Abstract
Novel therapeutics against multidrug-resistant Neisseria gonorrhoeae are urgently needed. Gonococcal lipooligosaccharide (LOS) often expresses lacto-N-neotetraose (LNnT), which becomes sialylated in vivo, enhancing factor H (FH) binding and contributing to the organism’s ability to resist killing by complement. We previously showed that FH domains 18–20 (with a D-to-G mutation at position 1119 in domain 19) fused to Fc (FHD1119G/Fc) displayed complement-dependent bactericidal activity in vitro and attenuated gonococcal vaginal colonization of mice. Gonococcal LOS phase-variation can result in loss of LNnT expression. Loss of sialylated LNnT, although associated with a considerable fitness cost, could decrease efficacy of FHD1119G/Fc. Similar to N. meningitidis, gonococci also bind FH domains 6 and 7 through Neisseria surface protein A (NspA). Here we show that a fusion protein comprising FH domains 6 and 7 fused to human IgG1 Fc (FH6,7/Fc) bound to 15 wild-type antimicrobial resistant isolates of N. gonorrhoeae and to each of six lgtA gonococcal deletion mutants. FH6,7/Fc mediated complement-dependent killing of 8 of the 15 wild-type gonococcal isolates and effectively reduced the duration and burden of vaginal colonization of three gonococcal strains tested in wild-type mice, including two strains that resisted complement-dependent killing, but on which FH6,7/Fc enhanced C3 deposition. FH/Fc lost efficacy when Fc was mutated to abrogate C1q binding and in C1q−/− mice, highlighting the requirement of the classical pathway for its activity. Targeting gonococci with FH6,7/Fc provides an additional immunotherapeutic approach against multidrug-resistant gonorrhea.
Keywords: complement, Neisseria gonorrhoeae, factor H, immunotherapy, Fc fusion protein
Introduction
Gonorrhea is caused by the gram-negative bacterium, Neisseria gonorrhoeae. About 78 million cases of gonorrhea occur world-wide, annually (1). Gonorrhea infects mucosal epithelia primarily and commonly manifests as cervicitis, urethritis, proctitis, and conjunctivitis. Infections at these sites, if left untreated, can lead to local complications including endometritis, salpingitis, tuboovarian abscess, bartholinitis, peritonitis, and perihepatitis in women, periurethritis and epididymitis in men, and ophthalmia neonatorum in newborns. Disseminated gonococcal infection is an uncommon event whose manifestations include skin lesions, tenosynovitis, septic arthritis, and rarely, endocarditis or meningitis.
Over the years N. gonorrhoeae has become resistant to almost every antibiotic that has been used for treatment (2). Strains that are resistant or show decreased susceptibility to ceftriaxone have been reported on almost every continent (3–6). The recent emergence of azithromycin-resistant isolates in several countries (5, 7) could render the first-line therapy, ceftriaxone plus azithromycin, recommended by the Centers for Disease Control and Prevention (8), ineffective in the near future.
In light of rapidly emerging multidrug-resistant N. gonorrhoeae worldwide, development of safe and effective vaccines and novel therapeutics against gonorrhea is a high priority (9). An approach for developing new and effective therapeutics against gonorrhea is to target key bacterial virulence mechanisms. One of these, is the ability of N. gonorrhoeae to sialylate the lacto-N-neotetraose (LNnT) glycan extension from heptose I (HepI) on lipooligosaccharide (LOS) (10, 11). Sialylation of LNnT LOS enhances binding of the complement inhibitor, factor H (FH) (12), via the three C-terminal domains of factor H (domains 18, 19 and 20) (12–14). Previously, we targeted this key virulence mechanism by constructing a chimeric protein comprising FH domains 18–20 fused to IgG Fc (FH18–20/Fc) and showed that this immunotherapeutic possessed complement-dependent bactericidal activity against gonococci in vitro and shortened the duration and diminished bacterial loads in the mouse model of vaginal colonization (15).
Phase-variation of LOS glycans occurs in vivo (11) as a result of slipped-stand mispairing of Neisserial LOS glycosyltransferase (lgt) genes (16–19) and results in gonococci not remaining sialylated throughout their life cycle, yet they are able to survive in the unsialylated state, at least temporarily, until they are re-sialylated (20). Phase variation ‘off’ of lgtA results in HepI substitution with lactose, which decreases binding and efficacy of FH18–20/Fc. Although rare, an example of a clinical gonococcal isolate that cannot express LNnT because of a genetic deletion of three genes in the lgt locus, has been reported (21).
Numerous pathogens also bind FH through domains 6 and 7 (reviewed in (22)) and we have previously characterized Neisserial surface protein A (NspA) on N. meningitidis as a ligand for FH domains 6 and 7 (23). A fusion protein that consists of FH domains 6 and 7 fused to IgG Fc (FH6,7/Fc) is efficacious against N. meningitidis (24), nontypeable Haemophilus influenzae (25) and group A streptococci (26) in rodent models. Gonococcal NspA also binds to human FH domains 6 and 7 (27) and in this report we examine the efficacy of FH6,7/Fc against N. gonorrhoeae to counter the threat of antimicrobial resistance.
Materials and Methods
Bacterial strains.
Strains F62 (28), Ctx-r(Spain) (similar to strain F89) (3), H041 (also known as WHO reference strain X) (6, 29), MS11 (11), UMNJ60_06UM (abbreviated to ‘NJ-60’) (30) and FA1090 (31) have all been described previously. Strains Ctx-r(Spain), H041 and NJ-60 are resistant to ceftriaxone. To genetically construct lgtA mutants, the LOS glycosyltransferase A (lgtA) was insertionally inactivated with a kanamycin-resistance marker (lgtA::kan) cloned into a unique StyI site in lgtA, as described previously (23, 32). Opacity protein-negative mutants of MS11 (33) and FA1090 (34) (all opa genes deleted) have been described previously. Supplemental Table S1 summarizes the characteristics of the strains listed above. Genotypic/phenotypic characteristics of nine N. gonorrhoeae isolates that were isolated as part of the Gonococcal Isolate Surveillance Project (GISP) site in California in 2012 (4, 35) are listed in Supplemental Table S2.
IgG and IgM depleted normal human serum (human complement).
Serum was obtained from normal healthy adult volunteers with no history of gonococcal or meningococcal infection who provided informed consent. Participation was approved by the University of Massachusetts Institutional Review Board for the protection of human subjects. Serum was obtained from whole blood that was clotted at 25 °C for 30 min followed by centrifugation at 1500 g for 20 min at 4 °C. To study the effects of the FH6,7/Fc proteins without confounding by natural anti-gonococcal antibodies present in NHS, we depleted IgG and IgM from freshly collected human serum, as described previously (36). Briefly, EDTA (final concentration 10 mM) and NaCl (final concentration 1 M) were added to freshly prepared human serum and treated sera was passed first over anti-human IgM agarose (Sigma), followed by passage through protein G-Sepharose; both columns were equilibrated in PBS containing 10 mM EDTA and 1 M NaCl. NaCl was added to minimize loss of C1q during passage of serum through the anti-human IgM column. The flow-through was collected, spin concentrated and dialyzed against PBS/0.1 mM EDTA to its original volume using a 10-kDa cutoff Amicon Ultra-15 centrifugal filter device (Millipore, Bedford, MA), sterilized by passage through a 0.22-μm filter (Millipore), aliquoted and stored at −70ºC. Hemolytic activity was confirmed using a total complement hemolytic plate assay (The Binding Site Inc., Birmingham, U.K).
Expression and purification of FH/Fc fusion proteins in CHO cells.
Cloning, expression in CHO cells and purification from cell culture supernatants of a chimeric protein comprising human FH (HuFH) domains 6 and 7 or domains 18–20 (D1119G) fused to human IgG1 Fc has been described previously (25, 37). Protein concentrations were determined using the BCA protein Assay kit (Pierce); mass was determined by Coomassie Blue staining of proteins separated by SDS-PAGE. The role of classical pathway activation in efficacy of FHD1119G/Fc and FH6,7/Fc was examined by introducing two amino acid point mutations – D270A and K322A – in the Fc region, which abrogates C1q binding (38, 39).
Antibodies.
Sheep anti-human C3c-FITC was obtained from AbD Serotec (cat. # AHP031F), and anti-human IgG FITC was from Sigma. Both antibodies (Abs) were used at a dilution of 1:200 in HBSS++ and 1% bovine serum albumin (BSA) (HBSS++/BSA) in flow cytometry assays.
Flow cytometry.
Binding of FH/Fc to bacteria and C3 fragments deposited on bacteria were measured by flow cytometry as described previously (24). Data were acquired on a LSRII flow cytometer and data were analyzed using FlowJo software.
Bactericidal assay.
Bactericidal assays with FH/Fc were performed as described previously (15), except that growth media lacked CMP-Neu5Ac. Approximately 2000 colony forming units (CFUs) of N. gonorrhoeae were incubated with 20% human complement (IgG and IgM depleted normal human serum) in the presence or the absence of the FH/Fc fusion protein (concentration indicated for each experiment). The final volume of the bactericidal reaction mixture was 150 µl. Aliquots of 25-µl reaction mixtures were plated onto chocolate agar in duplicate at the beginning of the assay (t0) and again after incubation at 37°C for 30 min (t30). Survival was calculated as the number of viable colonies at t30 relative to t0.
Opsonophagocytosis assay.
Opsonophagocytic killing of gonococci with freshly isolated human polymorphonuclear leukocytes (PMNs) was performed as described previously (15). Briefly, heparinized venous blood was obtained from a healthy adult volunteer in accordance with a protocol approved by the Institutional Review Board. PMNs were isolated using Mono-Poly resolving medium (MP Biomedicals), according to the manufacturer’s instructions. Isolated PMNs were washed and suspended in HBSS without added divalent cations, counted, and diluted to 1 × 107/ml in HEPES-buffered RPMI 1640 medium supplemented with l-glutamine and 1% heat-inactivated FBS. To measure survival of gonococci in the presence of PMNs, Opa protein–negative mutants of N. gonorrhoeae strain FA1090 and MS11 were added to 1 × 106 PMNs at a multiplicity of infection (MOI) of 1 (1 bacterium to 1 PMN). Opa-negative (Opa−) N. gonorrhoeae was used because select Opa proteins serve as ligands for human carcinoembryonic Ag–related cell adhesion molecule (CEACAM)3 that is expressed by PMNs and results in phagocytosis (40). FH6,7/Fc was added at a concentration of 67 μg/ml, followed by 10% human complement (prepared as described above). The reaction mixtures were incubated for 60 min at 37°C in a shaking water bath. Bacteria were serially diluted and plated at 0 and 60 min on chocolate agar plates. Percent survival of gonococci in each reaction was calculated as a ratio of CFUs at 60 min to CFUs at the start of the assay (0 min).
Mouse vaginal colonization model.
Use of animals in this study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Massachusetts Medical School. Female BALB/c mice 6–8 weeks of age (Jackson Laboratories) in the diestrus phase of the estrous cycle were started on treatment (that day) with 0.1 mg Premarin (Pfizer) in 200 µl of water given subcutaneously on each of three days; −2, 0 and +2 days (before, the day of and after inoculation) to prolong the estrus phase of the reproductive cycle and promote susceptibility to Ng infection. Antibiotics (vancomycin, colistin, neomycin, trimethoprim and streptomycin) ineffective against Ng were also used to reduce competitive microflora (41). Mice were infected on Day 0 with either strain OC7, MS11 or FA1090 (inoculum specified for each experiment). Mice were treated daily with 10 µg FH6,7/Fc intravaginally from day 0 till the conclusion of the experiment, or were given a corresponding volume of PBS (vehicle controls). In the experiments with FA1090 and MS11, we also included a group of mice that received FHD1119G/human Fc (25) to evaluate the comparative efficacies of the two FH/Fc molecules. We have previously shown that FHD1119G fused to mouse IgG2a Fc was efficacious against N. gonorrhoeae F62 (25).
Efficacy of FH6,7/Fc and FHD1119G/Fc against strain FA1090 were also evaluated in C1q deficient mice (C1q−/− mice) on the C57BL/6 background (42) and in transgenic mice that expressed human FH and human C4BP on the BALB/c background (43). FH/C4BP transgenic mice expressed levels of human FH and C4BP that are comparable to those found in normal human serum and show similar responses to a variety of stimuli as wild-type BALB/c mice.
Statistical analyses.
Experiments that compared clearance of N. gonorrhoeae in independent groups of mice estimated and tested three characteristics of the data (44): Time to clearance, longitudinal trends in mean log10 CFU and the cumulative CFU as area under the curve (AUC). Statistical analyses were performed using mice that initially yielded bacterial colonies on Days 1 and/or 2. Median time to clearance was estimated using Kaplan-Meier survival curves; times to clearance were compared between groups using the Mantel-Cox log-rank test. Mean log10 CFU trends over time were compared between groups using a linear mixed model with mouse as the random effect using both a random intercept and a random slope. A quadratic or cubic function in time was determined to provide the best fit; random slopes were linear in time. A likelihood ratio test was used to compare nested models (with and without the interaction term of group and time) to test whether the trend differed over time between the two groups. The mean AUC (log10 CFU versus time) was computed for each mouse to estimate the bacterial burden over time (cumulative infection); the means under the curves were compared between groups using the nonparametric two-sample Wilcoxon rank-sum (Mann-Whitney) test because distributions were skewed or kurtotic. The Kruskal-Wallis equality-of-populations rank test was also applied to compare more than two groups in an experiment.
Results
Binding of FH6,7/Fc to non-sialylated wild-type N. gonorrhoeae and their lgtA deletion mutants.
FH domains 6 and 7 bind to Neisserial surface protein A (NspA) on N. meningitidis (23). Based on the ability of N. gonorrhoeae NspA to bind to factor H like protein 1 (FHL-1; contains FH domains 1 through 7) and FH domains 5 through 8 fused to Fc (27), we concluded that similar to N. meningitidis NspA, domains 6 and 7 were also required for binding to gonococcal NspA. To address efficacy of FH6,7/Fc against non-sialylated N. gonorrhoeae, first we examined binding of FH6,7/Fc by flow cytometry to six strains of non-sialylated N. gonorrhoeae that included three ceftriaxone-resistant isolates (Ctx-r(Sp), HO41 and NJ-60) and the isogenic lgtA mutants of all six strains (Fig.1). The lgtA mutants lack the LNnT acceptor site for sialic acid. Binding of FH domains 6 and 7 to meningococcal / gonococcal NspA increases with LOS truncation (23, 27), as was observed here for the lgtA mutants of strains MS11, NJ-60 and FA1090 that showed reproducible increases in FH6,7/Fc binding compared to their wild-type counterparts. Wild-type F62, CTX-r(Spain) and H041 bound similar amounts of FH6,7/Fc as their respective lgtA mutants (Fig. 1).
Figure 1.
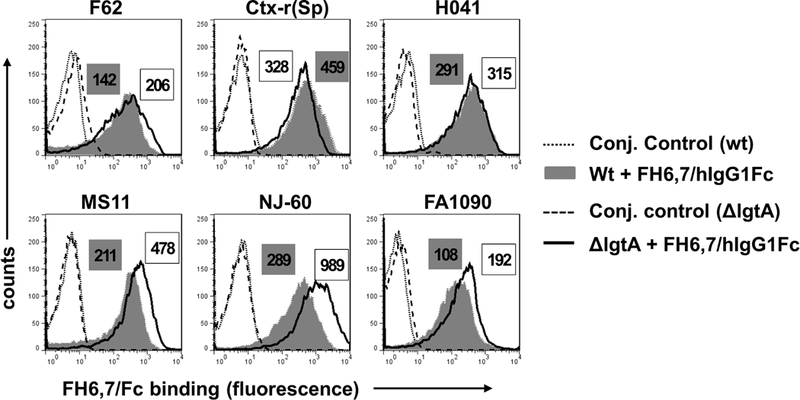
Binding of FH6,7/Fc (10 µg/ml) to six strains of N. gonorrhoeae and their lgtA mutants. FH6,7/Fc binding to wild-type (wt) N. gonorrhoeae strains F62, Ctx-r(Spain), H041, MS11, UMNJ60_06UM (abbreviated to ‘NJ-60’) and FA1090 by flow cytometry is shown by the grey shaded histograms and binding to their respective lgtA mutants is shown by the histograms depicted by solid lines. Conjugate controls for wt strains and lgtA mutants are shown by dotted and dashed lines, respectively. Numbers alongside histograms in grey shaded boxes and clear boxes represents the median fluorescence of binding to wt strains and lgtA mutants, respectively. In every instance, the median fluorescence of the conjugate controls was less than 10. X-axis, fluorescence on a log10 scale; Y-axis, counts. One representative experiment of two reproducible independently performed experiments is shown.
Bactericidal activity of FH6,7/Fc against non-sialylated wild-type N. gonorrhoeae and their lgtA deletion mutants.
The ability of FH6,7/Fc to mediate complement-dependent killing of the strains shown in Fig. 1 was evaluated in bactericidal assays using human serum depleted of IgG and IgM as the complement source. FH6,7/Fc showed bactericidal activity (>90% killing) against wild-type F62, Ctx-r(Spain), H041 and NJ-60 and their corresponding lgtA mutants (Fig. 2). In contrast, neither the wild-type nor the lgtA mutants of MS11 or FA1090 showed any significant killing (>70% survival) in the presence of FH6,7/Fc and complement.
Figure 2.
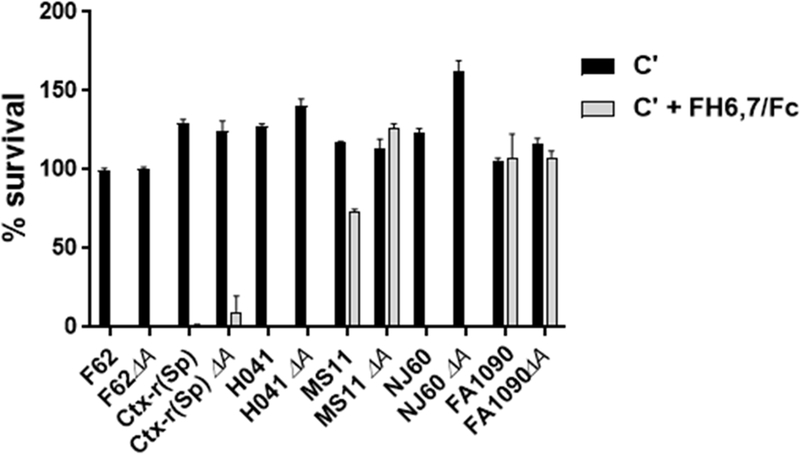
Complement-dependent bactericidal activity of FH6,7/Fc against wild-type N. gonorrhoeae strains and their lgtA mutants. The six strains of N. gonorrhoeae and their lgtA mutants listed on the X-axis were incubated with either 20% complement (C´; normal human serum (NHS) immunodepleted of IgG and IgM) alone (black bars), or C´ plus FH6,7/Fc (33 µg/ml) (grey shaded bars) and percent survival (Y-axis) calculated as CFU that survived at 30 min relative to CFU at 0 min. Each bar shows the mean (range) of two independently performed experiments.
C3 fragment deposition and opsonophagocytic killing of non-sialylated N. gonorrhoeae resistant to complement-dependent killing in the presence of FH6,7/Fc.
FHD1119G/Fc mediates complement activation and C3 fragment deposition on N. gonorrhoeae strains that resist direct killing by FHD1119G/Fc and this results in enhanced opsonophagocytosis (15). iC3b deposits on microbes and engages complement receptor 3 (CR3) on phagocytes that results in bacterial uptake and killing (45, 46). We measured FH6,7/Fc mediated C3 fragment deposition on the 2 wild-type strains, MS11 and FA1090, and their lgtA mutants that had resisted direct killing by FH6,7/Fc and complement (Fig. 2). As shown in Fig. 3, the addition of FH6,7/Fc to complement enhanced C3 deposition (measured as median fluorescence) on wild-type MS11 and the corresponding MS11 lgtA mutant, ~13- and ~15-fold respectively, compared to complement alone. In contrast, FH6,7/Fc enhanced C3 deposition only ~3-fold on wild-type FA1090. Consistent with greater binding of FH6,7/Fc to FA1090 lgtA mutant compared to wild-type, we observed a ~7-fold increase in C3 deposition on the lgtA mutant by FH6,7/Fc compared to complement alone.
Figure 3.
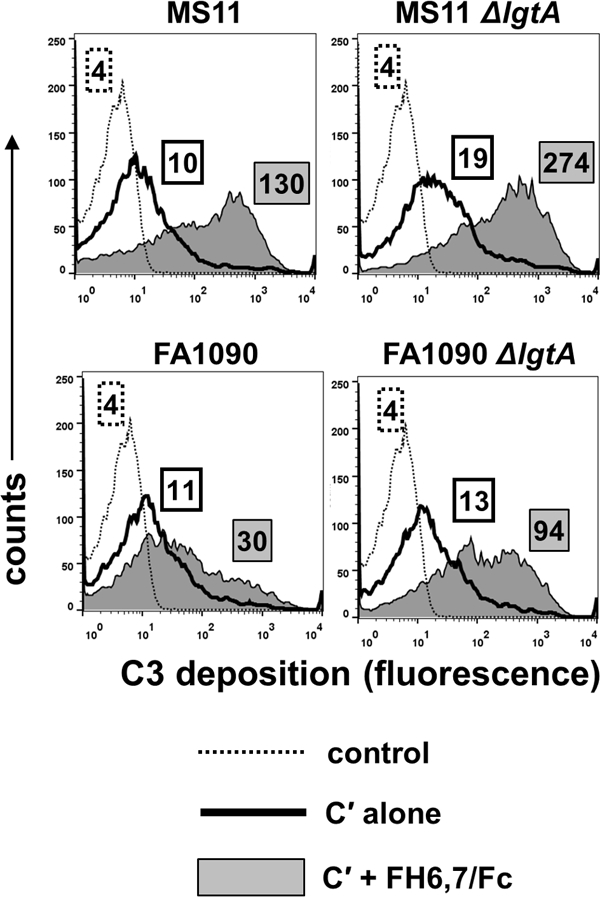
C3 fragment deposition on N. gonorrhoeae mediated by complement (C´) alone and C´ + FH6,7/Fc. Strains MS11, FA1090 and their lgtA mutants were incubated with either 20% C´ alone, or C´ plus FH6,7/Fc for 30 min at 37 °C and C3 fragments (C3b and iC3b) deposited on bacteria measured by flow cytometry using anti-human C3c FITC. X-axis, fluorescence (log10 scale); Y-axis, counts. Numbers in boxes with dotted outlines, boxes with solid outlines and grey shaded boxes represent median fluorescence of conjugate controls, C3 deposition in the presence of C´ alone or C´ plus FH6,7/Fc, respectively.
We next determined whether increased C3 deposition in the presence of FH6,7/Fc facilitated opsonophagocytosis by human PMNs (Supplemental Figure S1). Opacity protein (Opa)-negative mutants of MS11 and FA1090 were used to abrogate opsonic interactions between select Opa proteins and CEACAM3 on human PMNs. Despite greater C3 deposition on MS11 and FA1090, we noted only marginal killing by human PMNs when both FH6,7/Fc and active complement were present.
Binding and bactericidal activity of FH6,7/Fc against wild-type clinical isolates of N. gonorrhoeae.
We tested binding and efficacy of FH6,7/Fc against 9 recent clinical isolates of N. gonorrhoeae that were recovered by the GISP site in California in 2012 (4, 35); three (SD5, SD15 and SF2) displayed reduced susceptibility to ceftriaxone (0.125 µg/ml) and shared the same NG-MAST type (1407, Supplemental Table S2). The nine GISP isolates bound FH6,7/Fc to varying degrees (Fig. 4A). Four of the nine isolates (OC7, OC14, SD3 and SF6) were killed (<50 % survival) in the presence of complement and FH6,7/Fc (Fig. 4B). There was a trend towards less killing with lower FH/Fc binding (P=0.07; SD5 (included in the analysis) was an outlier). Substantial variation occurred in the binding of FH6,7/Fc to the 3 isolates that shared NG-MAST type 1407; however, percent survival in the bactericidal assay compared to control was >80% for the three isolates (Fig. 4). Collectively, these data show that FH6,7/Fc binds to and kills the tested N. gonorrhoeae strains to varying degrees.
Figure 4.
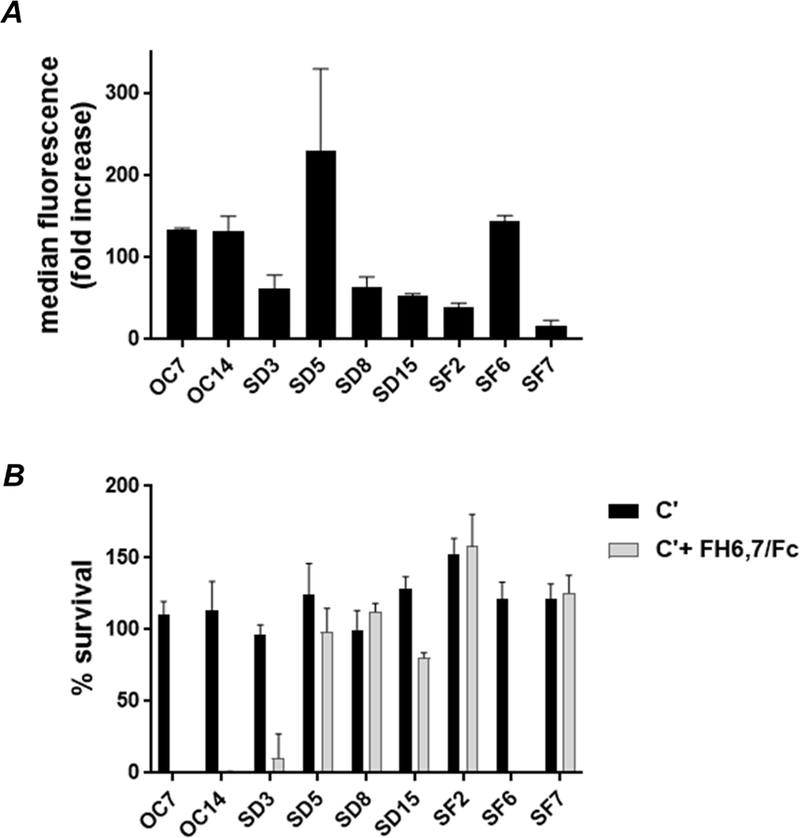
Binding to and complement (C´)-dependent bactericidal activity mediated by FH6,7/Fc against nine clinical strains of N. gonorrhoeae. A. Binding of FH6,7/Fc to N. gonorrhoeae. Binding of FH6,7/Fc (10 µg/ml) to each of the nine gonococcal isolates listed on the X-axis (identified in the Table) was measured by flow cytometry. Binding (Y-axis) is shown as fold-increase over conjugate controls. The mean (range) of at least two separate experiments is shown. B. Complement-dependent bactericidal activity mediated by FH6,7/Fc. The nine strains of N. gonorrhoeae were incubated with either 20% C´ alone (black bars) or C´ plus FH6,7/Fc (33 µg/ml) (grey shaded bars) and percent survival (Y-axis) at 30 min was measured. Each bar represents the mean (range) of at least two independently performed experiments.
Efficacy of FH6,7/Fc against N. gonorrhoeae in the BALB/c mouse vaginal colonization model.
We next evaluated the efficacy of FH6,7/Fc against N. gonorrhoeae in the mouse vaginal colonization model of gonorrhea. We tested three strains that differed in their abilities to resist killing by FH6,7/Fc and complement. Strain OC7 was highly sensitive to killing in vitro (0% survival; Fig. 4), while MS11 and FA1090 were resistant, showing ~75% survival and 100% survival, respectively (Fig. 2).
Treatment of OC7-infected mice (n=10) with FH6,7/Fc administered intravaginally at a dose of 10 µg daily, significantly shortened the duration of infection (Fig 5A). Ten control (PBS-treated) animals remained infected on day 8, while 90% of FH6,7/Fc treated mice cleared infection by day 5; 100% by day 6 (P<0.0001). Fig. 5B shows that the burden of infection declined more rapidly in FH6,7/Fc treated mice after 4 days. FH6,7/Fc was also more efficacious in reducing the Area Under Curve (AUC), a measure of overall bacterial burden over time (P<0.0001; Fig. 5C).
Figure 5.
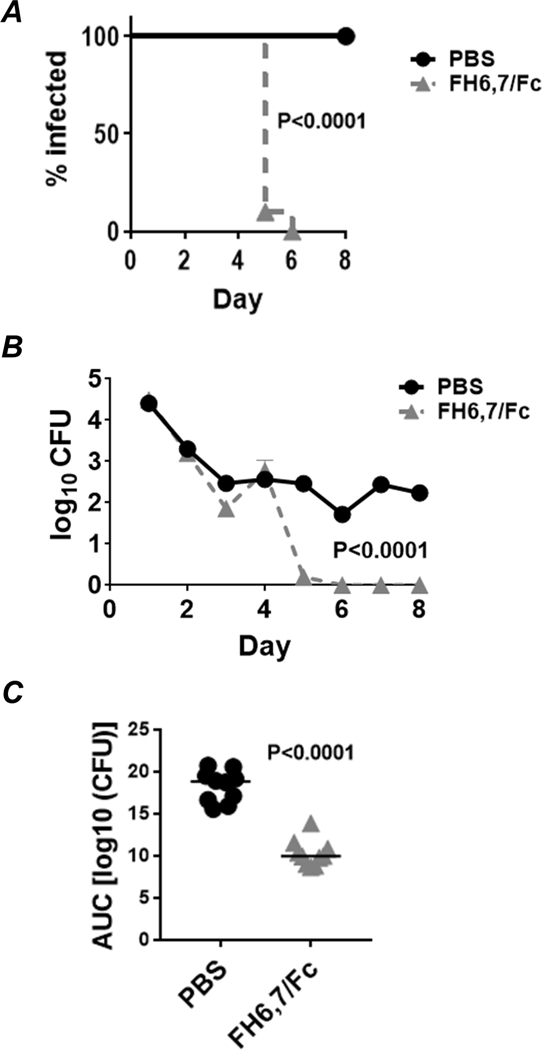
Efficacy of FH6,7/Fc against N. gonorrhoeae OC7 in the mouse vaginal colonization model of gonorrhea. Premarin®-treated wild-type BALB/c mice were given 6 × 105 CFU strain OC7 intravaginally on Day 0. Mice (n=10/group) were given either FH6,7/Fc or PBS (control) daily, beginning on Day 0 through Day 8, when the experiment was terminated. A. Kaplan Meier curves showing time to clearance of infection. P<0.0001 by Mantel-Cox log-rank test. B. Log10 CFU versus time. X-axis, day; Y-axis, log10 CFU. C. Bacterial burdens consolidated over time (Area Under the Curve [log 10 CFU] analysis) for the two groups. Comparison was made using the Mann-Whitney (non-parametric) test.
MS11 and FA1090 are both resistant to complement-mediated killing by FH6,7/Fc in vitro (Fig. 2) and we were uncertain whether FH6,7/Fc would show activity against these two isolates in vivo. We speculated that FHD1119G/Fc would be effective in vivo against MS11 based on its known bactericidal activity against MS11 and its ability to mediate opsonophagocytic killing of FA1090 (15), therefore we included a group of mice that received FHD1119G/Fc intravaginally as a positive control.
FH6,7/Fc and FHD1119G/Fc were both effective against FA1090. As shown in Fig. 6A–C the difference in median times to clearance (Fig. 6A) between the PBS and FH6,7/Fc groups were significant (P<0.0001), even though they differed by only 1 day (8 and 7 days for the PBS and FH6,7/Fc groups, respectively) because low levels of bacteria (102 to 103 CFU/swab) persisted in the FH6,7/Fc-treated animals on day 6 (Fig 6B). The median time to clearance for the FHD1119G/Fc group was 5.5. days. However, both FH6,7/Fc and FHD1119G/Fc performed comparably when log10 CFU versus time (Fig. 6B) and AUC (Fig. 6C) were measured.
Figure 6.
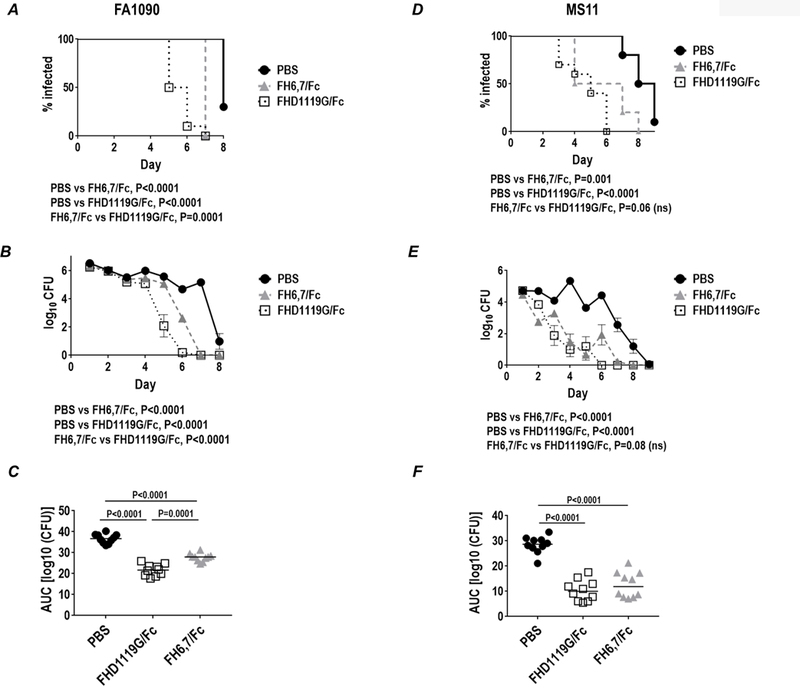
Efficacy of FH6,7/Fc and FHD1119G/Fc against strains MS11 and FA1090 in the mouse vaginal colonization model. Premarin®-treated wild-type BALB/c mice (n=10/group) were given 2.5 × 107 CFU strain FA1090 (panels A-C) or 3.4 × 105 CFU strain MS11 (panels D-F) intravaginally on Day 0 and treated intravaginally daily with 10 µg/d of either FH6,7/Fc (dashed grey lines and triangles) or FHD1119G/Fc (dotted black lines and non-filled squares), or 10 µl PBS (vehicle control; solid black line and circles), from Days 0 to 8 (for strain FA1090) or from Days 0 to 9 (for strain MS11). Vaginas were swabbed daily to enumerate CFU. A and D. Kaplan Meier curves showing time to clearance of infection. Groups were compared using the Mantel-Cox (log-rank) test. Significance was set at 0.017 (Bonferroni’s correction for comparisons across three groups). B and E. Log10 CFU versus time. X-axis, day; Y-axis, log10 CFU. C and F. Bacterial burdens consolidated over time (Area Under the Curve [log 10 CFU] analysis) for the two groups. The three groups were comparisons using the non-parametric Kruskal-Wallis equality of populations rank test. The χ2 with ties (two degrees of freedom) was 25.1 (P=0.0001) for C and 19.2 (P=0.0001) for F. Pairwise comparisons across groups was made with the Two-sample Wilcoxon rank-sum (Mann-Whitney) test.
Both FH/Fc molecules were also efficacious and performed similarly against strain MS11 (Fig. 6D–F). They significantly reduced the duration (median times to clearance for PBS, FH6,7/Fc and FHD1119G/Fc were 8.5, 5.5 and 5 days, respectively; Fig. 6D) and overall burden of infection as measured by AUC was reduced compared to the PBS control group (P<0.0001 for PBS vs FHD1119G/Fc and P=0.001 for PBS vs FH6,7/Fc; Fig. 6F).
FH/Fc requires an intact classical pathway for efficacy.
To assess the role of classical pathway activation in efficacy of FH/Fc, we used C1q−/− mice. We infected either C57BL/6 wild-type mice or C1q−/− mice (n=10/group) with FA1090 and treated them with either saline, FHD1119G/Fc or FH6,7/Fc (each given at 10 µg intravaginally daily, starting on day 0, through day 10). As shown in Fig. 7, loss of C1q abrogated activity of both FH/Fc molecules, suggesting that engagement of C1q by Fc is critical for activity of FH/Fc.
Figure 7.
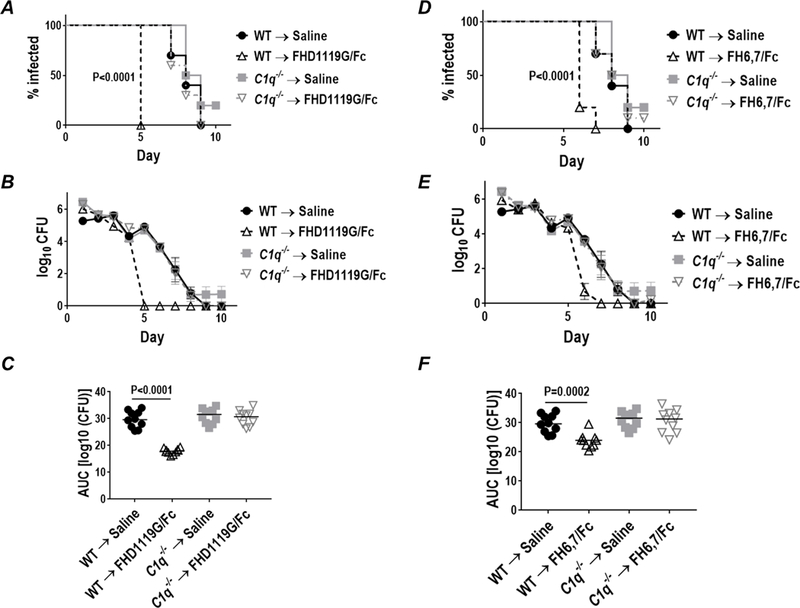
FH/Fc requires an intact classical pathway for efficacy. Wildtype C57BL/6 mice or C1q−/− mice on the C57BL/6 background (n=10 mice/group) were infected with 4 × 107 CFU N. gonorrhoeae strain OC7 and treated with either saline, FHD1119G/Fc or FH6,7/Fc (both at 10 µg/d starting on the day of infection through day 10). Efficacy of FHD1119G/Fc is shown in A–C, while efficacy of FH6,7/Fc is shown in graphs D–F. Note that the saline treated controls are the same for both sets of data; results with FHD1119G/Fc and FH6,7/Fc are shown separately for clarity. A and D. Kaplan Meier curves showing time to clearance of infection. Groups were compared using the Mantel-Cox (log-rank) test. Significance was set at 0.008 (Bonferroni’s correction for comparisons across four groups). B and E. Log10 CFU versus time. C and F. Bacterial burdens consolidated over time (Area Under the Curve [log 10 CFU] analysis). The four groups were compared using the non-parametric Kruskal-Wallis equality of populations rank test. The χ2 with ties (three degrees of freedom) was 22.4 (P<0.0001) for C and 18.5 (P=0.0003) for F. Pairwise comparisons across groups was made with the Two-sample Wilcoxon rank-sum (Mann-Whitney) test.
Efficacy of FH6,7/Fc against N. gonorrhoeae in factor H (FH) / C4b-binding protein (C4BP) dual transgenic mice.
N. gonorrhoeae bind to the complement inhibitors, factor H (FH) and C4b-binding protein (C4BP) in a human-specific manner (13, 47). Thus, complement activation on N. gonorrhoeae mediated by the FH/Fc molecules is expected to occur in an uninhibited manner in wild-type mice, whose FH and C4BP do not bind to gonococci. In humans, FH/Fc molecules must surmount the complement-dampening effects of human FH and human C4BP bound to the bacterial surface in order to be efficacious as immunotherapeutics. To model this, we tested the efficacy of FH6,7/Fc against strain OC7 (sensitive to killing by both FH/Fc molecules in serum bactericidal assays) in transgenic mice that express human FH and C4BP (FH/C4BP dual Tg mice) (Fig. 8; 10 animals/group)). To reiterate the role of the classical pathway for efficacy of FH6,7/Fc, we tested FH6,7/Fc that contained two mutations to abrogate C1q binding: D270A and K322A. Because we expected lower efficacy of FH6,7/Fc in FH/C4BP Tg mice compared to wildtype mice (Fig. 5), the Fc mutant protein also served as a control for specificity. As shown in Fig 8, FH6,7/Fc was efficacious against OC7 in Tg mice. The Fc mutant molecule that did not engage C1q was inactive, further emphasizing a role for classical pathway activation for anti-gonococcal activity of FH6,7/Fc.
Figure 8.
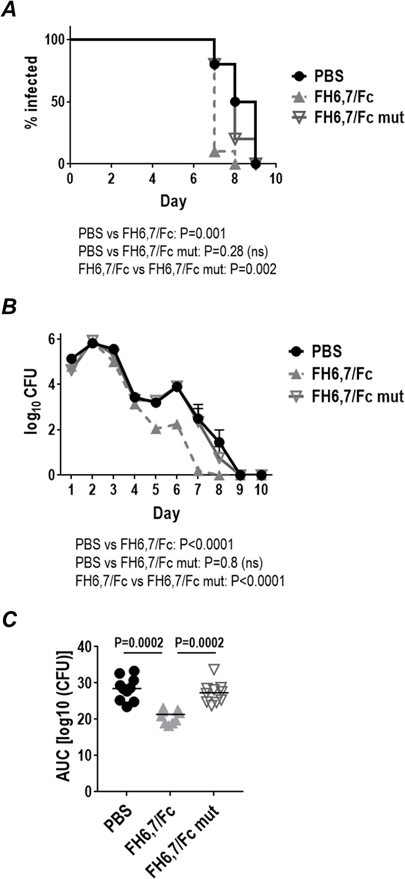
Efficacy of FH6,7/Fc against N. gonorrhoeae strain OC7 in human FH/C4BP transgenic (Tg) mice. Premarin®-treated FH/C4BP dual Tg mice (n=10/group) were given 6.2 × 107 CFU strain OC7 intravaginally on Day 0 and treated intravaginally daily with 10 µg/d of either FH6,7/Fc (dashed grey lines and triangles) or FH6,7/Fc with D270A/K322A mutations that abrogates C1q binding (solid grey lines and open inverted triangles) or 10 µl/d PBS (control; solid black line and circles) from Day 0 to Day 10. Vaginas were swabbed daily to enumerate CFU. A Kaplan Meier curves showing time to clearance of infection. Groups were compared using the log-rank (Mantel-Cox) test. Significance was set at 0.017 (Bonferroni’s correction for comparisons across three groups). B. Log10 CFU versus time. X-axis, day; Y-axis, log10 CFU. C. Bacterial burdens consolidated over time (Area Under the Curve [log 10 CFU] analysis). The three groups were compared using the non-parametric Kruskal-Wallis equality of populations rank test. The χ2 with ties (two degrees of freedom) was 19.8 (P=0.0001). Pairwise comparisons across groups was made with the Two-sample Wilcoxon rank-sum (Mann-Whitney) test.
We also tested efficacy of FH/Fc against strain FA1090; this strain was chosen because, unlike OC7, it binds high levels of C4BP (48, 49) and is resistant to complement-dependent killing by both FHD1119G/Fc (15) and FH6,7/Fc in vitro and therefore provides a stringent test of FH/Fc efficacy. As shown in Fig. 9 (left graph), both FH6,7/Fc and FHD1119G/Fc shortened the duration of infection compared to PBS-treated control mice (median duration of infection for PBS-, FH6,7/Fc- and FHD1119G/Fc-treated mice were 8, 7 and 6 d, respectively). While FHD1119G/Fc significantly shortened the duration of FA1090 colonization, the differences between the PBS and active FH6,7/Fc groups did not reach statistical significance. Inactivated FH6,7 (mutated Fc) showed no decrease in median time to clearance compared to PBS controls. Fig. 9 (middle graph) shows the log10 CFU versus time. Again, D1119G/Fc showed the greatest activity, while FH6,7/Fc only marginally enhanced clearance. The AUC analyses that indicates the overall burden of infection showed that both FH6,7/Fc and FHD1119G/Fc significantly decreased AUCs compared to PBS-treated mice. However, the FHD1119G/Fc-treated group showed a significantly lower AUC compared to FH6,7/Fc-treated group, suggesting superior efficacy compared to FH6,7/Fc against wild-type N. gonorrhoeae. Activity of FHD1119G/Fc and FH6,7/Fc against FA1090 in FH/C4BP Tg mice was repeated in a separate experiment (Supplemental Figure S2) with similar results.
Figure 9.
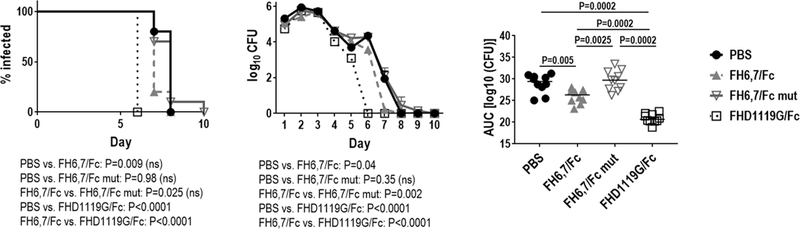
Efficacy of FH6,7/Fc and FHD1119G/Fc against FA1090 in the mouse vaginal colonization model using FH/C4BP dual transgenic (Tg) mice. Premarin®-treated FH/C4BP dual Tg mice (n=10/group) were given 7 × 107 CFU strain FA1090 intravaginally on Day 0 and treated intravaginally daily with 10 µg/d of either CHO-cell produced FH6,7/Fc (dashed grey lines and triangles), plant-produced FH6,7 fused to mutated Fc (D270A/K322A) (FH6,7/Fc mut; solid grey lines with inverted non-filled triangles) or FHD1119G/Fc (dotted black lines and non-filled squares) or 10 µl/d PBS (control; solid black line and circles) from Day 0 to Day 10. Vaginas were swabbed daily to enumerate CFU. The graph on the left shows Kaplan Meier curves for time to clearance of infection. Groups were compared using the log-rank (Mantel-Cox) test. Significance was set at 0.008 (Bonferroni’s correction for comparisons across three groups). The middle graph shows log10 CFU versus time. X-axis, day; Y-axis, log10 CFU. The graph on the right shows bacterial burdens consolidated over time (Area Under the Curve [log 10 CFU] analysis) for the two groups. The three groups were compared using the non-parametric Kruskal-Wallis equality of populations rank test. The χ2 with ties (three degrees of freedom) was 28.5 (P<0.0001). Pairwise comparisons across groups were made with the Mann-Whitney test.
Discussion
We have shown previously that FHD1119G/Fc binds to sialylated gonococci and reduces the duration and burden of N. gonorrhoeae infection in the mouse vaginal colonization model when administered topically (15). Gonococci are heterogeneously sialylated in the female genital tract (50), attributable, in part, to sialidases elaborated by coexisting bacteria, such as Gardnerella vaginalis and Prevotella bivia (51). Further, phase variation of LOS as occurs in vivo (11) could result in loss of expression of sialylatable LNnT (e.g., lgtA ‘off’). In this study, we have shown that FH6,7/Fc, which targets NspA (27) in these strains and does not require sialylated LOS for binding, also reduces the duration and burden of N. gonorrhoeae in the mouse model. Although unsialylated gonococcal strains are often reduced in fitness (10, 11, 52–54), an immunotherapeutic agent that remains effective against strains that do not express sialylated LNnT and therefore show reduced binding to FHD1119G/Fc, would serve as an effective adjunct to FHD1119 therapy. However, it was not possible to test efficacy of FH6,7/Fc against gonococci that express only lactose from HepI in this animal model because lgtA mutants infect mice for only 3–4 days (our unpublished observations). Therefore, testing the efficacy of FH/Fc molecules in vivo was restricted to wild-type isolates that express sialylatable LNnT on LOS. Not surprisingly, FHD1119G/Fc showed greater efficacy than FH6,7/Fc against all wild-type isolates whose LOSs become sialylated in vivo, which is consistent with our prior observations of decreased binding upon LOS sialylation of a chimeric protein that comprised FH domains 6–10 fused to Fc (55). Lack of efficacy of FH6,7/Fc relative to FHD1119G/Fc was evident in FH/C4BP Tg mice colonized with strain FA1090 (Fig. 9 and Supplemental Fig. S2).
Combinations of drugs are often used to prevent the development of resistance. Therapeutic use of FHD1119G/Fc and FH6,7/Fc in combination would diminish binding of FH (14) to two separate sites on the gonococcal surface in addition to exerting Fc function. In order to achieve resistance, gonococci would have to evolve away from displaying both FH binding sites thereby losing fitness. Given the promising efficacy data when each of the FH/Fc molecules are used individually, the use of these two molecules in combination may provide additive or synergistic killing, especially when LOS may phase-vary in vivo, and merits further investigation.
Here, using two independent approaches we show that efficacy of FH/Fc depends on C1q engagement by Fc. First, Fc mutations (D270A/K322A) that abrogated C1q binding rendered FH/Fc ineffective. Second, FH/Fc lost activity in C1q−/− mice. Strain FA1090 resists killing by both FH6,7/Fc and FHD1119G/Fc in direct complement-dependent bactericidal assays that employ human complement (15). It is worth noting that the ‘serum resistant’ phenotype of N. gonorrhoeae is confined to human and in some strains, chimpanzee serum, in large part because N. gonorrhoeae bind only to human complement inhibitors such as FH and C4BP (13, 47). Thus, in wild-type mice, whose FH and C4BP do not bind to N. gonorrhoeae, complement activation is unimpeded and possibly bactericidal against strains that resist human complement thereby explaining why in these mice FH6,7/Fc was efficacious against MS11 and FA1090, both of which resisted killing by human complement and FH6,7/Fc (Fig. 6).
We have shown previously that FHD1119G/Fc enhances C3 deposition and mediates killing by human PMNs in a complement-dependent manner (15). FH6,7/Fc also enhanced human C3 deposition on two wild-type strains that resist direct killing by complement, more so on MS11 than on FA1090 (Fig. 3). However, both strains were killed only marginally by human PMNs when FH6,7/Fc and active complement were present. Our experiments that used human FH/C4BP transgenic mice (43) for challenge with strain FA1090 that is highly resistant to human complement were intended to raise the barrier for efficacy of the fusion proteins. While FHD1119G/Fc overcame human FH and C4BP-mediated complement inhibition on the bacterial surface and reduced the duration and burden of wild-type FA1090 infection in FH/C4BP dual transgenic mice, FH6,7/Fc showed minimal efficacy in this situation, consistent with its lack of bactericidal and opsonic activity in the presence of human complement inhibitors.
Several human pathogens have evolved to bind to human FH using the same FH domains that interact with human cell surfaces (22). Binding of FH/Fc to human cells could activate complement and cause unwanted tissue damage. To mitigate this in FH18–20/Fc, we introduced a mutation (D to G at position 1119) in domain 19 to Fc to yield FHD1119G/Fc, which did not lyse anti-CD59-treated human RBCs (15). FH6,7/Fc also does not lyse anti-CD59-treated human RBCs in a complement-dependent manner (26). We have also not noted any short term renal (increases in creatinine) or hematologic (increase in LDH or decrease in hematocrit) or adverse side effects following systemic administration of either of these FH/Fc molecules to mice (our unpublished observations). However, further tissue cross reactivity and toxicology will be necessary during the pre-clinical development of these immunotherapeutic molecules.
A recent report showed that a monoclonal antibody against antibiotic resistant Acinetobacter baumanii was synergistic with colistin in enhancing bacterial clearance and evading sepsis (56). Similarly, the possibility that binding and complement activation on N. gonorrhoeae by FH/Fc may synergize with conventional antibiotics to reduce the duration and burden of infection also merits investigation. In summary, we have shown efficacy of two FH/Fc fusion proteins that target distinct epitopes against N. gonorrhoeae. These proteins may prove to be useful adjunctive immunotherapeutic agents in the prevention and treatment of multidrug-resistant gonococcal isolates that have emerged globally. The approach to combat infections with FH/Fc may also be useful against other human pathogens, as shown by proof-of-concept studies against N. meningitidis, nontypeable Haemophilus influenzae and group A streptococci (24–26).
Supplementary Material
Acknowledgments
We thank Nancy Nowak and Samuel Fountain for technical assistance, and Drs. Magnus Unemo (WHO Collaborating Centre for Gonorrhoea and other STIs, Department of Laboratory Medicine, Microbiology, Örebro University Hospital, Örebro, Sweden) and Makoto Ohnishi (National Institute of Infectious Diseases, Tokyo, Japan) for strain H041, Dr. Carmen Ardanuy (Microbiology Department, Hospital Universitari de Bellvitge-Universitat de Barcelona-IDIBELL, L’Hospitalet de Llobregat, Barcelona, Spain) for strain Ctx-r(Spain), Dr. Daniel Stein for MS11 Opa-, Dr. Janne Cannon for FA1090 Opa-, and Dr. Xia-Hong Su (Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, P. R. China) for strain UMNJ60_06UM.
1 This work was supported by grants from the National Institutes of Health / National Institutes of Allergy and Infectious Diseases, AI111728 (to J.S. and S.R.), AI118161 (to S.R.), AI114790 and AI132296 (to P.A.R and S.R.).
Nonstandard Abbreviations used:
- FH
factor H
- LOS
lipooligosaccharide
- wt
wild-type
- LNnT
lacto-N-neotetraose
- HBSS
Hanks’ balanced salt solution
References
- 1.Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, and Temmerman M. 2015. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS One 10: e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unemo M, and Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27: 587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camara J, Serra J, Ayats J, Bastida T, Carnicer-Pont D, Andreu A, and Ardanuy C. 2012. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother 67: 1858–1860. [DOI] [PubMed] [Google Scholar]
- 4.Gose S, Nguyen D, Lowenberg D, Samuel M, Bauer H, and Pandori M. 2013. Neisseria gonorrhoeae and extended-spectrum cephalosporins in California: surveillance and molecular detection of mosaic penA. BMC Infect Dis 13: 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz AR, Komeya AY, Kirkcaldy RD, Whelen AC, Soge OO, Papp JR, Kersh EN, Wasserman GM, O’Connor NP, O’Brien PS, Sato DT, Maningas EV, Kunimoto GY, and Tomas JE. 2017. Cluster of Neisseria gonorrhoeae Isolates With High-level Azithromycin Resistance and Decreased Ceftriaxone Susceptibility, Hawaii, 2016. Clin Infect Dis 65: 918–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, and Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55: 3538–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole JG, Fulcher NB, and Jerse AE. 2010. Opacity proteins increase Neisseria gonorrhoeae fitness in the female genital tract due to a factor under ovarian control. Infect Immun 78: 1629–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Workowski KA, and Berman S. 2010. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep 59: 1–110. [PubMed] [Google Scholar]
- 9.Alirol E, Wi TE, Bala M, Bazzo ML, Chen XS, Deal C, Dillon JR, Kularatne R, Heim J, Hooft van Huijsduijnen R, Hook EW, Lahra MM, Lewis DA, Ndowa F, Shafer WM, Tayler L, Workowski K, Unemo M, and Balasegaram M. 2017. Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines. PLoS Med 14: e1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider H, Cross AS, Kuschner RA, Taylor DN, Sadoff JC, Boslego JW, and Deal CD. 1995. Experimental human gonococcal urethritis: 250 Neisseria gonorrhoeae MS11mkC are infective. J Infect Dis 172: 180–185. [DOI] [PubMed] [Google Scholar]
- 11.Schneider H, Griffiss JM, Boslego JW, Hitchcock PJ, Zahos KM, and Apicella MA. 1991. Expression of paragloboside-like lipooligosaccharides may be a necessary component of gonococcal pathogenesis in men. J Exp Med 174: 1601–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ram S, Sharma AK, Simpson SD, Gulati S, McQuillen DP, Pangburn MK, and Rice PA. 1998. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med 187: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngampasutadol J, Ram S, Gulati S, Agarwal S, Li C, Visintin A, Monks B, Madico G, and Rice PA. 2008. Human Factor H Interacts Selectively with Neisseria gonorrhoeae and Results in Species-Specific Complement Evasion. J Immunol 180: 3426–3435. [DOI] [PubMed] [Google Scholar]
- 14.Shaughnessy J, Ram S, Bhattacharjee A, Pedrosa J, Tran C, Horvath G, Monks B, Visintin A, Jokiranta TS, and Rice PA. 2011. Molecular characterization of the interaction between sialylated Neisseria gonorrhoeae and factor H. J Biol Chem 286: 22235–22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaughnessy J, Gulati S, Agarwal S, Unemo M, Ohnishi M, Su XH, Monks BG, Visintin A, Madico G, Lewis LA, Golenbock DT, Reed GW, Rice PA, and Ram S. 2016. A Novel Factor H-Fc Chimeric Immunotherapeutic Molecule against Neisseria gonorrhoeae. J Immunol 196: 1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berrington AW, Tan YC, Srikhanta Y, Kuipers B, van der Ley P, Peak IR, and Jennings MP. 2002. Phase variation in meningococcal lipooligosaccharide biosynthesis genes. FEMS Immunol Med Microbiol 34: 267–275. [DOI] [PubMed] [Google Scholar]
- 17.Jennings MP, Srikhanta YN, Moxon ER, Kramer M, Poolman JT, Kuipers B, and van der Ley P. 1999. The genetic basis of the phase variation repertoire of lipopolysaccharide immunotypes in Neisseria meningitidis. Microbiology 145 (Pt 11): 3013–3021. [DOI] [PubMed] [Google Scholar]
- 18.Tan A, Atack JM, Jennings MP, and Seib KL. 2016. The Capricious Nature of Bacterial Pathogens: Phasevarions and Vaccine Development. Front Immunol 7: 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang QL, and Gotschlich EC. 1996. Variation of gonococcal lipooligosaccharide structure is due to alterations in poly-G tracts in lgt genes encoding glycosyl transferases. J Exp Med 183: 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apicella MA, Mandrell RE, Shero M, Wilson M, Griffiss JM, Brooks GF, Fenner C, Breen CF, and Rice PA. 1990. Modification by sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J Infect Dis 162: 506–512. [DOI] [PubMed] [Google Scholar]
- 21.Erwin AL, Haynes PA, Rice PA, and Gotschlich EC. 1996. Conservation of the lipooligosaccharide synthesis locus lgt among strains of Neisseria gonorrhoeae: requirement for lgtE in synthesis of the 2C7 epitope and of the beta chain of strain 15253. J Exp Med 184: 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ram S, Shaughnessy J, DeOliveira RB, Lewis LA, Gulati S, and Rice PA. 2016. Utilizing complement evasion strategies to design complement-based antibacterial immunotherapeutics: Lessons from the pathogenic Neisseriae. Immunobiology 221: 1110–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, and Ram S. 2010. The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement. PLoS Pathog 6: e1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaughnessy J, Vu DM, Punjabi R, Serra-Pladevall J, DeOliveira RB, Granoff DM, and Ram S. 2014. Fusion protein comprising factor H domains 6 and 7 and human IgG1 Fc as an antibacterial immunotherapeutic. Clin Vaccine Immunol 21: 1452–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong SM, Shaughnessy J, Ram S, and Akerley BJ. 2016. Defining the binding region in factor H to develop a therapeutic factor H-Fc fusion protein against nontypeable Haemophilus influenzae Front Cell Infect Microbiol [DOI] [PMC free article] [PubMed]
- 26.Blom AM, Magda M, Kohl L, Shaughnessy J, Lambris JD, Ram S, and Ermert D. 2017. Factor H-IgG Chimeric Proteins as a Therapeutic Approach against the Gram-Positive Bacterial Pathogen Streptococcus pyogenes. J Immunol [DOI] [PMC free article] [PubMed]
- 27.Lewis LA, Carter M, Burbage J, Chaves B, Beluchukwu A, Gulati S, Rice PA, and Ram S. 2012. The role of gonococcal Neisserial surface protein A in serum resistance and comparison of its factor H binding properties with that of its meningococcal counterpart. In XVIIIth International Pathogenic Neisseria Conference Frosch M, Rudel T, and Vogel U, eds, Wuerzburg, Germany: 389. [Google Scholar]
- 28.Shafer WM, Joiner K, Guymon LF, Cohen MS, and Sparling PF. 1984. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J Infect Dis 149: 175–183. [DOI] [PubMed] [Google Scholar]
- 29.Unemo M, Golparian D, Sanchez-Buso L, Grad Y, Jacobsson S, Ohnishi M, Lahra MM, Limnios A, Sikora AE, Wi T, and Harris SR. 2016. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother 71: 3096–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakraborti S, Lewis LA, Cox AD, St Michael F, Li J, Rice PA, and Ram S. 2016. Phase-Variable Heptose I Glycan Extensions Modulate Efficacy of 2C7 Vaccine Antibody Directed against Neisseria gonorrhoeae Lipooligosaccharide. J Immunol 196: 4576–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hitchcock PJ, Hayes SF, Mayer LW, Shafer WM, and Tessier SL. 1985. Analyses of gonococcal H.8 antigen: surface location, inter- and intrastrain electrophoretic heterogeneity, and unusual two- dimensional electrophoretic characteristics. J. Exp. Med 162: 2017–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ram S, Cox AD, Wright JC, Vogel U, Getzlaff S, Boden R, Li J, Plested JS, Meri S, Gulati S, Stein DC, Richards JC, Moxon ER, and Rice PA. 2003. Neisserial lipooligosaccharide is a target for complement component C4b: Inner core phosphoethanolamine residues define C4b linkage specificity. J Biol Chem 278: 50853–50862. [DOI] [PubMed] [Google Scholar]
- 33.Stein DC, LeVan A, Hardy B, Wang LC, Zimmerman L, and Song W. 2015. Expression of Opacity Proteins Interferes with the Transmigration of Neisseria gonorrhoeae across Polarized Epithelial Cells. PLoS One 10: e0134342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis LA, Ram S, Prasad A, Gulati S, Getzlaff S, Blom AM, Vogel U, and Rice PA. 2008. Defining targets for complement components C4b and C3b on the pathogenic neisseriae. Infect Immun 76: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen D, Gose S, Castro L, Chung K, Bernstein K, Samuel M, Bauer H, and Pandori M. 2014. Neisseria gonorrhoeae strain with reduced susceptibilities to extended-spectrum cephalosporins. Emerg Infect Dis 20: 1211–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ray TD, Lewis LA, Gulati S, Rice PA, and Ram S. 2011. Novel blocking human IgG directed against the pentapeptide repeat motifs of Neisseria meningitidis Lip/H.8 and Laz lipoproteins. J Immunol 186: 4881–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beernink PT, Shaughnessy J, Ram S, and Granoff DM. 2010. Impaired immunogenicity of a meningococcal factor H-binding protein vaccine engineered to eliminate factor h binding. Clin Vaccine Immunol 17: 1074–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duncan AR, and Winter G. 1988. The binding site for C1q on IgG. Nature 332: 738–740. [DOI] [PubMed] [Google Scholar]
- 39.Michaelsen TE, Sandlie I, Bratlie DB, Sandin RH, and Ihle O. 2009. Structural difference in the complement activation site of human IgG1 and IgG3. Scand J Immunol 70: 553–564. [DOI] [PubMed] [Google Scholar]
- 40.Sarantis H, and Gray-Owen SD. 2012. Defining the roles of human carcinoembryonic antigen-related cellular adhesion molecules during neutrophil responses to Neisseria gonorrhoeae. Infect Immun 80: 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jerse AE, Wu H, Packiam M, Vonck RA, Begum AA, and Garvin LE. 2011. Estradiol-Treated Female Mice as Surrogate Hosts for Neisseria gonorrhoeae Genital Tract Infections. Front Microbiol 2: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, and Walport MJ. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet 19: 56–59. [DOI] [PubMed] [Google Scholar]
- 43.Ermert D, Shaughnessy J, Joeris T, Kaplan J, Pang CJ, Kurt-Jones EA, Rice PA, Ram S, and Blom AM. 2015. Virulence of Group A Streptococci Is Enhanced by Human Complement Inhibitors. PLoS pathogens 11: e1005043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gulati S, Zheng B, Reed GW, Su X, Cox AD, St Michael F, Stupak J, Lewis LA, Ram S, and Rice PA. 2013. Immunization against a Saccharide Epitope Accelerates Clearance of Experimental Gonococcal Infection. PLoS Pathog 9: e1003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehlers MR 2000. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect 2: 289–294. [DOI] [PubMed] [Google Scholar]
- 46.van der Flier A, and Sonnenberg A. 2001. Function and interactions of integrins. Cell Tissue Res 305: 285–298. [DOI] [PubMed] [Google Scholar]
- 47.Ngampasutadol J, Ram S, Blom AM, Jarva H, Jerse AE, Lien E, Goguen J, Gulati S, and Rice PA. 2005. Human C4b-binding protein selectively interacts with Neisseria gonorrhoeae and results in species-specific infection. Proc Natl Acad Sci U S A 102: 17142–17147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gulati S, Agarwal S, Vasudhev S, Rice PA, and Ram S. 2012. Properdin is critical for antibody-dependent bactericidal activity against Neisseria gonorrhoeae that recruit C4b-binding protein. J Immunol 188: 3416–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ram S, Cullinane M, Blom A, Gulati S, McQuillen D, Monks B, O’Connell C, Boden R, Elkins C, Pangburn M, Dahlback B, and Rice PA. 2001. Binding of C4b-binding Protein to Porin: A molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med 193: 281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McQuillen DP, Gulati S, Ram S, Turner AK, Jani DB, Heeren TC, and Rice PA. 1999. Complement processing and immunoglobulin binding to Neisseria gonorrhoeae determined in vitro simulates in vivo effects. J Infect Dis 179: 124–135. [DOI] [PubMed] [Google Scholar]
- 51.Ketterer MR, Rice PA, Gulati S, Kiel S, Byerly L, Fortenberry JD, Soper DE, and Apicella MA. 2016. Desialylation of Neisseria gonorrhoeae Lipooligosaccharide by Cervicovaginal Microbiome Sialidases: The Potential for Enhancing Infectivity in Men. J Infect Dis 214: 1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gulati S, Schoenhofen IC, Whitfield DM, Cox AD, Li J, St Michael F, Vinogradov EV, Stupak J, Zheng B, Ohnishi M, Unemo M, Lewis LA, Taylor RE, Landig CS, Diaz S, Reed GW, Varki A, Rice PA, and Ram S. 2015. Utilizing CMP-Sialic Acid Analogs to Unravel Neisseria gonorrhoeae Lipooligosaccharide-Mediated Complement Resistance and Design Novel Therapeutics. PLoS Pathogens 11: e1005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis LA, Gulati S, Burrowes E, Zheng B, Ram S, and Rice PA. 2015. alpha-2,3-Sialyltransferase Expression Level Impacts the Kinetics of Lipooligosaccharide Sialylation, Complement Resistance, and the Ability of Neisseria gonorrhoeae to Colonize the Murine Genital Tract. MBio 6: e02465–02414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu H, and Jerse AE. 2006. Alpha-2,3-sialyltransferase enhances Neisseria gonorrhoeae survival during experimental murine genital tract infection. Infect Immun 74: 4094–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agarwal S, Ram S, Ngampasutadol J, Gulati S, Zipfel PF, and Rice PA. 2010. Factor H facilitates adherence of Neisseria gonorrhoeae to complement receptor 3 on eukaryotic cells. J Immunol 185: 4344–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nielsen TB, Pantapalangkoor P, Luna BM, Bruhn KW, Yan J, Dekitani K, Hsieh S, Yeshoua B, Pascual B, Vinogradov E, Hujer KM, Domitrovic TN, Bonomo RA, Russo TA, Lesczcyniecka M, Schneider T, and Spellberg B. 2017. Monoclonal Antibody Protects Against Acinetobacter baumannii Infection by Enhancing Bacterial Clearance and Evading Sepsis. J Infect Dis 216: 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


