Abstract
Depression is characterized by low positive emotionality (PE) and high negative emotionality (NE), as well as asymmetries in resting electroencephalography (EEG) alpha power. Moreover, frontal asymmetry has itself been linked to PE, NE, and related constructs. However, little is known about associations of temperamental PE and NE with resting EEG asymmetries in young children and whether this association changes as a function of development. In a longitudinal study of 254 three-year old children, we assessed PE and NE at age 3 using a standard laboratory observation procedure. Frontal EEG asymmetries were assessed at age 3 and three years later at age 6. We observed a significant three-way interaction of preschool PE and NE and age at assessment for asymmetry at F3-F4 electrode sites, such that children with both low PE and high NE developed a pattern of increasingly lower relative left-frontal cortical activity over time. In addition, F7-F8 asymmetry was predicted by a PE by time interaction, such that the frontal asymmetry in children with high PE virtually disappeared by age 6. Overall, these findings suggest that early temperament is associated with developmental changes in frontal asymmetry, and that the combination of low PE and high NE predicts the development of the pattern of frontal symmetry that is associated with depression.
Keywords: positive emotionality, negative emotionality, development, resting eeg, frontal asymmetry, depression, children
Many vulnerability factors for depression have been identified, but few studies have examined associations among them or how one influences the development of others. There is increasing interest in examining biobehavioral systems (e.g., positive and negative valence systems) across multiple levels of analysis to inform understanding of the development of psychopathology (Insel et al., 2010). In this paper, we consider two sets of vulnerability factors for depression that represent different units of analysis - temperamental emotionality (behavior) and resting frontal EEG asymmetry (physiology) - and explore whether temperament is associated with developmental changes in asymmetries in early childhood.
Depression and Temperament
Depression is associated with high levels of negative emotionality (NE) and low levels of positive emotionality (PE) (Klein, Kotov, & Bufferd, 2011; Kotov, Gamez, Schmidt, & Watson, 2010). NE is a temperament trait that refers to a tendency to experience sadness, fear, anger, and reactivity to stress, whereas PE refers to the tendency to experience joy, engagement with the environment, and sensitivity to reward. In addition to cross-sectional associations, longitudinal research indicates that low PE and high NE predict the first onset of depression and/or increases in depression symptoms (Goldstein, Kotov, Perlman, Watson, & Klein, (in press); Hakulinen et al., 2015). While either trait alone can predict depression, some researchers have reported that they interact such that depression is associated with the combination of low PE and high NE (Gershuny & Sher, 1998; Joiner & Lonigan, 2000; Vasey et al., 2013).
Depression and Resting EEG
Depression has also been associated with asymmetries in EEG alpha power measured at rest (Allen & Reznik, 2015; Thibodeau, Jorgensen, & Kim, 2006). Power in the alpha frequency band is thought of as the inverse of cortical activity (Laufs et al., 2003). Lower relative left-frontal cortical activity is found in currently depressed individuals (Henriques & Davidson, 1991; Thibodeau et al., 2006), persists after remission from depression (Henriques & Davidson, 1990), is present in offspring of depressed mothers (Goldstein et al., 2016), and predicts subsequent onset of major depressive disorder (Nusslock et al., 2011). In Davidson’s (1994) influential model, lower relative left-frontal activity is thought to index a reduced propensity to engage in approach behavior or a disposition to withdrawal behavior.
PE, NE, and Resting EEG asymmetries
There is a modest literature examining relationships of trait NE and PE with resting EEG asymmetries in adults. Higher NE and/or lower PE have been associated with lower relative left-frontal cortical activity (Jacobs & Snyder, 1996, but see Schmidtke & Heller, 2004), and these associations might be strongest for those who exhibit the greatest discrepancy between PE and NE (Tomarken, Davidson, Wheeler, & Doss, 1992). Relatedly, self-reports of trait behavioral activation system sensitivity, which overlaps conceptually with PE, are associated with greater relative left-frontal activity, and behavioral inhibition system sensitivity, a construct related to NE, is associated with lower relative left-frontal activity (Coan & Allen, 2003; Harmon-Jones & Allen, 1997; Hewig, Hagemann, Seifert, Naumann, & Bartussek, 2006; Sutton & Davidson, 1997, but see Wacker, Chavanon, & Stemmler, 2010). It should be noted, however, that not all components of NE are associated with lower relative left-frontal activity. Anger, which has been posited to be an approach emotion, appears to be related to higher relative left-frontal activity (Harmon-Jones, Gable, & Peterson, 2010).
Frontal asymmetry is also associated with response to emotionally salient stimuli in laboratory settings. For instance, in adults, lower relative left-frontal activity has been shown to predict increased negative affect in response to emotional film clips (Tomarken, Davidson, & Henriques, 1990) and decreased sensitivity to reward (Shankman, Klein, Tenke, & Bruder, 2007). However, less is known about how NE or PE are related to EEG asymmetries in children.
Temperament and Resting EEG in Children
In children, most studies of temperament and resting EEG asymmetries have focused on Kagan’s construct of temperamental behavioral inhibition (Kagan, Reznick, Clarke, Snidman, & Garcia-Coll, 1984), which emphasizes the fearfulness component of NE, but also includes aspects of low PE and high constraint (Laptook et al., 2008; 2010). In these studies, behaviorally inhibited children have generally exhibited lower relative left-frontal cortical activity (Calkins, Fox, & Marshall, 1996; Hane, Fox, Henderson, & Marshall, 2008), whereas uninhibited (or exuberant) children had greater relative left-frontal activity (Fox et al., 1995).
Only a few studies have examined associations of NE and PE with frontal asymmetry in children, generally failing to find significant associations (Shankman et al., 2005, 2011). Shankman et al. (2005) classified three-year-old children as either low or high on PE using a behavioral observation measure; three years later, 29 of the children received a resting EEG assessment. The groups did not differ on frontal asymmetry. In an earlier article using the sample in this paper, Shankman et al. (2011) examined resting EEG, PE, and NE in 329 three-year-olds. Again, they found no evidence for associations between PE or NE with frontal asymmetry. However, few studies have examined associations of PE and NE with frontal asymmetries as a function of development, which may clarify why results differ in children compared to adults.
Developmental changes of neurobiological systems of PE and NE
The lack of longitudinal research in youth is problematic as marked neural changes occur during development, including to cortical and subcortical regions that are linked to processes associated with PE and NE (Sowell et al., 2004). Structural and functional imaging studies of the regions and networks involved in the processing of emotions broadly, and reward (associated with PE) and punishment (associated with NE) more specifically, suggest that different brain regions develop along varying trajectories (often following an inverted U shape) which peak at different ages for different regions (Giedd & Rapoport, 2010; Somerville & Casey, 2010). Specifically, structures like the prefrontal cortex (important for emotion processing, cognitive control, motivation, and modulation of contingency learning) reach maturity later than subcortical regions such as the amygdala (associated with emotion) or the ventral striatum (associated with reward/punishment processing). In addition to differences in structure, task elicited activity and functional connectivity between areas related to PE and NE often differ with age (Galvan et al., 2006; Heller, Cohen, Dreyfuss, & Casey, 2016; Van Der Schaaf, Warmerdam, Crone, & Cools, 2011).
Moreover, there is evidence that frontal asymmetry changes in early childhood. First, the test-retest stability of EEG asymmetries in children is modest (McLaughlin, Fox, Zeanah, & Nelson, 2011; Müller, Kühn-Popp, Meinhardt, Sodian, & Paulus, 2015; Vuga, Fox, Cohn, Kovacs, & George, 2008). Additionally, some investigators have reported changes in the correlates of frontal asymmetry. Specifically, the associations of frontal asymmetry with temperamental behavioral inhibition, early life stressors, parenting, and maternal depression vary in magnitude across childhood (Diego, Field, Jones, & Hernandez-Reif, 2006; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Goldstein et al., 2016; McLaughlin et al., 2011).
To address the issue of change in asymmetries and the impact of development on the relationship between asymmetry, NE and PE requires longitudinal studies with multiple EEG assessments spaced over time. In the only study of young children to examine NE and resting EEG on several occasions, Lusby et al. (2016) found that the relationship between NE and EEG changed over time. At 3 months of age, higher NE was associated with lower relative left-frontal cortical activity in offspring of depressed mothers, but by 12 months of age the effect was reversed such that higher NE was associated with greater relative left-frontal activity. This study suggests that the relationship between NE and EEG frontal asymmetry may change considerably in early childhood and highlights the need for multiple assessments across development. To our knowledge, no previous studies have examined associations between PE and frontal asymmetry repeatedly across development.
The Present Study
In the current study, we assessed PE, NE and resting frontal asymmetry in 3-year-old children and then repeated the EEG assessment at age 6. As depression may be associated with high NE and low PE, as well as lower relative left-frontal activity, we hypothesized that NE would be positively, and PE negatively, associated with lower relative left-frontal activity. Moreover, as depression may be associated with an interaction of low PE and high NE, we examined whether this combination of traits was associated with the lowest level of relative left-frontal activity. Based on suggestive evidence that frontal asymmetry changes over time, we anticipated that PE, NE, or their combination may be associated with frontal asymmetry in different ways across development. Lastly, some researchers view anger as an approach-related emotion, whereas fear and sadness are withdrawal-related emotions (Harmon-Jones et al., 2010; Lewis & Ramsay, 2005). Therefore, we conducted additional analyses in which we examined the anger, sadness, and fearfulness components of NE separately.
Methods
Recruitment, sample demographics, and assessments have been described previously (Olino, Klein, Dyson, Rose, & Durbin, 2010; Shankman et al., 2011). An unselected community sample of 559 3-year-old children living on Long Island, New York were recruited for a longitudinal study of the development of temperament and psychopathology. At the age 3 baseline assessment, resting EEG and the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith, Reilly, Lemery, Longley, & Prescott, 1995), were administered. Approximately, 3 years later when the children were age 6, resting EEG was re-assessed. The participating parent was given a thorough overview of the study procedures and then provided written consent at both time points. All procedures were approved by Stony Brook Universities, Committees on Research Involving Human Subjects.
At baseline, 404 children completed both EEG and the Lab-TAB, of whom 333 completed EEG at follow-up. Conventionally, in EEG frontal asymmetry research, left-handed or ambidextrous children are excluded as their hemispheric lateralization is unclear. Handedness was determined by observing which hand/foot a child used to throw a ball, draw a circle, pretend to use scissors, open a jar, kick a ball, and look through a paper towel (Longoni & Orsini, 1988). As such, 70 children were removed. An additional child’s EEG data were lost due to technical problems and one child was removed for being an outlier (this child’s EEG data was an order of magnitude above the largest value observed in any other child). As we regard temperamental emotionality and EEG asymmetries as vulnerabilities to depression, we excluded seven children who had already been diagnosed with depression via the Preschool Age Psychiatric Assessment (PAPA; Egger & Angold, 2004). Therefore, the final sample for this study is 254 children. Children with complete data were similar to those missing data regarding racial/ethnic minority status (11.8% vs. 14.1% non-Caucasian and/or Hispanic; χ2 (1, N = 559) = .64, p > .05), parental marital status (94.5% vs. 94.2% married; χ2 (1, N = 517) = .02, p > .05), and the proportion of families where both parents held a college degree (37.2% vs. 29.5% with four-year degrees; χ2 (1, N = 545) = 3.64, p > .05). However, those missing data were more likely to be male; (57.7% vs. 49.2% male; χ2 (1, N = 559) = 4.02, p = .05). Gender was not associated with asymmetry when included as a covariate so it was dropped from final models. In the final sample, children were a mean 3.52 (SD=0.26) years of age at baseline and 6.02 (SD = 0.38) years at follow-up.
Measures
Laboratory Temperament Assessment Battery (Lab-TAB)
The Lab-TAB consists of a series of age-appropriate, standardized episodes that assess temperament-relevant emotional displays and behavior (Gagne, Van Hulle, Aksan, Essex, & Goldsmith, 2011; Goldsmith et al., 1995). Lab-Tab scores are associated with other methods of assessing temperament and are moderately stable over time (Durbin et al., 2007; Dyson et al., 2015). Children participated in 12 episodes, which were video-recorded and separated by brief breaks to reduce carryover effects.
Each episode is described in order of administration, along with the types of displays it tends to elicit. Risk room (behavioral inhibition/fear; activity level): Children are presented with ambiguous novel stimuli (e.g. a Halloween mask, a staircase, a black box) and allowed to explore. Tower of Patience (inhibitory control; interest): the child and experimenter build a tower, but the experimenter stalls by taking progressively more time to place the next block. Arc of Toys (PE; NE): the child is given permission to play independently with toys for 5 minutes and then has to clean up. Stranger Approach (behavioral inhibition/fear): the child is left alone in a room and a male research assistant enters and slowly walks toward the child. Make that Car Go (PE; interest): the child and experimenter race remote controlled cars. Transparent Box (persistence; interest; NE): the child picks a toy, which is then put in a box. The child is given a set of nonworking keys to open the box. Later, the child is offered the correct key. Exploring new Objects (behavioral inhibition/fear): the child is presented with novel stimuli, including objects in a cage that could be rodents, a mechanical spider, and sticky gel balls. The experimenter encourages the child to play. Pop up Snakes (PE): the experimenter shows the child a trick-container that is spring-loaded with “snakes.” The experimenter encourages the child to scare the parent. Impossibly Perfect Green Circles (NE; persistence): the experimenter asks the child to draw circles, but mildly criticizes the child after each circle is drawn. Popping Bubbles (PE; interest): the child and experimenter play with a bubble maker. Snack Delay (inhibitory control): the child is instructed not to eat until a bell is rung. Box Empty (NE): the child is given a wrapped gift box that is empty. The experimenter then gives the child toys to keep.
Behavioral coders were required to achieve 80% or higher agreement with an expert rater on all codes within an episode before coding independently (Dyson et al., 2015). Specific facial, vocal, and bodily indicators of affect and behavior (anger, fear, sadness, positive affect/exuberance, interest/engagement) were separately coded and aggregated to form the PE and NE composite variables. More specifically, each instance of facial, bodily, and vocal positive and negative affect was rated on a 3-point intensity scale (low, moderate, and high) and summed within episodes. Then an average was taken across episodes in each channel (e.g. an average fear facial score derived from each episode). Lastly, scores for affect in each channel were standardized and used to create an average for all affect indicators. For example, a participant’s fear score was calculated by standardized facial fear + standardized vocal fear + standardized bodily fear and divided by 3. Interest/engagement was assessed using a 4-point scale (none, low, moderate, and high) for each episode, which were then summed across all episodes. It was rated based on children’s vocalizations and behavior during the episode. Anger, fear, and sadness were combined to produce NE, and positive affect and interest/engagement were combined to produce PE. To reduce positive skew, NE was natural log transformed. PE and NE were found to possess adequate internal consistency (Cronbach alphas = .82 for both PE and NE) and inter-rater reliability (inter-class correlations = .89 for PE and .74 for NE). Inter-rater reliability was established on independent coders’ ratings of each episode for 35 participants. The descriptive statistics for and correlations among the temperament data can be found in Table 1. The results suggest a wide range of emotional/behavioral responses in the sample. Additionally, all three facets are strongly related to NE, but the associations amongst the facets vary considerably. Specifically, sadness is significantly associated with anger and fear, but fear and anger were not significantly correlated.
Table 1.
Descriptive statistics and correlations for temperament variables
| M | SD | Range | PE | NE | Anger | Fear | |
|---|---|---|---|---|---|---|---|
| PE | .10 | 1.64 | −5.09 to 4.83 | ||||
| NE | .55 | .26 | 0 to 1.32 | ||||
| −0.09 | |||||||
| Anger | .56 | .33 | 0 to 1.69 | ||||
| 0.03 | .64** | ||||||
| Fear | .66 | .37 | 0 to 1.76 | −0.09 | .70** | 0.05 | |
| Sadness | .53 | .29 | 0 to 1.75 | ||||
| −0.09 | .70** | .37** | .24** |
NE and its components were log transformed to reduce skew.
Resting EEG assessment
EEG was recorded in a sound attenuating chamber for 6 minutes while children sat at rest alternating between 1 minute blocks of eyes open and closed. EEG was recorded using a 32 electrode channel Lycra cap following the 10/20 labeling system (American Electroencephalographic Society, 1994). An extra electrode served as a reference on the nose. Blinks were captured by two electrodes above and below the left eye, one to the left of the left eye, and one to the right of the right eye. The Active Two system (Biosemi, Amsterdam, The Netherlands) was used to collect data at a sampling rate of 512 Hz. A 0.16-40 Hz bandpass filter was applied to all channels.
For further offline processing, Biosemi data was converted to Neuroscan 4.1 (Charlotte, NC) using PolyRex (Kayser, 2003). Data was segmented into 1.024-s epochs with each epoch overlapping by 50% to maximization data retention. Artifacts caused by blinks or other obvious movements were removed from the segmented data by visual inspection. A fast Fourier transform was applied to the data to compute power spectra. The alpha band was defined as ranging from 6-10 Hz, which is recommended for this age range (Marshall, Bar-Haim, & Fox, 2002). Average of alpha power was for each channel. Descriptive statistics for EEG power at each recording electrode are presented in Table 2. Intraclass correlations for the stability of asymmetry scores (Table 2) were similar, but somewhat smaller, than previous studies.
Table 2.
Average alpha power at each electrode site and Intraclass correlations for frontal asymmetry
| Medial | M | (SD) | Lateral | M | (SD) |
|---|---|---|---|---|---|
| F4 age 3 | 1.87 | (0.38) | F8 age 3 | 1.60 | (0.33) |
| F3 age 3 | 1.86 | (0.36) | F7 age 3 | 1.63 | (0.33) |
| F4 age 6 | 1.66 | (0.37) | F8 age 6 | 1.37 | (0.35) |
| F3 age 6 | 1.67 | (0.41) | F7 age 6 | 1.37 | (0.36) |
| F3-4 (open) | .08 | F7-8 (open) | .20*** | ||
| F3-4 (closed) | .07 | F7-8 (closed) | .26*** |
Frontal alpha for each electrode site at each age (averaged across eye recording condition). F corresponds to frontal electrodes; odd numbers correspond to the left hemisphere and even numbers correspond to the right hemisphere. The intraclass correlation are given for both eyes open and closed conditions.
p< .01
p<.001
Data analysis
Frontal asymmetry was computed by taking the difference of natural log transformed pairs of frontal electrodes (medial, F3-4 and lateral, F7-8 pairs) calculated as Ln(right) – Ln(left). Scores are interpreted so that positive scores indicate greater relative left cortical activity, negative scores indicate lower relative left cortical activity, and scores close to zero represent similar levels of left and right activity. To examine asymmetries, we used multi-level modeling (MLM; Mplus version 7.31) with maximum-likelihood estimation. This analytic approach is common in the asymmetry literature in part because it is more powerful than repeated measures ANOVA (Bagiella, Sloan, & Heitjan, 2000) and in our case, allows for a more precise model of age and the interval between assessments. In cross-sectional studies of frontal asymmetry, a two-level model may be sufficient to account for within subject variance related to recording conditions. However, our data was clustered within recording sessions and within subjects, requiring an additional level. Therefore, we used a three-level model to account for clustered data within recording sessions (alternating blocks of different eye conditions at each time) and within each participant (two time points per child). In our omnibus model, the data were structured in “long format” such that asymmetry scores for eye condition (open or closed), electrode position (F3-4 or F7-8), and assessment time point (age 3 or 6) were stacked. The first level predictor was eye condition (open vs. closed), the second level predictor was participant age at assessment and the third level predictors were NE, PE, and their interaction. Full factorial omnibus models were specified, resulting in 32 predictors (the full factorial design allows for testing developmental effects in two- or three-way cross level interactions between age at assessment, NE, and PE).
The age of each child at the assessment was used as the time variable. Age was mean centered to the samples’ average age at baseline. Age was a random effect variable, so that the effect of age on hemispheric asymmetries could vary for each participant (Singer & Willet, 2003). PE, NE, and eye condition were treated as fixed effects variables.
We initially ran an omnibus model for frontal asymmetry that collapsed across the F3-F4 and F7-F8 electrode pairs. However, it is often found that effects differ for different electrode pairs (Shankman et al., 2011); therefore, if the electrode pair variable was significant in interactions with substantive variables we planned to run follow-up models separating the electrode pairs. In tables of follow-up MLMs only substantive variables are shown. Follow-up analyses of significant interactions used Preacher’s approach (Preacher, Curran, & Bauer, 2006). For a three-way interaction, values are selected for two independent variables (W1 and W2) such that the relationship of a third independent variable (X) to the outcome variables can be interpreted at specified levels of W1 and W2. For instance, NE and PE can be specified as low or high so the effect of age on FA is then interpreted as a function of the level and combination of NE and PE.1 We also ran models examining the facets of NE (sadness, fear, and anger) for electrode pairs that were predicted by NE in the primary analyses.2
Results
Overall Frontal Models
The overall MLM for frontal asymmetry can be found in Table 3. There were no significant main effects for temperament or age. However, significant interactions with electrode position suggest that the effects of age and temperament for frontal asymmetry can be best interpreted by examining each electrode pair separately. Therefore, separate MLMs for F3-F4 and F7-F8 were conducted.
Table 3.
Effects of temperament and age on frontal asymmetry
| Full Model | |||
|---|---|---|---|
| Estimate | SE | p-val | |
| Intercept | −0.028 | 0.009 | 0.004 |
| Elec1 | −0.049 | 0.011 | <0.001 |
| Eye 2 | 0.018 | 0.011 | 0.10 |
| Elec*Eye | 0.004 | 0.022 | 0.86 |
| Age | 0.004 | 0.005 | 0.43 |
| Age*Elec | 0.013 | 0.006 | 0.04 |
| Age*Eye | −0.004 | 0.006 | 0.52 |
| Age*Elec*Eye | −0.007 | 0.012 | 0.55 |
| PE | −0.008 | 0.006 | 0.17 |
| NE | −0.008 | 0.037 | 0.83 |
| PE*NE | −0.029 | 0.024 | 0.23 |
| Age*PE | 0.008 | 0.003 | 0.01 |
| Age*NE | −0.018 | 0.019 | 0.33 |
| Age*PE*NE | 0.013 | 0.013 | 0.32 |
| Elec*PE | −0.003 | 0.007 | 0.62 |
| Elec*NE | −0.038 | 0.044 | 0.38 |
| Elec*PE*NE | 0.02 | 0.029 | 0.49 |
| Eye*PE | 0.002 | 0.007 | 0.74 |
| Eye*NE | −0.004 | 0.044 | 0.92 |
| Eye*PE*NE | 0.016 | 0.029 | 0.58 |
| Elec*Eye*PE | −0.005 | 0.014 | 0.69 |
| Elec*Eye*NE | −0.002 | 0.087 | 0.98 |
| Elec*Eye*PE*NE | −0.014 | 0.057 | 0.81 |
| Age*Elec*PE | 0.003 | 0.004 | 0.41 |
| Age*Elec*NE | 0.07 | 0.024 | 0.003 |
| Age*Elec*PE*NE | −0.073 | 0.016 | <0.001 |
| Age*Eye*PE | <0.001 | 0.004 | 0.93 |
| Age*Eye*NE | 0.02 | 0.024 | 0.41 |
| Age*Eye*PE*NE | −0.006 | 0.016 | 0.69 |
| Age*Elec*Eye*PE | 0.005 | 0.008 | 0.49 |
| Age*Elec*Eye*NE | −0.004 | 0.048 | 0.93 |
| Age*Elec*Eye*PE*NE | 0.019 | 0.032 | 0.55 |
Elec refers to a dichotomous variable coded to designate between the F3-4 and. F7-8 electrode pairs.
refers to whether the activity was measured when eyes were open or closed, which was alternated in one minute blocks for all subjects.
Frontal Asymmetry NE, PE, and age
The MLM for F3-F4 found that the main effects of age, PE, and NE, were not significant (Table 4). The age by NE interaction was significant, but this was qualified by a significant three-way age by PE by NE interaction. Follow-up simple slopes were calculated at 1 SD above and below the mean of NE and PE (Figure 1). Follow-up analyses indicate that children with both low PE and high NE developed a pattern of increasingly lower relative left-frontal cortical activity with age (b=−0.039, SE=0.012, p=.001). However, age was not associated with frontal asymmetry for any other combination of PE and NE. Specifically, the effect of age on frontal asymmetry was not significant for those with high PE and high NE (b=0.010, SE=0.013, p=0.45); high PE and low NE (b=0.003, SE=0.012, p=.82); or low PE and low NE (b=0.015, SE=0.013, p=.23). We further examined the effects of PE/NE at ages 3 and 6. At age 3, neither children with low PE (b=0.054, SE=0.052, p=.33), nor those with high PE (b=−0.043, SE=0.054, p=.44) differed on frontal asymmetry as a function of NE. At age 6, children with high PE again did not differ on frontal asymmetry as a function of NE (b=−0.008, SE=0.076, p=.92). However, among six-year-olds with low PE, those with high NE had lower relative left-frontal cortical activity compared to those with low NE (b=−0.213, SE=0.076, p< .005).
Table 4.
Effects of temperament and age on frontal asymmetry by each electrode pair
| F3-F4 | F7-F8 | |||||
|---|---|---|---|---|---|---|
| Estimate | SE | p-val | Estimate | SE | p-val | |
| Age | −0.003 | 0.006 | 0.64 | 0.010 | 0.007 | 0.13 |
| PE | −0.006 | 0.006 | 0.32 | −0.010 | 0.008 | 0.21 |
| NE | 0.005 | 0.036 | 0.88 | −0.025 | 0.052 | 0.64 |
| PE * NE | −0.030 | 0.024 | 0.21 | −0.025 | 0.034 | 0.46 |
| Age * PE | 0.006 | 0.004 | 0.13 | 0.010 | 0.004 | 0.02 |
| Age * NE | −0.046 | 0.023 | 0.05 | 0.014 | 0.026 | 0.60 |
| Age * PE * NE | 0.037 | 0.016 | 0.02 | −0.017 | 0.017 | 0.33 |
In the follow-up models of each electrode pair, eye condition was included as a main effect and in all possible interaction combinations; however, as eye condition did not influence the results we did not include them in the follow-up table for brevity.
Figure 1. NE by PE by age effects predicting frontal asymmetry.
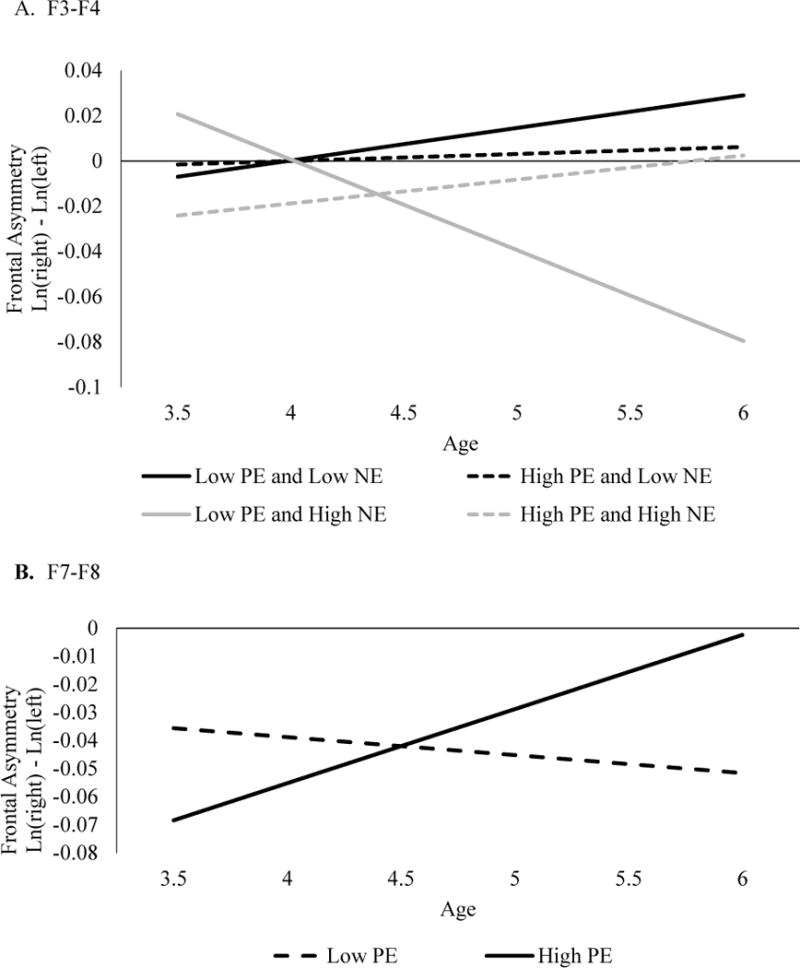
On the y-axis, positive values represent greater left than right activity, values closer to zero represent relatively equal activity in each hemisphere, and negative values represent greater right than left activity (or lower relative left activity).
The model for F7-F8 revealed that main effects of age, PE, and NE, were not significant (Table 4). However, there was a significant age by PE interaction (Figure 1). Follow-up analyses indicated that for children exhibiting high PE, frontal asymmetry shifted from lower relative left-frontal activity to a nearly even balance in cortical activity across hemispheres with age. (b=0.026, SE=0.010, p<.01). Frontal asymmetry in children with low PE did not differ as a function of age (b=−0.006, SE=0.009, p=.55). At age 3, children with low PE did not significantly differ from those with high PE (b=−0.010, SE=0.008, p=.21). Similarly, at age 6, children with low PE did not significantly differ from those with high PE (b=0.014, SE=0.009, p=.13).
Asymmetry and Facets of NE
Next, we ran follow-up MLMs of the NE facets for F3-4, as there is evidence that anger is associated with a different pattern of asymmetry than other components of NE. We ran three separate models predicting F3-4 asymmetry, replacing NE with either anger, sadness, or fear (Table 5). For the F3-4 sadness model, there was a significant three-way age X PE X sadness interaction (Figure 2). Follow-up analyses indicated that children with low PE and high sadness at age 3 developed a pattern of increasingly lower relative left-frontal cortical activity with age (b=−0.031, SE=0.011, p=.004), mirroring the age X PE X overall NE interaction described above.
Table 5.
MLMs examining facets of NE on F3-F4 asymmetry
| Anger Model | Estimate | SE | p-val |
|---|---|---|---|
| Age | −0.005 | 0.006 | 0.41 |
| PE | −0.006 | 0.006 | 0.27 |
| Anger | 0.035 | 0.028 | 0.20 |
| PE * Anger | −0.028 | 0.019 | 0.14 |
| Age * PE | 0.007 | 0.004 | 0.08 |
| Age * Anger | −0.042 | 0.018 | 0.02 |
| Age * PE * Anger | 0.026 | 0.013 | 0.04 |
| Fear Model | |||
|
| |||
| Age | −0.004 | 0.006 | 0.48 |
| PE | −0.005 | 0.006 | 0.34 |
| Fear | −0.011 | 0.025 | 0.65 |
| PE * Fear | −0.009 | 0.015 | 0.53 |
| Age * PE | 0.006 | 0.004 | 0.13 |
| Age * Fear | 0.001 | 0.017 | 0.95 |
| Age * PE * Fear | <0.001 | 0.01 | 0.99 |
| Sadness Model | |||
|
| |||
| Age | −0.003 | 0.006 | 0.63 |
| PE | −0.006 | 0.006 | 0.31 |
| Sadness | −0.026 | 0.033 | 0.44 |
| PE * Sadness | −0.008 | 0.021 | 0.69 |
| Age * PE | 0.006 | 0.004 | 0.12 |
| Age * Sadness | −0.021 | 0.022 | 0.34 |
| Age * PE * Sadness | 0.031 | 0.014 | 0.02 |
These models were identical to the primary analyses except that NE was replaced with one of its components. Eye condition was also included as in the primary analyses, but as eye condition did not influence the results we did not include them in the table for brevity.
Figure 2.
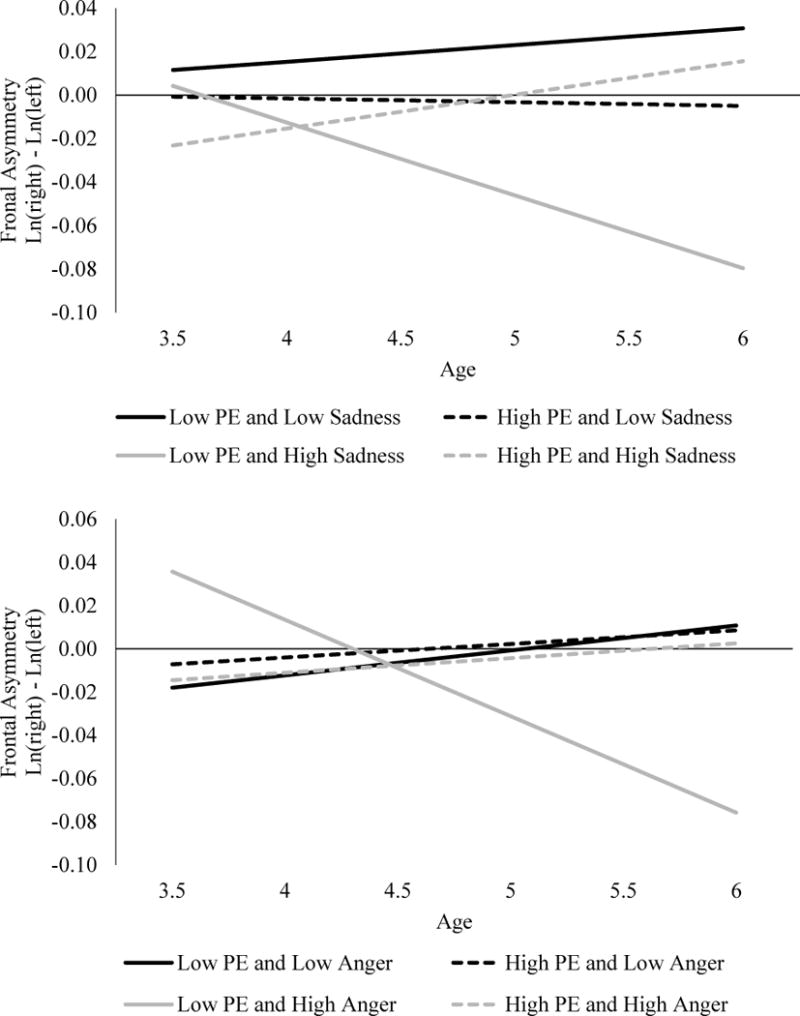
Sadness and anger interactions on F3-4
For the anger model predicting F3-4, there was also a significant three-way age X PE X anger interaction (Figure 2). Follow-up analyses indicated that children with low PE and high anger developed a pattern of increasingly lower relative left-frontal activity with age (b=−0.037, SE=0.012, p=.002). This is similar to the sadness and overall NE models at F3-F4. None of the effects were significant in the fear model3, suggesting that anger and sadness facets drive the overall NE effect at F3-F4.
Discussion
We examined the associations of PE and NE with changes in frontal asymmetry during early childhood. There was a significant three-way PE X NE X age interaction with frontal asymmetry at F3-F4. Preschool-aged children with low PE and high NE exhibited a pattern of increasingly lower relative left-frontal cortical activity over time. At age 3, temperament was not associated with frontal asymmetry, but by age 6, children who had exhibited both low PE and high NE at age 3 differed from peers with other temperament profiles in displaying a pattern of lower relative left-frontal activity, which can be interpreted as indicating reduced approach system/enhanced withdrawal system sensitivity.
When we decomposed NE into fearfulness, anger, and sadness, we found the same PE X NE interaction pattern for sadness and anger, but not fearfulness. While lower relative left-frontal asymmetry is not typically associated with anger (Harmon-Jones et al., 2010), these studies have not focused on early childhood, use very different measures, and do not examine the interaction of anger and low PE. In young children, sadness and anger may be expressed in similar types of situations (e.g. after a desired goal is unattainable; Lewis & Ramsay, 2005). More importantly, expressions of anger and sadness are difficult to distinguish, and are often blended, in young children (Camras, Sullivan, & Michel, 1993). Lastly, the absence of an effect for fearfulness (and BI) may have occurred as associations between BI and frontal asymmetry vary over development (Fox et al., 2001), and may not be evident in the period we examined.
At the F7-F8 electrode pair, we observed a two-way interaction between PE and age such that children with high PE at age 3 exhibited a pattern of lower relative left-frontal activity that attenuated over time, exhibiting virtually no evidence of asymmetry by age 6. In the asymmetry literature, it is not unusual to find different effects at different electrode pairs. Although this differs slightly from the effects at F3-F4, the two findings are complimentary. At the F7-F8 pair, higher PE was associated with a frontal asymmetry that became increasingly higher in the left relative to the right hemisphere; at F3-F4 lower PE in the context of high NE predicted increasingly lower relative left-frontal activity. In a meta-analysis, Thibodeau et al. (2006) found that F3-F4 was more sensitive to depression than F7-F8. Greater sensitivity to individual differences may explain why the low PE X high NE interaction was observed only at F3-F4.
The results of the current study suggest that temperamental NE and PE are associated with change in frontal asymmetry during early childhood. This is consistent with Lusby et al. (2016), who found that the relationship between NE and frontal asymmetry changed, and indeed reversed, direction over the first year of life. Similarly, previous studies have suggested that the strength of associations between frontal asymmetry and related temperament constructs such as behavioral inhibition may change over the course of development (Calkins et al., 1996). Furthermore, there is some indication that children’s frontal asymmetry may change in a nonlinear fashion over development (McLaughlin et al., 2011). Additionally, MRI studies of regions related to PE and NE may also exhibit non-linear change in structure and function (Giedd & Rapoport, 2010; Sowell et al., 2004). Taken together, the current and previous findings suggest that frontal asymmetry changes in complex ways during early childhood, such that associations between frontal asymmetry and other variables may be evident at some ages but not others. There may be a number of reasons for these changes. First, hemispheric volume asymmetry changes over time. In infants, the left hemisphere including the frontal cortex has been found to be larger than the right; however, in older children and adults this pattern reverses such that the right may be larger (Brain Development Cooperative Group, 2012; Giedd et al., 1996; Gilmore et al., 2007). Additionally, children’s behavior may influence their environment which in turn influences frontal asymmetry. Children with greater withdrawal tendencies may engage in less social interactions and therefore be exposed to fewer rewarding experiences (Coplan, DeBow, Schneider, & Graham, 2009). Additional longitudinal research, which uses three or more time points and includes measures of temperament and asymmetry at each time point are needed to further clarify directional nature of the association between frontal asymmetry in early childhood and its links with temperament.
These findings also have implications for understanding psychopathology, particularly by demonstrating the benefits of studying vulnerability factors at multiple levels and indicating that these factors can predict other factors in a dynamic way over the course of development. Interestingly, the results of the present study, which focuses on early temperament, converge with a previous study from our group (Goldstein et al., 2016) that focused on the offspring of depressed mothers, in that both sets of vulnerability factors independently predicted the emergence of lower relative left-frontal activity, the pattern of asymmetry most consistently associated with depression, over the course of early childhood.
This study has several strengths, including a longitudinal design, large sample, and utilization of a standardized behavioral observation assessment of temperamental emotionality. Importantly, this is also the first study to examine both NE and PE with resting EEG at multiple time points during early childhood. Additionally, we also examined multiple facets of NE. However, the study also has several limitations. Our results and the results of other investigators suggest that frontal asymmetry may change in complex ways during development and two assessments may not be sufficient to capture these changes (Fox et al., 2001; McLaughlin et al., 2011). Moreover, our sample was demographically homogenous so results may not generalize to other samples. Additionally, our results are based upon three-way interactions, which can be difficult to replicate, although the three-way interactions themselves were hypothesized. Lastly, our analyses only included assessment of personality at one time point, which prevents us from examining the possibility of bidirectional relationships between temperament and EEG over time. As we mentioned above, future studies that do include many assessments of both EEG and temperament will be beneficial.
Overall, our results suggest that temperament may be associated with changes in EEG asymmetries in early childhood. Specifically, NE and PE may, separately or in combination, predict different trajectories of EEG asymmetries. The number of longitudinal studies that examine cross-level relationships of vulnerability factors are limited, making it difficult to bridge levels of analysis (Schwartz, Lilienfeld, Meca, & Sauvigné, 2016), and our results suggest that development further complicates these linkages. Resting EEG and perhaps other neural markers change over the course of development; therefore, as researchers continue to examine biobehavioral constructs at multiple levels it is critical to conduct longitudinal developmental research to clarify cross-level relationships.
Acknowledgments
*This work was supported by National Institute of Mental Health grant R01 MH069942 (Klein).
Footnotes
In a previous paper, we found that maternal history of depression was associated with change in frontal asymmetry over time in an overlapping sample (Goldstein et al., 2016). To ensure that the effects in the current analyses were not driven by maternal depression, we ran additional models which included maternal depression and a maternal depression by age interaction as covariates. The results were largely unchanged by these covariates, suggesting that main effects of PE and NE, and their interactions with age, were not driven by maternal history of depression.
Parietal asymmetry has also been linked to depression; although, the evidence tends to be more inconsistent and it is less frequently studied (Thibodeau et al., 2006). Our group has previously found a relationship between temperament and parietal asymmetry (Shankman et al., 2005, 2011); therefore, we also ran additional models examining NE and PE with parietal asymmetry. We did find interactions between age and NE, but it was limited to P7-P8, whereas P3-P4 exhibited no associations with temperament. When conducting follow-up analyses at the P7-P8 pair, participants with low NE exhibited a pattern of greater relative left cortical activity that attenuated with age.
We also conducted additional models examining Behavioral Inhibition with asymmetries over time (for a description of how behavioral inhibition was scored from the Lab-TAB in this sample see Laptook et al., 2010). We did not find significant main or interaction effects with time, which parallels our results for the fearfulness effects.
References
- American Electroencephalographic Society. American electroencephalographic society guidelines in electroen- cephalography, evoked potentials, and polysomnography. Journal. Journal of Clinical Neurophysiology. 1994;11:1–142. [PubMed] [Google Scholar]
- Allen JJB, Reznik SJ. Frontal EEG asymmetry as a promising marker of depression vulnerability: summary and methodological considerations. Current Opinion in Psychology. 2015;4:93–97. doi: 10.1016/j.copsyc.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagiella E, Sloan RP, Heitjan DF. Mixed-effects models in psychophysiology. Psychophysiology. 2000;37(1):13–20. doi: 10.1017/S0048577200980648. [DOI] [PubMed] [Google Scholar]
- Brain Development Cooperative Group. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: The NIH MRI study of normal brain development. Cerebral Cortex. 2012;22(1):1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67(2):523–540. doi: 10.1111/j.1467-8624.1996.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Camras LA, Sullivan J, Michel G. Do infants express discrete emotions? Adult judgments of facial, vocal, and body actions. Journal of Nonverbal Behavior. 1993;17(3):171–186. doi: 10.1007/BF00986118. [DOI] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40(1):106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Coplan RJ, DeBow A, Schneider BH, Graham AA. The social behaviours of inhibited children in and out of preschool. The British Journal of Developmental Psychology. 2009;27(Pt 4):891–905. doi: 10.1348/026151008X396153. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Development and Psychopathology. 1994;6(4):741–758. [Google Scholar]
- Diego MA, Field T, Jones NA, Hernandez-Reif M. Withdrawn and intrusive maternal interaction style and infant frontal EEG asymmetry shifts in infants of depressed and non-depressed mothers. Infant Behavior and Development. 2006;29(2):220–229. doi: 10.1016/j.infbeh.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson MW, Olino TM, Durbin CE, Goldsmith HH, Bufferd SJ, Miller AR, Klein DN. The structural and rank-order stability of temperament in young children based on a laboratory-observational measure. Psychological Assessment. 2015;27(4):1388–1401. doi: 10.1037/pas0000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Angold A. The Preschool Age Psychiatric Assessment (PAPA): A structured parent interview for diagnosing psychiatric disorders in preschool children. In: DelCarmen-Wiggins R, Carter A, editors. Handbook of infant, toddler, and preschool mental health assessment. 1st. New York: Oxford University Press New York; 2004. pp. 223–243. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72(1):1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, Stewart S. Frontal activation asymmetry and social competence at four years of age. Child Development. 1995;66(6):1770–1784. doi: 10.1111/j.1467-8624.1995.tb00964.x. [DOI] [PubMed] [Google Scholar]
- Gagne JR, Van Hulle CA, Aksan N, Essex MJ, Goldsmith HH. Deriving childhood temperament measures from emotion-eliciting behavioral episodes: Scale construction and initial validation. Psychological Assessment. 2011;23(2):337–353. doi: 10.1037/a0021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershuny BS, Sher KJ. The relation between personality and anxiety: findings from a 3-year prospective study. Journal of Abnormal Psychology. 1998;107(2):252–262. doi: 10.1037/0021-843X.107.2.252. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron. 2010;67(5):728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cerebral Cortex. 1996;6(4):551–559. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, Gerig G. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. Journal of Neuroscience. 2007;27(6):1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH, Reilly J, Lemery KS, Longley S, Prescott A. Laboratory temperament assessment battery: Preschool version. Madison, WI: Department of Psychology,University of Wisconsin - Madison; 1995. [Google Scholar]
- Goldstein BL, Kotov R, Perlman G, Watson D, Klein DN. Trait and facet-level predictors of first-onset depressive and anxiety disorders in a community sample of adolescent girls. Psychological Medicine. 2017 doi: 10.1017/S0033291717002719. [DOI] [PubMed]
- Goldstein BL, Shankman SA, Kujawa A, Torpey-Newman DC, Olino TM, Klein DN. Developmental changes in electroencephalographic frontal asymmetry in young children at risk for depression. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2016;57(9):1075–1082. doi: 10.1111/jcpp.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakulinen C, Elovainio M, Pulkki-Råback L, Virtanen M, Kivimaki M, Jokela M, Jokela M. Personality and depressive symptoms: Individual participant meta-analysis of 10 cohort studies. Depression and Anxiety. 2015;32(7):461–470. doi: 10.1002/da.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane AA, Fox NA, Henderson HA, Marshall PJ. Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology. 2008;44(5):1491–1496. doi: 10.1037/a0012855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJB. Behavioral activation sensitivity and resting frontal EEG asymmetry: covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology. 1997;106(1):159–163. doi: 10.1037/0021-843X.106.1.159. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biological Psychology. 2010;84(3):451–462. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Heller AS, Cohen AO, Dreyfuss MFW, Casey BJ. Changes in cortico- subcortical and subcortico-subcortical connectivity impact cognitive control to emotional cues across development. Social Cognitive and Affective Neuroscience. 2016;11(12):1910–1918. doi: 10.1093/scan/nsw097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. Journal of Abnormal Psychology. 1990;99(1):22–31. doi: 10.1037/0021-843X.99.1.22. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100(4):535–545. doi: 10.1037/0021-843X.100.4.535. [DOI] [PubMed] [Google Scholar]
- Hewig J, Hagemann D, Seifert J, Naumann E, Bartussek D. The relation of cortical activity and BIS/BAS on the trait level. Biological Psychology. 2006;71(1):42–53. doi: 10.1016/j.biopsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN, Garvey MA, Heinssen RK, Pine DS, Quinn KJ, Wang PS. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jacobs GD, Snyder D. Frontal brain asymmetry predicts affective style in men. Behavioral Neuroscience. 1996;110(1):3–6. doi: 10.1037/0735-7044.110.1.3. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Lonigan CJ. Tripartite Model of Depression and Anxiety in Youth Psychiatric Inpatients: Relations With Diagnostic Status and Future Symptoms. Journal of Clinical Child Psychology. 2000;29(3):372–382. doi: 10.1207/S15374424JCCP2903_8. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, Garcia-Coll C. Behavioral Inhibition to the Unfamiliar. Child Development. 1984;55(6):2212. doi: 10.2307/1129793. [DOI] [Google Scholar]
- Kayser J. Polygraphic recording data exchange— PolyRex. New York, NY: New York State Psychiatric Institute, Department of Biopsychology; 2003. [Google Scholar]
- Klein DN, Kotov R, Bufferd SJ. Personality and depression: Explanatory models and review of the evidence. Annual Review of Clinical Psychology. 2011;7(1):269–295. doi: 10.1146/annurev-clinpsy-032210-104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychological Bulletin. 2010;136(5):768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Burkhouse KL. Vulnerability to depression in youth: Advances from affective neuroscience. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2017;2(1):28–37. doi: 10.1016/j.bpsc.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laptook RS, Klein DN, Durbin CE, Hayden EP, Olino TM, Carlson G. Differentiation between low positive affectivity and behavioral inhibition in preschool-age children: A comparison of behavioral approach in novel and non-novel contexts. Personality and Individual Differences. 2008;44(3):758–767. doi: 10.1016/j.paid.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laptook RS, Klein DN, Olino TM, Dyson MW, Carlson G. Low positive affectivity and behavioral inhibition in preschool-age children: A replication and extension of previous findings. Personality and Individual Differences. 2010;48(5):547–551. doi: 10.1016/j.paid.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, Krakow K. EEG-correlated fMRI of human alpha activity. NeuroImage. 2003;19(4):1463–1476. doi: 10.1016/S1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay D. Infant emotional and cortisol responses to goal blockage. Child Development. 2005;76(2):518–530. doi: 10.1111/j.1467-8624.2005.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longoni AM, Orsini L. Lateral preferences in preschool children: a research note. Journal of Child Psychology and Psychiatry. 1988;29(4):533–539. doi: 10.1111/j.1469-7610.1988.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Lusby CM, Goodman SH, Yeung EW, Bell MA, Stowe ZN. Infant EEG and temperament negative affectivity: Coherence of vulnerabilities to mothers’ perinatal depression. Development and Psychopathology. 2016;28(4pt1):895–911. doi: 10.1017/S0954579416000614. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113(8):1199–1208. doi: 10.1016/S1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Fox NA, Zeanah CH, Nelson CA. Adverse rearing environments and neural development in children: the development of frontal electroencephalogram asymmetry. Biological Psychiatry. 2011;70(11):1008–15. doi: 10.1016/j.biopsych.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller BCN, Kühn-Popp N, Meinhardt J, Sodian B, Paulus M. Long-term stability in children’s frontal EEG alpha asymmetry between 14-months and 83-months. International Journal of Developmental Neuroscience. 2015;41:110–114. doi: 10.1016/j.ijdevneu.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, Harmon-Jones E, Alloy LB, Coan JA, Abramson LY. Cognitive vulnerability and frontal brain asymmetry: Common predictors of first prospective depressive episode. Journal of Abnormal Psychology. 2011;120(2):497–503. doi: 10.1037/a0022940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: associations in a large community sample. Journal of Abnormal Psychology. 2010;119(3):468. doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, Kramer MD. A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. Journal of Abnormal Psychology. 2013;122(3):902–916. doi: 10.1037/a0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31(4):437–448. doi: 10.3102/10769986031004437. [DOI] [Google Scholar]
- Schmidtke JI, Heller W. Personality, affect and EEG: predicting patterns of regional brain activity related to extraversion and neuroticism. Personality and Individual Differences. 2004;36(3):717–732. doi: 10.1016/S0191-8869(03)00129-6. [DOI] [Google Scholar]
- Schwartz SJ, Lilienfeld SO, Meca A, Sauvigné KC. The role of neuroscience within psychology: A call for inclusiveness over exclusiveness. American Psychologist. 2016;71(1):52–70. doi: 10.1037/a0039678. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: A biobehavioral study. Journal of Abnormal Psychology. 2007;116(1):95–104. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Torpey DC, Olino TM, Dyson MW, Kim J, Tenke CE. Do positive and negative temperament traits interact in predicting risk for depression? A resting EEG study of 329 preschoolers. Development and Psychopathology. 2011;23(2):551–562. doi: 10.1017/S0954579411000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Tenke CE, Bruder GE, Durbin CE, Hayden EP, Klein DN. Low positive emotionality in young children: Association with EEG asymmetry. Development and Psychopathology. 2005;17(1):85–98. doi: 10.1017/s0954579405050054. http://doi.org/10.10170S0954579405050054. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willet JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20(2):271–277. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8(3):204–210. doi: 10.1111/j.1467-9280.1997.tb00413.x. [DOI] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology. 2006;115(4):715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Henriques JB. Resting frontal brain asymmetry predicts affective responses to films. Journal of Personality and Social Psychology. 1990;59(4):791–801. doi: 10.1037/0022-3514.59.4.791. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Doss RC. Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. Journal of Personality and Social Psychology. 1992;62(4):676–687. doi: 10.1037/0022-3514.62.4.676. [DOI] [PubMed] [Google Scholar]
- Van Der Schaaf ME, Warmerdam E, Crone EA, Cools R. Distinct linear and non-linear trajectories of reward and punishment reversal learning during development: Relevance for dopamine’s role in adolescent decision making. Developmental Cognitive Neuroscience. 2011;1(4):578–590. doi: 10.1016/j.dcn.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasey MW, Harbaugh CN, Lonigan CJ, Phillips BM, Hankin BL, Willem L, Bijttebier P. Dimensions of temperament and depressive symptoms: Replicating a three-way interaction. Journal of Research in Personality. 2013;47(6):908–921. doi: 10.1016/j.jrp.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuga M, Fox NA, Cohn JF, Kovacs M, George CJ. Long-term stability of electroencephalographic asymmetry and power in 3 to 9 year-old children. International Journal of Psychophysiology. 2008;67(1):70–77. doi: 10.1016/j.ijpsycho.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Chavanon ML, Stemmler G. Resting EEG signatures of agentic extraversion: New results and meta-analytic integration. Journal of Research in Personality. 2010;44(2):167–179. doi: 10.1016/j.jrp.2009.12.004. [DOI] [Google Scholar]


