Abstract
Conscientiousness is a personality trait associated with many important life outcomes, but little is known about the mechanisms that underlie it. We investigated its neural correlates using functional connectivity analysis in fMRI, which identifies brain regions that act in synchrony. We tested the hypothesis that a broad network resembling a combination of the salience and ventral attention networks, which we provisionally label the goal priority network (GPN), is a neural correlate of Conscientiousness. Self‐ and peer‐ratings of Conscientiousness were collected in a community sample of adults who underwent a resting‐state fMRI scan (N = 218). An independent components analysis yielded five components that overlapped substantially with the GPN. We examined synchrony within and between these GPN subcomponents. Synchrony within one of the components—mainly comprising regions of anterior insula, dorsal anterior cingulate cortex, and dorsolateral prefrontal cortex—was significantly associated with Conscientiousness. Connectivity between this component and the four other GPN components was also significantly associated with Conscientiousness. Our results support the hypothesis that variation in a network that enables prioritization of multiple goals may be central to Conscientiousness.
Keywords: Conscientiousness, functional connectivity, goal priority network, resting state fMRI, rs‐fcMRI, salience network, ventral attention network
1. GOAL PRIORITY NETWORK AS A NEURAL SUBSTRATE OF CONSCIENTIOUSNESS
Conscientiousness is a personality trait that describes the shared variance in traits such as self‐discipline, orderliness, industriousness, organization, and responsibility (DeYoung, 2015; John, Naumann, & Soto, 2008). Personality traits are relatively stable individual differences in emotion, motivation, cognition, and behavior. Conscientiousness is one of the so‐called “Big Five” personality dimensions, which capture the major patterns of covariation among more specific personality traits. Questionnaire assessments of Conscientiousness predict a variety of important life outcomes, including academic success, job performance, physical health, and mortality (Ozer & Benet‐Martínez, 2006). Conscientious people are less likely to smoke, eat an unhealthy diet, or abuse drugs and more likely to exercise and maintain healthy social relationships (Roberts, Lejuez, Krueger, Richards, & Hill, 2014). Low Conscientiousness is also linked to risk for a number of mental disorders, especially externalizing disorders involving impulsivity or substance abuse (DeYoung, Carey, Krueger, & Ross, 2016; Kotov, Gamez, Schmidt, & Watson, 2010). Despite the importance of conscientiousness in our everyday lives, we know surprisingly little about the psychological and neurobiological processes that support it. Here we test, for the first time, a hypothesis that was previously developed based on a review of existing research (Allen & DeYoung, 2016; DeYoung, 2015), namely, that a broad network of brain regions involved in prioritizing goals is a key neural correlate of Conscientiousness.
Prioritizing goals effectively is a cognitive process likely to be crucial for Conscientiousness that may explain why conscientious people appear to be good at resisting distractions and disruptive impulses. A recent study found that Conscientiousness predicted success in a task that required flexible adjustment of the priority of multiple response steps, accounting for 19% of interindividual variance in performance (Stock & Beste, 2015). Individuals higher in Conscientiousness were more efficient in situations that required decisions regarding the order of operations and where performance benefitted from addressing operations in the proper serial order, rather than simultaneously. The other Big Five traits (Neuroticism, Extraversion, Openness/Intellect, and Agreeableness) were not associated with task performance.
What neural systems might be crucial for goal prioritization, and, in turn, what neural systems are related to Conscientiousness? The hypothesis we tested was predicated on several lines of evidence. First, several studies indicate that regional volume within the lateral prefrontal cortex (PFC), including dorsal regions, is positively correlated with Conscientiousness (DeYoung et al., 2010; Jackson et al., 2011; Kapogiannis et al., 2013; though see Bjørnebekk et al., 2013; Hu et al., 2011; Liu et al., 2013 for nonreplications). The lateral PFC is an obvious candidate related to Conscientiousness because of its involvement in rule following and goal maintenance (Bunge & Zelazo, 2006, Paxton, Barch, Racine, & Braver, 2008). However, the function most often associated with dorsolateral PFC is working memory, which is unrelated to Conscientiousness (DeYoung et al., 2005, 2009; Nee et al., 2013). Working memory appears to be one of the major contributors to intelligence or IQ, which is also unrelated, or even weakly negatively related, to Conscientiousness (Ackerman & Heggestad, 1997; Conway, Kane, & Engle, 2003; DeYoung, 2011). Thus, when a neural substrate of Conscientiousness is identified it seems likely to be distinct from the neural substrates of working memory and intelligence, despite involving the lateral PFC.
This puzzle may be resolved by the observation that, although multiple large‐scale neural networks have nodes in lateral and even dorsolateral PFC, only one of these networks is primarily associated with working memory and intelligence. In this study, we identified connectivity networks empirically in our sample and classified them based on maps of functional connectivity networks derived from a study of 1,000 healthy adults given resting‐state fMRI scans (Yeo et al., 2011). Using cluster analysis to identify neural activity that co‐varies throughout the cortex, Yeo et al. (2011) identified two stable solutions, one with seven large networks and one with 17. Especially in the seven‐network solution, these networks corresponded fairly clearly to networks previously identified in task‐based fMRI research, allowing them to apply meaningful labels. It is well established that very similar functional networks are evident at rest and during tasks (Laird et al., 2011; Smith et al., 2009).Their seven network solution included the “frontoparietal control network” (FPCN), which encompasses most of the dorsolateral PFC and is strongly implicated in working memory and intelligence (Hampson, Driesen, Skudlarski, Gore, & Constable, 2006; Jung & Haier, 2007). However, another network also included a region of dorsolateral PFC in a similar location to those previously linked to Conscientiousness: this they labeled the “ventral attention network” (VAN).
The VAN traditionally centers around the temporoparietal junction (TPJ) and right ventrolateral parietal networks (Vossel, Geng, & Fink, 2014). However, the network identified by Yeo et al. (2011) as “VAN” is more extensive than what has typically been studied as the VAN. Their more extensive VAN includes not only an additional node in dorsolateral PFC but also portions of insula and dorsal anterior cingulate cortex (dACC) that are typically described as the salience network (Menon & Uddin, 2010). Based on overlapping functionalities between salience networks and the traditional VAN, we refer to this larger composite identified by Yeo et al. (2011) as the goal priority network (GPN) for clarity and brevity.
The traditional VAN is responsible for reorienting attention in response to salient stimuli (Fox et al., 2006). When salient distractors arise, the VAN helps redirect attention back to the task at hand or to unexpected task‐relevant stimuli. The right inferior frontal gyrus in particular appears to be crucial for inhibiting actions that are inappropriate given the operative goal (Sharp et al., 2010). Fox et al.'s (2006) emphasis on salience illustrates the VAN's natural connection to the salience network, which appears to be crucial for integrating information about external stimuli with emotional, motivational, and interoceptive information to determine salience (Seeley et al., 2007; Uddin, 2015). It seems sensible that these two networks are joined as part of a larger network, the GPN, which detects relevant stimuli and determines whether or not they should influence behavior based on operative goals. Highly conscientious people should be capable of determining both which stimuli should be salient, given their goals, and which stimuli that are spontaneously salient (due to affective significance or prediction error) and worth paying attention to.
A recent review (Allen & DeYoung, 2016) identifies previous studies that reported functional or structural neural associations with conscientiousness. Many of these associations fall within what we are calling the GPN, not only in lateral PFC, but also in the insula and dACC (though some of the latter studies used the Barratt Impulsivity Scale, which is strongly negatively related to Conscientiousness but was not designed specifically to measure the Big Five dimension). Several studies found that Conscientiousness is negatively correlated with insula volume (Churchwell & Yurgelun‐Todd, 2013; Liu et al., 2013; Nouchi et al., 2016; Riccelli et al., 2017), and an fMRI study found that impulsivity was negatively related to activity of the anterior insula and adjacent lateral frontal cortex during trials requiring inhibition (Farr, Hu, Zhang, & Li, 2012). A previous study, using the current sample, found that externalizing behavior problems (which are linked to low Conscientiousness) were associated with connectivity in neural networks that involved the insula (Abram et al., 2015; this study did not investigate the Big Five). Several other studies have linked Conscientiousness to the dACC and adjacent medial PFC (Brown, Manuck, Flory, & Hariri, 2006; Matsuo et al., 2009; Whittle et al., 2008).
This study is the first direct test of Allen and DeYoung's (2016) hypothesis that the GPN is a key substrate of Conscientiousness. Specifically, we hypothesized that Conscientiousness would be associated with resting‐state functional connectivity in empirically derived functional networks that overlap with parts of the broad GPN identified by Yeo et al. (2011). To identify these networks, we used independent components analysis (ICA). ICA is a data‐driven technique that identifies groups of voxels in the brain that tend to vary synchronously in their activation levels across time. This implies that they are functionally connected and involved in coordinated operations. These groups of voxels are referred to as intrinsic connectivity networks (ICNs). ICNs derived from resting‐state fMRI data are likely to be behaviorally meaningful because similar networks are observed across individuals and because the networks that emerge in resting‐state data are often identifiable as the same networks that have been identified in fMRI studies of activation during specific tasks (Andrews‐Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Smith et al., 2009).
A major advantage of ICA is that it allows identification and exclusion of patterns of covariation among voxels that are due to artifacts rather than neural activity. We used parameters for our ICA that have been optimized for the study of individual differences by maximizing the test–retest reliability of the identified nonartefactual components (Poppe et al., 2013). In other words, connectivity patterns in these ICNs tend to be stable over time. The ICA approach was preferable to using a mask of the entire GPN as identified by Yeo et al. (2011) for several reasons: (a) it identifies patterns of covariation specific to our sample, (b) it allows ICNs to overlap, which is a more realistic depiction of functional brain organization than the nonoverlapping parcellations of Yeo et al. because a particular brain region can be involved in multiple networks (cf. Marquand, Haak, & Beckmann, 2017), and (c) it fragments larger networks like the GPN into empirically defined, functionally coherent subnetworks (ICNs), which may be differentially related to Conscientiousness.
We examined two different types of variables derived from the ICNs that overlapped with GPN. The coherence of each ICN refers to how strongly the voxels within it are correlated within an individual (and across time). Each ICN is identified in the whole sample based on typical patterns of correlation, but individuals vary in terms of how strongly any given ICN coheres. Interconnectivity between ICNs refers to the degree to which the timecourse of activation in one ICN is correlated with the timecourse in another ICN. An interconnectivity variable can be derived for any pair of ICNs. We investigated associations of Conscientiousness with both the coherence and interconnectivity of ICNs that overlapped with the GPN. We hypothesized that individuals who score higher in Conscientiousness would have higher levels of coherence and interconnectivity within the GPN.
2. METHODS
2.1. Participants
A community sample (N = 306, age range: 20–39 years, all right handed) was collected as part of a study of neurobiological correlates of decision‐making and personality. Participants were recruited via Craigslist and fliers posted in public areas. A total of 218 participants were retained for the current analysis (51% female, mean age = 25.8 years, SD = 4.6). During recruitment, potential participants were excluded for reasons of MRI safety (e.g., metallic implants) or if they had ever been diagnosed with neurological or serious psychiatric conditions or were currently using psychotropic medications. Participants were not excluded for alcohol or illicit drug use; however, if participants acknowledged current and substantial drug or alcohol dysfunction or disruptions in their daily activities, they were excluded.
Participants who did not have the necessary data were excluded from this study. Exclusions were mostly due to: attrition (did not return for MRI scan, n = 6), poor quality data (n = 9), incomplete scanning sessions (n = 11), incomplete behavioral assessments (n = 5), and excessive movement during the scan (defined as average absolute displacement above 0.5 mm or any single instance of X, Y, or Z coordinate displacement above 2.75 mm, n = 57). This stringent motion exclusion criterion was used to avoid biasing resting fMRI correlations, as recommended by previous reports (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). Previous research suggests that including motion as a control parameter in analyses is inadequate for ameliorating subject‐level motion effects (Power et al., 2014). Exclusions due to excessive movement during the scan did not alter the range of Conscientiousness scores. The University of Minnesota institutional review board approved this study and all associated protocols. Participants completed the behavioral portion during one visit and then returned for an additional MRI session. All participants provided written informed consent at the beginning of each visit.
2.2. Measures
2.2.1. Personality questionnaires
All participants in the study sample completed the Big Five Inventory (BFI) and the Big Five Aspect Scales (BFAS). The BFI consists of 44 items measuring the Big Five (John, Naumann, & Soto, 2008). The BFAS consists of 100 items, with 10 items measuring each of the two major subfactors (“aspects”) within each of the Big Five (DeYoung, Quilty, & Peterson, 2007). (At the request of a reviewer, we tested whether our results differed significantly between the two aspects. They did not; hence, we report only on total Conscientiousness scores.) Additionally, approximately 65% of the sample had at least 2 peer ratings (including peer‐report versions of the BFI and BFAS) and 70% of the sample had at least 1 peer rating. Peer ratings were obtained by giving participants 3 informant packets that included a stamped and pre‐addressed envelope with instruction to have them completed by people who knew them well (participants received additional compensation when the peer ratings were returned). When multiple peer‐ratings were available, they were averaged to create a single peer‐rating score. The BFI and BFAS were averaged to create composite Big Five scores, and self‐ and peer ratings were averaged when both were available. Agreement between self and peers for the Big Five was good, with correlations ranging from .56 to .66; the correlation for Conscientiousness was .58.
2.2.2. Intelligence
All participants in the study sample completed four subtests of the Wechsler Adult Intelligence Scale – Fourth Edition (WAIS‐IV), which were used to estimate full scale IQ per the WAIS‐IV manual (Wechsler, 2008). The subtests were block design, matrix reasoning, vocabulary, and similarities. IQ was included as a covariate in the analysis because it is often weakly negatively correlated with Conscientiousness and it is widely correlated with resting state connectivity (Cole, Yarkoni, Repovš, Anticevic, & Braver, 2012; Song et al., 2008; Wang, Song, Jiang, Zhang, & Yu, 2011). Consistent with previous findings, there was a significant negative relation between IQ and Conscientiousness in the current sample (r = −.16, p < .001). See Table 1 for associations between IQ and other personality traits.
Table 1.
Descriptives and zero‐order correlations among behavioral variables
| Variables | C | A | N | O | E | IQ |
|---|---|---|---|---|---|---|
| Conscientiousness | ‐ | |||||
| Agreeableness | .34 | ‐ | ||||
| Neuroticism | −.40 | −.42 | ‐ | |||
| Openness | −.18 | .10 | −.03 | ‐ | ||
| Extraversion | .29 | .20 | −.33 | .13 | ‐ | |
| IQ | −.16 | −.03 | −.03 | .31 | −.15 | ‐ |
| Mean | 3.54 | 3.94 | 2.59 | 3.88 | 3.57 | 113.4 |
| SD | 0.50 | 0.47 | 0.64 | 0.48 | 0.61 | 15.67 |
Note. N = 218. All correlations >.13 in absolute value are significant at p < .05.
2.3. Image acquisition and preprocessing
Resting‐state functional MRI scans were acquired using a 3 T Siemens Trio scanner (Erlangen, Germany) at the University of Minnesota's Center for Magnetic Resonance Research. Participants were instructed to stay awake during the scan and to gaze at a basic fixation cross projected on a screen inside the bore. Participants were instructed to click a button when the fixation cross changed from gray to white, or vice versa, which occurred five times during the 5 min scan (at 1 min, 1′30″, 2′45″, 3′45″, and 4′30″). This minimal task ensured that the participants remained awake while minimizing eye movements for the duration of the scan (Fair et al., 2007; Fox et al., 2009; Fox & Grecius, 2010). It is possible that individuals scoring higher in Conscientiousness were more likely to focus harder on the minimal task than individuals scoring lower. Nonetheless, our hypothesis regarding the neural network related to Conscientiousness can still be supported, even in the context of a task that involves Conscientiousness‐related behavior—just as one can detect the neural correlates of intelligence effectively in fMRI of difficult cognitive tasks (Choi et al., 2008). The same large networks are generally present in both resting and task‐based fMRI, although different tasks cause them to change their shape to some degree (Krienen, Yeo, & Buckner, 2014). Our minimal task should cause minimal change relative to a resting scan with no task.
Scan sequence parameters were as follows: gradient‐echo echo‐planar imaging of 150 volumes; repetition time (TR) = 2 s; echo time (TE) = 28 ms; flip angle = 80°; voxel size = 3.5 × 3.5 × 3.5 mm. A high‐resolution T1‐weighted MPRAGE was collected for registration purposes. Preprocessing was completed using FMRIB Software Library (FSL 4.1.9), including brain extraction, motion correction, grand mean intensity normalization of the 4D dataset, high‐pass temporal filtering (at a filtering threshold of 0.1 Hz), and registration of functional images to high‐resolution T1‐weighted structural images (Wisner, Atluri, Lim, & MacDonald, 2013; Wisner, Patzelt, Lim, & MacDonald, 2013). Motion regression was completed as the final step.
2.4. Independent components analysis
2.4.1. Independent components analysis
ICNs were extracted using a meta‐ICA procedure to optimize the reliability of the resulting network variables (Abram et al., 2015; Poppe et al., 2013). Twenty‐five group‐level, probabilistic, spatial ICAs were completed using the MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) function in FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC). Each ICA used a unique randomly generated set of 80 participants as inputs (thus, a different subset in a random order for each ICA); all the subjects were not included in each ICA due to software and hardware limitations and to reduce the likelihood of overfitting. We chose to extract 60 components based on analyses conducted by Poppe et al. (2013). The 60 components from each ICA were temporally concatenated into a single file, which was then used as the input to a meta‐level MELODIC analysis (meta‐ICA) to derive the 60 most consistent group‐level components. We then applied the group‐level components to the subject‐level data using a dual regression procedure to obtain spatial maps and corresponding timeseries for each individual based on the group‐level maps from the meta‐ICA (Abram et al., 2015; Beckmann, Mackay, Filippini, & Smith, 2009; Filippini et al., 2009; Wisner et al., 2013; Zuo et al., 2010).
2.4.2. Component selection
Procedures outlined by Kelly et al. (2010) were used to visually identify 31 artefactual components, including those which appeared to reflect cardiac function, respiration, nonneural fluctuations, white matter tracts, or movement. The remaining 29 nonartifactual components (ICNs) were normalized by their maximum value, then thresholded at a z max > 0.30 (Poppe et al., 2013) and binarized to compare them to the maps of Yeo et al. (2011) and to apply them as masks to subject‐level maps. We identified which of the 29 ICNs corresponded most closely to the GPN as identified by Yeo et al. (2011; https://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation_Yeo2011) in their seven‐network, parcellated, resting functional connectivity map (note that they labeled this network “VAN”). We used AFNI (Analysis of Functional NeuroImages; https://afni.nimh.nih.gov/afni) to reorient and change the resolution of the seven Yeo et al. networks to match the orientation and resolution (2 mm) of our ICN maps (Cox, 1996). We then calculated the percentage of cortical overlap for each of our 29 ICNs with each of their networks (also known as the “association index”; Dice, 1945). The ICNs with the highest overlap with their “VAN” network were then visually inspected to verify that they corresponded well to this network. This procedure yielded five GPN ICNs (Figure 1). They occupy a larger area than Yeo et al.'s network (Figure 2a), but this is to be expected because our ICNs, being derived from spatial ICA, can overlap, whereas the maps of Yeo et al. depict nonoverlapping parcellations. Nonetheless, the locations of our five ICNs are generally centered on regions of Yeo et al.'s “VAN” network.
Figure 1.
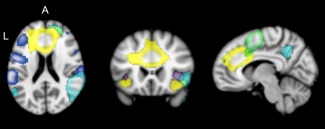
Intrinsic connectivity networks (ICNs) associated with the goal priority network (GPN); ICN‐1 = yellow, ICN‐2 = purple, ICN‐3 = dark blue, ICN‐4 = cyan, ICN‐5 = green (MNI coordinates x, y, z = 8, 22, 20) [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2.
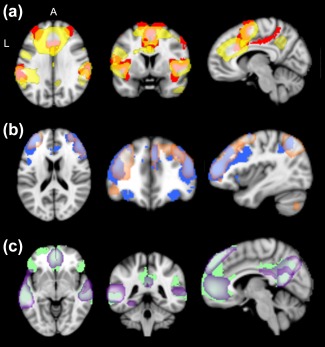
(a) GPN ICNs (yellow) compared to Yeo et al.'s (2011) VAN (red; MNI Coordinates x, y, z = 8, 4, 30); (b) FPCN ICNs (orange) compared to Yeo et al.'s (2011) FPCN (blue; MNI coordinates x, y, z = 34, 34, 20); (c) DN ICNs (purple) compared to Yeo et al.'s (2011) DN (green; MNI coordinates x, y, z = 1, 38, 0). VAN = ventral attention network; FPCN = frontoparietal control network; DN = default network [Color figure can be viewed at http://wileyonlinelibrary.com]
ICN‐1 primarily contained regions of dACC, anterior insula, and middle and superior frontal gyri. ICN‐2 primarily contained regions of the ACC, posterior insula, and temporal operculum. ICN‐3 primarily contained regions in the dACC, lateral PFC, and TPJ. ICN‐4 contained primarily regions in the right temporal cortex, right lateral PFC, and right insula. ICN‐5 contained primarily regions of the supplemental motor cortex and caudal superior frontal gyrus.
To determine the specificity of our findings to the GPN, we also examined variance in ICNs corresponding to the two other large‐scale networks that are extensively represented in the lateral prefrontal cortex in the Yeo et al. (2011) map and that are, therefore, most important to rule out as alternative correlates of Conscientiousness: the FPCN and the default network (DN). The same percentage overlap procedure described for GPN yielded three FPCN ICNs and five DN ICNs (Figure 2b,c). The coherence values from these eight ICNs were used as covariates when examining associations between GPN coherence and Conscientiousness. This procedure was particularly important for the coherence variables because they are all highly intercorrelated, which is likely to indicate either some artefactual source of shared variance or a general tendency toward synchronized brain activity that is not specific to any given neural network or region. The interconnectivity variables do not show these high levels of intercorrelation. We also tested whether Conscientiousness was associated with coherence and interconnectivity in FPCN and DN.
2.4.3. Functional connectivity metrics
Connectivity within each ICN (coherence) was computed as the average correlation of the time‐series of each voxel in a given ICN with the mean time‐series for all voxels in that ICN for that subject (using the subject‐specific spatial maps derived via dual‐regression). Connectivity between ICNs (interconnectivity) was computed as the correlation (Fisher z‐transformed) between the mean time series of each pair of ICNs for each subject (using the subject‐specific timeseries derived via dual‐regression).
2.4.4. Motion parameter
Head motion in resting‐state functional connectivity MRI studies can cause artefacts that produce spurious patterns in correlation between regions of interest (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). Although ICA can account for motion aggregated at the group level (by identifying components reflecting artefactual patterns of covariance due to movement, which can then be excluded) it does not guarantee that individual differences in movement are not biasing values in nonartefactual components. Because of the susceptibility of intervoxel correlations to movement we included an index of motion as a control variable. We used root‐mean‐squared (RMS) movement as our index of motion, which is computed by calculated the root‐mean‐squared head position change. This summary statistic accounts for the average movement or change across six movement parameters, translational displacement across the X, Y, and Z axes and rotational displacements of pitch, yaw, and roll. Motion was not associated with Conscientiousness (r = −.08, p = .24).
3. RESULTS
Descriptives and zero‐order correlations among behavioral variables are presented in Table 1.
3.1. Coherence
To test the hypothesis that Conscientiousness is associated with coherence of GPN networks, we examined partial correlations controlling for age, sex, IQ, motion, and the eight ICNS in FPCN and DN (Figure 3). ICN‐1 coherence was correlated with Conscientiousness (partial r = .22, p = .001; see Figure 4a for scatterplot), and this effect remained significant after Bonferroni correction for multiple tests. (We corrected only for the multiple tests of association with Conscientiousness because these are the only tests where we risk supporting our hypothesis through Type I error; associations with the other Big Five traits are included in Figure 3 only as a test of whether effects were specific to Conscientiousness.) As can be seen in Figure 4a, one participant was an outlier on ICN‐1 Coherence. However, removing this participant did not substantively change the result; without this participant the partial correlation was r = .20, p = .005. This effect also remained significant after controlling for the other four Big Five traits (partial r = .19, p = .007). ICN‐1 coherence also correlated with Neuroticism (partial r = −.14, p = .049) but this result did not remain significant when correcting for multiple tests or after controlling for the other four Big Five traits (partial r = −.02, p = .773).
Figure 3.
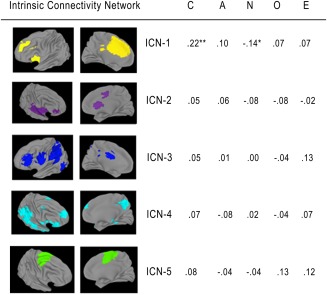
Partial correlations between the Big Five and ICN coherence in the goal priority network, controlling for age, sex, IQ, motion, and components in the FPCN and DN. N = 218. *p < .05, **p < .01. FPCN = frontoparietal control network; DN = default network; ICN = intrinsic connectivity network [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
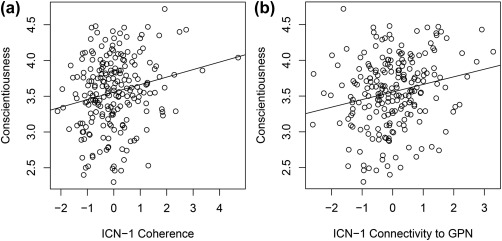
(a) Relation between Conscientiousness and ICN‐1 coherence (residualized). Higher coherence scores reflect a more synchronous network. (b) Relation between Conscientiousness and ICN‐1 connectivity to other GPN ICNs (residualized). Higher scores of connectivity reflect more synchrony between ICN‐1 and the rest of the GPN
Next we tested the specificity of the association of conscientiousness with the GPN, with the hypothesis that Conscientiousness would not be associated with the FPCN or DN. We computed partial correlations of Conscientiousness with FPCN and DN coherence, controlling for sex, age, IQ, motion, and ICNs from GPN and either FPCN and DN (Table 2). FPCN coherence was not significantly related to conscientiousness, whereas coherence within one of the DN ICNs (encompassing the superior temporal gyrus and TPJ) was negatively correlated with Conscientiousness (partial r = −.17, p = .017), but this effect was not significant after correcting for multiple tests.1 (At the request of a reviewer, we extended our test of specifity to the other 16 nonartefactual networks identified in ICA, which included multiple recognizable visual, sensory/motor, and limbic networks. Controlling for sex, age, IQ, motion, and the five GPN coherence variables, none of the remaining networks were even nominally significantly correlated with Conscientiousness, all r < .13, p > .06.)
Table 2.
Partial correlations between the Big Five and coherence of components in FPCN and DN, controlling for age, sex, IQ, motion, and components in GPN and either FPCN or DN
| C | A | N | O | E | |
|---|---|---|---|---|---|
| FPCN component | |||||
| rFPCN | .05 | .04 | −.02 | .03 | −.06 |
| lFPCN | .05 | −.02 | .00 | .11 | .08 |
| dlPFC/frontal pole | −.03 | .05 | .01 | .04 | −.02 |
| DN component | |||||
| Precuneus/angular gyrus | −.02 | .07 | −.09 | .06 | .07 |
| STG/TPJ | −.17* | .01 | .02 | .09 | .00 |
| Precuneus | .02 | .10 | −.07 | .01 | −.01 |
| mPFC | −.09 | −.08 | .13 | −.22** | .01 |
| Dorsal mPFC | −.13 | −.17* | .07 | .00 | −.03 |
Note. C = Conscientiousness; A = Agreeableness; N = Neuroticism; O = Openness/Intellect; E = Extraversion; GPN = goal priority network; FPCN = frontoparietal control network; DN = default network; rFPCN = right frontoparietal control network; lFPCN = left frontoparietal control network; dlPFC = dorsolateral prefrontal cortex; STG = superior temporal gyrus; TPJ = temporoparietal junction; mPFC = medial prefrontal cortex.
N = 218. * p < .05, *p < .01. Correlations with FPCN components control for DN and vice versa.
3.2. Interconnectivity
We next tested our hypothesis that Conscientiousness would be associated with interconnectivity between GPN ICNs. As shown in Table 3, we calculated partial correlations of Conscientiousness with the ten interconnectivity variables for the five GPN ICNs, controlling for age, sex, IQ, and motion. Significant correlations were evident for interconnectivities of GPN ICN‐1 with all four of the other GPN ICNs. Of note, GPN ICN‐1 is the same ICN for which coherence was significantly related to Conscientiousness. Only the connectivity between ICN‐1 and ICN‐3 would remain significant after Bonferroni correction, but Bonferroni correction is inappropriate here given the nonindependence of the interconnectivity variables involved, which, as a group, represent the connectivity of ICN‐1 with the rest of the GPN. We therefore created a composite variable, taking the average of those four interconnectivities to represent ICN‐1–GPN connectivity. This variable was significantly correlated with Conscientiousness (partial r = .22, p = .002; see Figure 4b), and it remained significant even after additionally controlling for the other four Big Five traits (r = .15, p = .026). ICN‐1–GPN connectivity was not significantly related to any of the other Big Five traits, except Neuroticism (r = −.16, p = .017), and this effect was not significant after controlling for Conscientiousness (r = −.06, p = .383).
Table 3.
Partial correlations of the Big Five with GPN interconnectivity, controlling for age, sex, motion, and IQ
| Connectivity | C | A | N | O | E |
|---|---|---|---|---|---|
| ICN‐1–ICN‐2 | .17* | .06 | −.13 | −.07 | .00 |
| ICN‐1–ICN‐3 | .19** | .07 | −.16* | −.01 | .06 |
| 1CN‐1–ICN‐4 | .15* | .03 | −.15* | −.07 | −.04 |
| ICN‐1–ICN‐5 | .14* | .05 | −.07 | −.03 | .08 |
| ICN‐2–ICN‐3 | .04 | .06 | −.07 | .08 | .06 |
| ICN‐2–ICN‐4 | .02 | .09 | −.13 | .05 | .16* |
| ICN‐2–ICN‐5 | −.06 | −.01 | −.04 | .02 | .04 |
| ICN‐3–ICN‐4 | −.04 | .03 | −.07 | .08 | .07 |
| ICN‐3–ICN‐5 | .05 | −.03 | .04 | .04 | .03 |
| ICN‐4–ICN‐5 | .01 | .05 | −.08 | .04 | .12 |
Note. N = 218. * p < .05, **p < .01.
Table 3 also shows that associations with GPN interconnectivity were largely specific to Conscientiousness. Three exceptions were that Extraversion was significantly related to the interconnectivity of ICN‐2 and ICN‐4, and Neuroticism was significantly related to the interconnectivity of ICN‐1 and ICN‐3 and also ICN‐1 and ICN‐4. However, none of these associations remained significant after controlling for the other Big Five traits.
To test the specificity of the association of Conscientiousness with interconnectivity in the GPN, we examined associations with interconnectivity variables among all pairs of FPCN ICNs and all pairs of DN ICNs, controlling for age, sex, IQ, and motion. None of the FPCN or DN interconnectivities was significantly correlated with Conscientiousness.2
3.3. Variance in Conscientiousness explained by GPN variables
The above results indicate that the GPN is associated with conscientiousness, but they do not show how much variance in conscientiousness is accounted for by all of our significant GPN predictors as a set. To test this, we used blocked regression to assess the total variance explained by the significant GPN predictors from the coherence and interconnectivity analyses. The first block included only covariates (IQ, age, sex, and motion) and accounted for 8% of the variance in Conscientiousness. The second block added all five significant GPN variables (ICN‐1 coherence and all interconnectivity values involving ICN‐1) and accounted for an additional 6% of the variance in Conscientiousness (ΔR 2 = .06, F (5, 208) = 2.73, p = .021).
3.4. Exploratory analyses
Additional exploratory analyses were conducted to see if the interconnectivity of ICN‐1 with any FPCN or DN ICN was related to conscientiousness. Given the association of Conscientiousness with ICN‐1 and with its functional connections to the rest of the GPN, Conscientiousness might also be related to ICN‐1's connections to other important brain networks. Of the eight additional interconnectivity variables, only two were related to Conscientiousness, interconnectivity of ICN‐1 with a DN ICN primarily including superior temporal gyrus and TPJ (partial r = .16, p = .018) and with a FPCN ICN primarily including left dlPFC and frontal pole and the parietal angular gyrus (partial r = .14, p = .044). Only the former remained significant when additionally controlling for the other Big Five traits (r = .16, p = .019). This was the same DN ICN for which Conscientiousness was associated with coherence, and it also had the highest overlap of any of the DN components with Yeo et al.'s (2011) VAN.
4. DISCUSSION
Despite the well‐established importance of Conscientiousness as a trait, relatively little is known about the psychological and neural processes that support it. The present research provided the first direct test of a hypothesis developed based on the relatively sparse neuroscience research targeting Conscientiousness and related constructs (Allen & DeYoung, 2016; DeYoung, 2015), namely that Conscientiousness is related to functioning of the GPN, a broad neural network incorporating both the VAN and the salience network. Supporting our hypothesis, coherence (i.e., within‐network connectivity) of one of five GPN ICNs correlated with Conscientiousness (ICN‐1). This ICN included the dACC, anterior insula, and middle and superior frontal gyri and was positively related to Conscientiousness, meaning that more synchrony within this network was associated with higher Conscientiousness scores. Additionally, interconnectivity between this network and the other four GPN ICNs were positively associated with Conscientiousness. Together, these five variables accounted for 6% of the variance in Conscientiousness. Thus, as synchrony within the GPN increases, Conscientiousness also increases. This suggests that ICN‐1 and its GPN connections may be particularly important for maintenance of motivational stability and nonimmediate goal attainment. Coherence and interconnectivity in other networks were generally not associated with Conscientiousness, suggesting that the GPN may be a relatively specific neural correlate.
Our pattern of results highlights connections between the dlPFC, insula, and dACC. ICN‐1 contained all of these regions, which suggests that they probably form a core GPN subnetwork. As discussed in our introduction, task‐based fMRI suggests that the GPN detects potentially relevant stimuli in the environment and then determines whether or not to act on the stimuli based on relevance to one's goals. It is plausible that individuals high in Conscientiousness have a more functionally integrated GPN that is both (1) better at determining which stimuli should be salient given their goals and (2) better at determining if spontaneous distractor stimuli are worth paying attention to (in the context of nonimmediate goals).
Interestingly, the subnetwork involving dlPFC, insula, and dACC appears to have been identified by Yeo et al. (2011) in their 17‐network parcellation. This more fine‐grained parcellation tended to divide broad neural networks from the 7‐network parcellation into smaller parts (e.g., the DN split into three well‐documented subsystems; Andrews‐Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010). In this parcellation, the network we are calling GPN split into two distinct networks. One of those subnetworks extended into some brain regions covered by the FPCN in the 7‐network parcellation and was the closest match spatially to our ICN‐1, with a higher percentage spatial overlap than any of the other networks from either parcellation (Figure 5). Like our ICN‐1, this network includes the right ventrolateral PFC, anterior insula, and dACC plus adjacent medial frontal cortex. This network encompasses several of the regions typically labeled as parts of the VAN and salience network; however, it emphasizes frontal areas, encompassing only small regions of TPJ. This smaller network may be a good representation of the core of the network that we called the GPN, enabling prioritization of goals and, hence, conscientious behavior.
Figure 5.
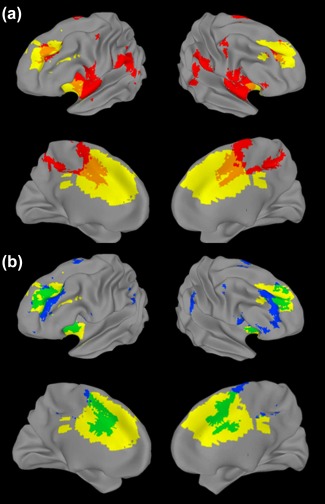
Inflated brain map comparing our ICN‐1 (yellow) to networks identified by Yeo et al. (2011). (a) Broad ventral attention network (VAN) from their 7‐network parcellation (red, overlap indicated in orange). (b) Network most closely approximating our ICN‐1, from their 17‐network parcellation (blue, overlap indicated in green) [Color figure can be viewed at http://wileyonlinelibrary.com]
In this study, however, it was not just coherence within ICN‐1 that predicted Conscientiousness, but also interconnectivity between this ICN and the other four GPN ICNs (whereas connectivity of ICN‐1 with ICNs in the FPCN and DN was not for the most part significant). This pattern suggests that, although the subnetwork of the GPN represented by ICN‐1 is particularly important for Conscientiousness, the larger GPN is also relevant, and should continue to be a target of research on the neural substrates of Conscientiousness.
In reviewing research on associations of regional brain volume with Conscientiousness, Allen and DeYoung (2016) found that the pattern of findings suggested that Conscientiousness is associated with greater lateral PFC volume but reduced volume in other areas of the GPN. This suggests that it may be the balance of power within the GPN that is most important for Conscientiousness (given the principle of neural Darwinism, in which larger populations of neurons are capable of exerting more influence than smaller ones). If so, connectivity in the GPN should be important partly because the capacity of the lateral PFC to represent goals, suppress disruptive impulses, and maintain an effective order of operations should depend on its ability to influence other brain structures that generate disruptive impulses or otherwise interfere with prioritizing goals effectively.
Previous studies using different imaging modalities have found evidence emphasizing the importance of connectivity between lateral prefrontal cortex and the insula. A structural connectivity study of white matter in 556 older adults found that Conscientiousness was positively associated with white matter coherence in the uncinate fasciculus, a white matter tract connecting the insula to frontal regions (Lewis et al., 2016). An fMRI study of the stop‐signal task, in which participants must override actions after they have already been cued, found that a measure of impulsivity associated with low Conscientiousness predicted less functional connectivity between insula and dlPFC (Farr et al., 2012). Further, researchers have proposed that the salience network serves a specific function in executive control: maintaining sustained attention on a task and directing attention to task‐relevant stimuli (Dosenbach et al., 2006; Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008). One interpretation of this previous research, consistent with our proposal regarding the role of the GPN in Conscientiousenss, is that the insula frequently generates salience signals for stimuli that may be distractors, while regions of lateral PFC are involved in suppressing distraction and maintaining current goal pursuit.
Although some previous studies have reported results congruent with the findings presented here, one study found a positive association of scores on a cognitive failures questionnaire with interconnectivity between a cingulo‐opercular network resembling our ICN‐1 and a posterior parietal network (Bey, Montag, Reuter, Weber, & Markett, 2015). In terms of item content, at face value the cognitive failures questionnaire appears to resemble low Conscientiousness (which would render their finding contrary to ours in the direction of association), but it is also positively correlated with Neuroticism (Broadbent, Cooper, FitzGerald, & Parkes, 1982). Because of uncertainties regarding how the cognitive failures construct maps on to the Big Five, it is difficult to compare the finding of Bey et al. (2015) to ours. Further, the small size of their sample (N = 71) increases the likelihood that the discrepancy is merely due to sampling error.
A potential limitation of our research is that our functional connectivity metrics were derived from a 5 min resting state scan. Previous research suggests that the overall reliability of resting state functional connectivity can be improved by increasing scanning time from 5 to 13 min (Birn et al., 2013). However, this finding was based on a global estimate of reliability (average of reliability across the entire brain), and the authors noted it was most relevant to seed‐based approaches. In contrast, dual regression ICA generally produces connectivity estimates with higher reliability, and our ICA method was specifically designed to maximize test–retest reliability (Poppe et al., 2013; Zuo & Xing, 2014). Finally, local rather than global reliability is generally high even at scan lengths as short as 5 min, in regions of the brain that are not affected by susceptibility artifacts (Mueller et al., 2015). In short, most of the neural variables we examined in this study should be adequately reliable, but it would nonetheless be desirable to replicate our results in other data with longer scan durations. It would also be of interest to determine whether using a resting scan without the minimal task that we included would alter results.
5. CONCLUSION
This study is the first to test the hypothesis that a specific neural network is a major neural correlate of Conscientiousness (Allen & DeYoung, 2016; DeYoung, 2015). Our results indicate that Conscientiousness is related to a broad network that encompasses both the VAN and the salience network, which we provisionally labeled the goal priority network. Future research will be necessary to further validate our interpretation of the function of this broad network and to elucidate exactly what functions it carries out that contribute to conscientiousness. Particularly important for Conscientiousness was a subnetwork of the GPN encompassing dlPFC, anterior insula, and dACC plus adjacent medial frontal cortex, and we speculate that this may reflect the ability of goal representations in dlPFC to control motivational salience processes in the other two regions.
Although our sample was large by the standards of neuroimaging research and had the advantage of being a community sample, it would nonetheless be a good idea in future to attempt to replicate our finding in other datasets. We note, however, that our study had an advantage for studying personality that many others do not, namely the extensiveness of its assessment of the Big Five. Rather than relying on a single brief self‐report measure of the Big Five, we used two questionnaires, one of which assessed the two major subfactors of each trait. Further, for the majority of the sample, we also had peer‐ratings of the Big Five using these two questionnaires. Rigor and accuracy of measurement is just as important for personality as it is for the brain, and the frequent use of suboptimal personality measures is likely to attenuate many true effects, potentially to the point where they cannot be reliably detected.
Discovering the neural substrate of Conscientiousness has many potential applications. Understanding why some people are better able to inhibit impulses or to direct their attention towards non‐immediate goals may lead to treatments for impulse‐control disorders or interventions for underachieving youth. Functional connectivity is malleable through experience (functional connectivity changes over the course of development) and through pharmacological treatment (Vaidya & Gordon, 2013). It may be possible to improve GPN function via cognitive training, behavioral interventions, or pharmacological treatments, which in turn has the potential to make life better and more satisfying for people who suffer from a lack of Conscientiousness.
ACKNOWLEDGMENTS
This research was supported by grants to CGD from the National Institute on Drug Abuse (R03 DA029177‐01A1) and to CGD, AR, and ARR from the National Science Foundation (SES‐1061817; GRF‐00039202). Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. The authors declare no conflict of interest.
Rueter AR, Abram SV, MacDonald AW III, Rustichini A, DeYoung CG. The goal priority network as a neural substrate of Conscientiousness. Hum Brain Mapp. 2018;39:3574–3585. 10.1002/hbm.24195
Funding information National Institute on Drug Abuse, Grant/Award Number: R03 DA029177‐01A1; National Science Foundation, Grant/Award Numbers: SES‐1061817, GRF‐00039202
Footnotes
Other research has found an association between Openness/Intellect and connectivity in the DN (Beaty et al., 2016), which we did not replicate. One possible reason for this nonreplication is that Beaty et al. (2016) used a different measure of connectivity, namely, a graph‐theory metric of efficiency among nodes in the DN selected from prior research.
In a previously published study using this sample, some facets of Agreeableness were found to be associated with interconnectivity in the DN (Allen, Rueter, Abram, Brown, & DeYoung, 2017). Given the number of tests that would be required, with attendant low statistical power, we did not conduct additional exploratory analysis using the other Big Five traits in this study.
REFERENCES
- Abram, S. V. , Wisner, K. M. , Grazioplene, R. G. , Krueger, R. F. , MacDonald, A. W. , & DeYoung, C. G. (2015). Functional coherence of insula networks is associated with externalizing behavior. Journal of Abnormal Psychology, 124(4), 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman, P. L. , & Heggestad, E. D. (1997). Intelligence, personality, and interests: Evidence for overlapping traits. Psychological Bulletin, 121(2), 219–245. [DOI] [PubMed] [Google Scholar]
- Allen, T. , & DeYoung, C. (2016). Personality neuroscience and the five‐factor model. Oxford Handbooks Online. [Google Scholar]
- Allen, T. A. , Rueter, A. R. , Abram, S. V. , Brown, J. S. , & DeYoung, C. G. (2017). Personality and neural correlates of mentalizing ability. European Journal of Personality, 31(6), 599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna, J. R. , Reidler, J. S. , Sepulcre, J. , Poulin, R. , & Buckner, R. L. (2010). Functional‐anatomic fractionation of the brain's default network. Neuron, 65(4), 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, R. E. , Kaufman, S. B. , Benedek, M. , Jung, R. E. , Kenett, Y. N. , Jauk, E. , … Silvia, P. J. (2016). Personality and complex brain networks: The role of openness to experience in default network efficiency. Human Brain Mapping, 37(2), 773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann, C. F. , Mackay, C. E. , Filippini, N. , & Smith, S. M. (2009). Group comparison of resting‐state fMRI data using multi‐subject ICA and dual regression. NeuroImage, 47, S148. [Google Scholar]
- Bey, K. , Montag, C. , Reuter, M. , Weber, B. , & Markett, S. (2015). Susceptibility to everyday cognitive failure is reflected in functional network interactions in the resting brain. NeuroImage, 121, 1–9. [DOI] [PubMed] [Google Scholar]
- Birn, R. M. , Molloy, E. K. , Patriat, R. , Parker, T. , Meier, T. B. , Kirk, G. R. , … Prabhakaran, V. (2013). The effect of scan length on the reliability of resting‐state fMRI connectivity estimates. NeuroImage, 83, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnebekk, A. , Fjell, A. M. , Walhovd, K. B. , Grydeland, H. , Torgersen, S. , & Westlye, L. T. (2013). Neuronal correlates of the five factor model (FFM) of human personality: Multimodal imaging in a large healthy sample. NeuroImage, 65, 194–208. [DOI] [PubMed] [Google Scholar]
- Broadbent, D. E. , Cooper, P. F. , FitzGerald, P. , & Parkes, K. R. (1982). The Cognitive Failures Questionnaire (CFQ) and its correlates. British Journal of Clinical Psychology, 21(1), 1–16. [DOI] [PubMed] [Google Scholar]
- Brown, S. M. , Manuck, S. B. , Flory, J. D. , & Hariri, A. R. (2006). Neural basis of individual differences in impulsivity: Contributions of corticolimbic circuits for behavioral arousal and control. Emotion, 6(2), 239–245. [DOI] [PubMed] [Google Scholar]
- Bunge, S. A. , & Zelazo, P. D. (2006). A brain‐based account of the development of rule use in childhood. Current Directions in Psychological Science, 15(3), 118–121. [Google Scholar]
- Choi, Y. Y. , Shamosh, N. A. , Cho, S. H. , DeYoung, C. G. , Lee, M. J. , Lee, J.‐M. , … Lee, K. H. (2008). Multiple bases of human intelligence revealed by cortical thickness and neural activation. Journal of Neuroscience, 28(41), 10323–10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell, J. C. , & Yurgelun‐Todd, D. A. (2013). Age‐related changes in insula cortical thickness and impulsivity: Significance for emotional development and decision‐making. Developmental Cognitive Neuroscience, 6, 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, M. W. , Yarkoni, T. , Repovš, G. , Anticevic, A. , & Braver, T. S. (2012). Global connectivity of prefrontal cortex predicts cognitive control and intelligence. Journal of Neuroscience, 32(26), 8988–8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway, A. R. , Kane, M. J. , & Engle, R. W. (2003). Working memory capacity and its relation to general intelligence. Trends in Cognitive Sciences, 7(12), 547–552. [DOI] [PubMed] [Google Scholar]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- DeYoung, C. G. (2011). Intelligence and personality. Cambridge Handbook of Intelligence, 711–737. [Google Scholar]
- DeYoung, C. G. (2015). Cybernetic Big Five Theory. Journal of Research in Personality, 56, 33–58. [Google Scholar]
- DeYoung, C. G. , Carey, B. E. , Krueger, R. F. , & Ross, S. R. (2016). Ten aspects of the Big Five in the Personality Inventory for DSM–5. Personality Disorders: Theory, Research, and Treatment, 7(2), 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung, C. G. , Hirsh, J. B. , Shane, M. S. , Papademetris, X. , Rajeevan, N. , & Gray, J. R. (2010). Testing predictions from personality neuroscience brain structure and the Big Five. Psychological Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung, C. G. , Peterson, J. B. , & Higgins, D. M. (2005). Sources of openness/intellect: Cognitive and neuropsychological correlates of the fifth factor of personality. Journal of Personality, 73(4), 825–858. [DOI] [PubMed] [Google Scholar]
- DeYoung, C. G. , Quilty, L. C. , & Peterson, J. B. (2007). Between facets and domains: 10 aspects of the Big Five. Journal of Personality and Social Psychology, 93(5), 880–896. [DOI] [PubMed] [Google Scholar]
- DeYoung, C. G. , Shamosh, N. A. , Green, A. E. , Braver, T. S. , & Gray, J. R. (2009). Intellect as distinct from openness: Differences revealed by fMRI of working memory. Journal of Personality and Social Psychology, 97(5), 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice, L. R. (1945). Measures of the amount of ecologic association between species. Ecology, 26(3), 297–302. [Google Scholar]
- Dosenbach, N. U. F. , Fair, D. A. , Cohen, A. L. , Schlaggar, B. L. , & Petersen, S. E. (2008). A dual‐networks architecture of top‐down control. Trends in Cognitive Sciences, 12(3), 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach, N. U. F. , Visscher, K. M. , Palmer, E. D. , Miezin, F. M. , Wenger, K. K. , Kang, H. C. , … Petersen, S. E. (2006). A core system for the implementation of task sets. Neuron, 50(5), 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair, D. A. , Schlaggar, B. L. , Cohen, A. L. , Miezin, F. M. , Dosenbach, N. U. F. , Wenger, K. K. , … Petersen, S. E. (2007). A method for using blocked and event‐related fMRI data to study “resting state” functional connectivity. NeuroImage, 35(1), 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr, O. M. , Hu, S. , Zhang, S. , & Li, C. R. (2012). Decreased saliency processing as a neural measure of Barratt impulsivity in healthy adults. NeuroImage, 63(3), 1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini, N. , MacIntosh, B. J. , Hough, M. G. , Goodwin, G. M. , Frisoni, G. B. , Smith, S. M. , … Mackay, C. E. (2009). Distinct patterns of brain activity in young carriers of the APOE‐epsilon4 allele. Proceedings of the National Academy of Sciences of the United States of America, 106(17), 7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , Corbetta, M. , Snyder, A. Z. , Vincent, J. L. , & Raichle, M. E. (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences, 103(26), 10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , & Greicius, M. (2010). Clinical applications of resting state functional connectivity. Frontiers in Systems Neuroscience, 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , Zhang, D. , Snyder, A. Z. , & Raichle, M. E. (2009). The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology, 101(6), 3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson, M. , Driesen, N. R. , Skudlarski, P. , Gore, J. C. , & Constable, R. T. (2006). Brain connectivity related to working memory performance. Journal of Neuroscience, 26(51), 13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, X. , Erb, M. , Ackermann, H. , Martin, J. A. , Grodd, W. , & Reiterer, S. M. (2011). Voxel‐based morphometry studies of personality: Issue of statistical model specification—effect of nuisance covariates. NeuroImage, 54(3), 1994–2005. [DOI] [PubMed] [Google Scholar]
- Jackson, J. , Balota, D. A. , & Head, D. (2011). Exploring the relationship between personality and regional brain volume in healthy aging. Neurobiology of Aging, 32(12), 2162–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, O. P. , Naumann, L. P. , & Soto, C. J. (2008). Paradigm shift to the integrative big‐five trait taxonomy: History, measurement, and conceptual issues In John O.P., Robins R. W., & Pervin L. A. (Eds.), Handbook of personality: Theory and research (pp. 114–158). New York, NY: Guilford Press. [Google Scholar]
- Jung, R. E. , & Haier, R. J. (2007). The Parieto‐Frontal Integration Theory (P‐FIT) of intelligence: Converging neuroimaging evidence. Behavioral and Brain Sciences, 30(02), 135–154. [DOI] [PubMed] [Google Scholar]
- Kapogiannis, D. , Sutin, A. , Davatzikos, C. , Costa, P. , & Resnick, S. (2013). The five factors of personality and regional cortical variability in the Baltimore longitudinal study of aging. Human Brain Mapping, 34(11), 2829–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, R. E. , Alexopoulos, G. S. , Wang, Z. , Gunning, F. M. , Murphy, C. F. , Morimoto, S. S. , … Hoptman, M. J. (2010). Visual inspection of independent components: Defining a procedure for artifact removal from fMRI data. Journal of Neuroscience Methods, 189(2), 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov, R. , Gamez, W. , Schmidt, F. , & Watson, D. (2010). Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta‐analysis. Psychological Bulletin, 136(5), 768–821. [DOI] [PubMed] [Google Scholar]
- Krienen, F. M. , Yeo, B. T. T. , & Buckner, R. L. (2014). Reconfigurable task‐dependent functional coupling modes cluster around a core functional architecture. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1653), 20130526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Fox, P. M. , Eickhoff, S. B. , Turner, J. A. , Ray, K. L. , McKay, D. R. , … Fox, P. T. (2011). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience, 23(12), 4022–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, G. J. , Cox, S. R. , Booth, T. , Maniega, S. M. , Royle, N. A. , Hernández, M. V. , … Deary, I. J. (2016). Trait conscientiousness and the personality meta‐trait stability are associated with regional white matter microstructure. Social Cognitive and Affective Neuroscience, nsw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W.‐Y. , Weber, B. , Reuter, M. , Markett, S. , Chu, W.‐C. , & Montag, C. (2013). The Big Five of Personality and structural imaging revisited: A VBM ‐ DARTEL study. Neuroreport, 24(7), 375–380. [DOI] [PubMed] [Google Scholar]
- Marquand, A. F. , Haak, K. V. , & Beckmann, C. F. (2017). Functional corticostriatal connection topographies predict goal directed behaviour in humans. bioRxiv, 169151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo, K. , Nicoletti, M. , Nemoto, K. , Hatch, J. P. , Peluso, M. A. M. , Nery, F. G. , & Soares, J. C. (2009). A voxel‐based morphometry study of frontal gray matter correlates of impulsivity. Human Brain Mapping, 30(4), 1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V. , & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function, 214(5–6), 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, S. , Wang, D. , Fox, M. D. , Pan, R. , Lu, J. , Li, K. , … Liu, H. (2015). Reliability correction for functional connectivity: Theory and implementation. Human Brain Mapping, 36(11), 4664–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee, D. E. , Brown, J. W. , Askren, M. K. , Berman, M. G. , Demiralp, E. , Krawitz, A. , & Jonides, J. (2013). A meta‐analysis of executive components of working memory. Cerebral Cortex, 23(2), 264–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouchi, R. , Takeuchi, H. , Taki, Y. , Sekiguchi, A. , Kotozaki, Y. , Nakagawa, S. , … Kawashima, R. (2016). Neuroanatomical bases of effortful control: Evidence from a large sample of young healthy adults using voxel‐based morphometry. Scientific Reports, 6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer, D. J. , & Benet‐Martínez, V. (2006). Personality and the prediction of consequential outcomes. Annual Review of Psychology, 57(1), 401–421. [DOI] [PubMed] [Google Scholar]
- Paxton, J. L. , Barch, D. M. , Racine, C. A. , & Braver, T. S. (2008). Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex, 18(5), 1010–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe, A. B. , Wisner, K. , Atluri, G. , Lim, K. O. , Kumar, V. , & MacDonald, A. W. III , (2013). Toward a neurometric foundation for probabalistic independent component analysis of fMRI data. Cognitive, Affective, & Behavioral Neuroscience, 13(3), 641–659. [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Mitra, A. , Laumann, T. O. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccelli, R. , Toschi, N. , Nigro, S. , Terracciano, A. , & Passamonti, L. (2017). Surface‐based morphometry reveals the neuroanatomical basis of the five‐factor model of personality. Social Cognitive and Affective Neuroscience, 12(4), 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, B. W. , Lejuez, C. , Krueger, R. F. , Richards, J. M. , & Hill, P. L. (2014). What is conscientiousness and how can it be assessed? Developmental Psychology, 50(5), 1315–1330. [DOI] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, D. J. , Bonnelle, V. , Boissezon, X. D. , Beckmann, C. F. , James, S. G. , Patel, M. C. , & Mehta, M. A. (2010). Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences, 107(13), 6106–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Fox, P. T. , Miller, K. L. , Glahn, D. C. , Fox, P. M. , Mackay, C. E. , … Beckmann, C. F. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences, 106(31), 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, M. , Zhou, Y. , Li, J. , Liu, Y. , Tian, L. , Yu, C. , & Jiang, T. (2008). Brain spontaneous functional connectivity and intelligence. NeuroImage, 41(3), 1168–1176. [DOI] [PubMed] [Google Scholar]
- Stock, A.‐K. , & Beste, C. (2015). Conscientiousness increases efficiency of multicomponent behavior. Scientific Reports, 5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin, L. Q. (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16(1), 55–61. [DOI] [PubMed] [Google Scholar]
- Vaidya, C. J. , & Gordon, E. M. (2013). Phenotypic variability in resting‐state functional connectivity: Current status. Brain Connectivity, 3(2), 99–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel, S. , Geng, J. J. , & Fink, G. R. (2014). Dorsal and ventral attention systems distinct neural circuits but collaborative roles. Neuroscientist, 20(2), 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Song, M. , Jiang, T. , Zhang, Y. , & Yu, C. (2011). Regional homogeneity of the resting‐state brain activity correlates with individual intelligence. Neuroscience Letters, 488(3), 275–278. [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (2008). Wechsler adult intelligence scale ‐ Fourth edition (WAIS‐IV). San Antonio, TX: NCS Pearson. [Google Scholar]
- Whittle, S. , Yücel, M. , Fornito, A. , Barrett, A. , Wood, S. J. , Lubman, D. , … Allen, N. B. (2008). Neuroanatomical correlates of temperament in early adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 47(6), 682–693. [DOI] [PubMed] [Google Scholar]
- Wisner, K. M. , Atluri, G. , Lim, K. O. , & MacDonald, A. W. (2013). Neurometrics of intrinsic connectivity networks at rest using fMRI: Retest reliability and cross‐validation using a meta‐level method. NeuroImage, 76, 236–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner, K. M. , Patzelt, E. H. , Lim, K. O. , & MacDonald, A. W. (2013). An intrinsic connectivity network approach to insula‐derived dysfunctions among cocaine users. American Journal of Drug and Alcohol Abuse, 39(6), 403–413. [DOI] [PubMed] [Google Scholar]
- Yeo, B. T. , Krienen, F. M. , Sepulcre, J. , Sabuncu, M. R. , Lashkari, D. , Hollinshead, M. , … Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, X.‐N. , Kelly, C. , Adelstein, J. S. , Klein, D. F. , Castellanos, F. X. , & Milham, M. P. (2010). Reliable intrinsic connectivity networks: Test‐retest evaluation using ICA and dual regression approach. NeuroImage, 49(3), 2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, X.‐N. , & Xing, X.‐X. (2014). Test‐retest reliabilities of resting‐state fMRI measurements in human brain functional connectomics: A systems neuroscience perspective. Neuroscience & Biobehavioral Reviews, 45, 100–118. [DOI] [PubMed] [Google Scholar]


