Abstract
Gut microbiota influence the host immune system and are associated with various diseases. In recent years, postmenopausal bone loss has been suggested to be related to gut microbiota. In the present study, we investigated the treatment effect of the probiotic Bacillus subtilis C-3102 (C-3102) on bone mineral density (BMD) and its influence on gut microbiota in healthy postmenopausal Japanese women. Seventy-six healthy postmenopausal Japanese women were treated with a placebo or C-3102 spore-containing tablets for 24 weeks. When compared with the placebo, C-3102 significantly increased total hip BMD (placebo = 0.83 ± 0.63%, C-3102 = 2.53 ± 0.52%, p=0.043). There was a significant group-by-time interaction effect for urinary type I collagen cross-linked N-telopeptide (uNTx) (p=0.033), a marker of bone resorption. Specifically, the C-3102 group showed significantly lower uNTx when compared with the placebo group at 12 weeks of treatment (p=0.015). In addition, in the C-3102 group, there was a trend towards a decrease in the bone resorption marker tartrate-resistant acid phosphatase isoform 5b (TRACP-5b) when compared with the placebo group at 12 weeks of treatment (p=0.052). The relative abundance of genus Bifidobacterium significantly increased at 12 weeks of treatment compared with the baseline in the C-3102 group. The relative abundance of genus Fusobacterium was significantly decreased in the C-3102 group at 12 and 24 weeks of treatment compared with the baseline. These data suggested that C-3102 improves BMD by inhibiting bone resorption and modulating gut microbiota in healthy postmenopausal women.
Keywords: probiotics, osteoporosis, bone, microbiota
INTRODUCTION
Osteoporosis is a disease characterized by low bone mineral density (BMD) and increased susceptibility to fractures. Osteoporotic fractures are associated with a worsening of health-related quality of life [1] and increased mortality risk [2]. Since BMD is an important predictor of osteoporotic fractures [3], preventing loss of BMD is important in reducing the risk of fracture. A Japanese population-based cohort study (Research on Osteoarthritis Against Disability; ROAD) estimated that approximately 13 million Japanese people, aged 40 years and older, are affected by osteoporosis, and more than 70% of them are women [4]. In postmenopausal women, estrogen deficiency induces a decrease in BMD by enhancing osteoclastogenesis and increasing the rate of bone turnover [5]. Postmenopausal women with osteoporosis are commonly treated with an antiresorptive agent or selective estrogen receptor modulator (SERM); however, these agents have various side effects [6, 7], poor compliance, and high costs. Dietary supplements such as vitamin D and calcium are ingested to prevent osteoporosis, but the effect on BMD improvement is not sufficient [8, 9]. A more effective and safe method to improve BMD is required.
In recent years, gut microbiota have been shown to be associated with various diseases (allergies, autoimmune diseases, obesity, inflammatory bowel disease, etc.) [10]. Previous studies have suggested that gut microbiota are also related to postmenopausal osteoporosis: bone loss was not observed in germ-free mice with induced estrogen depletion after treatment with gonadotropin-releasing hormone agonists [11]. In addition, an antibiotic prevented bone loss in rats after ovariectomy (OVX) induced osteoporosis [12]. Furthermore, it has been suggested that certain probiotics may be advantageous for bone health: Lactobacillus paracasei treatment in OVX mice has been reported to increase BMD [13]. In humans, ingestion of a multispecies probiotic supplement for six months improved bone metabolism in postmenopausal women [14]. In addition, ingestion of fermented soybean (natto) made using Bacillus subtilis, which produces high amounts of vitamin K2, improved undercarboxylated osteocalcin (ucOC), the marker of fracture risk, in healthy men and women [15]. Although these studies suggest that probiotics may improve BMD, their effects are still not sufficient in humans.
Bacillus subtilis strain C-3102 (C-3102) are aerobic spore-forming bacteria used in probiotics. In an in vitro gastrointestinal model study, 99% of the ingested C-3102 were still alive when passed through a model of the small intestine from the stomach and 8% germinated [16]. In addition, in an open human trial, ingestion of supplements containing C-3102 spores increased the relative abundance of Bifidobacterium and decreased the number of Enterobacteriaceae [17]. Moreover, in an open-label, single-arm pilot study, 13 weeks ingestion of supplements containing C-3102 spores decreased serum levels of tartrate-resistant acid phosphatase-5b (TRACP-5b), a marker of bone resorption, in postmenopausal women (unpublished data). These results suggested that C-3102 has the potential to improve the intestinal environment and increase BMD, but the actual effect is unknown.
In the present randomized, placebo-controlled, double-blind clinical trial, we investigated the effect of C-3102 on BMD, bone metabolism, and gut microbiota in healthy postmenopausal Japanese women.
MATERIALS AND METHODS
Participants
Sample size estimation was performed using power analysis and information from a preliminary study (unpublished information). We estimated that 34 participants would be required for each group to detect a difference in TRACP-5b, assuming an α level of 0.05 and 80% power. Assuming a drop-out rate of 10%, we calculated a final sample size of 76 participants (38 per group). Healthy, postmenopausal Japanese women aged 50–69 years were recruited from the general public and registered for the study at Orthomedico Inc. (Tokyo, Japan) from December 2015 to April 2016. Participants underwent a medical examination at Takara Clinic, Medical Corporation Seishinkai (Tokyo, Japan): BMD was measured at the lumbar spine and total hip. Participants were excluded from the study based on the following criteria: 1) cessation of menses less than two years from the study date; 2) diagnosed with osteoporosis; 3) previously diagnosed with a chronic disease (cardiovascular disease, impaired hepatic or renal function, cerebrovascular disease, diabetes, rheumatoid arthritis, dyslipidemia, high blood pressure, irritable bowel syndrome); 4) using medication, including traditional Chinese medicine; 5) taking dietary supplements known to affect bone metabolism (Ca, Mg, vitamin D, vitamin K, soy isoflavone, etc.) once a week or more; 6) consuming natto (soybeans fermented by Bacillus subtilis) four times a week or more; 7) having any allergies; and 8) participation in other clinical trials in the past three months. Written informed consent was obtained from all individual participants included in the study.
Study design
A randomized, placebo-controlled, double-blind clinical trial was conducted to investigate the effect of C-3102 on BMD. The experimental periods were divided into two weeks of observation before treatment and 24 weeks of treatment. Seventy-six participants were assigned an individual trial identification number and randomly allocated into two groups of 38 with stratification by BMD, bone turnover marker, age, and body mass index (BMI). The participants were assigned a treatment code by the study coordinator, and all staff and participants were blind to the treatment allocation throughout the trial. After two weeks of observation, participants ingested three test tablets each day after breakfast. Participants were instructed to maintain their normal living practices, including diet and exercise, and to record medication used during the study period. The participants visited the clinic every 12 weeks for a medical examination. Safety was monitored by assessing the hematology, clinical chemistry, and vital signs of each participant, in addition to physical examinations at each clinic visit and recording adverse events. This study was approved by the ethics committee of Takara Clinic, Medical Corporation Seishinkai, and carried out in accordance with the principles of the Helsinki Declaration (2013).
Test materials
A pre-culture was prepared using soybean oil residue inoculated with C-3102. Dried soybeans were then fermented using the pre-culture and used in the test tablets, in addition to excipients, emulsifiers, and a lubricant. Each tablet weighed 0.19 g. Placebo tablets were prepared using the same method, except C-3102 soybean fermentation extract was replaced with dextrin. Ultimately, 3 tablets contained 3.4 × 109 CFU of C-3102. Table 1 shows the composition of test samples per day. Adherence was monitored by tablet count (number dispensed and returned) and by the participants’ daily records (number of tablets taken).
Table 1. Nutritional composition of test samples (daily doses).
| Placebo | C-3102 | ||
|---|---|---|---|
| Energy | (kcal) | 2.21 | 2.19 |
| Fat | (g) | 0.01 | 0.01 |
| Protein | (g) | <0.001 | 0.06 |
| Carbohydrate | (g) | 0.53 | 0.46 |
| Vitamin D | (μg) | N.D. | N.D. |
| Vitamin K2 | (μg) | N.D. | 5.52 |
| Isoflavone | (mg) | N.D. | 0.34 |
| Phosphorus | (mg) | 0.02 | 0.81 |
| Calcium | (mg) | 0.01 | 0.36 |
| Magnesium | (mg) | 0.002 | 0.338 |
| C-3102 spore | (CFU) | N.D. | 3.4 × 109 |
N.D.: Not detected.
Dietary assessment
Dietary patterns during the study period were estimated using a brief-type self-administered diet history questionnaire (BDHQ) [18]. The daily intake of total energy, fat, protein, carbohydrates, calcium, magnesium, phosphorus, vitamin D, vitamin C, and vitamin K was estimated from the dietary intake of 58 food and beverage items using a purpose-built computer algorithm for the BDHQ, which was based on the Standard Tables of Food Composition in Japan [19].
BMD measurements
BMD was measured at the lumbar spine (L2–L4) and the left hip using dual-energy X-ray absorptiometry (Hologic Discovery DXA system, Hologic Inc., Waltham, MA, USA). Measurements were assessed at the first visit to the hospital and at 24 weeks of the trial. The Hologic APEX software, version 3.3.0.1, was used to analyze the DXA scans. Quality control was performed using a calibration phantom before each measurement.
Biochemical analysis
Fasting blood samples and urine were collected at baseline and at 12 weeks and 24 weeks of the trial. Blood samples were centrifuged at 3,000 rpm for 10 min, and serum samples from each participant were stored at −80°C. Serum TRACP-5b was measured using an Osteolinks TRAP-5b kit (DS Pharma Biomedical Co., Ltd., Osaka, Japan), and serum bone alkaline phosphatase (BAP) was measured using Access Ostase (Beckman Coulter, Inc., Fullerton, CA, USA). Serum intact parathyroid hormone (iPTH) was measured using Elecsys PTH (Roche Diagnostics, Mannheim, Germany). Urinary type I collagen cross-linked N-telopeptide (uNTx) was measured using an Osteomark NTx kit (Alere, Tokyo, Japan) and corrected using urinary creatinine levels for standardization.
Fecal sampling and DNA extraction
Fecal samples were collected with a test container (Sarstedt K.K., Tokyo, Japan) before each visit to the clinic and were kept at −30°C for later use. Frozen samples were thawed at room temperature, and then 0.2 mg of fecal sample were aliquoted into a plastic tube. Samples were washed in 1.0 ml PBS and subjected to centrifugation at 14 ,000 × g for 3 min. Pellets were resuspended in 500 µl extraction buffer (166 mM Tris/HCl, 66 mM EDTA, 8.3% SDS, pH 9.0) and 500 µl TE buffer-saturated phenol. Glass beads (300 mg, 0.1 mm diameter) were added to the suspension, and the mixture was vortexed vigorously for 60 s using a Multi-beads Shocker (Yasui Kikai Corporation, Osaka, Japan). After centrifugation at 14, 000 × g for 5 min, 400 µl of the supernatant were extracted using phenol-chloroform-isoamyl alcohol, and DNA was precipitated with isopropanol. The samples were washed in 70% ethanol and dissolved in TE buffer. A High Pure PCR Template Preparation Kit (Roche, Tokyo, Japan) was used for further purification according to the manufacturer’s recommendations. The eluted DNA was preserved at 4°C.
Sequencing and data processing
Sequencing of the gene encoding 16S rRNA was performed using a MiSeq V2 kit, according to the manufacturer’s instructions (http://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf). Briefly, the V4 region of the bacterial 16S rDNA was amplified by PCR with forward and reverse primers (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG GTGCCAGCMGCCGCGGTAA-3′ and 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG GACTACHVGGGTATCTAATCC-3′, respectively), 5 ng of DNA from a fecal sample, and a TaKaRa Ex Taq HS kit (Takara Bio, Kusatsu, Shiga, Japan). The PCR products were purified using Agencourt AMPure XP (Beckman Coulter, Inc., Brea, CA, USA) and then amplified using a Nextera Index kit (Illumina, San Diego, CA, USA). After the second PCR, amplified products were purified using Agencourt AMPure XP. Sequencing was conducted using a paired-end to 2 × 150-bp cycle run on an Illumina MiSeq system using MiSeq Reagent Kit v2 (300 cycles).
16S rDNA-based taxonomic and diversity analysis
QIIME v.1.8.0 was used to filter and analyze the attained sequences [20]. Quality filtering was performed using the provided fastq files, and sequences that produced a quality score of under 29 were removed. Chimeric sequences were removed using USEARCH, and assignment to operational taxonomic units (OTUs) was carried out using open-reference OTU picking with a 97% threshold of pairwise identity. OTUs containing less than five sequences were removed before the remaining OTUs were classified taxonomically using the Greengenes reference database (http://greengenes.secondgenome.com/downloads/database/13_5). Alpha diversity (Chao1, number of observed species, and Shannon diversity index) within two groups (placebo and C-3102) was estimated using QIIME. In further analysis, the classified OTUs were excluded using a cutoff of 0.01% in mean relative abundance at baseline in at least one group.
Statistical analysis
Statistical analysis was performed using IBM SPSS ver. 23.0 (IBM Japan, Ltd., Tokyo, Japan). Differences in baseline characteristics between groups were tested using the Student’s t-test. One-factor analysis of covariance (ANCOVA) was performed to evaluate the percentage change from baseline in BMD between groups with baseline values as covariates. Changes in bone turnover markers were compared using a linear mixed model of repeated measures, including group, time, and group-by-time interaction as fixed effects and participants as random effects. Alpha diversity and the relative abundances of gut bacterial genera at 12 and 24 weeks of treatment within the groups were compared with the baseline values by Wilcoxon signed-rank test with Bonferroni correction. Spearman rank correlation was performed to determine the correlation between the relative abundances of gut bacterial genera and BMD or bone turnover markers. All tests were two-sided with a significance level of 5%.
RESULTS
Subjects
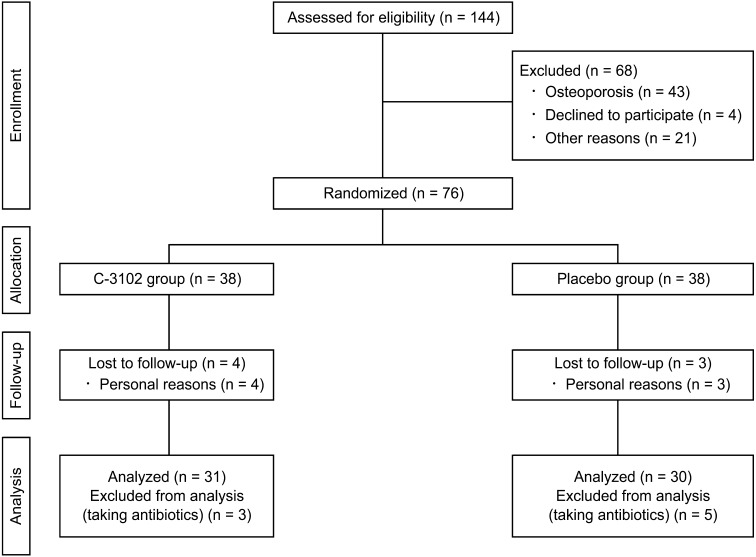
A flow chart of the trial is shown in Fig. 1. A total of 144 postmenopausal women agreed to participate in the study and underwent screening. Sixty-eight participants were excluded from the trial due to a doctor’s inquiries and medical examination (n=21), withdrawal from participation (n=4), or diagnosis of osteoporosis based on criteria for primary osteoporosis [21] (n=43). Seventy-six participants were eligible, and 38 participants were randomly allocated to each of the placebo and C-3102 groups. During the treatment period, seven participants dropped out due to personal reasons. Thirty-five participants in the placebo group and 34 participants in the C-3102 group completed the full 24-week trial period. Eight participants who took antibiotics during the treatment period were excluded from the analysis due to the effects of the antibiotics on C-3102 and gut microbiota. Ultimately, 30 participants in the placebo group and 31 participants in the C-3102 group were analyzed. The overall mean compliance rate was 99.5% ± 0.1% (placebo = 99.6% ± 0.2%, C-3102 = 99.5% ± 0.2%). No adverse effects were reported during the study period.
Fig. 1.
Study flowchart for participants.
Baseline characteristics of the participants are shown in Table 2. No significant differences were observed between the C-3102 and placebo groups in age, BMI, BMD, or bone turnover markers.
Table 2. Baseline clinical characteristics of the C-3102 and placebo groups.
| Placebo group (n=30) |
C-3102 group (n=31) |
pvalue | ||
|---|---|---|---|---|
| Age (years) | 57.8 ± 5.4 | 57.5 ± 4.3 | 0.84 | |
| Body mass index (kg/m2) | 22.1 ± 2.7 | 22.2 ± 3.3 | 0.89 | |
| Bone mineral density (g/cm2) | ||||
| L2–L4 | 0.895 ± 0.088 | 0.887 ± 0.074 | 0.70 | |
| Total hip | 0.790 ± 0.065 | 0.778 ± 0.070 | 0.49 | |
| TRACP-5b (mU/dl) | 446 ± 136 | 438 ± 101 | 0.79 | |
| BAP (mg/l) | 14.2 ± 4.2 | 14.1 ± 5.1 | 0.96 | |
| Urinary NTx (nmol/mmol·Cr) | 46.8 ± 14.8 | 54.1 ± 21.9 | 0.13 | |
| Intact PTH (pg/ml) | 50.9 ± 16.6 | 56.3 ± 18.5 | 0.24 | |
Values are means ± SDs. p values were determined by Student’s t-test.
Table 3 shows the daily intake of energy, fat, carbohydrates, protein, Ca, Mg, P, vitamin C, vitamin D, and vitamin K during the treatment period. The C-3102 group had a significantly lower daily intake of fat when compared with the placebo group at baseline and 12 weeks of treatment. No significant differences were observed between the C-3102 and placebo groups in minerals and vitamins related to bone metabolism (Ca, Mg, P, vitamin C, vitamin D, and vitamin K) during the trial.
Table 3. Daily intake of energy, fat, carbohydrates, protein, minerals, and vitamins related to bone metabolism during the treatment period.
| Group | Treatment period |
|||
|---|---|---|---|---|
| Baseline | 12 weeks | 24 weeks | ||
| Energy (kcal/day) | Placebo | 1,718 ± 469 | 1,638 ± 337 | 1,662 ± 460 |
| C-3102 | 1,574 ± 536 | 1,463 ± 402 | 1,470 ± 453 | |
| Fat (g/day) | Placebo | 56 ± 16 | 57 ± 14 | 55 ± 17 |
| C-3102 | 49 ± 19* | 49 ± 18* | 46 ± 17 | |
| Carbohydrates (g/day) | Placebo | 227 ± 67 | 204 ± 50 | 218 ± 67 |
| C-3102 | 208 ± 83 | 186 ± 58 | 193 ± 66 | |
| Protein (g/day) | Placebo | 64 ± 17 | 63 ± 16 | 63 ± 21 |
| C-3102 | 63 ± 27 | 57 ± 17 | 59 ± 23 | |
| Calcium (mg/day) | Placebo | 506 ± 185 | 499 ± 181 | 480 ± 180 |
| C-3102 | 480 ± 243 | 478 ± 189 | 464 ± 268 | |
| Magnesium (mg/day) | Placebo | 231 ± 68 | 220 ± 50 | 226 ± 67 |
| C-3102 | 228 ± 85 | 212 ± 54 | 215 ± 79 | |
| Phosphorus (mg/day) | Placebo | 971 ± 279 | 955 ± 253 | 947 ± 317 |
| C-3102 | 938 ± 406 | 881 ± 271 | 895 ± 399 | |
| Vitamin C (mg/day) | Placebo | 109 ± 49 | 95 ± 30 | 113 ± 48 |
| C-3102 | 108 ± 53 | 90 ± 44 | 107 ± 46 | |
| Vitamin D (mg/day) | Placebo | 10 ± 5 | 12 ± 5 | 11 ± 6 |
| C-3102 | 14 ± 16 | 12 ± 9 | 12 ± 11 | |
| Vitamin K (mg/day) | Placebo | 254 ± 108 | 235 ± 80 | 250 ± 108 |
| C-3102 | 253 ± 135 | 242 ± 107 | 226 ± 120 | |
Values are means ± SDs. Significant differences were determined by Student’s t-test. *p<0.05 vs. placebo group.
BMD
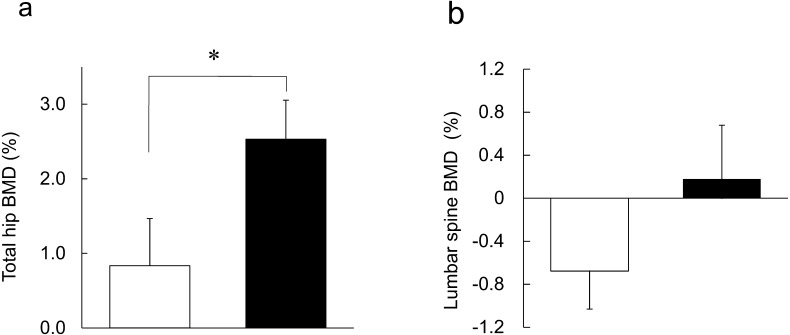
Figure 2 shows the mean relative change from baseline to 24 weeks in BMD at the lumbar spine and the total hip. In the C-3102 group, total hip BMD significantly increased when compared with the placebo group (Fig. 2a; placebo = 0.83 ± 0.63%, C-3102 = 2.53 ± 0.52%, p=0.043). Lumbar spine BMD was not significantly different between the two groups (Fig. 2b; placebo = −0.68 ± 0.35%, C-3102 = 0.18 ± 0.50%, p=0.112).
Fig. 2.
Treatment effects on bone mineral density in the placebo and C-3102 groups.
Mean ± SEM relative changes from baseline to 24 weeks of treatment in BMD in the (a) total hip and (b) lumbar spine (L2–L4) area. Data are shown for the placebo group (open bar) and C-3102 group (closed bar). Significant differences were determined by ANCOVA. *p=0.043, vs. placebo group.
Bone turnover markers
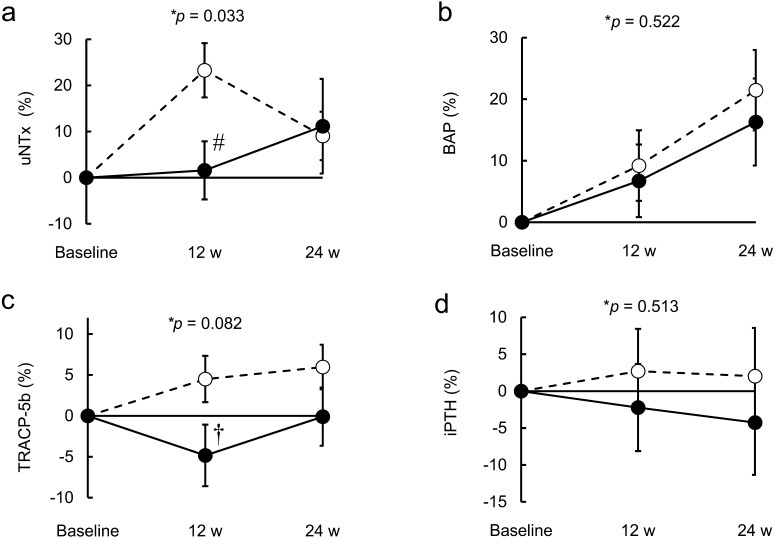
Figure 3 shows the mean relative change from baseline to 24 weeks in bone turnover markers. A trend-level interaction effect of group-by-time was observed for TRACP-5b (p=0.082), a marker of bone resorption. In the C-3102 group, there was a trend towards a decrease in TRACP-5b when compared with the placebo group at 12 weeks of treatment (Fig. 3c; placebo = 4.5 ± 2.8%, C-3102 = −4.8 ± 3.8%, p=0.054). There was a significant group-by-time interaction effect for uNTx (p=0.033), also a marker of bone resorption. Specifically, the placebo group showed significantly higher uNTx when compared with the C-3102 group at 12 weeks of treatment (Fig. 3a; placebo = 23.3 ± 5.9%, C-3102 = 1.6 ± 6.3%, p=0.015). The data suggests that C-3102 inhibits bone resorption at 12 weeks of treatment. However, there was no significant difference between the placebo and C-3102 groups in these two bone resorption markers at 24 weeks of treatment (uNTx: placebo = 9.1 ± 5.3%, C-3102 = 11.2 ± 10.3%, p=0.857; TRACP-5b: placebo = 6.0 ± 2.7%, C-3102 = −0.10 ± 10.3%, p=0.183). There was no significant group-by-time interaction effect for BAP as a marker of bone formation, but the effect of time was significant (p=0.522 and p<0.001, respectively). There was no significant group-by-time interaction effect for iPTH, which is one of the main regulators of calcium and bone metabolism (p=0.513).
Fig. 3.
Mean percent change from baseline in bone turnover markers.
Mean ± SEM relative changes from baseline to 24 weeks of treatment in bone turnover markers. (a) uNTx, (b) BAP, (c) TRACP-5b, (d) iPTH. Data are shown for the placebo group (broken line) and C-3102 group (solid line). The fixed effects of treatment, time, and group-by-time interaction on bone turnover markers were analyzed using a linear mixed model of repeated measures. *Group-by-time interaction effect. #p=0.015, vs. placebo group at 12 weeks using Student’s t-test. †p=0.054, vs. placebo group at 12 weeks using Student’s t-test.
Gut microbiota analysis
To investigate the influence of C-3102 on gut microbiota, fecal samples were analyzed by high-throughput sequencing. In the C-3102 group, the Chao1 (species richness) and Shannon (evenness) indices significantly decreased after 24 weeks of treatment when compared with the baseline (Table 4). Furthermore, at the genus level, C-3102 treatment resulted in a significant change in the relative abundance of 11 bacterial genera when compared with the baseline in the C-3102 group (Table 5). Among them, the relative abundance of genus Bifidobacterium significantly increased in the C-3102 group at 12 weeks of treatment when compared with the baseline, and genus Fusobacterium significantly decreased in the C-3102 group at 12 and 24 weeks of treatment when compared with the baseline. It was suggested that C-3102 modulates host-gut microbiota; however, these bacterial genera did not significantly correlate with BMD or bone turnover markers (data not shown).
Table 4. Alpha diversity of gut microbiota.
| Placebo |
C-3102 |
|||||
|---|---|---|---|---|---|---|
| Baseline | 12 weeks | 24 weeks | Baseline | 12 weeks | 24 weeks | |
| Observed species | 1,056 ± 68 | 1,196 ± 72 | 1,032 ± 62 | 1,180 ± 72 | 1,140 ± 89 | 943 ± 63† |
| Shannon | 6.15 ± 0.20 | 6.60 ± 0.17 | 6.16 ± 0.18 | 6.48 ± 0.18 | 6.17 ± 0.22 | 5.97 ± 0.20# |
| Chao1 | 1,662 ± 109 | 1,834 ± 115 | 1,588 ± 99 | 1,809 ± 116 | 1,711 ± 135 | 1,440 ± 99# |
Values are means ± SEMs. Significant differences were determined by Wilcoxon signed-rank test with Bonferroni correction. #p<0.05, vs. baseline. †p<0.1, vs. baseline.
Table 5. Relative abundance of gut microbiota that significantly increased or decreased from baseline.
| Phylum | Family | Genus | Group | Relative abundance (%) | ||
|---|---|---|---|---|---|---|
| Baseline | 12 weeks | 24 weeks | ||||
| Actinobacteria | Bifidobacteriaceae | Bifidobacterium | Placebo | 7.84 ± 2.24 | 5.62 ± 1.79 | 5.80 ± 1.48 |
| C-3102 | 3.95 ± 1.14 | 10.82 ± 2.60# | 6.63 ± 2.28 | |||
| Coriobacteriaceae | Collinsella | Placebo | 0.22 ± 0.06 | 1.46 ± 0.40# | 0.51 ± 0.13# | |
| C-3102 | 0.23 ± 0.06 | 1.37 ± 0.49# | 0.68 ± 0.24# | |||
| Eggerthella | Placebo | 0.04 ± 0.01 | 0.13 ± 0.03# | 0.08 ± 0.03 | ||
| C-3102 | 0.03 ± 0.02 | 0.09 ± 0.03# | 0.04 ± 0.01 | |||
| Firmicutes | Christensenellaceae | Unknown | Placebo | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.05 ± 0.02 |
| C-3102 | 0.04 ± 0.01 | 0.02 ± 0.01# | 0.03 ± 0.01 | |||
| Lachnospiraceae | Dorea | Placebo | 1.96 ± 0.30 | 1.39 ± 0.22 | 1.20 ± 0.24 | |
| C-3102 | 1.82 ± 0.34 | 1.00 ± 0.23# | 0.93 ± 0.18# | |||
| Other | Placebo | 0.87 ± 0.11 | 1.52 ± 0.24# | 0.83 ± 0.12 | ||
| C-3102 | 1.60 ± 0.28 | 1.95 ± 0.56 | 1.18 ± 0.35# | |||
| Turicibacteraceae | Turicibacter | Placebo | 0.06 ± 0.03 | 0.03 ± 0.01 | 0.03 ± 0.01 | |
| C-3102 | 0.08 ± 0.03 | 0.04 ± 0.01 | 0.02 ± 0.01# | |||
| Veillonellaceae | Megamonas | Placebo | 1.42 ± 0.87 | 0.33 ± 0.24 | 1.01 ± 0.73 | |
| C-3102 | 1.09 ± 0.90 | 0.25 ± 0.24 | 0.06 ± 0.06 | |||
| Fusobacteria | Fusobacteriaceae | Fusobacterium | Placebo | 0.07 ± 0.03 | 0.03 ± 0.01 | 0.24 ± 0.17 |
| C-3102 | 0.13 ± 0.06 | 0.02 ± 0.01# | 0.06 ± 0.04# | |||
| Proteobacteria | Alcaligenaceae | Sutterella | Placebo | 0.17 ± 0.05 | 0.14 ± 0.05 | 0.37 ± 0.10 |
| C-3102 | 0.24 ± 0.11 | 0.33 ± 0.21 | 0.51 ± 0.30# | |||
| Enterobacteriaceae | Other | Placebo | 0.13 ± 0.12 | 0.96 ± 0.68 | 0.63 ± 0.44 | |
| C-3102 | 0.10 ± 0.07 | 0.15 ± 0.06# | 0.83 ± 0.61 | |||
Values are means ± SEMs. Significant differences were determined by Wilcoxon signed-rank test with Bonferroni correction. #p<0.05 vs. baseline.
DISCUSSION
In the present study, we investigated the effect of C-3102 on BMD, bone metabolism, and gut microbiota in healthy postmenopausal Japanese women in a randomized, placebo-controlled, double-blind clinical trial. We found that C-3102 significantly increased total hip BMD. In addition, C-3102 was suggested to inhibit bone resorption and modulate gut microbiota.
A longitudinal cohort study of multinational women revealed that total hip BMD decreases at a rate of 1.4% per year in postmenopausal woman [22]. In the present study, the percent difference in total hip BMD (percent change of BMD in the C-3102 group minus that in the placebo group) at 24 weeks of treatment was 1.70%. Thus, C-3102 is considered to be useful in preventing bone loss in postmenopausal women.
It has been reported that six months of whole body vibration training increased total hip BMD in postmenopausal women (percent difference in total hip BMD = 1.55%) [23]. Furthermore, a one-year treatment of 1,200 mg calcium per day increased total hip BMD in elderly women (percent difference in total hip BMD = 1.46%) [24]. Although it is difficult to directly compare our results with these studies due to different subject backgrounds and study periods, C-3102 could improve total hip BMD in postmenopausal women as effectively as these interventions.
Since BMD is determined by the dynamic balance between bone formation and bone resorption, we investigated the change in bone formation and bone resorption markers. uNTx is a fragment of type I collagen generated during bone resorption and hence it is considered to reflect osteoclast activity [25]. A significant group-by-time interaction effect for relative changes in uNTx indicated that the change in uNTx over time differed between the groups. C-3102 inhibited bone resorption by suppressing osteoclast activity, as the C-3102 group showed significantly lower uNTx than the placebo group at 12 weeks of treatment. TRACP-5b, also a bone resorption marker, is an Osteoclast-specific enzyme and hence is considered to reflect osteoclast number [25]. There was a trend towards a decrease in the relative change of TRACP-5b in the C-3102 group when compared with the placebo group at 12 weeks of treatment. The smaller sample size than that calculated by power analysis (n=34 per group) may result in insufficient power to detect a difference between the C-3102 group and the placebo group. Thus, C-3102 appeared to suppress not only osteoclast activity but also osteoclast number.
Analysis of the bone formation marker showed there was no significant group-by-time interaction effect for BAP: only the effect of time was significant. This result suggests that C-3102 does not influence bone formation. We suggest C-3102 inhibits bone resorption by suppressing osteoclast activity without influencing bone formation and thus improves BMD.
We then investigated the causes of improvement in BMD in the C-3102 group. It is possible that the natural ingredients, such as vitamin K2 and soy isoflavone in fermented soybean, in the C-3102 tablets contribute to the improvement in BMD. In the present study, the test tablets contained 5.52 µg of vitamin K2 and 0.34 mg of soy isoflavone (aglycon and glycoside) per daily dose. Vitamin K2 mediates the carboxylation of osteocalcin, which is the most abundant non-collagenous protein of the bone extracellular matrix. The minimum effective dose of vitamin K2 for improving BMD in postmenopausal osteoporotic women is 45 mg per day [26]; therefore, the vitamin K2 in the C-3102 tablets is not expected to have contributed to the improvement in BMD in the present study. Soy isoflavone has a hormone-like effect, and this is considered to improve bone metabolism [27]. A meta-analysis of clinical trials reported that ingestion of 82 mg soy isoflavone per day increases BMD in postmenopausal women [28]. In the present study, the effect of 0.34 mg of soy isoflavone contained in the C-3102 tablets is expected to have been small. We suggest that the natural ingredients in fermented soybean contained in the C-3102 tablets do not contribute to improvement of BMD.
It is possible that C-3102 indirectly improved BMD via modulation of gut microbiota. A comparison of gut microbiota in osteoporosis patients, osteopenia patients, and normal controls suggested that the alpha diversity index is inversely correlated with BMD [29]. In the present study, alpha diversity indices significantly decreased at 24 weeks of treatment when compared with the baseline in the C-3102 group. Thus, improvement in BMD after ingestion of C-3102 may be associated with decreased alpha diversity.
In the C-3102 group, the relative abundance of genus Bifidobacterium, members of which are commonly used as probiotics, significantly increased at 12 weeks of treatment when compared with the baseline. This result is consistent with our previous study in which C-3102 increased the relative abundance of Bifidobacterium in healthy men and women [17]. It has been reported that treatment with Bifidobacterium longum increases BMD in OVX-induced osteoporotic rats by suppressing bone resorption [30]. Furthermore, ingestion of multispecies probiotics, including Bifidobacterium longum, decreased serum levels of C-terminal telopeptide (CTx), a marker of bone resorption, in postmenopausal women [14]. We suggest that the improvement in BMD in postmenopausal women after ingestion of C-3102 may be related to the increase in the relative abundance of genus Bifidobacterium in gut microbiota.
The relative abundance of genus Fusobacterium, including pathogenic species such as Fusobacterium nucleatum, significantly decreased in the C-3102 group at 12 and 24 weeks of treatment when compared with the baseline. It has been reported that there is a positive correlation between the abundance of Fusobacterium nucleatum and inflammatory cytokine (TNF-α) expression in adenoma patients [31]. In addition, it has been reported that oral infection with Fusobacterium nucleatum induces alveolar bone loss and increases inflammatory cytokines (TNF-α and IL-1β) in mice [32]. Since inflammatory cytokines are considered to be osteoclastogenic cytokines [33], C-3102 may inhibit bone resorption by decreasing the relative abundance of the genus Fusobacterium, followed by improvement in inflammatory cytokines. However, there was no significant correlation between the relative abundance of these bacterial genera and BMD or bone turnover markers, suggesting that bone metabolism is not related to a single bacterial genus.
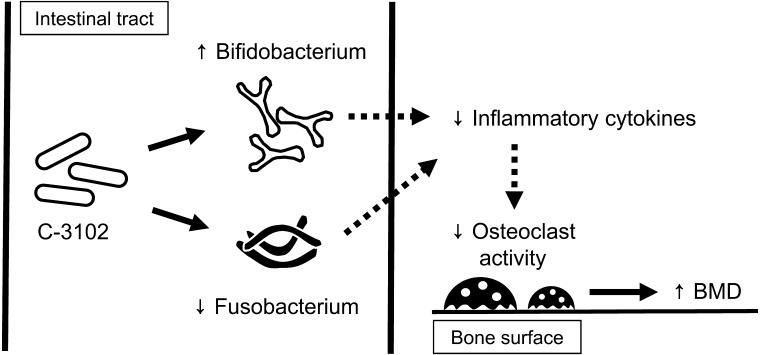
In recent years, it has been proposed that gut microbiota regulate bone metabolism via effects on the host immune system, and a new research field called “osteo-microbiology” has been suggested [34]. A hypothetical mechanism of action for C-3102 on bone mineral density is shown in Fig. 4. Further studies are needed to elucidate the mechanisms of the effect of improvement in BMD caused by ingestion of C-3102.
Fig. 4.
Schematic illustration of the hypothetical mechanisms of action of C-3102 on BMD.
C-3102 modulates gut microbiota such as by increasing the genus Bifidobacterium or decreasing the genus Fusobacterium. Modulation of gut microbiota may lead to an increase in BMD through reduction of inflammatory cytokines and hence a reduction in osteoclast activity.
The present study has several limitations. First, it was targeted at healthy postmenopausal women; further studies are needed to investigate whether C-3102 improves BMD in osteoporotic patients, including men and young women. Second, no significant differences were observed in the bone resorption markers at 24 weeks of treatment even though C-3102 is suggested to inhibit bone resorption. A longer study period will be required in order to evaluate the effect of C-3102 on bone resorption. Third, neither the placebo nor the C-3102 group reached the required sample size due to exclusion of the antibiotic recipients from the analysis; therefore, in some of the data the statistical power was too low to detect a significant difference.
In conclusion, the present study showed that treatment with C-3102 improves BMD by inhibiting bone resorption and modulating gut microbiota in healthy postmenopausal Japanese women. To our knowledge, this is the first report of improvement of BMD by a probiotic treatment in humans. Our findings suggests that C-3102 could be useful in preventing bone loss in healthy postmenopausal women.
Acknowledgments
We would like to thank Yasunori Nakamura for comments that greatly improved the manuscript, Kyoko Morimoto and Megumi Ogiwara for technical support, and Kyoko Kaneko and Masatsugu Akashi of Asahi Calpis Wellness Co., Ltd., for producing the placebo and test tablets.
References
- 1.Al-Sari UA, Tobias J, Clark E. 2016. Health-related quality of life in older people with osteoporotic vertebral fractures: a systematic review and meta-analysis. Osteoporos Int 27: 2891–2900. [DOI] [PubMed] [Google Scholar]
- 2.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. 2009. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 301: 513–521. [DOI] [PubMed] [Google Scholar]
- 3.Fujiwara S, Kasagi F, Masunari N, Naito K, Suzuki G, Fukunaga M. 2003. Fracture prediction from bone mineral density in Japanese men and women. J Bone Miner Res 18: 1547–1553. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura N, Muraki S, Oka H, Mabuchi A, En-Yo Y, Yoshida M, Saika A, Yoshida H, Suzuki T, Yamamoto S, Ishibashi H, Kawaguchi H, Nakamura K, Akune T. 2009. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab 27: 620–628. [DOI] [PubMed] [Google Scholar]
- 5.Riggs BL, Khosla S, Melton LJ., 3rd2002. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23: 279–302. [DOI] [PubMed] [Google Scholar]
- 6.de Villiers TJ, Chines AA, Palacios S, Lips P, Sawicki AZ, Levine AB, Codreanu C, Kelepouris N, Brown JP. 2011. Safety and tolerability of bazedoxifene in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled phase 3 trial. Osteoporos Int 22: 567–576. [DOI] [PubMed] [Google Scholar]
- 7.Kennel KA, Drake MT. 2009. Adverse effects of bisphosphonates: implications for osteoporosis management. Mayo Clin Proc 84: 632–637, quiz 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reid IR, Bolland MJ, Grey A. 2014. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet 383: 146–155. [DOI] [PubMed] [Google Scholar]
- 9.Tai V, Leung W, Grey A, Reid IR, Bolland MJ. 2015. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 351: h4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemente JC, Ursell LK, Parfrey LW, Knight R. 2012. The impact of the gut microbiota on human health: an integrative view. Cell 148: 1258–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, Jones RM, Pacifici R. 2016. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest 126: 2049–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams S, Wakisaka A, Zeng QQ, Barnes J, Seyedin S, Martin G, Wechter WJ, Liang CT. 1998. Effect of minocycline on osteoporosis. Adv Dent Res 12: 71–75. [DOI] [PubMed] [Google Scholar]
- 13.Ohlsson C, Engdahl C, Fåk F, Andersson A, Windahl SH, Farman HH, Movérare-Skrtic S, Islander U, Sjögren K. 2014. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One 9: e92368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jafarnejad S, Djafarian K, Fazeli MR, Yekaninejad MS, Rostamian A, Keshavarz SA. 2017. Effects of a multispecies probiotic supplement on bone health in osteopenic postmenopausal women: a randomized, double-blind, controlled trial. J Am Coll Nutr 36: 497–506. [DOI] [PubMed] [Google Scholar]
- 15.Tsukamoto Y, Ichise H, Kakuda H, Yamaguchi M. 2000. Intake of fermented soybean (natto) increases circulating vitamin K2 (menaquinone-7) and gamma-carboxylated osteocalcin concentration in normal individuals. J Bone Miner Metab 18: 216–222. [DOI] [PubMed] [Google Scholar]
- 16.Hatanaka M, Nakamura Y, Maathuis AJ, Venema K, Murota I, Yamamoto N. 2012. Influence of Bacillus subtilis C-3102 on microbiota in a dynamic in vitro model of the gastrointestinal tract simulating human conditions. Benef Microbes 3: 229–236. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki H, Watabe J, Takeuchi H, Tadano Y, Masuda S, Maruta K. 2004. Effect of Bacillus subtilis C-3102 intakes on the composition and metabolic activity of fecal microflora of humans. Chounai saikingaku zasshi 18: 93–99 (In Japanese). [Google Scholar]
- 18.Kobayashi S, Murakami K, Sasaki S, Okubo H, Hirota N, Notsu A, Fukui M, Date C. 2011. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr 14: 1200–1211. [DOI] [PubMed] [Google Scholar]
- 19.Science and Technology Agency.2005. Standard table of food composition in Japan. 5th revised and enlarged edition. Printing Bureau of the Ministry of Finance, Tokyo, Japan (In Japanese). [Google Scholar]
- 20.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soen S, Fukunaga M, Sugimoto T, Sone T, Fujiwara S, Endo N, Gorai I, Shiraki M, Hagino H, Hosoi T, Ohta H, Yoneda T, Tomomitsu T, Japanese Society for Bone and Mineral Research and Japan Osteoporosis Society Joint Review Committee for the Revision of the Diagnostic Criteria for Primary Osteoporosis.2013. Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab 31: 247–257. [DOI] [PubMed] [Google Scholar]
- 22.Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, Lo JC, Johnston JM, Cauley JA, Danielson ME, Neer RM. 2008. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 93: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. 2004. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res 19: 352–359. [DOI] [PubMed] [Google Scholar]
- 24.Zhu K, Devine A, Dick IM, Wilson SG, Prince RL. 2008. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: a five-year randomized controlled trial. J Clin Endocrinol Metab 93: 743–749. [DOI] [PubMed] [Google Scholar]
- 25.Henriksen K, Tanko LB, Qvist P, Delmas PD, Christiansen C, Karsdal MA. 2007. Assessment of osteoclast number and function: application in the development of new and improved treatment modalities for bone diseases. Osteoporos Int 18: 681–685. [DOI] [PubMed] [Google Scholar]
- 26.Orimo H, Fujita T, Onomura T, Inoue T, Kushida K, Shiraki M. 1992. Clinical evaluation of soft capsule menatetrenone (Ea-0167) in the treatment of osteoporosis: late phase II dose study. J New Rem Clin 41: 1249–1279 (In Japanese). [Google Scholar]
- 27.Setchell KD, Cassidy A. 1999. Dietary isoflavones: biological effects and relevance to human health. J Nutr 129: 758S–767S. [DOI] [PubMed] [Google Scholar]
- 28.Taku K, Melby MK, Kurzer MS, Mizuno S, Watanabe S, Ishimi Y. 2010. Effects of soy isoflavone supplements on bone turnover markers in menopausal women: systematic review and meta-analysis of randomized controlled trials. Bone 47: 413–423. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Wang Y, Gao W, Wang B, Zhao H, Zeng Y, Ji Y, Hao D. 2017. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ 5: e3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei AN, Abdul-Majeed S, Ahmad Z, Ibrahim Z, Jamaluddin R. 2015. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate Sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. BioMed Res Int 2015: 897639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCoy AN, Araújo-Pérez F, Azcárate-Peril A, Yeh JJ, Sandler RS, Keku TO. 2013. Fusobacterium is associated with colorectal adenomas. PLoS One 8: e53653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polak D, Wilensky A, Shapira L, Halabi A, Goldstein D, Weiss EI, Houri-Haddad Y. 2009. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: bone loss and host response. J Clin Periodontol 36: 406–410. [DOI] [PubMed] [Google Scholar]
- 33.Zupan J, Jeras M, Marc J. 2013. Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts. Biochem Med (Zagreb) 23: 43–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohlsson C, Sjögren K. 2015. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab 26: 69–74. [DOI] [PubMed] [Google Scholar]