SA, Khadka S, Stevens MC, Austad CS, Raskin SA, Tennen H, Wood RM, Fallahi CR, Potenza MN, Pearlson GD, 2016. A preliminary prospective study of an escalation in ‘maximum daily drinks’, fronto-parietal circuitry and impulsivity-related domains in young adult drinkers. Neuropsychopharmacology 41, 1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worhunsky PD, Potenza MN, Rogers RD, 2017. Alterations in functional brain networks associated with loss-chasing in gambling disorder and cocaine-use disorder. Drug Alcohol Depend 178, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worhunsky PD, Stevens MC, Carroll KM, Rounsaville BJ, Calhoun VD, Pearlson GD, Potenza MN, 2013. Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychol. Addict. Behav 27, 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Calhoun VD, Worhunsky PD, Xiang H, Li J, Wall JT, Pearlson GD, Potenza MN, 2015. Functional network overlap as revealed by fMRI using sICA and its potential relationships with functional heterogeneity, balanced excitation and inhibition, and sparseness of neuron activity. Plos One 10, e0117029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Potenza MN, Calhoun VD, Zhang R, Yip SW, Wall JT, Pearlson GD, Worhunsky PD, Garrison KA, Moran JM, 2016. Large-scale functional network overlap is a general property of brain functional organization: reconciling inconsistent fMRI findings from general-linear-model-based analyses. Neurosci. Biobehav. Rev 71, 83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, Worhunsky PD, Xu JS, Morie KP, Constable RT, Malison RT, Carrol KM, Potenza MN, 2018Gray-matter relationships to diagnostic and transdiagnostic features of drug and behavioral addictions. Addict. Biol 23, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Butelman R, Kreek MJ, 2005. Biological clocks may modulate drug addiction. Eur. J. Hum. Genet 13, 1101–1103. [DOI] [PubMed] [Google Scholar]
- Zar JH, 1999. Biostatistical Analysis 4th Edition, Prentice Hall, Upper Saddle River, NJ. [Google Scholar]
- Zhang S, Hu S, Chao HH, Ide JS, Luo X, Farr OM, Li CSR, 2014. Ventromedial prefrontal cortex and the regulation of physiological arousal. Soc. Cogn. Affect. Neur 9, 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Luo X, Farr OM, Li CSR, 2012. Cerebral correlates of skin conductance responses in a cognitive task. Neuroimage 62, 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu SE, Hu JP, Wu PL, Chao HH, Li CSR, 2015a. Barratt impulsivity and neural regulation of physiological arousal. Plos One 10, e0129139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CR, 2017. Functional connectivity parcellation of the human thalamus by independent component analysis. Brain Connect 7, 602–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CSR, 2012. Functional networks for cognitive control in a stop signal task: Independent component analysis. Hum. BrainMapp 33,89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Tsai SJ, Hu S, Xu J, Chao HH, Calhoun VD, Li CS, 2015b. Independent component analysis of functional networks for response inhibition: Inter-subject variation in stop signal reaction time. Hum. Brain Mapp 36, 3289–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang S, Ide JS, Hu S, Zhornitsky S, Wang W, Dong G, Tang X, Chiang-shan RL, 2018. Dynamic network dysfunction in cocaine dependence: Graph theoretical metrics and stop signal reaction time. NeuroImage Clin 18, 793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]


