Abstract
Highly expressed in the mammalian brain and widely distributed across the genome, MeCP2 is a key player in recognizing modified DNA and interpreting the epigenetic information encoded in different DNA methylation/hydroxymethylation patterns. Alterations in sequence or copy number of the X-linked human MECP2 gene cause either Rett syndrome (RTT) or MECP2 duplication syndrome. Alterations in MECP2 levels have also been identified in patients with autism. To fully understand the significant role of MECP2 in regulating the development and function of the nervous system, it is important to study all aspects of MeCP2 function. Stimulus-induced MeCP2 phosphorylation has been shown to influence the proliferation and differentiation of neural progenitor cells, synaptic scaling, excitatory synaptogenesis, and animal behavior. However, all of the previous functional evidence is from studying phospho-dead mutations. In addition, the relationship between phosphorylation events at multiple sites on the MeCP2 protein is not well understood. Here, we report the generation of a phospho-mimic knockin Mecp2 mouse line. At the synaptic and behavioral levels, the phospho-mimic Mecp2 mice show phenotypes opposite to those observed in phospho-dead mutation at the same phosphorylation site. Moreover, we report opposite phenotypes between phospho-mutants of two sites on the MeCP2 protein. Our new data further confirm the functional significance of specific MeCP2 phosphorylation event and support the opposing regulatory role between different MeCP2 phosphorylation events.
Introduction
Methyl-CpG binding protein 2 (MeCP2) is a key player in recognizing modified DNA (methylation and hydroxymethylation) and interpreting the epigenetic information encoded in different DNA methylation/hydroxymethylation patterns. MeCP2 was initially identified as a protein that specifically binds to methylated DNA in vitro and represses transcription from methylated promoters both in vitro and in vivo (Lewis et al., 1992; Nan et al., 1997). Later work from the Zoghbi group showed that MeCP2 might activate transcription of some genes, while repress others (Chahrour et al., 2008). Since it is highly expressed throughout the entire mammalian brain (Akbarian et al., 2001; Shahbazian et al., 2002; Ballas et al., 2009; Maezawa et al., 2009; Maezawa and Jin, 2010), it is not surprising that MeCP2 plays an important role in the development and function of the nervous system. Alterations in sequence, copy number, and expression level of the X-linked human MECP2 gene have been linked to Rett syndrome (RTT), MECP2 duplication syndrome, and autism (Nagarajan et al., 2006; Xi et al., 2007; Nagarajan et al., 2008; Ramocki et al., 2009).
MeCP2 is almost as abundant as the histones in the mouse brain, and is widely distributed across the entire genome (Skene et al., 2010). MeCP2 is similar to histones, the core components of chromatin, in three ways. First, both are present at abundant level in the nucleus. Second, both are widely distributed across the mammalian genome. Third, both can be modified post-translationally to influence gene transcription and subsequent cellular functions. Those properties allow MeCP2 to serve as a molecular switch on the chromatin to integrate diverse extracellular signals and generate adaptive transcriptional/functional outputs. Such adaptive and/or plastic responses are a hallmark of the nervous system across the molecular, synaptic, circuit, and behavioral levels. While the functions of histone modifications and its underlying mechanisms have been studied extensively, research to define the nature of MeCP2 modification, to characterize its function, and to reveal its underlying mechanism is still at the early stages.
MeCP2 phosphorylation was first discovered by the Greenberg group in a study designed to assess the potential role of MeCP2 in neuronal activity-dependent transcription regulation (Chen et al., 2003). The first identified and most studied neuronal activity-induced phosphorylation site on MeCP2 is serine 421 (S421). Past research in the field has firmly established neuronal activity-induced phosphorylation at S421 as an important module for regulating MeCP2 function in post-mitotic neurons (Chen et al., 2003; Zhou et al., 2006; Geranton et al., 2007; Murgatroyd et al., 2009; Deng et al., 2010; Cohen et al., 2011; Hutchinson et al., 2011; Li et al., 2011; Hutchinson et al., 2012; Zhong et al., 2012). Opposite to the dynamics of S421 phosphorylation, S80 is constitutively phosphorylated in resting neurons, and undergoes dephosphorylation upon membrane depolarization (Tao et al., 2009). Except for an opposing role against S421 phosphorylation in regulating locomotor activity (Tao et al., 2009), the in vivo function of S80 phosphorylation remains largely unknown.
Since the first report of neuronal activity-induced MeCP2 phosphorylation, most studies have focused on identifying the sites of phosphorylation and studying the potential function of these phosphorylation events in post-mitotic neurons. Recently, we discovered that S421 is also phosphorylated in adult neural progenitor cells (aNPC) isolated from the hippocampus. Interestingly, the stimulus, the regulation and the function of S421 phosphorylation in aNPCs are completely different from those in post-mitotic neurons. In aNPCs, MeCP2 S421 phosphorylation is induced by growth factors, linked to cell cycle, directly regulated by aurora kinase B, and plays critical roles in regulating the proliferation and differentiation of aNPCs through the Notch signaling pathway (Li et al., 2014). These new findings further generalize MeCP2 phosphorylation as a common regulatory module in cellular functions.
While previous studies have implicated a role for MeCP2 phosphorylation in regulating learning and memory (as measured by fear conditioning and Morris water maze tests), excitatory synaptogenesis, and synaptic scaling, all of them have focused on the effect of loss of MeCP2 phosphorylation by studying the phospho-dead mutant mice. Thus, it is necessary to analyze the phospho-mimic mutant mice to formally rule out the possibility that phenotypes of the phospho-mutants are caused by the missense mutation, but not the loss of phosphorylation. Moreover, the relationship between different phosphorylation sites on the MeCP2 protein is less well understood. To begin addressing those two questions, we have generated the Mecp2S421E mice to mimic phosphorylation at S421. Our initial characterization of the Mecp2S421E mice revealed synaptic and behavioral phenotypes that are opposite to those observed in the phospho-dead mutant Mecp2S421A;S424A mice. In addition, we performed more analysis on the previously reported Mecp2S80A mice, and revealed phenotypes in neural progenitor cell proliferation/differentiation that are opposite to those observed in the phospho-dead mutant Mecp2S421A;S424A mice. Our new data further confirm the functional significance of specific MeCP2 phosphorylation event and support the opposing regulatory role between different MeCP2 phosphorylation events.
Materials and Methods
Animals
All Mecp2 phospho-mutant knockin mice were generated through the conventional strategy of homologous recombination in mouse embryonic stem cells. All the experiments were performed using male mice. Mecp2S421E/y and Mecp2S80A/y mice have been backcrossed to C57BL/6J (http://www.jax.org/strain/000664) background for more than 10 generations. Mice were housed in 12hr light (6am–6pm)/12 hr dark (6pm–6am) cycle. Mice were housed <5 mice/cage. All protocols were approved by the Institutional Animal Care and Use Committee at University of Wisconsin-Madison.
Western blot analyses of S421 phosphorylation
Anti-MeCP2 (Abcam), and anti-phospho-S421 (custom made by Covance) were used. Brains were quickly dissected out from 10–12 weeks old Mecp2S421E/y and wild type littermate mice and homogenized in RIPA buffer containing both the protease inhibitor cocktail (Roche) and the protein phosphatase inhibitor cocktail (Roche). Lysate was run on 10% NuPAGE Bis-Tris gel (Invitrogen), and transferred to Protran BA 85 nitrocellulose membranes (Whatman). The membrane was incubated with appropriate primary and IR dye-conjugated secondary antibodies (Thermo Scientific), and scanned by the Odyssey infrared imaging system.
Primary culture of hippocampal neuron
Mecp2S421E/+ female and wild type male were mated to generate Mecp2S421E/y and Mecp2+/y pups. Genotyping assay was performed as described previously (Li et al., 2011). Dissociated hippocampal neurons were isolated from postnatal day 0–1 pups of both genotypes. The hippocampal neurons were isolated using the Papain Dissociation System (Worthington Biochemical Corporation LK003153). For the electrophysiology and AMPA receptor surface staining experiment, hippocampal neurons are plated at a density of 2.5× 104 cm−2 on 12 mm coverslips coated with 100ug/ml Poly-L-Lysine (Sigma P2636), and cultured in the Neurobasal A (Invitrogen 10888) containing 2% B27 (vol/vol, Invitrogen 12587-010), 1% FBS (vol/vol, Invitrogen 26140), 25 μM Glutamate and 0.5 mM GlutaMax (Invitrogen 35050-061). Half of the media was changed every 4 days with the Neurobasal A containing 2% B27 and 0.5 mM GlutaMax. 10 μM Ara-c (Sigma C1768) was added to the culture media in order to inhibit glial cell growth on 8 DIV. In experiments involving drug treatments, the treatments started at 16 DIV. 10 μM Ara-c was added starting from 8 DIV. The neurons were harvested on 18 DIV.
Analysis of excitatory synaptogenesis in cultured hippocampal and cortical neurons
Dissociated hippocampi neurons were plated at a density of 1 × 104/cm2 on poly-L-lysine (Sigma# P2636) coated coverslips. Culture medium was Neurobasal (Invitrogen #21103-049) containing 2% B27 (Invitrogen #12587-010), 1% N2 (invitrogen#17502-048), and 0.5 mM GlutaMax (Invitrogen#35050-061). Cultured hippocampal and cortical neurons were fixed at DIV (days in vitro) 21 with 4% PFA and 4% sucrose in PBS, washed with PBS, permeabilized with Tritonx-100 in PBS, blocked with 10% Normal Donkey Serum in PBS (all at room temperature), and incubated at 4 °C overnight with mouse anti-MAP2 (Millipore#05-346, 1:300), rabbit anti-PSD95 (Cell Signaling#2507, 1:200) and goat anti-VGLUT1 (Santa Cruz#sc-13320, 1:200) antibodies in PBS containing 10% normal donkey serum. The next day, after being washed in PBS, the coverslips were incubated with secondary antibodies, including AffiniPure F(ab′)2 Fragment donkey IgG(H+L) conjugated with DyLight-488 (anti-goat),-549 (anti-rabbit), -649 (anti-mouse) respectively (1:200, Jackson immunoResearch Laboratories). Coverslips were then washed in PBS and stained with DAPI before being mounted on glass slides with FLUOROMOUNT G (SouthernBiotech#0100-01).
Image analysis
Images were captured on a Nikon A1RSI confocal microscope. For each experimental series, all the images were acquired with identical settings for laser power, photomultiplier gain and offset. Z-stacks were collapsed in a maxmum projection and analyzed with NIH ImageJ software. For pre- and post-synaptic puncta quantification of the primary neuronal cell culture, only primary dendritic branches were included in the analysis. VGLUT1 and PSD95 images were thresholded using constant settings for each experiment, and converted to binary images. Thresholds were chosen such that all recognizable punctate structures were included into the analysis. VGLUT1 and PSD95 puncta were identified by the “partial analysis” function in ImageJ, with the minimal size 0.2 μm2 for VGLUT1 and 0.1 μm2 for PSD95. VGLUT1 puncta with overlap or directly adjacent PSD95 puncta were scored as colocalized VGLUT1/PSD-95 puncta. Puncta density was measured as the puncta number per μm of dendritic branch.
Electrophysiology
Whole-cell patch-clamp was carried out at room temperature. The recording chamber was continuously perfused with external recording solution contained (in mM): 120 NaCl, 3 KCl, 15 Hepes, 1 MgCl2, 2 CaCl2, 20 Glucose (pH7.4, 300 ± 5 mmol/kg). In order to record the AMPA receptors mediated mEPSCs only, the bath solution also contained 1 μM TTX, 20 μM Bicuculline and 50 μM D-AP5. The patch pipette (3 to 5 MΩ) solution contained (in mM) 140 K-gluconate, 7.5 KCl, 10 Hepes-K, 0.5 EGTA-K, 4 Mg-ATP and Li-GTP (pH7.4, 290± 5 mmol/kg). Hippocampal culture neurons were visualized using an Olympus Optical (Tokyo, Japan) BX51WI microscope. Pyramidal neurons were chosen according to the cell morphology (Turrigiano et al., 1998). The mEPSCs were recorded 3 min after the whole-cell voltage clamp configuration was established and lasted for 5 min on a gap free mode. The neurons were held at −70 mV. Whole-cell capacitance compensation was applied. Recordings with resting membrane potential (Vr) higher than −50mV and series resistance (Rs) greater than 15 MΩ were excluded. Raw data were amplified with a Multiclamp 700B amplifier, acquired with pClamp10.2 software (Axon Instruments, Sunnyvale, CA). Signals were filtered at 2 Hz and sampled at 10 kHz by Digidata 1440A (Axon Instruments, Sunnyvale, CA). mEPSCs were analyzed using the Template Search tool of the Clampfit10.2 software (Axon Instruments). In order to create the template, several well-shape mEPSCs traces were picked from the wild type hippocampal pyramidal neurons recording and averaged to the template window. Only the first 200 evens were used to do the data analysis. The mEPSCs events were accepted manually. All group data are shown as mean ± standard error of the mean (SEM).For the mEPSCs average event and the cumulative curve preparation, all the events from all of the recorded neurons were included. The average mEPSCs event was gained by using the concatenate files command and average traces command of Clampfit10.2. The sample traces and the average event traces were transferred out, and the traces were made by using SigmaPlot11.0 software (Scientific Computing).
Mouse behavioral tests
10–12 weeks old Mecp2S421E/y mice and their wild type littermates were used in the fear conditioning test. 5 months old Mecp2S80A/y mice and their wild type littermates were used in the Morris water maze test. All protocols were approved by the Institutional Animal Care and Use Committee at University of Wisconsin-Madison.
For fear conditioning test, mice were placed into a shock chamber and allowed to explore for 2 min. Then, mice received a mild footshock (0.5 mA). 2 min later, the same footshock was delivered again. 1 min after the end of the second shock, mice were returned to their home cages. The context test was performed 22 hr after the training. During the test, mice were placed back into the same training chamber, and monitored by an overhead camera in the chamber for 5 min. All events in the fear conditioning test were programmed and data recorded through the FreezeFrame2 and FreezeView software from (Actimetrics Software, Wilmette, IL, USA).
For Morris water maze, mice were trained to locate a hidden platform (1 cm below the surface of the water) in a circular pool (1.20 m in diameter) of opaque water using distal visual cues. Mice were given 2 blocks of 4 training trials a day for 4 consecutive days. During a training trial, each mouse was released into the pool from 1 of 4 starting positions in a random order. The location of the hidden platform remained constant throughout training. Time to find the platform was measured in each trial. If a mouse did not find the platform within 60 sec, it was gently guided by hand onto the platform and allowed to remain there for 10 sec. After 4 days, the probe test was performed with no platform. Each mouse was allowed to search the platform for 60 sec, while an overhead camera recorded its movement. Time spent searching in each quadrant by a mouse was used to characterize its search behavior. In the visible platform test, the platform was marked by a flag.
Isolation and culture of adult NPCs
NPCs used in this study were isolated from the DG of 6 to 8-week-old male Mecp2S80A/y mice and wild-type (WT) littermate controls based on published methods3. Briefly, DG was microdissected from 400 um coronal sections of forebrain. After enzymatic digestion using MACS Neural Tissue Dissociation kit (Miltenyi Biotech), DMEM/F-12 (Invitrogen) containing 10% FBS (Invitrogen) were added into each sample for stopping digestion. After filtering through a 70-μm m cell strainer (BD Biosciences) and washing with DMEM/F-12, the single-cell suspension was collected and cultured with proliferation medium: Neurobasal medium (Invitrogen) containing 20 ng/ml basic fibroblast growth factor (FGF-2, Waisman Biomanufacturing), 20 ng/ml epidermal growth factor (EGF, Peprotech), B27 supplement (Invitrogen), Penicillin Streptomycin (Invitrogen), and L-glutamine(Invitrogen) in a 5% CO2 incubator at 37°C. Half of the medium was replaced every two days.
Proliferation and differentiation assays of adult NPCs
Proliferation and differentiation of aNPCs were analyzed using published method4. Only early passage cells (between passage 4 and 10) and the same passage numbers of wild type and Mecp2S80A/y cells were used for the assay. For each experiment, stereological counting (Stereo Investigator, MBF Bioscience) of immunofluorescence positive cells from duplicated wells were analyzed, and results were averaged as one data point (n = 1). At least 3 independent experiments (n = 3) were performed and used for statistical analyses for each analysis.
In cell proliferation assay, we dissociated aNPCs with 0.05% Trypsin-EDTA (Invitrogen) for no longer than 1 min, stopped the digest with Trypsin inhibitor (Sigma-Aldrich) and finally plated the cells on poly-L-lysine/laminin-coated cover slips at a density of 100,000 cells/well in proliferation medium (see above). At 20 h post-plating, 5 μM M 5-bromo-2′-deoxyuridine (BrdU, Sigma-Aldrich) was added into the culture medium for 8 hours, after which the cells were fixed with 4% paraformaldehyde for 30 min at room temperature. To detect BrdU incorporation, fixed cells were pretreated with 1M HCl for 30 min at 37°C, and then washed with borate buffer, pH 8.5, for 30 min. We then followed our standard immunocytochemistry protocol.
For the differentiation assay, aNPCs were similar treated and plated as in the proliferation assay, but at a density of 50,000 cells/well. At 24 h post-plating, cells were changed into differentiation medium: Neurobasal medium with 1% B27 supplement, 1% Penicillin Streptomycin (Invitrogen),2 mM L-glutamine(Invitrogen), 5 μM M forskolin (Sigma-Aldrich), 1 μM M retinoic acid (Sigma-Aldrich). Half of the medium was changed with fresh differentiation medium for four days, followed by fixation with 4% paraformaldehyde for 30 min and standard immunocytochemistry protocol.
Statistical analysis
No statistical methods were used to predetermine sample sizes, but the samples sizes we used were consistent with those generally employed in the field. Data was first tested for normality using the D’Agostino-Pearson omnibus test when necessary. Comparisons between two groups were analyzed by unpaired t-test with Welch’s correction. Multiple comparisons in the same data set were analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. Data from multiple groups with multiple treatments were analyzed by two-way ANOVA followed by Tukey’s multiple comparisons test. For Morris water maze data, two way ANOVA with repeated measures was performed to reveal any significant interaction between genotype and quadrant, which was followed by a Tukey-HSD post hoc test to complete the pairwise comparison of the percentage of time spent by each genotype in each quadrant. P < 0.05 was considered to be statistically significant. Statistical processing was performed using Microsoft Excel and GraphPad Prism Software.
Results
Generation and characterization of the Mecp2S421E mice
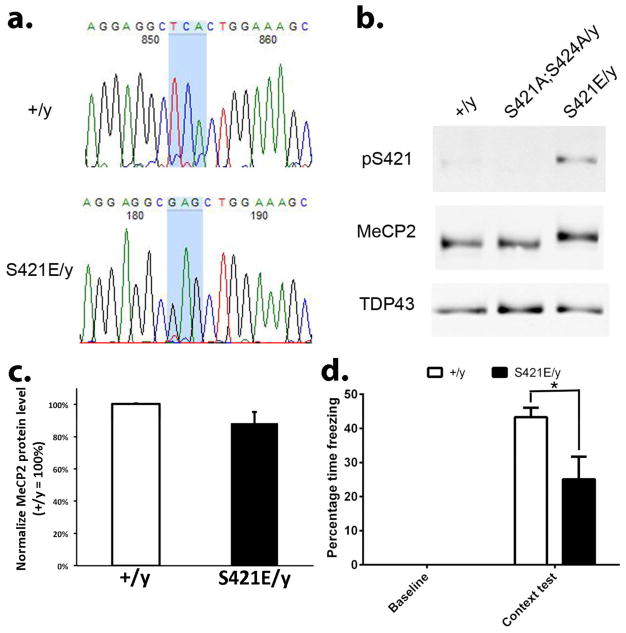
To complement previous studies of Mecp2 phospho-dead mutant mice and unequivocally determine the functional significance of MeCP2 S421 phosphorylation, we generated Mecp2S421E mice with serine to glutamic acid mutation at the 421th amino acid of the endogenous Mecp2 locus, as the serine to glutamic acid change has been widely used to mimic phosphorylation at serine residues. The Mecp2S421E mice were generated using conventional knockin stretagy through homologous recombination in mouse embryonic stem cells. Sequencing of the genomic DNA isolated from the Mecp2S421E/y mice revealed the presence of the mutated codon, but not the wild type codon observed in wild type samples (Fig. 1a). More importantly, an antibody that specifically recognizes S421 phosphorylation (pS421) detected a weak signal in wild type brain lysate, no signal in the Mecp2S421A;S424A/y brain lysate, and a strong signal in Mecp2S421E/y brain lysate (Fig. 1b). This pS421 antibody has been well characterized and reported in multiple previous studies (Zhou et al., 2006; Deng et al., 2010; Li et al., 2011; Li et al., 2014). Finally, an antibody that recognizes MeCP2 detected an upshift of the MeCP2 band in the Mecp2S421E/y brain lysate as compared with the those in the wild type and the Mecp2S421A;S424A/y brain lysates. This upshift reflects a slower migration of the MeCP2 protein in the gel caused by the phosphorylation event at S421, which has been reported in multiple previous studies (Chen et al., 2003; Zhou et al., 2006; Li et al., 2011). Importantly, the total MeCP2 protein level was similar between the wild type and Mecp2S421E/y mice (Fig. 1c). Together, these results strongly suggest we have generated an Mecp2 S421 phospho-mimic mouse line.
Figure 1. Generation and characterization of the Mecp2S421E/y mice.
(a) Genomic DNA from wild type (+/y) and Mecp2S421E/y mice (S421E/y) was amplified by PCR and sequenced, showing the wild type sequence TCA (encoding serine) is mutated to GAG (encoding glutamie acid) in the Mecp2S421E/y mice. (b) Western blot analysis with antibodies specific for phospho-S421 (pS421) and total MeCP2 in brain nuclear fraction from Mecp2+/y, Mecp2S421A;S424A/y, and Mecp2S421E/y mice. A nuclear protein TDP43 was used as a sample loading control. (c) Quantification of the total MeCP2 protein level in wild type and Mecp2S421E/y cortex (n=3 mice in each genotype, p=0.18). (d) The Mecp2S421E/y mice show a deficit in the hippocampus-dependent contextual fear learning/memory (n=4 mice in wild type and n=5 mice in Mecp2S421E/y, p=0.05). *p<0.05, Bar graphs represent mean ± s.e.m.
Impaired fear learning and memory and reduced excitatory synaptogenesis in the Mecp2S421E/y mice
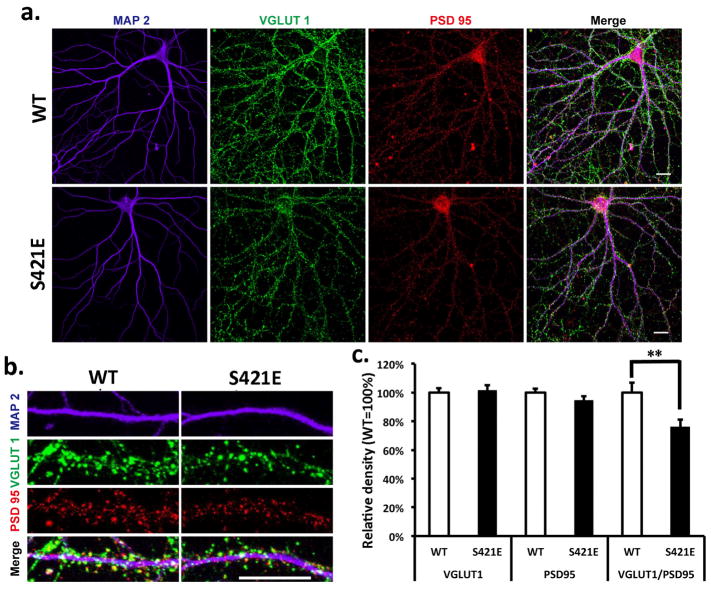
We have previously reported enhanced fear learning and memory in the Mecp2S421A;S424A/y (phospho-dead) mice (Li et al., 2011). For comparison, we tested the Mecp2S421E/y (phospho-mimic) mice in the fear conditioning test. Contrary to what was seen in the Mecp2S421A;S424A/y mice, the Mecp2S421E/y mice froze significantly less than the wild type mice (25% in Mecp2S421E/y vs. 43% in wild type littermates) in the context test (Fig. 1d), suggesting a deficit in fear learning and memory in the phospho-mimic mice. While the sample size was small (5 Mecp2S421E/y mice and 4 wild type mice), the difference nonetheless reached statistical significance (p=0.05). Since the Mecp2S421A;S424A/y mice also showed increased excitatory synaptogenesis (Li et al., 2011), we examined the development of excitatory synapses in hippocapal neurons isolated from the the Mecp2S421E/y mice. Primary hippocampal neurons were prepared from postnatal day 0–1 Mecp2S421E/y and wild type littermate pups, and cultured separately for 21 days in vitro (DIV). Triple immunostaining of VGLUT1 (excitatory presynaptic marker), PSD95 (postsynaptic marker), and MAP2 (dendritic marker) was performed on these cultures to determine the density of excitatory synapses. Result from this experiment revealed a 24% reduction of the density of excitatory synapses (i.e. number of co-localized VGLUT1/PSD95 puncta per unit length of MAP2 labeled neurites) in the Mecp2S421E/y mice (Fig. 2). This result is consistent with the the deficit in fear learning and memory observed in these mice. It is interesting to note that such a reduction of the density of excitatory synapses does not appear to be caused by a decrease in either pre-synaptic (as indicated by VGLUT1 puncta) or post-synaptic (as indicated by PSD95 puncta) density. Rather, it appears to be the result of the loss of co-localization of the VGLUT1 and PSD95 puncta. Future studies are needed to precisely define the underlying mechanism.
Figure 2. Reduced synaptogenesis in the Mecp2S421E/y mice.
(a) Representative images of wild type (WT) and Mecp2S421E/y (S421E) primary hippocampal neurons labeled with MAP2, VGLUT1, and PSD95. (b) Representative images of the primary dendrites of WT and S421E primary hippocampal neurons labeled with MAP2, VGLUT1, and PSD95. (c) Quantification of the density of excitatory synapses as measured by the colocalization of pre- and post-synaptic densities in hippocampal neurons isolated from the WT and S421E mice (n=52 in each genotype, p=0.729 for VGLUT1, p=0.195 for PSD95, p=0.007 for colocalization of VGLUT1 and PSD95). **p<0.01, Bar graphs represent mean ± s.e.m.
Mimicking phosphorylation at S421 specifically affects TTX-induced synaptic scaling up, but not bicuculline-induced synaptic scaling down
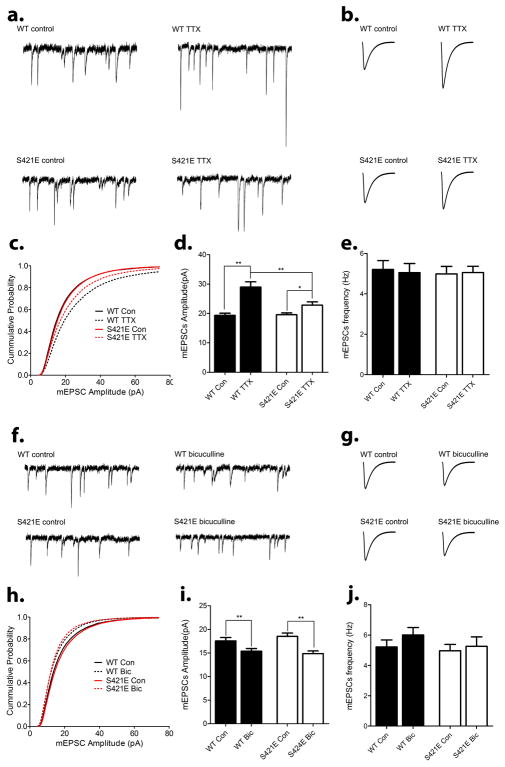
Our previous work has shown that loss of phosphorylation at S421 affects bicuculline-induced synaptic scaling down, but not TTX-induced synaptic scaling up (Zhong et al., 2012). To determining how mimicking phosphorylation at S421 influence synaptic scaling, hippocampal neurons were isolated from postnatal day 0–1 (P0-1) Mecp2S421E/y pups and their wild type littermates and cultured for 16 days in vitro (16 DIV). Cultures from both genotypes were then treated for 48–72 hours with either tetrodotoxin (TTX), or bicuculline, or left untreated. Consistent with previous studies, the amplitudes of miniature excitotary postsynaptic currents (mEPSCs) in wild type neurons increased significantly in response to the TTX treatment (Fig. 3a–d) and decreased significantly in response to the bicuculline treatment (Fig. 3f–i). Although the amplitudes of mEPSCs in the Mecp2S421E/y neurons showed a modest increase after the TTX treatment, the increase was significantly less than that observed in the wild type (Fig. 3d). In contrast, the extent of bicuculline-induced reduction in the amplitudes of mEPSCs in the Mecp2S421E/y neurons was comparable to that observed in the wild type (Fig. 3i). These alterations in S421 phospho-mimic neurons are opposite to those previously reported in S421 phospho-dead neurons (Zhong et al., 2012).
Figure 3. Impaired TTX-induced synaptic scaling up and normal bicucullin-induced synaptic scaling down in hippocampal neurons isolated from the Mecp2S421E/y mice.
(a–c) Representative traces (a), average waveforms (b) and cumulative probability distribution (c) of miniature excitatory postsynaptic current (mEPSC) obtained through whole-cell patch clamp recording from hippocampal neurons isolated from wild type (WT) and Mecp2S421E/y (S421E) mice that were cultured for 18 days in vitro (18 DIV) and treated with either control solution (control) or 1 μm TTX (TTX) for 48 hours. (d–e) Quantification of mean mEPSC amplitute (d) and frequency (e) for each experimental group in (a–c). (n=74–80 cells per group). (f–h) Representative traces (f), average waveforms (g) and cumulative probability distribution (h) of miniature excitatory postsynaptic current (mEPSC) obtained through whole-cell patch clamp recording from hippocampal neurons isolated from wild type (WT) and Mecp2S421E/y (S421E) mice that were cultured for 18 days in vitro (18 DIV) and treated with either control solution (control) or 40 μm bicuculline (bicuculline) for 48 hours. (i–j) Quantification of mean mEPSC amplitute (i) and frequency (j) for each experimental group in (f–h). (n=52–58 cells per group). *p<0.05, **p<0.01. Bar graphs represent mean ± s.e.m.
Impaired spatial learning and memory in the Mecp2S80A/y mice
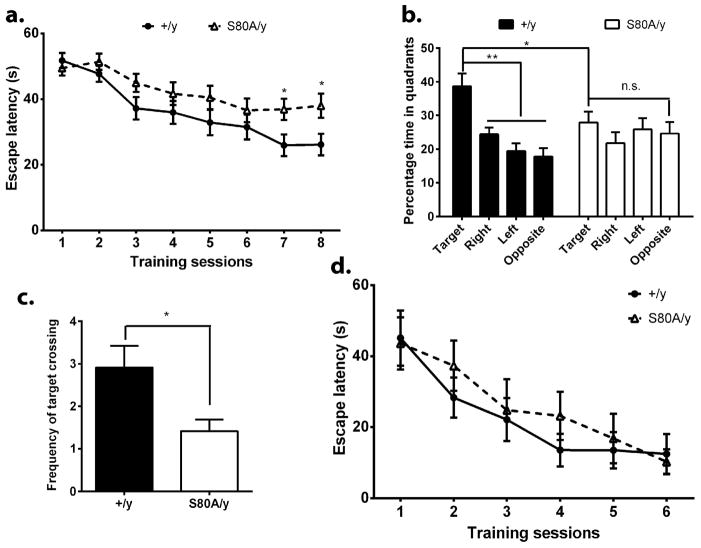
In post-mitotic neurons, membrane depolarization-triggered calcium influx leads to concurrent phosphorylation at S421 and dephosphorylation at S80 (Tao et al., 2009), suggesting that phosphorylation regulation at these two site (one at the N-terminus, the other at the C-terminus of the MeCP2 protein) need to be coordinated for normal neuronal functions. One hypothesis from this earlier observation is that the loss of S80 phosphorylation may lead to opposite phenotypes from the loss of S421 phosphorylation. To test that at the behavioral level, we analyzed the Mecp2S80A/y mice in the Morris water maze, a well-established hippocampus-dependent spatial learning and memory assay. The hidden platform version of the water maze test was used in this experiment. Throughout the training period, the wild type mice learned the location of the hidden platform equally well, as indicated by the persistent decrease in time needed to find and climb onto the platform (Fig. 4a). In the probe trials, the wild type mice remembered where the hidden platform was placed during training by spending significantly more time in the target quadrant than the other quadrants, indicating that they remembered the location of the platform (Fig. 4b). In contrast, the Mecp2S80A/y mice had trouble learning the location of the hidden platform, as indicated by the significantly more time needed to find and climb onto the platform at the end of the training session (Fig. 4a). Moreover, the Mecp2S80A/y mice did not remember the location of the platform, because they spent equal time in each quadrant (Fig. 4b) and crossed the location of the platform significantly less than the wild type mice (Fig. 4c). To rule out the possibility that the Mecp2S80A/y mice were blind, a separate cohort of the Mecp2S80A/y mice and their wild type littermates were trained in the visible platform version of the Morris water maze. Mice from both genotypes were able to learn the location of the visible platform effectively (Fig. 4d). Thus, the Mecp2S80A/y mice showed impaired spatial learning and memory, a phenotype opposite to that observed in the Mecp2S421A;S424A/y mice.
Figure 4. Impaired spatial learning and memory in the Mecp2S80A/y mice.
(a) Escape latency during the training sessions in the hidden-platform version of Morris water maze. The Mecp2S80A/y mice (S80A/y) did not learn as well as the wild type mice (+/y). (b) Percentage of time spent in each quadrant of the water maze during probe trial. While the +/y mice remembered the location of the hidden platform as indicated by their spending significantly more time in the target quadrant to search for the platform, the S80A/y mice did not. (c) The number of times mice crossing the location of the hidden platform used to be during the training sessions is significantly smaller in the S80A/y mice. (d) The S80A/y mice learned the location of the platform as efficiently as the +/y mice in the visible-platform version of the water maze, suggesting the learning dificit cannot be attributed to vision deficit. 8 Mecp2S80A/y mice and 7 wild type littermates were included in the Morris water maze test. *p<0.05, **p<0.01. Bar graphs represent mean ± s.e.m.
Increased proliferation and decreased neural differentiation in the Mecp2S80A/y aNPCs
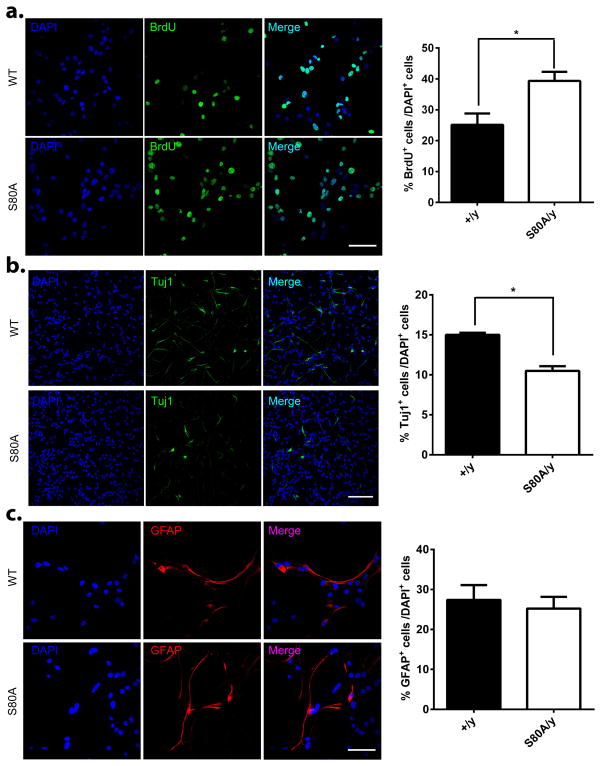
In addition to its important regulatory role in post-mitotic neurons, S421 phosphorylation has been shown to modulate the proliferation and differentiation of adult neural progenitor cells (aNPCs) (Li et al., 2014). To reveal the functional significance of S80 phosphorylation in aNPCs, we examined proliferation and differentiation in both wild type and Mecp2S80A/y aNPCs. Significantly more BrdU -labeled aNPCs were observed in phospho-mutant compared with WT cells (Fig. 5a), indicating an increased proliferation potential. Upon differentiation of aNPCs, significantly fewer Tuj1 positive cells were detected in Mecp2S80A/y as compared with the wild type cells (Fig. 5b), suggesting a reduced potential in neural differentiation. In contrast, no difference in glial differentiation was observed between the wild type and the Mecp2S80A/y aNPCs (Fig. 5c). Again, these phenotypes are opposite to those observed in the Mecp2S421A;S424A/y aNPCs (Li et al., 2014).
Figure 5. Increased proliferation and decreased neural differentiation in the Mecp2S80A/y aNPCs.
(a) Representative images and quantification of BrdU positive aNPCs isolated from wild type (+/y) and Mecp2S80A/y (S80A/y) adult hippocampus under proliferation condition with BrdU pulse labeling, followed by immunocytochemistry analysis using antibody specific for BrdU (p=0.04, n=3 in each genotype). BrdU labeling indicates actively dividing cells. (b) Representative immunocytochemistry images and percentage quantification of Tuj1 positive neurons differentiated from wild type (+/y) and Mecp2S80A/y (S80A/y) aNPCs (p=0.002, n=4 in each genotype). Tuj1 is a cell type specific marker for neurons. (c) Representative immunocytochemistry images and percentage quantification of GFAP positive astrocytes differentiated from wild type (+/y) and Mecp2S80A/y (S80A/y) aNPCs (p=0.68, n=3 in each genotype). GFAP is a cell type specific marker for astrocytes. Scale bars = 50 μm. *p<0.05, Bar graphs represent mean ± s.e.m.
Discussion
Previous studies from our group and others have provided abundant evidence for the significance of stimulus-induced MeCP2 phosphorylation in various aspects of neuronal development and function. Yet all of the evidence so far has come from phospho-dead mutant mice. We have generated the first phospho-mimic mutant mice (Mecp2S421E), and studied the effect of mimicking S421 phosphorylation on animal behavior, synaptogenesis, and synaptic scaling. In all assays used so far, mimicking S421 phosphorylation has led to phenotypes opposite to those caused by abolishing S421 phosphorylation (Table 1), which strongly suggests that the behavioral and synaptic phenotypes are caused by the presence/absence of phosphorylation, but not the missense mutation.
Table 1.
Opposite phenotypes observed in the S421 phospho-dead and phospho-mimic knockin mice.
| Phenotypic assays | S421A knockin mice (Li et al, 2011) | S421E knockin mice (this study) |
|---|---|---|
| Contextual Fear Memory | Increased | Decreased (Fig. 1c) |
| Synaptic scaling up | Normal | Reduced (Fig. 2) |
| Synaptic scaling down | Lost | Normal (Fig. 2) |
| Excitatory synaptogenesis | Increased | Decreased (Fig. 1d) |
In the meanwhile, we acknowledge that our definition of opposite is limited to the general direction of each phenotype. It remains to be seen whether any of these is mediated by opposite function at the molecular and cellular level. Moreover, it is important to point out that, while the serine to glutamic acid mutation is commonly used to mimic constitutive phosphorylation, there are significant caveats with this approach, because the mutation may either have confounding effect of protein structure or fail to recapitulate the physiological effects of phosphorylation. The interpretation of mimicking S421 phosphorylation is further compounded by the lack of a clear molecular effect that can be consistently verified. Thus, more detailed studies on the Mecp2S421E mutant mice in the future are needed to ascertain its role in mimicking S421 phosphorylation. Finally, we noticed a slight trend of lower (15% less) total MeCP2 protein level in the Mecp2S421E mutant brain (p=0.18) as compared to their wild type littermates. This could be due to the small sample size (3 mice in each genotype) and warrant further investigation in the future, since ~42% reduction of MeCP2 protein level has been reported to lead to behavioral phenotypes (Samaco et al., 2008).
Although multiple phosphorylation sites on the MeCP2 protein have been identified and studied, direct comparison between phenotypes modulated by different sites has been rare. In the current study, we phenotyped the Mecp2S80A mice in assays previously used to characterize the Mecp2S421A;S424A mice, thereby allowing direct comparison of the effect of those two phosphorylation events on animal behavior and neuronal development. Combining with results from three previous studies, our new data reveal an opposing regulatory role of S80 phosphorylation and S421 phosphorylation in functions ranging from the molecular, cellular, and animal behavioral levels (Table 2). Again, acknowledge that our definition of opposite is limited to the general direction of each phenotype. It remains to be seen whether any of these is mediated by opposite function at the molecular and cellular level. Nonetheless, these results raise a possibility that dynamic phosphorylation states at multiple sites on MeCP2 may form a combinatorial code, presenting another epigenetic regulatory module in addition to DNA methylation and histone codes. Future studies involving combinatorial phosphor mutations at different sites are needed to address such possibilities.
Table 2.
Opposite phenotypes observed in the S421 phospho-dead and S80A phospho-dead knockin mice.
| Phenotypic assays | S421A knockin mice (Li et al, 2011 and 2014; Tao et al, 2009) | S80A knockin mice (this study and Tao et al, 2009) |
|---|---|---|
| Morris water maze | Enhanced | Impaired (Fig. 3) |
| Baseline locomotor activity | Increased | Decreased |
| aNPC proliferation | Decreased | Increased (Fig. 4a) |
| aNPC neural differentiation | Increased | Decreased (Fig. 4b) |
| MeCP2 binding to gene promoters | Increased | Decreased |
Two previous studies have presented a puzzle on the role of MeCP2 in regulating adult neurogenesis. Smrt et al (Smrt et al., 2007) did not observe any significant change in aNPC proliferation/differentiation in the hippocampus of the Mecp2 knockout mice, while we showed decreased aNPC proliferation and increased neural differentiation in the hippocampus of the Mecp2S421A;S424A/y mice (Li et al., 2014). Our observation of increased prolifer ation and neural differentiation in the Mecp2S80A/y aNPC resolves that puzzle by revealing clearly opposing regulatory roles between S80 and S421 in the same biological process. In wild type cells, those forces balance each other out. In single mutant, the balance is lost, which results in corresponding phenotypes. In Mecp2 knockout cells, both forces are lost, which results in no overt phenotypes.
Although no RTT-causing mutations around the S421- or S80-equivalent sites in human MECP2 have been reported to date, a serine to proline missense mutation at S435 (S435P) in human MECP2 isoform 2 (the equivalent of S421 in mouse MeCP2 isoform 1 [Q9Z2D6-1]) is found 13 times in the Exome Aggregation Consortium (ExAC) database (http://exac.broadinstitute.org/gene/ENSG00000169057). In addition, a lysine to arginine missense mutation at L94 (L94R) in human MECP2 isoform 2 (2 amino acids away from the equivalent of S80 in mouse MeCP2 isoform 1) is found in the ExAC database. Since individuals affected by severe pediatric disease (RTT qualifies as a severe pediatric disease) are removed from the database, these mutations are unlikely to be associated with RTT. Nonetheless, in light of the wide-ranging phenotypes observed in the phospho-mutant Mecp2 mice, humans with missense mutations at these two key phosphorylation sites may have altered neural functions that have yet to be explored.
In summary, our new findings not only provide new insights into, but also establish new tools/approaches for future studies of the function of MeCP2 phosphorylation. Even if MeCP2 phosphorylation may not be directly relevant to any specific disease, it is still critical for a clear understanding of the molecular function of MeCP2 and its role in regulating neural functions in general.
Acknowledgments
This work was supported by NIH NICHD R01 HD064743 to Q.C. and U54HD090256 to the Waisman Center. H.L. was supported by a pre-doctoral fellowship from the Stem Cell and Regenerative Medicine Center at the University of Wisconsin-Madison. We thank Xiaoji Zhang for technical support.
Footnotes
Competing interest statement: The authors declare no competing financial interests.
References
- Akbarian S, Chen RZ, Gribnau J, Rasmussen TP, Fong H, Jaenisch R, Jones EG. Expression pattern of the Rett syndrome gene MeCP2 in primate prefrontal cortex. Neurobiol Dis. 2001;8:784–791. doi: 10.1006/nbdi.2001.0420. [DOI] [PubMed] [Google Scholar]
- Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Cohen S, Gabel HW, Hemberg M, Hutchinson AN, Sadacca LA, Ebert DH, Harmin DA, Greenberg RS, Verdine VK, Zhou Z, Wetsel WC, West AE, Greenberg ME. Genome-Wide Activity-Dependent MeCP2 Phosphorylation Regulates Nervous System Development and Function. Neuron. 2011;72:72–85. doi: 10.1016/j.neuron.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng JV, Rodriguiz RM, Hutchinson AN, Kim IH, Wetsel WC, West AE. MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nat Neurosci. 2010 doi: 10.1038/nn.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranton SM, Morenilla-Palao C, Hunt SP. A role for transcriptional repressor methyl-CpG-binding protein 2 and plasticity-related gene serum- and glucocorticoid-inducible kinase 1 in the induction of inflammatory pain states. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:6163–6173. doi: 10.1523/JNEUROSCI.1306-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson AN, Deng JV, Cohen S, West AE. Phosphorylation of MeCP2 at Ser421 contributes to chronic antidepressant action. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:14355–14363. doi: 10.1523/JNEUROSCI.2156-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson AN, Deng JV, Aryal DK, Wetsel WC, West AE. Differential Regulation of MeCP2 Phosphorylation in the CNS by Dopamine and Serotonin. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Meehan RR, Henzel WJ, Maurer-Fogy I, Jeppesen P, Klein F, Bird A. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69:905–914. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- Li H, Zhong X, Chau KF, Williams EC, Chang Q. Loss of activity-induced phosphorylation of MeCP2 enhances synaptogenesis, LTP and spatial memory. Nature neuroscience. 2011;14:1001–1008. doi: 10.1038/nn.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhong X, Chau KF, Santistevan NJ, Guo W, Kong G, Li X, Kadakia M, Masliah J, Chi J, Jin P, Zhang J, Zhao X, Chang Q. Cell cycle-linked MeCP2 phosphorylation modulates adult neurogenesis involving the Notch signaling pathway. Nat Commun. 2014;5:5601. doi: 10.1038/ncomms6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Jin LW. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:5346–5356. doi: 10.1523/JNEUROSCI.5966-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Swanberg S, Harvey D, LaSalle JM, Jin LW. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci. 2009;29:5051–5061. doi: 10.1523/JNEUROSCI.0324-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nature neuroscience. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Nagarajan RP, Hogart AR, Gwye Y, Martin MR, LaSalle JM. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006;1:e1–11. doi: 10.4161/epi.1.4.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan RP, Patzel KA, Martin M, Yasui DH, Swanberg SE, Hertz-Picciotto I, Hansen RL, Van de Water J, Pessah IN, Jiang R, Robinson WP, LaSalle JM. MECP2 promoter methylation and X chromosome inactivation in autism. Autism Res. 2008;1:169–178. doi: 10.1002/aur.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X, Campoy FJ, Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell. 1997;88:471–481. doi: 10.1016/s0092-8674(00)81887-5. [DOI] [PubMed] [Google Scholar]
- Ramocki MB, Peters SU, Tavyev YJ, Zhang F, Carvalho CM, Schaaf CP, Richman R, Fang P, Glaze DG, Lupski JR, Zoghbi HY. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann Neurol. 2009;66:771–782. doi: 10.1002/ana.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Fryer JD, Ren J, Fyffe S, Chao HT, Sun Y, Greer JJ, Zoghbi HY, Neul JL. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum Mol Genet. 2008;17:1718–1727. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian MD, Antalffy B, Armstrong DL, Zoghbi HY. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum Mol Genet. 2002;11:115–124. doi: 10.1093/hmg/11.2.115. [DOI] [PubMed] [Google Scholar]
- Skene PJ, Illingworth RS, Webb S, Kerr AR, James KD, Turner DJ, Andrews R, Bird AP. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrt RD, Eaves-Egenes J, Barkho BZ, Santistevan NJ, Zhao C, Aimone JB, Gage FH, Zhao X. Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiol Dis. 2007;27:77–89. doi: 10.1016/j.nbd.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Hu K, Chang Q, Wu H, Sherman NE, Martinowich K, Klose RJ, Schanen C, Jaenisch R, Wang W, Sun YE. Phosphorylation of MeCP2 at Serine 80 regulates its chromatin association and neurological function. Proc Natl Acad Sci U S A. 2009;106:4882–4887. doi: 10.1073/pnas.0811648106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Xi CY, Ma HW, Lu Y, Zhao YJ, Hua TY, Zhao Y, Ji YH. MeCP2 gene mutation analysis in autistic boys with developmental regression. Psychiatr Genet. 2007;17:113–116. doi: 10.1097/YPG.0b013e3280114a5c. [DOI] [PubMed] [Google Scholar]
- Zhong X, Li H, Chang Q. MeCP2 phosphorylation is required for modulating synaptic scaling through mGluR5. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:12841–12847. doi: 10.1523/JNEUROSCI.2784-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JA, Weitz CJ, Greenberg ME. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]