Abstract
It is unclear whether arterial healing occurs beyond 1 year following paclitaxel-coated stent implantation in peripheral artery disease. An 81-year-old woman with superficial femoral artery disease underwent endovascular therapy with a paclitaxel-coated stent. An angiography 21 months later revealed peri-stent contrast staining in the superficial femoral artery, and optical frequency domain imaging demonstrated incomplete stent apposition with significant positive vascular remodeling. High-resolution angioscopy detected positive vascular wall remodeling and in-stent yellow plaque more clearly than conventional angioscopy. Refractory superficial femoral arterial wall healing was apparent more than 20 months after paclitaxel-coated stent implantation.
Keywords: angioscopy, peripheral artery disease, refractory healing
Introduction
Recently published data in Japan shows that peripheral artery disease is prevalent even in patients without coronary artery disease.1) The Zilver PTX Stent (Cook Medical, Bloomington, IN, USA) is a polymer-free paclitaxel-coated self-expanding nitinol stent designed for femoropopliteal arteries. Previous randomized studies demonstrated the clinical effectiveness of long-term patency compared with that using a bare-metal stent.2) However, angioscopy and optical coherence tomography (OCT) revealed delayed arterial healing for up to 1 year after Zilver PTX implantation.3) It is unclear whether arterial healing occurs beyond 1 year following Zilver PTX implantation.
Angioscopy provides direct visualization of the thrombus, yellow plaque, stent malapposition, and neointimal coverage.4–6) Conventional angioscopy uses an optical fiber cable designed for coronary artery assessment; however, the image resolution and viewing angle are limited for analyzing large peripheral arteries. The Zemporshe angioscope (Taisho Biomed Instruments, Osaka, Japan) has a 0.48-megapixel equivalent resolution, and is equipped with a camera at the distal catheter tip with a 90 degree viewing angle. We report refractory arterial wall healing in the very long-term phase after Zilver PTX implantation using high-resolution angioscopy and optical frequency domain imaging (OFDI).
Case Report
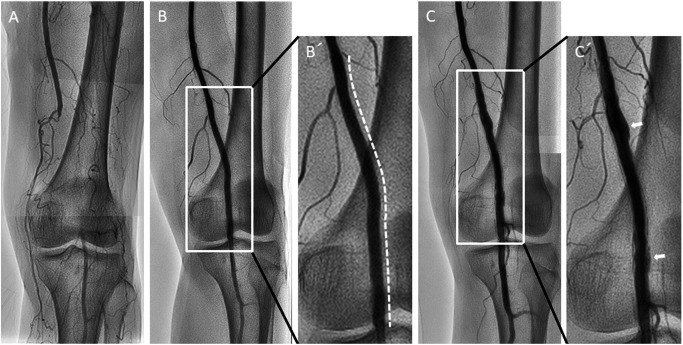
An 81-year-old woman with a total occlusive lesion in the left superficial femoral artery–popliteal artery (SFA–POP) (Fig. 1A) and chronic below-the-knee (BK) broad occlusive disease underwent endovascular therapy (EVT) for SFA–POP and BK lesions. With the patient’s agreement, given that she was suffering from skin lesions caused by scleroderma with steroid therapy, EVT with drug-eluting stent (DES) for SFA–POP lesion was performed. A 6×120 mm paclitaxel-coated Zilver PTX stent was implanted in the left SFA–POP lesion (Figs. 1B and 1B′). The stent was selected to fit a 5.7-mm vessel diameter measured using intravascular ultrasound. The left ankle–brachial index showed improvement from 0.55 to 1.14. The dorsal/plantar skin perfusion pressure increased from 16/17 mmHg to 70/76 mmHg. However, the patient experienced recurrence of limb ischemia with rest pain and cutaneous toe ulcers 21 months later. Angiography revealed isolated reocclusion of BK with SFA–POP stent patency. We successfully treated the BK lesions using angioplasty.
Fig. 1 (A) Angiogram showing total occlusive lesion in the left distal superficial femoral artery to proximal popliteal artery. (B/B′) A 6.0×120 mm Zilver PTX stent was implanted in the occlusive lesion (white dotted line). The stent size was selected to match the vessel size. (C/C′) Angiogram showing peri-stent staining (white arrows) at 21 months after Zilver PTX implantation.
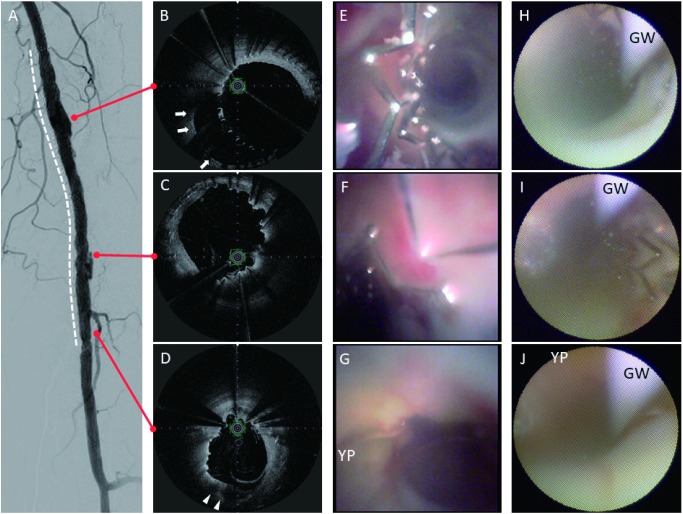
Peri-stent contrast staining (PSS) was observed around the SFA–POP stent site (Figs. 1C and 1C′). Furthermore, OFDI demonstrated incomplete stent apposition with significant positive vascular remodeling (Fig. 2). The vessel diameter was remodeled to 8.2 mm. Medial degeneration was observed at the positive remodeling site. Neointimal coverage was heterogeneous and uncovered stent struts were attached with fibrin thrombi. A heterogeneous lipid plaque was partially observed (Fig. 2D).
Fig. 2 (A) Digital subtraction image of the Zilver PTX in the femoropopliteal artery (white dotted line). (B–D) Optical frequency domain imaging showing significant positive vessel wall remodeling (white arrows) and heterogeneous low-intensity lipid plaque (white arrowheads). (E–G) Zemporshe angioscopy clearly revealing incomplete stent apposition and stent thrombus. Positive vascular wall remodeling and yellow plaque were more clearly visible compared with that using fiberoptic angioscopy. (H–J) Conventional fiberoptic angioscopy images had noticeably lower resolution and smaller viewing angles than those observed using Zemporshe angioscopy. YP: yellow plaque; GW: guidewire.
Conventional fiberoptic angioscopy (Forwardlooking, Taisho Biomed Instruments, Osaka, Japan) and high-resolution angioscopy were performed. Dextran was flushed using a temporary occlusion balloon guiding catheter (Optimo PPI, Tokai Medical Products, Aichi, Japan) during angioscopic examination. High-resolution angioscopy revealed heterogeneous neointimal coverage, uncovered stent struts, and stent thrombi (Figs. 2E–2G) more clearly than with conventional angioscopy (Figs. 2H–2J). Yellow plaque and a positively remodeled vascular wall were clearly detected using the Zemporshe angioscope (Fig. 2G). Yellow plaque sites matched the heterogeneous lipid plaque sites observed with OFDI, and heterogeneous endothelial coverage was observed in the positively remodeled vascular wall (Fig. 2E).
The imaging devices demonstrated incomplete stent apposition (ISA) and yellow plaque in the very late phase after Zilver PTX implantation. We decided to continue dual antiplatelet therapy to prevent very late stent thrombosis. Refractory femoropopliteal arterial wall healing was apparent 21 months after paclitaxel-coated stenting.
Discussion
We describe a case of refractory arterial wall healing after polymer-free paclitaxel-coated stent implantation in the femoropopliteal artery. Uncovered stent struts and heterogeneous neointimal coverage are more frequently observed after Zilver PTX implantation than with the use of a bare-metal stent for up to 1 year following implantation.3) ISA and PSS after DES implantation have been reported in coronary artery disease. PSS is defined as a contrast staining outside the stent contour extending to ≥20% of the stent diameter.7) This is the first report of very late phase PSS in the femoropopliteal artery observed using OFDI and high-resolution angioscopy.
In this case, ISA developed with positive vascular remodeling. OFDI images demonstrated up to 44% positive remodeling of the vascular diameter at 21 months after stenting. The process of positive remodeling after DES implantation remains unclear; however, several hypotheses have been suggested for coronary artery disease.8) Pathology studies have shown incomplete endothelialization, medial wall inflammatory changes, or hypersensitivity responses to the drug and/or polymer; these might induce positive remodeling after coronary DES implantation. Although the presence of a durable polymer has been suggested as one of the causes of neointimal heterogeneity with use of a paclitaxel-eluting stent,9) the Zilver PTX is polymer-free. Therefore, the presence of paclitaxel itself may be associated with neointimal heterogeneity with the use of the Zilver PTX stent. A previous OCT study demonstrated that the extra-stent lumen area was enlarged 6–12 months after Zilver PTX implantation.3) The enlargement was caused by dilatation of the external elastic membrane area over time because of localized inflammation.10) In this case, sustained positive remodeling was observed at a very late phase.
The Zemporshe high-resolution angioscope was developed for peripheral vascular use. It is equipped with a 0.48-megapixel equivalent resolution camera at the catheter tip and has higher quality imaging and a larger angular field compared with conventional fiberoptic angioscopes. Angioscopy imaging has been used for the assessment of vascular thrombus, plaque behavior, and stent neointimal coverage in peripheral artery disease.6) The Zemporshe angioscope enables circumferential intravascular wall observation in large peripheral arteries with high resolution. Yellow plaque and positively remodeled vascular wall through stent struts were particularly detected clearly with the Zemporshe angioscope. It can be potentially beneficial for histopathological observation of large vessels.
Dual antiplatelet therapy is recommended for at least 60 days after Zilver PTX implantation. PSS is reported to occur in 1.3% of cases after DES implantation in coronary artery disease. However, the PSS incidence rate is unclear in peripheral artery disease after DES implantation. Delayed vascular healing may be apparent beyond 1 year after DES implantation, although collagen disease with steroid therapy in this case could be involved. Delayed vascular healing induces late stent thrombosis. The Zilver PTX registry reported that stent thrombosis increased over time following EVT. Therefore, further study is required to determine the optimal duration of dual antiplatelet therapy. Moreover, long-term follow-up beyond 1 year is essential for patients receiving Zilver PTX implantation.
Conclusion
Refractory superficial femoral arterial wall healing was apparent more than 20 months after paclitaxel-coated stent implantation. High-resolution angioscopy detected positive vascular wall remodeling and in-stent yellow plaque more clearly than conventional angioscopy.
Disclosure Statement
There are no financial or other relations that could lead to a conflict of interests.
Author Contributions
Study conception: KI, KO
Data collection: KI, KO, TW
Analysis: TW, SN
Investigation: YS
Writing: IK, SH
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
References
- 1).Hokimoto S, Soejima H, Kojima S, et al. Distribution of ankle-brachial index among inpatients with cardiovascular disease: analysis using the Kumamoto University Hospital medical database. Ann Vasc Dis 2016; 9: 22-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX randomized trial. Circulation 2016; 133: 1472-83; discussion, 1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Tomoi Y, Kuramitsu S, Soga Y, et al. Vascular response after Zilver PTX stent implantation for superficial femoral artery lesions: serial optical coherence tomography findings at 6 and 12 months. J Endovasc Ther 2015; 22: 41-7. [DOI] [PubMed] [Google Scholar]
- 4).Sakai S, Mizuno K, Yokoyama S, et al. Morphologic changes in infarct-related plaque after coronary stent placement: a serial angioscopy study. J Am Coll Cardiol 2003; 42: 1558-65. [DOI] [PubMed] [Google Scholar]
- 5).Awata M, Kotani J, Uematsu M, et al. Serial angioscopic evidence of incomplete neointimal coverage after sirolimus-eluting stent implantation: comparison with bare-metal stents. Circulation 2007; 116: 910-6. [DOI] [PubMed] [Google Scholar]
- 6).Ishihara T, Iida O, Okamoto S, et al. Potential mechanisms of in-stent occlusion in the femoropopliteal artery: an angioscopic assessment. Cardiovasc Interv Ther 2017; 32: 313-7. [DOI] [PubMed] [Google Scholar]
- 7).Imai M, Kadota K, Goto T, et al. Incidence, risk factors, and clinical sequelae of angiographic peri-stent contrast staining after sirolimus-eluting stent implantation. Circulation 2011; 123: 2382-91. [DOI] [PubMed] [Google Scholar]
- 8).Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006; 48: 193-202. [DOI] [PubMed] [Google Scholar]
- 9).Awata M, Nanto S, Uematsu M, et al. Heterogeneous arterial healing in patients following paclitaxel-eluting stent implantation: comparison with sirolimus-eluting stents. JACC Cardiovasc Interv 2009; 2: 453-8. [DOI] [PubMed] [Google Scholar]
- 10).Cook S, Ladich E, Nakazawa G, et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation 2009; 120: 391-9. [DOI] [PubMed] [Google Scholar]