Abstract
Objective: To investigate the predictors of acute kidney injury (AKI) following surgery for abdominal aortic aneurysm.
Materials and Methods: Subjects were 642 non-hemodialysis patients (open aortic repair [OAR] group, n=453; endovascular aortic repair [EVAR] group, n=189) who underwent elective surgery between 2009 and 2015. AKI was assessed according to the Kidney Disease Improving Global Outcomes criteria. In-hospital mortality and incidence of AKI were compared between the OAR and EVAR groups. The effect of AKI on outcomes and predictors of AKI were examined in both groups.
Results: In-hospital mortalities were 0.7% (3/453) in the OAR group and 0.5% (1/189) in the EVAR group. The incidence of AKI increased in the OAR group (14.1% vs. 3.7%, P<0.01). In the OAR group, in-hospital mortality (0% vs. 4.7%, P<0.01) increased in patients with AKI. In the OAR group, hemoglobin level <10 g/dL, estimated glomerular filtration rate <60 mL/min/1.73 m2, operation time >300 min, history of ischemic heart disease, and amount of bleeding >1,000 mL were predictors of AKI. In the EVAR group, amount of transfusion>1,000 mL was a predictor of AKI, but AKI was not found to worsen outcomes.
Conclusion: AKI affected outcomes of OAR. Knowledge of predictors may optimize perioperative care.
Keywords: acute kidney injury, abdominal aortic aneurysm, open aortic repair, endovascular aortic repair
Introduction
Abdominal aortic aneurysm (AAA) is a serious cardiovascular disorder due to arteriosclerosis. Enlarged AAA is a life-threatening condition, and aortic repair is indicated for patients in whom the AAA diameter exceeds 55 mm or the aneurysm growth exceeds 10 mm/year.1) Open aortic repair (OAR) and endovascular aortic repair (EVAR) are established treatment options for AAA. EVAR has been reported to decrease in-hospital mortality and perioperative morbidities,2) and the anatomical indications have been expanding with the development of device technology.
Acute kidney injury (AKI) is a known postoperative complication of both OAR and EVAR. Postoperative AKI is associated with not only early mortality3–5) but also survival,4,6,7) cardiovascular events,8) and aneurysm-related death9) in the late follow-up period. Although EVAR may decrease the risk of AKI,3,5,10–14) the reported incidence rates of AKI widely range from 12.4% to 36.2% for elective OAR4,5,11,15,16) and 2.9% to 18.8% for elective EVAR.5,7,11,17) Complexity in research on AKI in this area might be caused by heterogenous manifestations, such as ruptured or unruptured, and the involvement of aneurysm requiring suprarenal aortic clamp. In addition, differences in AKI classification systems among studies make the comparison of research on AKI complicated. Several AKI classification systems include the Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE) criteria,18) the Acute Kidney Injury Network (AKIN) criteria,19) and the recently developed Kidney Disease Improving Global Outcomes (KDIGO) criteria.20) A few groups have recently reported AKI following surgical intervention for AAA using the KDIGO criteria.3,6,8,15) The use of this classification system, however, has not been standardized in the research field of vascular surgery.
In the present study, we aimed to analyze the incidences, effect on early outcomes, and predictors of AKI assessed using the KDIGO criteria, the most recent consensus AKI criteria, in elective OAR and EVAR for AAA.
Materials and Methods
Patient population
Between January 2009 and June 2015, a total of 803 patients underwent surgical intervention for AAA at Saitama Medical Center, Jichi Medical University, Saitama, Japan. One hundred patients who emergently or urgently underwent surgery for symptomatic or ruptured AAA were excluded. We also excluded 11 chronic hemodialysis patients and 50 patients who required suprarenal aortic clamp in OAR for juxta- or pararenal AAA. As a result, the study involved 642 non-hemodialysis patients who underwent elective OAR (n=453) and EVAR (n=189) as subjects. The OAR group included four patients treated with simple aneurysm resection for a solitary hypogastric artery aneurysm and eight patients treated with prosthesis replacement for a solitary common iliac artery aneurysm. The diagnosis of AAA was confirmed by computed tomography in all patients. The ethics committee of Saitama Medical Center, Jichi Medical University, approved the study (Reg. No. S17-142), and the need for individual informed consent was waived. Patients’ clinical charts and the computerized vascular surgery database were reviewed retrospectively.
Study design
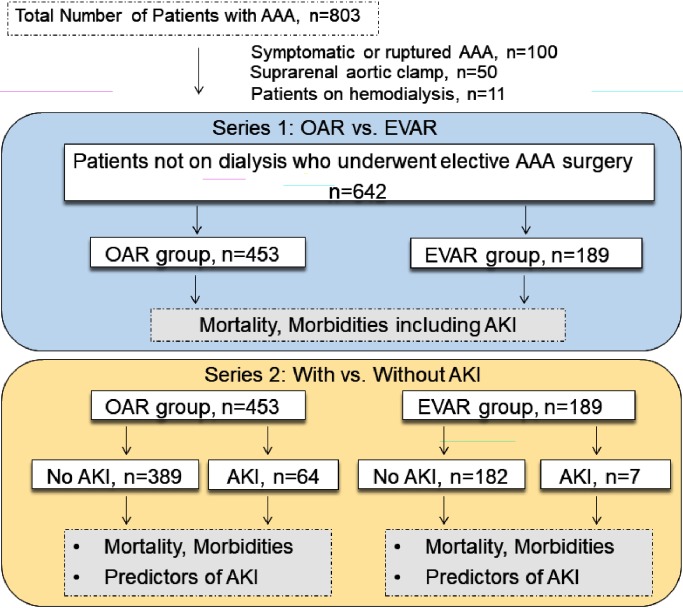
The overall study design is shown in Fig. 1. In the first series, comorbidities and characteristics, in-hospital mortality, and morbidities including AKI were compared between patients treated with OAR and EVAR. In the second series, in-hospital mortality and morbidities were compared between patients with and without AKI in the OAR and EVAR groups. Finally, we investigated predictors of AKI in both groups.
Fig. 1 Flow diagram of the study design and number of patients.
AAA: abdominal aortic aneurysm; OAR: open aortic repair; EVAR: endovascular aortic repair; AKI: acute kidney injury
Definition of AKI
Preoperative estimated glomerular filtration rate (eGFR) was estimated using the Modification of Diet in Renal Disease study equation for Japanese patients.21) We used the serum creatinine concentration at the time of admission as the preoperative value. Patients were considered to have AKI when they fulfilled the KDIGO criteria within 7 days of surgery: stage 1 denotes an increase in serum creatinine concentration by ≥0.3 mg/dL within 48 h or a 1.5–1.9-fold increase from the preoperative value; stage 2 denotes a 2.0–2.9-fold increase in serum creatinine concentration from the preoperative value; and stage 3 denotes a ≥3.0-fold increase in serum creatinine concentration from the preoperative value, an increase in serum creatinine concentration of ≥4.0 mg/dL, or initiation of renal replacement therapy.20,22) The urine output was not considered in the staging, as data on hourly urine output were not available from medical records. For patients with AKI, serum creatinine concentration at discharge was measured. AKI resolution was defined as an increase of <0.3 mg/dL in serum creatinine concentration at discharge from the preoperative serum creatinine concentration.
Surgical procedure
The surgical procedure to be performed was individually determined based on anatomical morphology and the age, comorbidities, and frailty status of patients. EVAR was preferably performed in elderly patients and those with prior abdominal operation. OAR using a Dacron graft was performed under general anesthesia via a transperitoneal or retroperitoneal approach. Autologous blood transfusion device was routinely used in OAR. In this series, no patients were treated with renal protection procedures, including renal artery perfusion and temporary bypass via a conduit from the subclavian artery, in OAR. EVAR was performed with commercially available devices in the operation room under general anesthesia except for those patients with severe chronic obstructive pulmonary disease (COPD) or interstitial pneumonia, who were at high risk for mechanical ventilation. For these patients, operations were performed under local anesthesia. In all cases, stent grafts were planned to be deployed below both renal arteries. For cases with hypogastric artery aneurysm, coil embolization was performed preoperatively except for those cases with short proximal neck. In these cases, stent graft deployment was performed simultaneously due to the risk of rupture of the hypogastric artery aneurysm.
Statistical analysis
All values are expressed as mean±standard deviation. Between-group differences in clinical variables were analyzed by χ2 or Fisher’s exact test or by unpaired t-test or Mann–Whitney U test, as appropriate. To identify predictors of postoperative AKI, multivariate forward stepwise logistic regression analysis was performed. Variables with a P value <0.1 in the univariate analysis were included in the logistic regression model. There were 20 common variables for the OAR and EVAR groups: age, male sex, Marfan syndrome, history of smoking, hypertension, diabetes, dyslipidemia, COPD, peripheral artery disease, history of ischemic heart disease, history of cerebrovascular disease, prior thoracic aortic surgery, maximum aortic diameter, left ventricular ejection fraction <40%, preoperative hemoglobin level <10 g/dL, eGFR <60 mL/min/1.73 m2, albumin level <4.0 g/mL, operation time >5 h, amount of bleeding >1,000 mL, and amount of transfusion>1,000 mL. Only four variables (bifurcated graft replacement, straight graft replacement, reconstruction of the inferior mesenteric artery, and reconstruction of the hypogastric artery) for the OAR group and only two variables (contrast material volume and vascular access complication) for the EVAR group were present. All statistical analyses were performed using IBM SPSS Statistics version 23.0 for Windows (IBM Corp., Armonk, NY, USA). P<0.05 was considered statistically significant.
Results
Series 1: Comparisons of characteristics and outcomes between the OAR and EVAR groups
First, we compared the basic information, comorbidities, and clinical characteristics of patients who underwent OAR and EVAR (Table 1). The patients treated with OAR were younger and more likely to have a prior history of aortic dissection and anatomically to have a hypogastric artery aneurysm than those treated with EVAR. Preoperative hemoglobin level was lower in the EVAR group. However, there were no differences between the groups in other clinical characteristics, including concomitant comorbidities, preoperative left ventricular ejection fraction, maximum aortic diameter, and laboratory data.
Table 1 Comorbidities and clinical characteristics of patients who underwent open and endovascular aortic repair.
| Open repair, N=453 | Endovascular repair, N=189 | P value | |
|---|---|---|---|
| Age, years, mean±SD | 70.8±7.9 | 77.5±7.6 | <0.01 |
| Male sex | 85.9% (389/453) | 84.1% (159/189) | 0.57 |
| Marfan syndrome | 0.9% (4/453) | 0% (0/189) | 0.45 |
| History of aortic dissection | 7.3% (33/453) | 1.6% (3/189) | <0.01 |
| Hypertension | 81.9% (371/453) | 79.4% (150/189) | 0.45 |
| Diabetes | 19.6% (89/453) | 11.6% (22/189) | 0.014 |
| Dyslipidemia | 44.2% (200/453) | 43.4% (82/189) | 0.86 |
| COPD | 10.2% (46/453) | 16.9% (32/189) | 0.017 |
| History of smoking | 75.7% (343/453) | 75.7% (143/189) | 0.98 |
| History of ischemic heart disease | 37.3% (169/453) | 40.2% (76/189) | 0.49 |
| History of cerebrovascular disease | 13.0% (59/453) | 9.0% (17/189) | 0.15 |
| Peripheral artery disease | 4.2% (19/453) | 2.1% (4/189) | 0.29 |
| Left ventricular ejection fraction<50% | 4.0% (18/453) | 1.1% (2/189) | 0.097 |
| Max diameter (mm), mean±SD | 51.0±11.7 | 52.1±9.2 | 0.28 |
| Mycotic aneurysm | 0.9% (4/453) | 0% (0/189) | 0.45 |
| Inflammatory aneurysm | 3.3% (15/453) | 1.6% (3/189) | 0.34 |
| Hypogastric artery aneurysm | 16.8% (76/453) | 9.5% (18/189) | 0.018 |
| Laboratory data | |||
| White blood cell (g/µL), mean±SD | 6,486±4,862 | 5,826±1,606 | 0.069 |
| Hemoglobin (g/dL), mean±SD | 12.9±1.8 | 12.5±1.9 | 0.029 |
| Hematocrit (%), mean±SD | 38.4±5.2 | 37.9±5.0 | 0.30 |
| Platelet (×103/µL), mean±SD | 20.9±6.1 | 19.9±5.8 | 0.37 |
| Albumin (g/mL), mean±SD | 4.1±0.6 | 4.0±0.6 | 0.52 |
| Creatinine (mg/dL), mean±SD | 1.0±0.5 | 1.0±0.4 | 0.39 |
| eGFR (mL/min/1.73 m2), mean±SD | 63.1±35.5 | 61.4±18.4 | 0.52 |
| eGFR <60 mL/min/1.73 m2 | 45.4% (205/453) | 48.7% (92/189) | 0.44 |
SD: standard deviation; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate
Table 2 shows the comparisons of operative variables and early outcomes between the two groups. As reported previously, operation time, amount of bleeding and transfusion, and prevalence of intraoperative transfusion were significantly higher in the OAR group. The inferior mesenteric and hypogastric arteries were reconstructed in 24.9% and 28.9% of patients who underwent OAR, respectively. In the present study, the average contrast material volume in the EVAR group was 134.2 mL.
Table 2 Operative variables and early outcomes of patients who underwent open and endovascular aortic repair.
| Open repair, N=453 | Endovascular repair, N=189 | P value | |
|---|---|---|---|
| Operative variables | |||
| Operation time (minutes), mean±SD | 274.9±84.9 | 224.7±59.1 | <0.01 |
| Amount of bleeding (mL), mean±SD | 404.0±351.7 | 115.6±130.9 | <0.01 |
| Amount of transfusion (mL), mean±SD | 366.1±667.9 | 71.2±228.0 | <0.01 |
| No transfusion | 62.0% (281/453) | 87.8% (166/189) | <0.01 |
| Amount of contrast (mL), mean±SD | NA | 134.2±51.0 | NA |
| Bifurcated graft replacement | 90.7% (411/453) | NA | NA |
| Straight graft replacement | 6.6% (30/453) | NA | NA |
| Reconstruction of IMA | 24.9% (113/453) | NA | NA |
| Reconstruction of hypogastric artery | 28.9% (131/453) | NA | NA |
| Outcomes | |||
| In-hospital mortality | 0.7% (3/453) | 0.5% (1/189) | 1.0 |
| Length of hospital stay (days), median (IQR) | 14 (12, 17) | 10 (9, 13) | <0.01 |
| Complications | |||
| Acute kidney injury | 14.1% (64/453) | 3.7% (7/189) | <0.01 |
| KDIGO Stage 1 | 12.1% (55/453) | 2.6% (5/189) | <0.01 |
| KDIGO Stage 2 | 1.1% (5/453) | 0.5% (1/189) | 0.81 |
| KDIGO Stage 3 | 0.9% (4/453) | 0.5% (1/189) | 1.0 |
| Need for temporary RRT | 0.2% (1/453) | 0% (0/189) | 1.0 |
| Need for permanent RRT | 0% (0/453) | 0% (0/189) | 1.0 |
| AKI restoration at discharge† | 78.7% (48/61) | 71.4% (5/7) | 0.66 |
| Paraplegia | 0.4% (2/453) | 0% (0/189) | 0.89 |
| Cerebral infarction | 0.4% (2/453) | 0% (0/189) | 0.89 |
| Prolonged ventilation>48 h | 0.2% (1/453) | 0% (0/189) | 1.0 |
| Re-exploration for bleeding | 0.9% (4/453) | 0% (0/189) | 0.46 |
| Mesenteric ischemia | 0.7% (3/453) | 0% (0/189) | 0.68 |
| Ileus | 4.0% (18/453) | NA | NA |
| Vascular access complication | NA | 1.6% (3/189) | NA |
| Type 1 endoleak | NA | 1.6% (3/189) | NA |
NA: not applicable; SD: standard deviation; IMA: inferior mesenteric artery; IQR: interquartile range; KDIGO: Kidney Disease Improving Global Outcomes; RRT: renal replacement therapy † AKI restoration was defined as serum creatinine level. Four in-hospital dead patients were excluded.
Hospital stay was longer in the OAR group; however, in-hospital mortalities were similar and less than 1% in both groups. The incidence of AKI assessed using the KDIGO criteria was significantly higher in the OAR group. Of note, only seven patients (3.7%) showed postoperative AKI following EVAR. Stage 1 had the highest incidence among all AKI stages in both groups, with 85.9% (55/64) in the OAR group and 71.4% (5/7) in the EVAR group. In the present study, the incidence of stage 2 and 3 AKI assessed using the KDIGO criteria was similar in both groups. One patient who underwent OAR was treated with temporary renal replacement therapy. No patients in the present study required permanent renal replacement therapy. AKI resolution was achieved in more than 70% of patients in both groups. No significant differences in the incidence of the remaining postoperative complications between the OAR and EVAR groups were observed.
Series 2: Effect of AKI on outcomes and risk factors for AKI
The patients were divided into two groups according to the presence or absence of AKI in the OAR and EVAR groups, and early outcomes were then compared between both groups. Table 3 shows in-hospital outcomes assessed by the presence or absence of AKI. In the OAR group, in-hospital mortality was significantly higher in patients with AKI (0% vs. 4.7%, P<0.01). The cause of in-hospital mortality in the OAR group included mesenteric ischemia in two patients and infective endocarditis in one patient. The cause of the single in-hospital mortality following EVAR was cerebral infarction. The median length of hospital stay was longer in patients with AKI in the OAR group. No differences in the incidence of the remaining postoperative complications were found in the OAR group. In the present study, AKI did not affect early outcomes in patients who underwent EVAR.
Table 3 In-hospital outcomes assessed by presence or absence of acute kidney injury.
| Open repair | Endovascular repair | |||||
|---|---|---|---|---|---|---|
| Non AKI n=389 | AKI n=64 | P value | Non AKI n=182 | AKI n=7 | P value | |
| In-hospital mortality | 0 (0/389) | 4.7% (3/64) | <0.01 | 0.5% (1/182) | 0% (0/9) | 1.0 |
| Length of hospital stay (days), median (IQR) | 14 (12, 17) | 17 (13, 23) | <0.01 | 12 (11, 15) | 9 (17, 25) | 0.431 |
| Complications | ||||||
| Paraplegia | 0.5% (2/389) | 0% (0/64) | 1.0 | 0% (0/182) | 0% (0/7) | 1.0 |
| Cerebral infarction | 0.3% (1/389) | 1.6% (1/64) | 0.66 | 0.5% (1/182) | 0% (0/7) | 1.0 |
| Prolonged ventilation>48 h | 0% (0/389) | 1.6% (1/64) | 0.30 | 0% (0/182) | 0% (0/7) | 1.0 |
| Reoperation for bleeding | 0.5% (2/389) | 3.1% (2/64) | 0.18 | 0% (0/182) | 0% (0/7) | 1.0 |
| Mesenteric ischemia | 0.3% (1/389) | 3.1% (2/64) | 0.073 | 0% (0/182) | 0% (0/7) | 1.0 |
| Ileus | 4.1% (16/389) | 3.1% (2/64) | 0.98 | NA | NA | NA |
| Vascular access complication | NA | NA | NA | 1.6% (3/182) | 0% (0/7) | 1.0 |
| Type 1 endoleak | NA | NA | NA | 1.6% (3/182) | 0% (0/7) | 1.0 |
AKI: acute kidney injury; IQR: interquartile range; NA: not applicable
Finally, we investigated the risk factors for AKI in both groups (Table 4). In the OAR group, renal dysfunction, defined as eGFR <60 mL/min/1.73 m2, hemoglobin level <10 g/dL, operation time >300 min, history of ischemic heart disease, and amount of bleeding >1,000 mL, was associated with AKI. In the EVAR group, amount of transfusion>1,000 mL was identified as the single risk factor for AKI. In this series, contrast material volume was not associated with the development of AKI.
Table 4 Logistic regression analysis for postoperative AKI.
| Factors associated with acute kidney injury | Odds ratio (95% CI) | P value |
|---|---|---|
| Open repair | ||
| eGFR<60 mL/min/1.73 m2 | 2.81 (1.53–5.17) | 0.001 |
| Hemoglobin<10 g/dL | 4.50 (1.10–6.34) | 0.001 |
| Operation time>300 min | 2.17 (1.21–3.91) | 0.010 |
| History of ischemic heart disease | 1.93 (1.09–3.42) | 0.023 |
| Bleeding>1,000 mL | 2.64 (1.10–6.34) | 0.029 |
| Endovascular repair | ||
| Transfusion>1,000 mL | 14.75 (1.17–186.02) | 0.037 |
CI: confidence interval; eGFR: estimated glomerular filtration rate
Discussion
AKI is a common but important complication following OAR or EVAR for AAA. Emergency surgery for ruptured aneurysm is associated with a much higher incidence rate of AKI (74% by the RIFLE criteria23) and 75.7% by the AKIN criteria24)). Similarly, suprarenal aortic clamp has been reported to predispose those who undergo OAR to AKI.25,26) In the present study, we focused on investigating patients who underwent elective OAR and EVAR. To the best of our knowledge, our study is the largest-scale single-center study that assessed postoperative AKI using the KDIGO criteria. This study showed that AKI increased in-hospital mortality and length of hospital stay in patients treated with elective OAR. In contrast, AKI was relatively rare in patients who underwent elective EVAR and did not affect the outcomes. AKI seems to be a reversible complication, and renal dysfunction was reversed in more than 70% of patients at discharge in both groups.
Postoperative AKI following surgery for AAA has been previously studied. However, assessing the severity of AKI in the literature is complicated, mainly because of the lack of consensus on the AKI classification system. Several studies have investigated AKI related to surgery for AAA using the RIFLE and AKIN criteria, the two most commonly used classification systems. The studies that used the RIFLE criteria showed that the incidences of postoperative AKI following surgery for infrarenal AAA ranged from 13.7% to 21.7% for elective OAR4,16) and 10.5% for elective EVAR.4) The studies that used the AKIN criteria showed incidences of AKI of 22.4% for elective OAR4) and from 13.1% to 18.8% for elective EVAR.4,6,17) Bang et al. compared the utilities of these two classification systems in elective OAR and EVAR for infrarenal AAA and reported that the AKIN criteria were more predictive of mortality in patients who underwent surgery for AAA.4) The Aneurysm Renal Injury Score (ARISe), which is based on the RIFLE criteria but tailored to aneurysm repair, is another AKI classification system recently advocated by Twine and Boyle.27)
Two groups have studied the incidence of AKI using the KDIGO classification system. Tang et al. reported that the incidence of AKI assessed by the KDIGO classification system in a cohort comprising 314 patients, including 54 patients with ruptured AAA.3) According to this study, the incidence of AKI was 27.1% (70/258) in patients treated with EVAR and 42.8% (24/56) in those treated with OAR.3) Our results showed that the incidence of AKI was 14.1% (64/453) in the OAR group and 3.7% (7/189) in the EVAR group. We consider that excluding patients undergoing urgent surgery might have led to the lower incidence of AKI both in the OAR and EVAR groups. Saratzis et al. studied the incidence of AKI after OAR and EVAR for an unruptured infrarenal AAA using the KDIGO criteria. According to this multicenter study, the incidence of AKI was 17.6% (167/947) in patients treated with EVAR and 17.3% (21/121) in those treated with OAR.8) Though our study and that of Saratzis et al. covered much of similar patient group, the incidence of AKI in our EVAR group was lower. The mean constant material volume of our study was 134.2 mL, which was comparable to that of 121 mL reported by Saratzis et al.8) We consider that the relatively smaller patient volume in our EVAR group might have been related to the lower incidence of AKI. Further large-scale multicenter studies using the KDIGO criteria will be needed to elucidate the severity, incidence, and clinical impact of postoperative AKI following aortic repair for AAA. Using ARISe, Castagno et al. recently reported that the incidence of postoperative AKI was 26.3% (75/285) and 5.5% (8/146) in patients who underwent elective OAR and EVAR, respectively.5) In a large-scale study, Zarkowsky et al. collected data on preoperative and postoperative serum creatinine concentration from the Vascular Quality Initiative database.7) They reported that only 2.9% of the 14,475 non-hemodialysis patients who underwent elective EVAR developed AKI, which was defined as an increase in serum creatinine concentration>0.5 mg/dL, and 0.4% newly required hemodialysis.7) These results were in accordance with our result that EVAR was associated with a lower incidence of postoperative AKI.
In the present study, we performed separate risk factor analyses for the OAR and EVAR groups, although the number of patients with AKI was relatively small in the EVAR group (n=7). With respect to preoperative risk factors, our results showed that eGFR <60 mL/min/1.73 m2, hemoglobin level <10 g/dL, and history of ischemic heart disease were risk factors for AKI in the OAR group. Preoperative renal dysfunction was reported to increase the risk of AKI after elective surgery for AAA.3,5,10) Tang et al. reported that preoperative eGFR >60 mL/min/1.73 m2 was associated with a 0.957-fold decreased risk of AKI in combined patient analysis for both OAR and EVAR.3) Castagno et al. similarly reported that chronic renal disease, defined as a preoperative serum creatinine concentration>1.2 mg/dL, was associated with a 2.53-fold increased risk of AKI in combined patient analysis for both OAR and EVAR.5) Consistent with these reports, our study showed that eGFR <60 mL/min/1.73 m2 was associated with a 2.81-fold increased risk of AKI in patients who underwent OAR. Tang et al. also reported that cardiovascular disease increased the risk of AKI in elective AAA repair,3) which is in accordance with our results. Other reported preoperative risk factors for AKI after elective aortic repair included smoking, hypertension, and arrhythmia.5)
Our study showed that postoperative AKI increased in-hospital mortality and length of hospital stay in patients who underwent elective OAR. Of note, patients without AKI who underwent OAR showed favorable outcomes with no in-hospital mortality (0/389). Prevention of AKI is important, especially in patients treated with OAR. With respect to intraoperative risk factors, our results showed that operation time >300 min and amount of bleeding >1,000 mL were associated with postoperative AKI in OAR and that amount of transfusion>1,000 mL was associated with postoperative AKI in EVAR. Tang et al. similarly reported that amount of intraoperative bleeding >1,000 mL increased the risk of AKI in elective AAA repair.3) To prevent the development of AKI, secure and careful surgical procedures that minimize intraoperative bleeding are needed in both OAR and EVAR. Other reported intraoperative or postoperative risk factors included intraoperative hypotension (<mean arterial pressure of 60 mmHg), postoperative low cardiac index (<2.4 L/min/m2),16) and postoperative vasopressor requirement.7) As reported previously,7,9) contrast material volume was not associated with postoperative AKI following EVAR.
The present study has several limitations. First, this single-center study was retrospectively conducted. A multicenter prospective study will be desirable to elucidate the risk factors for and clinical impact of AKI on aortic repair for AAA. Second, similar with most previous studies on AKI,3–5,7,9,10,13,16,17,23–25) our study did not use urine output criteria, which might have caused misclassification in some patients. Third, information on perioperative medications was not available in this series. However, preoperative medications including angiotensin-converting enzyme inhibitors, beta-blockers, and calcium channel blockers were reported to be unrelated to postoperative AAA in elective AAA repair.5)
Conclusion
Our study showed that the incidence of AKI assessed using the KDIGO criteria was higher in the OAR group, although in-hospital mortality was similar in both groups. The development of AKI was associated with prolonged hospital stay and increased in-hospital mortality in OAR. Preoperative hemoglobin level <10 g/dL, eGFR <60 mL/min/1.73 m2, operation time >300 min, history of ischemic heart disease, and amount of bleeding >1,000 mL were predictors of AKI. In the EVAR group, amount of transfusion>1,000 mL was a predictor of AKI, but AKI did not worsen early outcomes. Knowing these predictors may optimize perioperative patient management and postoperative care.
Disclosure Statement
The authors have no conflict of interest to disclose.
Author Contributions
Study conception: NK
Data collection: TN, MN, NK
Analysis: YS, NK
Investigation: TN, NK
Writing: TN, DH, NK
Funding acquisition: none
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
References
- 1).Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014; 35: 2873-926. [DOI] [PubMed] [Google Scholar]
- 2).Schermerhorn ML, Buck DB, O’Malley AJ, et al. Long-term outcomes of abdominal aortic aneurysm in the Medicare population. N Engl J Med 2015; 373: 328-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Tang Y, Chen J, Huang K, et al. The incidence, risk factors and in-hospital mortality of acute kidney injury in patients after abdominal aortic aneurysm repair surgery. BMC Nephrol 2017; 18: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Bang JY, Lee JB, Yoon Y, et al. Acute kidney injury after infrarenal abdominal aortic aneurysm surgery: a comparison of AKIN and RIFLE criteria for risk prediction. Br J Anaesth 2014; 113: 993-1000. [DOI] [PubMed] [Google Scholar]
- 5).Castagno C, Varetto G, Quaglino S, et al. Acute kidney injury after open and endovascular elective repair for infrarenal abdominal aortic aneurysms. J Vasc Surg 2016; 64: 928-33. e1. [DOI] [PubMed] [Google Scholar]
- 6).Saratzis A, Melas N, Mahmood A, et al. Incidence of acute kidney injury (AKI) after endovascular abdominal aortic aneurysm repair (EVAR) and impact on outcome. Eur J Vasc Endovasc Surg 2015; 49: 534-40. [DOI] [PubMed] [Google Scholar]
- 7).Zarkowsky DS, Hicks CW, Bostock IC, et al. Renal dysfunction and the associated decrease in survival after elective endovascular aneurysm repair. J Vasc Surg 2016; 64: 1278-85. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Saratzis A, Harrison S, Barratt J, et al. Intervention associated acute kidney injury and long-term cardiovascular outcomes. Am J Nephrol 2015; 42: 285-94. [DOI] [PubMed] [Google Scholar]
- 9).Toya N, Ohki T, Momokawa Y, et al. Risk factors for early renal dysfunction following endovascular aortic aneurysm repair and its effect on the postoperative outcome. Surg Today 2016; 46: 1362-9. [DOI] [PubMed] [Google Scholar]
- 10).Wald R, Waikar SS, Liangos O, et al. Acute renal failure after endovascular vs open repair of abdominal aortic aneurysm. J Vasc Surg 2006; 43: 460-6.e2. [DOI] [PubMed] [Google Scholar]
- 11).Pirgakis KM, Makris K, Dalainas I, et al. Urinary cystatin C as an early biomarker of acute kidney injury after open and endovascular abdominal aortic aneurysm repair. Ann Vasc Surg 2014; 28: 1649-58. [DOI] [PubMed] [Google Scholar]
- 12).Saratzis AN, Goodyear S, Sur H, et al. Acute kidney injury after endovascular repair of abdominal aortic aneurysm. J Endovasc Ther 2013; 20: 315-30. [DOI] [PubMed] [Google Scholar]
- 13).Ambler GK, Coughlin PA, Hayes PD, et al. Incidence and outcomes of severe renal impairment following ruptured abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 2015; 50: 443-9. [DOI] [PubMed] [Google Scholar]
- 14).Aziz F, Azab A, Schaefer E, et al. Endovascular repair of ruptured abdominal aortic aneurysm is associated with lower incidence of post-operative acute renal failure. Ann Vasc Surg 2016; 35: 147-55. [DOI] [PubMed] [Google Scholar]
- 15).Siemiatkowski A, Jablonowska A, Pietrewicz J, et al. Estimation of V-POSSUM and E-PASS scores in prediction of acute kidney injury in patients after elective open abdominal aortic aneurysm surgery. Ann Vasc Surg 2017; 42: 189-97. [DOI] [PubMed] [Google Scholar]
- 16).Tallgren M, Niemi T, Poyhia R, et al. Acute renal injury and dysfunction following elective abdominal aortic surgery. Eur J Vasc Endovasc Surg 2007; 33: 550-5. [DOI] [PubMed] [Google Scholar]
- 17).Statius van Eps RG, Nemeth B, Mairuhu RTA, et al. Determinants of acute kidney injury and renal function decline after endovascular abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 2017; 54: 712-20. [DOI] [PubMed] [Google Scholar]
- 18).Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8: R204-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11: R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120: c179-84. [DOI] [PubMed] [Google Scholar]
- 21).Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982-92. [DOI] [PubMed] [Google Scholar]
- 22).Sasabuchi Y, Kimura N, Shiotsuka J, et al. Long-term survival in patients with acute kidney injury after acute type A aortic dissection repair. Ann Thorac Surg 2016; 102: 2003-9. [DOI] [PubMed] [Google Scholar]
- 23).van Beek SC, Legemate DA, Vahl A, et al. Acute kidney injury defined according to the ‘Risk,’ ‘Injury,’ ‘Failure,’ ‘Loss,’ and ‘End-stage’ (RIFLE) criteria after repair for a ruptured abdominal aortic aneurysm. J Vasc Surg 2014; 60: 1159-67.e1. [DOI] [PubMed] [Google Scholar]
- 24).Kopolovic I, Simmonds K, Duggan S, et al. Risk factors and outcomes associated with acute kidney injury following ruptured abdominal aortic aneurysm. BMC Nephrol 2013; 14: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Sugimoto M, Takahashi N, Niimi K, et al. Long-term fate of renal function after open surgery for juxtarenal and pararenal aortic aneurysm. J Vasc Surg 2017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26).Wartman SM, Woo K, Yaeger A, et al. Outcomes after abdominal aortic aneurysm repair requiring a suprarenal cross-clamp. J Vasc Surg 2014; 60: 893-9. [DOI] [PubMed] [Google Scholar]
- 27).Twine CP, Boyle JR. Renal dysfunction after EVAR: time for a standard definition. J Endovasc Ther 2013; 20: 331-3. [DOI] [PubMed] [Google Scholar]