Abstract
Background:
One potential treatment strategy to enhance axon regeneration is transplanting Schwann Cells (SCs) that overexpress glial cell line-derived neurotrophic factor (GDNF). Unfortunately, constitutive GDNF overexpression in vivo can result in failure of regenerating axons to extend beyond the GDNF source, a phenomenon termed the “candy-store” effect. Little is known about the mechanism of this axon entrapment in vivo.
New Method:
We present a reproducible in vitro culture platform using a microfluidic device to model axon entrapment and investigate mechanisms by which GDNF causes axon entrapment. The device is comprised of three culture chambers connected by two sets of microchannels, which prevent cell soma from moving between chambers but allow neurites to grow between chambers. Neurons from dorsal root ganglia were seeded in one end chamber while the effect of different conditions in the other two chambers was used to study neurite entrapment.
Results:
The results showed that GDNF-overexpressing SCs (G-SCs) can induce axon entrapment in vitro. We also found that while physiological levels of GDNF (100 ng/mL) promoted neurite extension, supra-physiological levels of GDNF (700 ng/mL) induced axon entrapment.
Comparison with Existing Method:
All previous work related to the “candy-store” effect were done in vivo. Here, we report the first in vitro platform that can recapitulate the axonal entrapment and investigate the mechanism of the phenomenon.
Conclusions:
This platform facilitates investigation of the “candy-store” effect and shows the effects of high GDNF concentrations on neurite outgrowth.
Keywords: peripheral nerve injury, Schwann cells, neurite extension, gene therapy
Introduction
Severe peripheral nerve injury (PNI) can have a devastating impact on patients’ quality of life resulting in lifelong disabilities. Autologous nerve grafting has been the standard of care for PNI, despite its major disadvantage of donor site morbidity(Lundborg, 2000). Alternative methods, such as acellular nerve grafts, are ineffective for large gaps because of the lack of cells and regenerative factors within the graft(Fox et al., 2005). Even with proper surgical reconstruction, functional recovery can be incomplete(Moore et al., 2009). Although the peripheral nerve is capable of regeneration, the rate of axonal regeneration is slow at 1–2 mm/day(Grinsell and Keating, 2014). Patients suffering from proximal injuries, such as brachial plexus injuries, which can involve regeneration distances of up to a meter, may require 2–3 years for axons to reach target muscles(Lundborg, 2000). However, chronic denervation can result in quiescence of Schwann cells (SCs), which leads to their precipitous decline in growth factor expression and a progressive failure to promote axonal regeneration over time(Fox et al., 2005). In addition, essentially irreversible changes to muscle occur by 12–18 months(Kobayashi et al., 1997), at which point functional recovery is unlikely. To treat these patients, it is critical to devise strategies to enhance axonal growth and prevent chronic denervation of SCs to promote robust axon regeneration over long distances. One appealing strategy to improve recovery is to use exogenous growth factors and/or SCs with exogenous growth factor expression to enhance axonal regeneration.
Glial cell line-derived neurotrophic factor (GDNF) is one of these potent growth factors involved in the normal regenerative process after injury. GDNF has been shown to improve axonal regeneration in motor neurons(Scheib and Höke, 2013) and a subset of sensory neurons(Wu-Fienberg et al., 2014). In vitro studies have shown that GDNF forms a complex with GDNF family receptor alpha 1 (GFRα1) and acts as an axonal guidance signal during regeneration(Blits et al., 2004; Lundborg, 2003; Marquardt et al., 2015; Taylor et al., 2008). Although SCs intrinsically secrete GDNF following injury, they are unable to maintain this trophic support for neurons over long periods of time after injury(Höke et al., 2002). In rodents, injury-induced GDNF expression is insufficient to stimulate axonal regeneration after 2 months and decreases to baseline level by 6 months(Höke et al., 2002). Therefore, one viable strategy to increase the regeneration window is to develop effective delivery systems for GDNF over a long period of time.
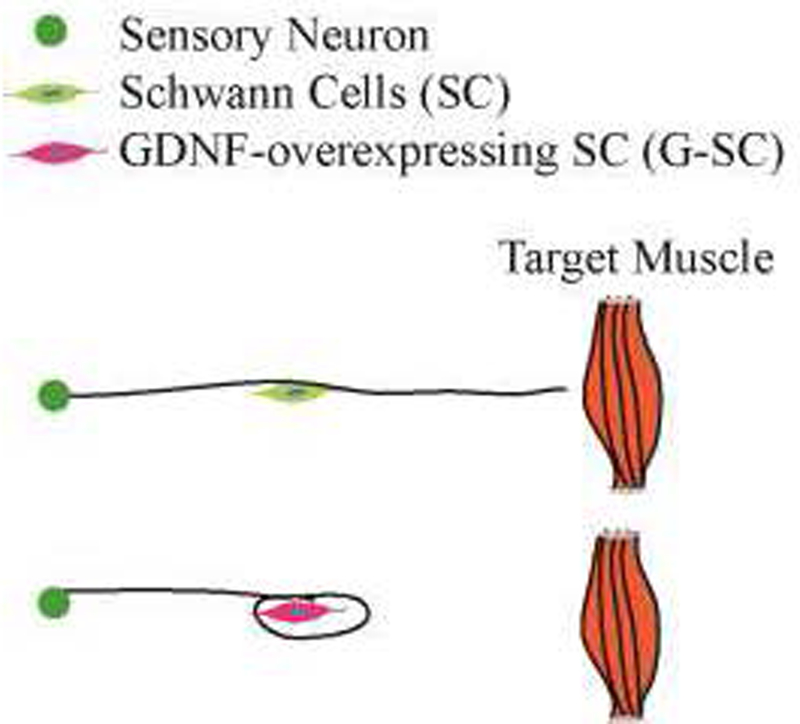
Various methods of delivery have been developed including protein delivery systems, gene therapy, and cell therapy. In particular, the use of adeno-associated and lenti-viral vectors that encode GDNF have been used in many studies. Our lab and others have previously studied lentiviral mediated overexpression of GDNF from SCs (GDNF-overexpression SCs, or G-SCs)(Blits et al., 2004; Eggers et al., 2013; Marquardt et al., 2015; Wu-Fienberg et al., 2014). However, excess GDNF production causes the “candy-store” effect, where axons do not extend beyond sources of GDNF expression and are entrapped at a site rich in growth factor(Blits et al., 2004; Eggers et al., 2013; Tannemaat et al., 2008) (Fig. 1). Axonal swirling and coils form at these GDNF sources and are composed of a large number of motor axons and densely packed SCs(Eggers et al., 2013; Tannemaat et al., 2008). Inside the axon coils, myelination is severely impaired as shown by a decrease in myelin basic protein expression(Eggers et al., 2013). These negative side effects create a major barrier against using GDNF to promote axonal regeneration.
Figure 1: Schematics of the Candy-Store Effect.
Axons normal grow past Schwann cells (SC) and reach towards target muscles (Top). When we constitutively overexpress GDNF in SCs (GDNF-SC), axons fail to extend beyond the GDNF source (Bottom), causing the Candy-store effect.
Understanding the “candy-store” effect is important for effectively using GDNF to promote axonal regeneration and reduce its negative impact. Studying the “candy-store” effect in vivo is challenging given the involvement of multiple cell types and the complex peripheral nerve environment. In contrast, in vitro investigation will enable us to conduct controlled studies of individual factors. However, standard in vitro culture platforms cannot spatially separate neurons from extending neurites for clear visualization. Alternatively, microfluidic devices have been extensively used to model nerve injury(Cohen et al., 2011; Marquardt and Sakiyama-Elbert, 2015; Park et al., 2006; Siddique and Thakor, 2014; Taylor et al., 2005), with the major advantage of easy fabrication of the topographical structures of the culture platform. Namely, microfluidic devices can have features on the micrometer scale, which is ideal for isolating neurite extensions from neuronal soma. Therefore, developing a microfluidic device for recapitulating axon entrapment will allow for investigating the mechanism of the “candy-store” effect.
In this study, we developed a microfluidic device to recapitulate the “candy-store” effect in vitro and examined the effects of G-SCs on neuronal axon entrapment. Neurite extension was quantified in neuron-SCs (normal SCs) and neuron-G-SCs (GDNF overexpressing) co-cultures. We also investigated the effects of different GDNF concentrations on axon entrapment. G-SCs resulted in significantly greater trapping compared to normal SCs indicating that G-SCs can entrap neurites in vitro. In addition, high GDNF concentrations led to a similar decrease in neurite extension suggesting that high GDNF levels alone are sufficient to entrap axons.
Materials and Methods
Fabrication of Microfluidic Device Master Mold:
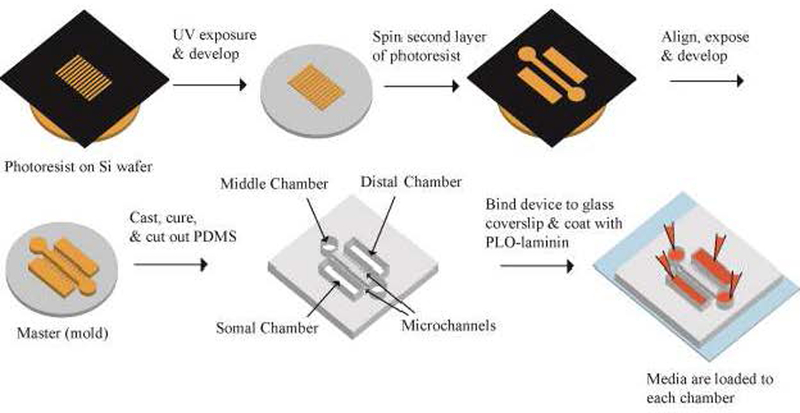
3-inch silicon wafers (Silicon Inc. ID, USA) were coated with SU-8 5 photoresists (MicroChem Corp, MA, USA) using a spin-coater. The wafers were then exposed under UV light under (or through) the first layer of photo mask that had the pattern for the microchannels (Fig. 2). After development, the wafers were coated with a layer of SU-8 2050 and exposed under the second photo mask, which contained the pattern for the cell culture chambers. After development, the wafers were exposed to trichloro(1,1,2,2-perfluorooctyl) silane (Millipore Sigma, MO, USA) under vacuum for 1 hour to form an antiadhesive layer.
Figure 2: Schematics of Microfludic Device Fabrication Process.
The master mold for the device consist of two layers of photoresist structures. The first layer defines the microchannels at 5 μm height. The second layer defines the culture chambers at 100 μm height. Soft lithography is used to make PDMS device, which is then assembled with glass coverslip as bottom and coated with PLO-laminin. The three chambers are connected by two sets of microchannels. The middle chamber is accessed through the two circular reservoirs.
Soft Lithography and Microfluidic Device:
Polydimethylsiloxane (PDMS, Sylgard 184, Dow Corning, MI, USA) was poured onto the silicon master mold in a petri dish with 1:10 weight ratio of curing agent to base and put under vacuum to remove air bubbles (Fig. 2). The PDMS was cured at 60°C overnight. The device was then cut out and bonded to a glass slide using a plasma cleaner (Plasma Clean PDC-001, Harrick Plasma Inc., NY, USA). After 20 minutes of UV sterilization, the completed device was coated with 0.01% poly-L-ornithine (PLO) for 1 hour at 37°C and laminin (1 mg/mL, Life Technologies, CA. USA) for 1 hour at 37°C.
FITC Conjugation and Protein Transport Quantifications:
Fluorescein isothiocyanate (FITC) conjugation was performed as directed by the manufacturer (Sigma). Porcine pepsin (35 kD, Sigma) was used as an alternative to GDNF (30.4 kD) for protein labeling because of their similar sizes. After completion of the conjugation, the FITC-labeled protein was in PBS solution with 1% bovine serum albumin (Sigma). Various volumes of FITC-labeled protein (1 μg/mL) in solution were then applied to the distal chamber with the expectation that higher volumes would increase protein transport to the somal chamber. After 24 hrs, the liquid from the somal chamber was collected and the fluorescence was measured at 488nm using a fluorescence spectrophotometer (SpectraMax M2e, Molecular Devices, CA, USA). The percent fluorescence was calculated as a percentage of somal chamber fluorescence intensity over distal chamber fluorescence intensity.
Isolation of Sensory Neurons and Culture Conditions:
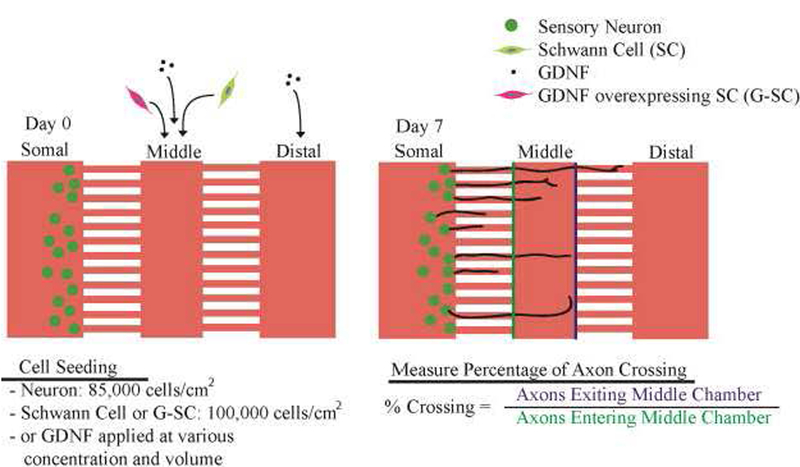
Sensory neurons were harvested from White Leghorn chicken (Texas A&M University, Texas, USA) embryos at Day 7–9. Dorsal root ganglia were isolated from embryos and dissociated using 0.25% trypsin-EDTA (Invitrogen, CA, USA) for 20 minutes at 37°C. Dissociated neurons were seeded in the somal chamber at 85,000 cells/cm2 and cultured in neurobasal medium with 2% B27 supplement, 1% penicillin-streptomycin (Pen/Strep), and 1% GlutaMAX (Invitrogen) (Fig. 3). Glial cell-line derived neurotrophic factor (GDNF, PeproTech, NJ, USA) was applied to the middle and/or distal chamber. Medium in the somal chamber was replaced with fresh medium every two days. Medium in the middle and distal chamber was also replaced every two days to maintain the GDNF level. By replacing media every two days in all chambers, the volumes of each chamber was maintained.
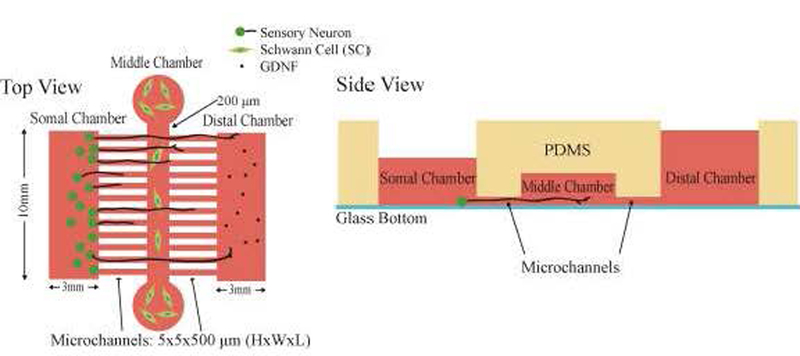
Figure 3: Schematics of the microfluidic device.
A) Top and B) side views of the microfluidic device. Both Somal and Distal chambers have a surface of 10 mm×3 mm (schematics are not drawn to scale). Middle chamber reservoirs are 5mm in diameter and the chamber dimensions are 100 mm × 200 μm × 100 μm (L×W×H). The two sets of microchannels dimensions are 5 × 5 × 500 (L×W×H) μm. Side view shows different volumes applied to each chamber. The volume applied to the middle chamber is the volume applied to the two circular reservoirs.
Schwann Cell Harvest and Culture:
SCs were harvested from sciatic nerves collected from adult male Lewis rats using previously described methods(Tao, 2013). To isolate the SCs, first nerve branches were placed in DMEM (Dulbecco’s Modified Eagle Medium) with sodium pyruvate (110 mg/L, Invitrogen), Collagenase (10 mg/mL, Thermo Fisher), and 0.25% trypsin for 30 min at 37 °C. After triturating the branches, they were centrifuged for 10 min at 500×g, and the cell pellet was washed with SC medium containing DMEM plus 10% fetal bovine serum (FBS, Life Technologies) and 1% Pen/Strep. The cells were then plated into a 100 mm petri dish coated with 0.1% poly-L-lysine (Sigma) and laminin. Cytarabine (Ara-C, 10 μM, Sigma) was added to the plate along with fresh SC medium on Day 3 after plating. Then the plate was supplemented with 2 μM forskolin (BioGems, CA, USA) with fresh SC medium and cultured for 1–2 weeks before use. G-SCs were generated based on a protocol previously developed (Wu-Fienberg et al., 2014). Briefly, SC cultures were transduced with lentiviral particles containing GDNF transfer plasmid before passage 7. Cells were incubated with 2 μg/mL of polybrene (Santa Cruz Biotechnology, TX, USA) in SC medium for 1 hour at 37°C, after which the lentiviral vectors containing GDNF are introduced (MOI = 35). Cells are then incubated with 2 μg/mL polybrene and vector for 20 hours at 37°C. G-SCs were tested using enzyme-linked immune-sorbent assay (ELISA, R&D Systems, MN, USA) for GDNF production according to previously published results (Wu-Fienberg et al., 2014). G-SCs were seeded in the middle chamber at 100,000 cells/cm2. G-SCs produced 106.2±6.8 ng/mL/day of GDNF in the middle chamber. Based on this GDNF production, approximately 744 ng/mL of GDNF was produced over 7 days. Therefore, we used 700 ng/mL of GDNF as the supra-physiological level to be applied in the middle chamber.
Immunocytochemistry and Data Quantification:
Immunocytochemistry (ICC) was performed on Day 6 after the start of neuronal culture. Briefly, the cells in the device were fixed using 4% paraformaldehyde (Sigma) for 20 min. After washing 3 times with PBS, the cells were permeabilized with 0.01% Triton X-100 (Sigma) for 20 min. The cells in the device were then blocked using 5% normal goat serum (NGS, Sigma) in PBS for 1 hr. Primary antibodies used included anti-neurofilament (1:200 with 2% NGS in PBS, clone 3A10, Developmental Studies Hybridoma Bank, IA, USA) for neurites and anti-S100β (1:400 with 2% NGS in PBS, Polyclonal Rabbit Ant-S100, Dako, CA, USA) for SCs. After incubating with primary antibodies overnight, the device was washed 3 times with PBS and then secondary antibodies were applied for 2 hrs. Hoechst 33342 was applied (1:1000 dilution) for 20 min after washing the device with PBS. The device was then imaged using a wide-field fluorescence microscope (Leica DMi8, Leica, IL, USA). After acquiring images of the middle chamber and microchannels, neurites were quantified as a percentage of axons crossing, namely, the number of neurites exiting the middle chamber was divided by the number of neurites entering the middle chamber (Fig. 5). Overlapping neurites in one microchannel are accounted for by tracing them for a short distance after exiting the microchannel, at which point the neurites disperse (typically 3–4 neurites, data not shown).
Figure 5: Schematics of culturing conditions.
Neurons are seeded on Day 0 and stained on Day 7 using immunocytochemistry (ICC), at which point the percentage of axons crossing is quantified. Different Schwann cells or GDNF concentrations are applied to the middle chamber and distal chamber (Table 1).
Statistical Analysis:
One-way ANOVA is performed on the sample groups using Minitab software. Scheffe’s post-hoc test with p<0.05 for significance was used. Data represented were shown as mean ± standard deviation. Data included N = 8 or more devices per condition; n=30–518 neurites measured per device.
Results
Microfluidic device development
One of the key features in the design of this microfluidic device was that it should enable cell signaling through exogenous growth factors and/or cell-cell interactions. Therefore, it was important to test protein transport from distal chamber to somal chamber in the microfluidic device to ensure that factors in the distal chamber could elicit a response in neurons separated by microchannels. We also sought to determine whether a growth factor in the distal chamber could provide axon guidance and/or promote survival of neurons seeded in the other chamber. We used pepsin (35 kDa), a similarly sized protein to GDNF (30.4 kDa), conjugated with fluorescein isothiocyanate (FITC) to examine the transport of proteins between the distal and somal chamber. FITC-pepsin was applied to the distal chamber and the fluorescence intensity in the somal chamber was measured over time. This allowed an estimation for the amount of GDNF that will reach the somal chamber for neurons to detect, and thus was used to determine the initial GDNF concentration that should be applied to the distal chamber to obtain a physiologically relevant level in the somal chamber.
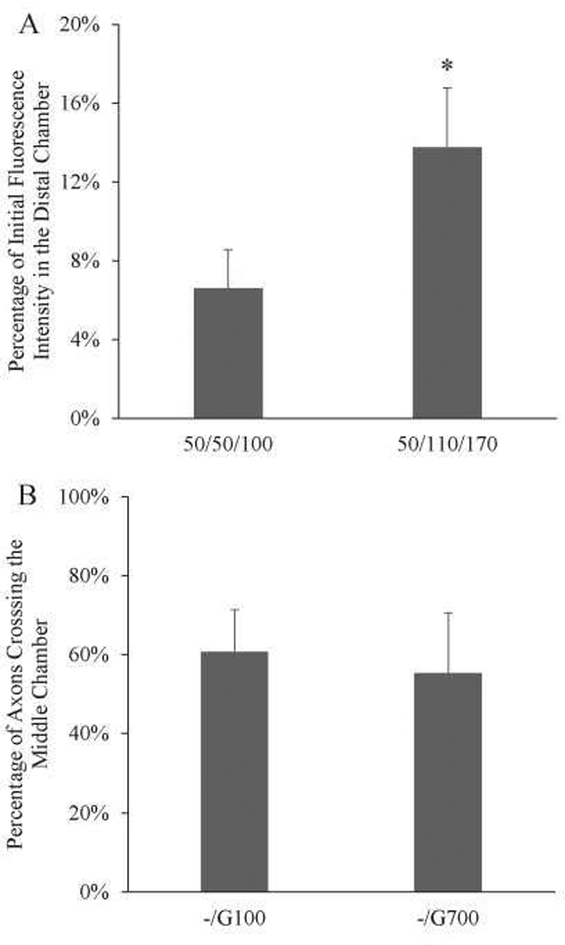
We tested whether protein transport can be controlled by modulating the volume in each chamber (and thus the pressure differential) such that fluid was only flowing unidirectionally from the distal to the somal chamber. Two different volume sets were applied to the device. The first set of conditions tested had 50 μL of liquid in the somal and the middle chamber, and 100 μL of liquid in the distal chamber (Fig. 4A, 50/50/100). After 24 hours, the fluorescence intensity in the somal chamber was 6.6±2.0% of the initial fluorescence intensity in the distal chamber. The second set of conditions had 110 μL of liquid in the middle chamber, and 170 μL in the distal chamber. The fluorescence intensity increased to 13.8±3.0% (Fig. 4A, 50/110/170). This demonstrated that protein concentration in the somal chamber can be controlled by varying the volume applied to the other two chambers.
Figure 4: Microfluidic device shows controlled protein transport and robust axon crossing.
A) Varying the loading volume in each chamber changes the amount of protein transported. 50/50/110 correspond to the volumes in somal/middle/distal chamber in μL. With a volume increase in the distal and middle chamber, the amount of fluorescence increased more than 2-fold (6.6% to 13.8%). *: p<0.05 N=4. B) Quantification of axons crossing the middle chamber with different conditions. Both conditions showed robust axons crossing (-/G100 at 64.6%±10.7%, -/G700 at 55.3%±15.2%). N = 8.
To test whether high GDNF concentrations can cause axon entrapment in vitro, we wanted to first establish a positive control that allows neurites to robustly cross the middle chamber. Table 1 shows the conditions applied to the middle and distal chambers. Either no GDNF, 100ng/mL, or 700ng/mL of GDNF was added to the respective chambers. No growth factor addition resulted in low neurite outgrowth into the middle chamber (<30 neurites/device). Even though the percentage of axon crossing was low under this condition, it did not represent the axonal entrapment effect but likely a result of poor neurite extension in the first place. We found that with 100 ng/mL and 700 ng/mL of GDNF in the distal chamber and 0 ng/mL in the middle and somal chamber, neurites were able to robustly grow across the middle chamber to reach the second set of microchannels (Fig. 4B). Both conditions showed at least 50% of axons crossing with an average of 261 neurites measured in each device. These two conditions were used positive controls to compare with potential axon trapping conditions.
Table 1.
List of conditions applied to the middle and distal chambers
| Middle Chamber | Distal Chamber | ||
|---|---|---|---|
| Schwaim Cells | None | ||
| GDNF-Overexpressing Schwann Cells | None | ||
| Control (No GDNF) | None | GDNF (100ng/mL) | GDNF (700ng/mL) |
| GDNF (100 ng/ML) | None | GDNF (100ng/mL) | GDNF (700ng/mL) |
| GDNF (700 ng/ML) | None | GDNF (100ng/mL) | GDNF (700ng/mL) |
GDNF-overexpressing Schwann cells show axon entrapment in the microfluidic device
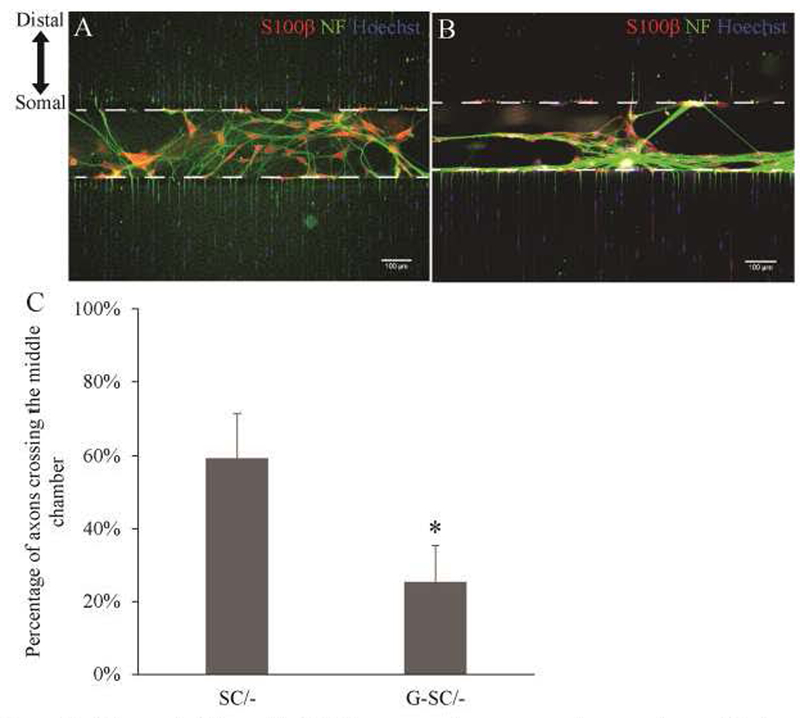
To mimic the in vivo axon entrapment in the microfluidic device, we evaluated the effects of placing G-SCs in the middle chamber, on neurite outgrowth compared to normal Schwan cells (Table 1). Briefly, we seeded DRG neurons in the somal chamber and normal SCs or G-SCs in the middle chamber (Fig. 5). We found that seeding normal SCs in the middle chamber resulted in robust axon growth resulting 59.2% axons crossing (Fig. 6C, SC/-), comparable to 100 ng/mL of GDNF in the distal chamber (Fig. 4B). In contrast, seeding G-SCs in the middle chamber significantly reduced the percentage of axons crossing to the distal chamber to 25.3% (Fig. 6C, G-SC/-). These results demonstrated that G-SCs can induce axon entrapment in vitro. It was also noted that neurites with G-SCs seeded in the middle chamber formed a dense network of fibers along the G-SCs (Fig. 6B), while neurites with normal SCs do not demonstrate this behavior (Fig. 6A). This resembled morphologies observed in previous in vivo studies where axons tend to form nerve coils at the sites of GDNF overexpression(Blits et al., 2004; Eggers et al., 2013).
Figure 6: GDNF-Overexpressing Schwann Cells causes axonal trapping in vitro.
A-B) Normal Schwann Cells (A) and G-SCs (B) seeded in the middle chamber. C) Quantification of axons crossing. SC results in 59.2±12.3%. G-SC results in 25.3±9.9%. N=8. *: p<0.00005
Effects of differential GDNF concentrations on neurite extensions
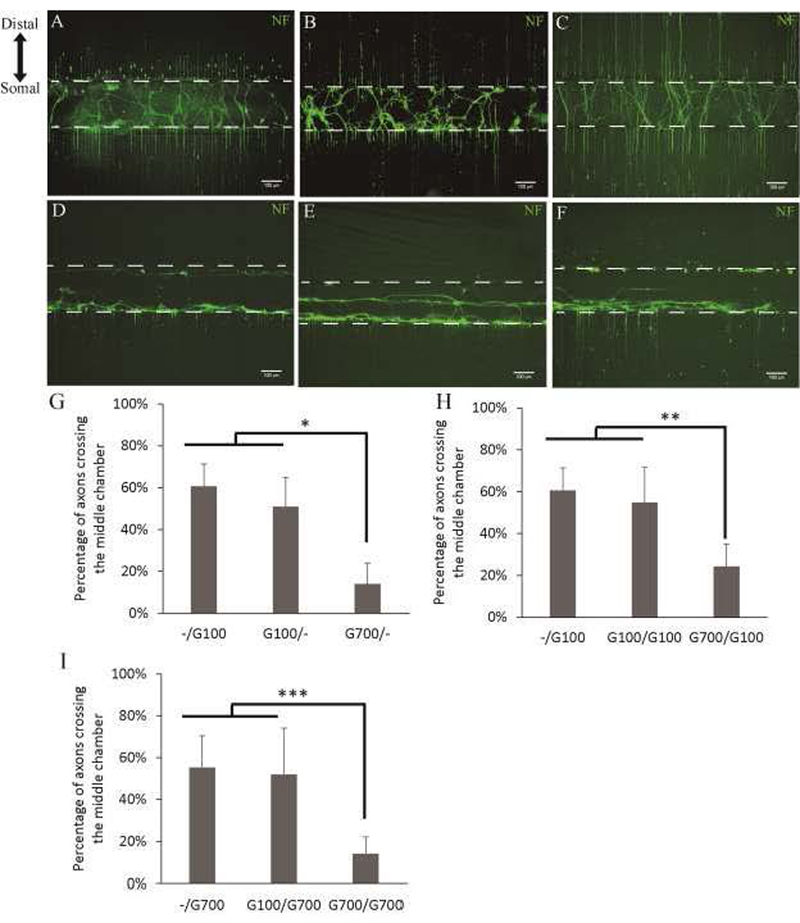
We wanted to examine the effects of different concentrations of GDNF in the middle and distal chambers (Table 1). Previous in vitro studies had shown that physiological levels of GDNF (100 ng/mL) improve neurite outgrowth(Marquardt and Sakiyama-Elbert, 2015). In addition, applying 100 ng/mL of GDNF to the distal chamber also promoted robust neurite outgrowth towards the distal chamber. Based on the GDNF production from G-SCs, approximately 744 ng/mL of GDNF was produced over 7 days. Therefore, we used 700 ng/mL of GDNF as the supra-physiological level. GDNF uptake by neurons was qualitatively assessed by ICC staining of GDNF. Cell treated with 700ng/mL of GDNF showed greater levels of antibody staining compared to untreated cells and cell treated with 100ng/mL of GDNF (Sup Fig.1). We found that supra-physiological levels of GDNF in the middle chamber significantly reduced the percentage of axons crossing the middle chamber (Fig. 7G). Applying 100 ng/mL of GDNF to the middle chamber with 700 ng/mL in the distal chamber did not reduce the percentage of axons crossing comparing to 100 ng/mL in the distal chamber and 0 ng/mL in the middle chamber (Fig. 7G, -/G100, G100/-). However, 700 ng/mL of GDNF in the middle chamber and 0 ng/mL in the distal chamber significantly reduced the percentage of axons crossing to 14.0% (Fig. 7D & G, G700/-). This suggests supra-physiological levels of GDNF can induce axon trapping in vitro. Immunostaining for neurofilament showed that neurites tend to stay in the middle chamber; however, we did not observe the formation of dense fibers networks observed with G-SCs. We also examined the effects of GDNF at 250 ng/mL and 500 ng/mL applied in the middle chamber and 0 ng/mL in the distal chamber (G250/-, G500/-). G250/- showed axons crossing at 40.6%±12.4%; while G500/- showed axons crossing at 45.8%±15.3%. This indicates the threshold concentration for GDNF to induce axon entrapment appears to be between 500 ng/mL and 700 ng/mL.
Figure 7: Effects of differential GDNF concentrations on neurite extensions.
A-C) Low (100ng/mL) GDNF applied to the middle chamber with varying concentrations in the distal chamber. A) G100/- at 50.9±14.0%; B) G100/G100 at 54.8±17.0%; C) G100/G700 at 51.8±22.2%. D-F) High (700ng/mL) GDNF applied to the middle chamber with varying concentrations in the distal chamber. D) G700/- at 14.0±10.0%; E) G700/G100 at 24.3±10.7%; F) G700/G700 at 14.1±8.1%. G-I) Quantification of axons crossing with varying concentrations of GDNF applied to the middle chamber and distal chamber. N=8. *: p<0.00005, **: p<0.05, ***: p<0.005.
Next, we wanted to see whether adding GDNF to the distal chamber can overcome the axon trapping induced by applying high levels GDNF to the middle chamber (Fig. 7H). When 100 ng/mL of GDNF was in both middle and distal chambers, 54.8% of axons were able to cross the middle chamber, similar the result observed with the same concentration of GDNF added to either just the distal or just the middle chamber (Fig. 7B, G100/G100). This suggests that axons were not trapped by a uniform distribution of GDNF at a physiological concentration. In contrast, applying supra-physiological levels of GDNF in the middle chamber and physiological GDNF levels to the distal chamber reduced the percentage of axons crossing to 24.3% (Fig. 7E & H, G700/G100). Therefore, physiological GDNF levels at a more distal site to the neurons cannot overcome axon trapping induced by high GDNF levels at a more proximal site. More importantly, when supra-physiological GDNF is applied to both the middle and distal chambers, only 14.1% of axons were able to cross the middle chamber (Fig. 7F & I, G700/G700), suggesting that high GDNF levels also cannot abolish axon trapping. Together, these data suggest that supra-physiological levels of GDNF have a direct effect on reducing neurite outgrowth.
Discussion
A number of studies have focused on the use of exogenous growth factors for treating peripheral nerve injuries. Growth factors, such as GDNF, are released by SCs in response to nerve injury and aid neuronal survival and axon regeneration(Springer et al., 1995). Many studies have shown that GDNF also acts as a directional cue for guidance of regenerating axons (Batchelor, 2002; Lin et al., 2011). In addition to its direct benefits, GDNF also contributes to the ability of SCs to promote repair after injury. The presence of GDNF enhances the ability of SCs to myelinate axons by inducing differentiation of a myelinating phenotype(Höke et al., 2003; Iwase et al., 2005; Jesuraj et al., 2014). As a result of their growth promoting benefits, various methods for the delivery of growth factors have been examined including using lentiviral(Tannemaat et al., 2008, 2007) or adenoviral injections(Kells et al., 2004), microspheres(Garbayo et al., 2009; Wood et al., 2012), and scaffolds and conduits(Madduri et al., 2010). Yet, there still remains much to be understood about the effects of growth factors on axon regeneration.
Unlike other growth factors(Albers et al., 1996, 1994; Tolwani et al., 2004), GDNF when overexpressed can cause axon entrapment within the zone of GDNF expression, and is thus dubbed the “candy-store” effect(Blits et al., 2004; Ee et al., 2017; Eggers et al., 2013; Shakhbazau et al., 2013; Tannemaat et al., 2008). This behavior was first shown by Blits et al., where adenoviral-mediated overexpression of GDNF near the motoneuron pool promoted axon sprouting but prevented directional growth of axons at denervated motoneuron pool. We and others have also observed a similar phenomenon when overexpressing GDNF after sciatic nerve injury with either viral expression or transgenic expression in SCs(Eggers et al., 2013; Marquardt et al., 2015). In order to resolve this issue, Eggers et al. generated a GDNF gradient in vivo using lentiviral vectors and found that even the lowest concentration of GDNF still entrapped axons. We and others have used tetracycline-inducible systems to temporally control GDNF overexpression and identified a critical window of time at which GDNF overexpression should be shut off (Marquardt et al., 2015; Shakhbazau et al., 2013). However, little is known about the mechanism of the “candy-store” effect and whether it acts directly on axons or indirectly via SCs. Evidence suggests SCs undergo phenotypic changes in the presence of high levels of GDNF that could affect axon growth(Eggers et al., 2013). A recent study also shows G-SCs affect fibroblasts, and this interaction results in significant ECM remodeling (Ee et al., 2017). However, all these studies have been done using in vivo models. The complexity of the in vivo environment presents a major challenge to eliciting the exact mechanism that underlies the “candy-store” effect. Here we developed a microfluidic device that enables us to conduct controlled studies of individual factors and provide spatial separation of neurons from extending neurites for clear visualization. This in vitro culture platform can help us understand why GDNF overexpression leads to the “candy-store” effect.
Previous studies have shown that G-SCs cause axon entrapment when transplanted in acellular nerve graft in sciatic nerve injury models(Marquardt et al., 2015). Similarly, our results show that G-SCs cause axon entrapment in the microfluidic device. This demonstrates that our in vitro platform is able to mimic the in vivo phenomenon. Previous studies have shown that GDNF overexpression causes SCs to undergo phenotypic changes marked by a decrease in myelin basic protein and an increase in p75 receptor expression(Eggers et al., 2013). In this study, we observed that neurites form dense fiber networks along G-SCs, whereas these dense fibers were not observed in the presence of normal SCs (Fig. 6A & B). This dense accumulation of axons is strikingly similar to previous in vivo results where elevated GDNF expression from virally-induced cells resulted in thick fiber coils formed at GDNF overexpressing locations(Blits et al., 2004; Ee et al., 2017; Eggers et al., 2013). Our recent study showed that GDNF overexpression during nerve regeneration in vivo caused extensive axonal sprouting (Ee et al., 2017). The evidence from these studies and our current studies suggests G-SCs play a major role in causing axon entrapment. Specifically, these cells can prevent neurites from extending beyond the region of elevated GDNF and induce the formation of fiber networks. GDNF gradients can guide axon regeneration from lower concentrations to higher concentrations and eventually lead to muscle cells as the final target in the peripheral nervous system. It has been shown that viral vector-induced GDNF expression in myoblasts improved motor neuron survival (Mohajeri et al., 1999). Making myoblast as a source of neurotrophic factor would be particularly useful when nerves were close enough to the muscles to create a gradient that the axons could sense. Therefore, it might also be valuable to explore using myoblast as the source of neurotrophic factor for future studies of regeneration near the muscle.
In addition to investigating G-SC’s effects on axon entrapment, we examined how exogenous GDNF affects neurite outgrowth in a microfluidic device. Previous studies have shown that GDNF applied at physiological levels (50 to 100 ng/mL) promoted axon growth (Jesuraj et al., 2014; Marquardt and Sakiyama-Elbert, 2015). In this study, our results indicated that GDNF applied at a supra-physiological level (700 ng/ml) decreased neurite outgrowth. This suggests that a high GDNF level may be sufficient to trap axons. It is interesting to note that high GDNF levels in the distal chamber did not make up for axon entrapment caused by high GDNF in the middle chamber, suggesting axon entrapment is activated when GDNF reaches a certain threshold concentration and cannot be overcome by similarly high levels downstream. We also observed that neurites treated with high GDNF protein levels did not show dense fiber network formation. Compared to trapping caused by G-SCs, this might suggest the important roles of G-SC and ECM remodeling in axon entrapment and that the “candy-store” effect constitutes two parts, where axon trapping is caused by GDNF directly while fiber networks form after ECM remodeling and may involve SCs.
Understanding both the benefits and negative impacts of using growth factors is critical developing safe therapeutics. Here, we have developed a microfluidic platform that allowed us to investigate a major negative side effect of overexpressing GDNF from SCs. We have shown that G-SCs can induce axon entrapment similar to what is seen in vivo. In addition, this system allowed us to examine the effect of exogenous GDNF on neurite outgrowth. We have shown that high levels of GDNF alone can significantly reduce neurite outgrowth due to entrapment. Overall, this platform may provide a valuable tool to further investigate the underlying molecular mechanisms of the “candy-store” effect. Together, these findings may prove valuable as they provide directions toward devising drug delivery methods that harness only the beneficial effects of GDNF to establish a robust treatment system for PNI in animal models. Clinically, this project has the potential to lead to therapies that prolong the regeneration window for long gap PNI.
Supplementary Material
Highlights.
Microfluidic device recapitulates the axon entrapment in the “candy-store” effect.
GDNF-overexpressing SCs induce axon entrapment in the device as seen in vivo.
High concentration of GDNF is sufficient to induce axon entrapment.
Acknowledgments
This work was supported by the National Institutes of Health [RO1NS051706]. This work was performed in part at the Nano Research Facility at Washington University in St. Louis and Nano and Molecular Science Lab at University of Texas at Austin. The authors thank Daniel Hunter for the histological micrographs.
Abbreviations:
- GDNF
glial cell line-derived neurotrophic factor
- G-SC
GDNF-overexpressing Schwann Cells
- PNI
peripheral nerve injury
- SC
Schwann cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers KM, Perrone TN, Goodness TP, Jones ME, Green MA, Davis BM, 1996. Cutaneous overexpression of NT-3 increases sensory and sympathetic neuron number and enhances touch dome and hair follicle innervation. J. Cell Biol 134, 487–497. 10.1083/jcb.134.2.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers KM, Wright DE, Davis BM, 1994. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J. Neurosci 14, 1422–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor P, 2002. Macrophages and Microglia Produce Local Trophic Gradients That Stimulate Axonal Sprouting Toward but Not beyond the Wound Edge. Mol. Cell. Neurosci 21, 436–453. 10.1006/mcne.2002.1185 [DOI] [PubMed] [Google Scholar]
- Blits B, Carlstedt TP, Ruitenberg MJ, De Winter F, Hermens WTJMC, Dijkhuizen PA, Claasens JWC, Eggers R, Van Der Sluis R, Tenenbaum L, Boer GJ, Verhaagen J, 2004. Rescue and sprouting of motoneurons following ventral root avulsion and reimplantation combined with intraspinal adeno-associated viral vector-mediated expression of glial cell line-derived neurotrophic factor or brain-derived neurotrophic factor. Exp. Neurol 189, 303–316. 10.1016/j.expneurol.2004.05.014 [DOI] [PubMed] [Google Scholar]
- Cohen MS, Orth CB, Kim HJ, Jeon NL, Jaffrey SR, 2011. Neurotrophin-mediated dendrite-to-nucleus signaling revealed by microfluidic compartmentalization of dendrites. Proc. Natl. Acad. Sci. U. S. A 108, 11246–11251. 10.1073/pnas.1012401108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ee X, Yan Y, Hunter DA, Schellhardt L, Sakiyama-Elbert SE, Mackinnon SE, Wood MD, 2017. Transgenic SCs expressing GDNF-IRES-DsRed impair nerve regeneration within acellular nerve allografts. Biotechnol. Bioeng 114, 2121–2130. 10.1002/bit.26335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers R, de Winter F, Hoyng SA, Roet KCD, Ehlert EM, Malessy MJA, Verhaagen J, Tannemaat MR, 2013. Lentiviral Vector-Mediated Gradients of GDNF in the Injured Peripheral Nerve: Effects on Nerve Coil Formation, Schwann Cell Maturation and Myelination. PLoS One 8 10.1371/journal.pone.0071076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper RM, 2004. Rapid Axoglial Signaling Mediated by Neuregulin and Neurotrophic Factors. J. Neurosci 10.1523/JNEUROSCI.1692-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox IK, Schwetye KE, Keune JD, Brenner MJ, Yu JW, Hunter DA, Wood PM, Mackinnon SE, 2005. Schwann-cell injection of cold-preserved nerve allografts. Microsurgery 25, 502–507. 10.1002/micr.20152 [DOI] [PubMed] [Google Scholar]
- Garbayo E, Montero-Menei CN, Ansorena E, Lanciego JL, Aymerich MS, Blanco-Prieto MJ, 2009. Effective GDNF brain delivery using microspheres-A promising strategy for Parkinson’s disease. J. Control. Release 135, 119–126. 10.1016/j.jconrel.2008.12.010 [DOI] [PubMed] [Google Scholar]
- Grinsell D, Keating CP, 2014. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. Biomed Res. Int. 2014 10.1155/2014/698256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höke A, Gordon T, Zochodne DWD, Sulaiman OAR, 2002. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp. Neurol 173, 77–85. 10.1006/exnr.2001.7826 [DOI] [PubMed] [Google Scholar]
- Höke A, Ho T, Crawford TO, LeBel C, Hilt D, Griffin JW, 2003. Glial cell line-derived neurotrophic factor alters axon schwann cell units and promotes myelination in unmyelinated nerve fibers. J. Neurosci 23, 561–567. 10.1002/jbm.a.30587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase T, Jung CG, Bae H, Zhang M, Soliven B, 2005. Glial cell line-derived neurotrophic factor-induced signaling in Schwann cells. J. Neurochem 94, 1488–1499. 10.1111/j.1471-4159.2005.03290.x [DOI] [PubMed] [Google Scholar]
- Jesuraj NJ, Marquardt LM, Kwasa JA, Sakiyama-Elbert SE, 2014. Glial cell line-derived neurotrophic factor promotes increased phenotypic marker expression in femoral sensory and motor-derived Schwann cell cultures. Exp. Neurol 257, 10–18. 10.1016/j.expneurol.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kells AP, Fong DM, Dragunow M, During MJ, Young D, Connor B, 2004. AAV-mediated gene delivery of BDNF or GDNF is neuroprotective in a model of Huntington disease. Mol. Ther 9, 682–688. 10.1016/j.ymthe.2004.02.016 [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Mackinnon SE, Watanabe O, Ball DJ, Gu XM, Hunter D. a, Kuzon WM, 1997. The effect of duration of muscle denervation on functional recovery in the rat model. Muscle Nerve 20, 858–66. [DOI] [PubMed] [Google Scholar]
- Lin Y-C, Ramadan M, Hronik-Tupaj M, Kaplan DL, Philips BJ, Sivak W, Rubin JP, Marra KG, 2011. Spatially controlled delivery of neurotrophic factors in silk fibroin-based nerve conduits for peripheral nerve repair. Ann. Plast. Surg 67, 147–155. 10.1097/SAP.0b013e3182240346 [DOI] [PubMed] [Google Scholar]
- Lundborg G, 2003. Nerve injury and repair - A challenge to the plastic brain, in: Journal of the Peripheral Nervous System. pp. 209–226. 10.1111/j.1085-9489.2003.03027.x [DOI] [PubMed] [Google Scholar]
- Lundborg G, 2000. A 25-year perspective of peripheral nerve surgery: Evolving neuroscientific concepts and clinical significance. J. Hand Surg. Am 25, 391–414. 10.1053/jhsu.2000.4165 [DOI] [PubMed] [Google Scholar]
- Madduri S, Feldman K, Tervoort T, Papaloïzos M, Gander B, 2010. Collagen nerve conduits releasing the neurotrophic factors GDNF and NGF. J. Control. Release 143, 168–174. 10.1016/j.jconrel.2009.12.017 [DOI] [PubMed] [Google Scholar]
- Marquardt LM, Ee X, Iyer N, Hunter D, Mackinnon SE, Wood MD, Sakiyama-Elbert SE, 2015. Finely Tuned Temporal and Spatial Delivery of GDNF Promotes Enhanced Nerve Regeneration in a Long Nerve Defect Model. Tissue Eng. Part A 21, 2852–64. 10.1089/ten.TEA.2015.0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt LM, Sakiyama-Elbert SE, 2015. GDNF preconditioning can overcome Schwann cell phenotypic memory. Exp. Neurol 265, 1–7. 10.1016/j.expneurol.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajeri MH, Figlewicz DA, Bohn MC, 1999. Intramuscular grafts of myoblasts genetically modified to secrete glial cell line-derived neurotrophic factor prevent motoneuron loss and disease progression in a mouse model of familial amyotrophic lateral sclerosis. Hum. Gene Ther 10, 1853–66. 10.1089/10430349950017536 [DOI] [PubMed] [Google Scholar]
- Moore AM, Ray WZ, Chenard KE, Tung T, Mackinnon SE, 2009. Nerve allotransplantation as it pertains to composite tissue transplantation. Hand 4, 239–244. 10.1007/s11552-009-9183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Vahidi B, Taylor AM, Rhee SW, Jeon NL, 2006. Microfluidic culture platform for neuroscience research. Nat. Protoc 1, 2128–36. 10.1038/nprot.2006.316 [DOI] [PubMed] [Google Scholar]
- Scheib J, Höke A, 2013. Advances in peripheral nerve regeneration. Nat. Rev. Neurol 9, 668–76. 10.1038/nrneurol.2013.227 [DOI] [PubMed] [Google Scholar]
- Shakhbazau A, Mohanty C, Shcharbin D, Bryszewska M, Caminade AM, Majoral JP, Alant J, Midha R, 2013. Doxycycline-regulated GDNF expression promotes axonal regeneration and functional recovery in transected peripheral nerve. J. Control. Release 172, 841–851. 10.1016/j.jconrel.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Siddique R, Thakor N, 2014. Investigation of nerve injury through microfluidic devices. J. R. Soc. Interface 11, 20130676 10.1098/rsif.2013.0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JE, Seeburger JL, He J, Gabrea A, Blankenhorn EP, Bergman LW, 1995. cDNA sequence and differential mRNA regulation of two forms of glial cell line-derived neurotrophic factor in Schwann cells and rat skeletal muscle. Exp Neurol 131, 47–52. ST–cDNA sequence and differential mRNA r. [DOI] [PubMed] [Google Scholar]
- Tannemaat MR, Boer GJ, Verhaagen J, Malessy MJA, 2007. Genetic modification of human sural nerve segments by a lentiviral vector encoding nerve growth factor. Neurosurgery 61, 1286–1294. 10.1227/01.neu.0000306108.78044.a2 [DOI] [PubMed] [Google Scholar]
- Tannemaat MR, Eggers R, Hendriks WT, De Ruiter GCW, Van Heerikhuize JJ, Pool CW, Malessy MJA, Boer GJ, Verhaagen J, 2008. Differential effects of lentiviral vector-mediated overexpression of nerve growth factor and glial cell line-derived neurotrophic factor on regenerating sensory and motor axons in the transected peripheral nerve. Eur. J. Neurosci 28, 1467–1479. 10.1111/j.1460-9568.2008.06452.x [DOI] [PubMed] [Google Scholar]
- Tao Y, 2013. Isolation and culture of Schwann cells. Methods Mol. Biol. 10.1007/978-1-62703-444-9_9 [DOI] [PubMed]
- Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL, 2005. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2, 599–605. 10.1038/nmeth777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. a, Braza D, Rice JB, Dillingham T, 2008. The incidence of peripheral nerve injury in extremity trauma. Am. J. Phys. Med. Rehabil 87, 381–385. 10.1097/PHM.0b013e31815e6370 [DOI] [PubMed] [Google Scholar]
- Tolwani RJ, Cosgaya JM, Varma S, Jacob R, Kuo LE, Shooter EM, 2004. BDNF overexpression produces a long-term increase in myelin formation in the peripheral nervous system. J. Neurosci. Res 77, 662–669. 10.1002/jnr.20181 [DOI] [PubMed] [Google Scholar]
- Wood MD, Kim H, Bilbily A, Kemp SWP, Lafontaine C, Gordon T, Shoichet MS, Borschel GH, 2012. GDNF released from microspheres enhances nerve regeneration after delayed repair. Muscle and Nerve 46, 122–124. 10.1002/mus.23295 [DOI] [PubMed] [Google Scholar]
- Wu-Fienberg Y, Moore AM, Marquardt LM, Newton P, Johnson PJ, Mackinnon SE, Sakiyama-Elbert SE, Wood MD, 2014. Viral transduction of primary Schwann cells using a Cre-lox system to regulate GDNF expression. Biotechnol. Bioeng 111, 1886–1894. 10.1002/bit.25247 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.