Abstract
Purpose: Sepsis is a systemic inflammatory response caused by infection. Curcumin is known to have antioxidant and anti-inflammatory activities. FM0807, a curcumin derivative, was investigated in the present study to determine its effect on cytokines and the possible molecular mechanism. Main methods: The experiments were carried out in lipopolysaccharide (LPS)-induced RAW 264.7 cells. Cell viability was measured by MTT assay. ELISA, Griess assays, fluorescence-based quantitative PCR, flow cytometric analysis, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) experiments, and Western blotting were carried out to assess the potential effects of FM0807 on LPS-induced RAW 264.7 cells. Significant findings: FM0807 had no cytotoxic effects on RAW 264.7 cells. Furthermore, pretreatment with FM0807 inhibited the inflammatory factor tumor necrosis factor-α (TNF-α), interleukin (IL) 1β (IL-1β), IL-6, and inducible nitric oxide synthase (iNOS) at the protein and gene levels. FM0807 also inhibited the production of reactive oxygen species (ROS) and apoptosis. In addition, the activation of the ROS/JNK (c-jun NH2-terminal kinase)/p53 signaling pathway was inhibited by FM0807 in RAW 264.7 cells in vitro. Conclusion: FM0807 has anti-inflammatory activity in vitro, which suggests a potential clinical application in sepsis. The anti-inflammatory activity of FM0807 may be mediated by the ROS/JNK/p53 signaling pathway.
Keywords: Curcumin, inflammatory cytokine, LPS, RAW 264.7, sepsis
Introduction
Sepsis is a systemic inflammatory response caused by a dysregulated host response to infection. Sepsis is one of the most serious and common causes of death in the intensive care unit (ICU). A meta-analysis reported that the hospital mortality was 17% for sepsis and 26% for severe sepsis from 1979 to 2015 in seven high-income countries. And a tentative extrapolation from high-income country data suggests global estimates of 31.5 million sepsis and 19.4 million severe sepsis cases, with potentially 5.3 million deaths annually [1]. The pathogenesis of sepsis is not very clear. Sepsis becomes to be a huge therapeutic challenge. In recent years, studies have shown that oxidative stress may be one of the factors in the cause of sepsis [2–4]. Under normal physiological conditions, the formation and clearance of reactive oxygen species (ROS) and reactive nitrogen species (RNS) are in dynamic state, ROS and RNS are pivotal to the defense against invading pathogens during sepsis by performing intracellular signaling for several cytokines and growth factors, participating in intracellular killing of bacterial pathogens in phagocytosis [5]. In infection, poisoning, trauma, and other pathological conditions, the body has a variety of ways to produce ROS and RNS. The oxidative stress and nitrosative stress come, which are caused by overwhelming production of ROS and RNS. ROS play a wide range of capabilities due to action in many ways and numerous places simultaneously [6]. ROS can directly play a toxic effect on the cells. In the process of oxidative stress, ROS can cause protein and nucleic acid to be oxidized, and can lead to mitochondrial membrane destruction, then the cells apoptosis. Caspases such as caspase-3 and caspase-9 play a vital role in apoptosis. Many studies showed that ROS can lead to activation of the mitogen-activated protein kinase (MAPK) signaling pathway [7,8]. There are three main MAPKs, including c-jun NH2-terminal kinases (JNK), extracellular signal-regulated kinases (ERKs), and p38 [9,10]. p53 is a potent inducer of apoptosis. Recently, many studies suggested that the ROS/JNK/p53 pathway plays a vital role in apoptosis [11,12]. ROS activates the MAPK signaling pathway to induce apoptosis and the production of a large number of inflammatory cytokines [13–15].
Curcumin has a wide range of pharmacological effects, such as antitumor, hypolipidemic, and anti-inflammatory effects [16–18]. However, its poor water-solubility and low bioavailability restrict its use. To address this problem, a number of derivatives and analogs of Cur have been developed in our lab, such as FM0807 (2-hydroxy-4-[(1E, 6E)-7-(4-hydroxy-3-methoxyphenyl)-3,5-dioxo-1,6-heptadien-1-yl]-2-methoxyphenyl ester), which incorporated a 2-hydroxy-benzoic acid chain into Cur that retains the β-diketone structure and exhibits better anti-inflammatory activity than that of Cur [19]. FM0807 can also be considered as the combined compound of Cur and aspirin (the structures of Cur, aspirin, and FM0807 are shown in Figure 1A–C). In the present study, a curcumin derivative, FM0807, (Figure 1C) was synthesized, and its anti-inflammatory activities were evaluated in RAW264.7 cells. To explore the molecular mechanisms underlying these effects, we also investigated the possible involvement of the ROS/JNK/p53 signaling pathway.
Figure 1. Chemical structures of Cur, aspirin, and FM0807 and inhibitory effects of FM0807 on LPS-induced cytokines.
(A) Chemical structure of Curcumin; (B) chemical structure of aspirin; (C) chemical structure of curcumin analog FM0807. Molecular formula: C28H24O8, molecular weight: 488.15 g.mol−1; (D) viability of RAW 264.7 after treatment of different concentrations of FM0807. At a given concentration, FM0807, had no significant effect on the cellular activity of RAW 264.7 compared with the control group; (E) the release of TNF-α; (F) the release of IL-1β; (G) the release of IL-6; (H) the release of nitrite. Values were mean ± S.D. (n=3); **P<0.01 compared with the control group; #P<0.01 and ##P<0.05 compared with the LPS-treated group. Abbreviations: IL, interleukin, LPS, lipopolysaccharide, TNF-α, tumor necrosis factor-α.
Materials and methods
Chemicals and reagents
The curcumin derivative FM0807 was synthesized by Clinical Pharmacology Research Center of Fujian Medical University. Lipopolysaccharide (LPS) (Escherichia coli 0111: B4) and DMSO were obtained from Sigma–Aldrich (St. Louis, MO, U.S.A.), and the Annexin-V FITC Apoptosis Detection Kit was purchased from Becton, Dickinson and Company (San Jose, CA, U.S.A.). Dulbecco’s modified Eagle’s medium (DMEM) and low-endotoxin FBS were purchased from Gibco Company (Grand Island, NY, U.S.A.). ELISA kits for tumor necrosis factor-α (TNF-α), interleukin (IL) 1β (IL-1β), and IL-6 were obtained from Santa Cruz Biotechnology (Dallas, TX, U.S.A.). Primary antibodies against p-JNK1/2, p-ERK1/2, JNK1/2, ERK1/2, p53, caspase-9, caspase-3, and β-actin were purchased from Cell Signaling Technology (Danvers, MA, U.S.A.). PMSF was purchased from Roche Company (Switzerland). The nitric oxide (NO) assay kit, BCA protein content determination kit, and reactive oxygen test kit were obtained from Beyotime Institute of Biotechnology (Shanghai, China).
Cell lines and sample treatment
The RAW 264.7 mouse macrophage cell line was purchased from Shanghai Institute of Cellular Biology of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 U/ml) in a humidified incubator containing 5% CO2 at 37°C. When grown to 70–80% confluence, the cells were subjected to passaging. The medium was replaced every 1–2 days. The concentration of the cell suspension was adjusted to 1 × 105/ml. Then, 1 ml samples of the suspension were added to 24-well plates and cultured in a humidified incubator containing 5% CO2 at 37°C. The cells were divided into six groups: the control group (the cells were untreated), the LPS group (the cells were treated with 0.5 μg/ml LPS), the positive control + LPS group (the cells were treated with 40 mM hydrocortisone plus 0.5 μg/ml LPS), and the FM0807+LPS groups (the cells were treated with 10, 20, or 40 μM FM0807 plus 0.5 μg/ml LPS). The RAW 264.7 cells were pretreated with different concentrations of FM0807 and hydrocortisone for 20 h before treatment with 0.5 μg/ml (final concentration) LPS for 4 h, with 200 µl of supernatant per well. Each group was represented by three wells, and the supernatants were preserved at −20°C.
Cell viability assay
RAW 264.7 cells were seeded in 96-well plates at a density of 1 × 105/ml in a volume of 200 µl/well. After incubation for 24 h at 37°C, the cells were treated with FM0807 at the indicated concentrations for 24 h, followed by the addition of 5 mg/ml MTT solution to each well, and the plates were further incubated for 4 h at 37°C. The supernatant was removed, and 150 µl of DMSO was added to each well to solubilize the water-insoluble purple formazan crystals. The absorbance at a wavelength of 570 nm was measured using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, U.S.A.). The percentage of viable cells was estimated and compared with that of untreated control cells.
Measurement of NO
The Griess assay was performed to detect NO [20]. RAW 264.7 cells were seeded in 96-well plates at a density of 1 × 105/ml and a volume of 200 µl/well. After incubation for 24 h at 37°C, the cells were treated with hydrocortisone and FM0807 at the indicated concentrations for 20 h in serum-free medium, prior to the addition of LPS (0.5 μg/ml). After 4 h, the culture supernatants were collected and reacted with Griess reagent (1% sulphanilamide/0.1% naphthyl ethylene diamine dihydrochloride/2% phosphoric acid) in a 96-well plate. Then, the optical density was detected at 540 nm with a microplate reader.
Determination of TNF-α, IL-1β, and IL-6 production
The production of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in the cell cultures was detected by ELISA. The culture medium was collected and centrifuged at 12000 rpm for 10 min. The supernatants were collected and used to detect TNF-α, IL-1β, and IL-6 levels with ELISA kits, according to the manufacturer’s protocol.
The transcription levels of TNF-α, IL-1β, IL-6, and inducible NO synthase
Total RNA was extracted using TRIzol reagent (Takara, Dalian, China) according to the manufacturer’s protocol. cDNA synthesis was performed using a reverse transcription kit (Takara, Dalian, China) according to the manufacturer’s instructions. For quantitative PCR, the cDNA was mixed with 12.5 μl of 2× SYBR Green Master Mix (BioEasy SYBR Green Real-Time PCR Kit, Bioer), 1 μl of primers (400 nM), and 9.5 μl of RNase-free water. The nucleotide sequences of the primers are shown in Table 1. Fluorescence-based quantitative PCR was performed using SYBR Premix II in an Applied Biosystems VR7500 Fast Dx Real-Time PCR Instrument. The 2ΔΔCT method was used to calculate relative quantities, as described previously, and β-actin was used as the internal reference gene.
Table 1. DNA Sequences of the primers used for real-time PCR.
| Gene | Primer | Sequence (5′–3′) |
|---|---|---|
| TNF-α | Forward | CAGGAGGGAGAACAGAAACTCCA |
| Reverse | CCTGGTTGGCTGCTTGCTTGAAAGC | |
| IL-1β | Forward | TCCAGGATGAGGACATGAGCAC |
| Reverse | GAACGTCACACACCAGCAGGTTA | |
| IL-6 | Forward | GCCAGAGTCCTTCAGAGAGA |
| Reverse | GGTCTTGGTCCTTAGCCACT | |
| iNOS | Forward | TAGGCAGAGATTGGAGGCCTTG |
| Reverse | GGGTTGTTGCTGAACTTCCAGTC | |
| COX-2 | Forward | CAGGCTGAACTTCGAAACA |
| Reverse | GCTCACGAGGCCACTGATACCTA | |
| β-actin | Forward | GCCACCAGTTCGCCATGGAT |
| Reverse | GCTTTGCACATGCCGGAGC |
ROS levels
ROS levels were monitored using a 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) cell-permeant probe [21]. In this method, DCFH-DA is hydrolyzed to generate nonfluorescent membrane-permeable DCFH, which is converted into impermeable fluorogenic dichlorofluorescein (DCF) under oxidative conditions. Briefly, the cells from different groups were collected. After plating in 24-well plates at a density of 60000 cells/well, the cells were washed twice and incubated with 10 μmol/l DCFH-DA at 37°C in 5% CO2 for 30 min and then washed with serum-free medium to remove the extracellular DCFH-DA. Fluorescence was then determined at a 488-nm excitation wavelength and a 525-nm emission wavelength.
Flow cytometric analysis of apoptosis
The cells were treated with hydrocortisone and FM0807 at the indicated concentrations for 20 h in serum-free medium, prior to the addition of LPS (0.5 μg/ml) for 4 h. The cells were then collected and stained with Annexin V and propidium iodide (PI) using Apoptosis Detection Kit for flow cytometric analysis. The flow cytometric analysis was performed on a BD FACSCanto II, and the data were analyzed with FlowJo 8.8.6 software (Tree Star Inc., Ashland, OR, U.S.A.). All experiments were performed with biological triplicates, and the data are representative of at least three independent experiments.
Western blotting
The cells were preincubated with or without the indicated concentrations of FM0807 and hydrocortisone for 20 h prior to exposure to LPS (0.5 μg/ml). After 4 h, the cells were harvested. The cells were washed with PBS and treated with trypsin-EDTA. The cell pellets were obtained by centrifugation at 3000 rpm for 5 min at 4°C, lysed in lysis buffer, and centrifuged at 12000 rpm for 5 min at 4°C to obtain whole-cell lysates. Protein concentrations were determined using a BCA protein assay kit (Beyotime, China). The proteins were electroblotted on to PVDF membranes after separation via SDS/PAGE (12% gel). The transferred membranes were blocked with TBS containing 5% nonfat dried milk and 0.1% Tween-20 at 4°C for 2 h. After blocking, the membranes were incubated with primary antibodies (1:1000) overnight at 4°C with gentle shaking. Then, the blots were washed three times with Tween-20/TBS and incubated with a horseradish peroxidase (HRP)-conjugated anti-rabbit IgG secondary antibody (1:1000) for 2 h at room temperature with gentle shaking. After the membranes were washed three times for 10 min in TBS containing 0.1% Tween-20, the bands were detected using ECL Western blotting detection reagents according to the manufacturer’s protocol. β-actin was used as the loading control. The band density was measured using ImageJ software.
Statistical analysis
All values are expressed as the mean ± S.D. Two groups were compared using an unpaired two-tailed t test. For multiple groups, the data were subjected to ANOVA. All experiments were repeated three times with similar results. The obtained data were statistically analyzed with SPSS 15.0 software. P-values less than 0.05 were considered statistically significant, and P-values less than 0.01 were considered notably statistically significant.
Results
The effect of FM0807 on the viability of RAW 264.7 cells
The effect of FM0807 on the viability of RAW 264.7 cells was assessed using an MTT assay. The results are presented in Figure 1D. FM0807, at a given concentration, had no significant effect on the cellular activity of RAW 264.7 cells compared with that of the control group. This finding demonstrated that FM0807 had no cytotoxic effects on RAW 264.7 cells.
The effects of FM0807 on cytokine production by LPS-induced RAW 264.7 cells
As shown in Figure 1E–H, after LPS induction for 4 h, the TNF-α, IL-1β, IL-6, and NO levels significantly increased compared with those of the control group (P<0.01). FM0807 significantly reversed the LPS-induced TNF-α, IL-1β, IL-6 and NO levels, with P<0.05 or P<0.01 for the 10, 20, and 40 μM treatments.
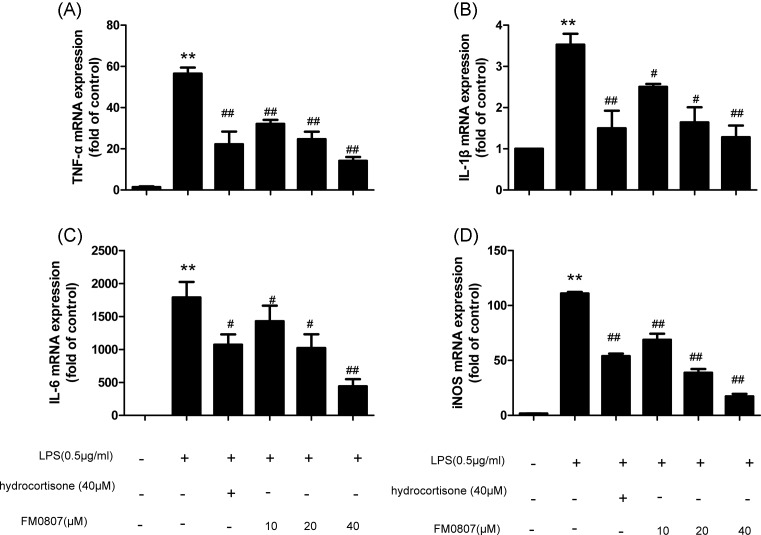
The relative transcription levels of TNF-α, IL-6, IL-1β, and iNOS
Compared with those of the control group, the transcription levels of TNF-α, IL-6, IL-1β, and iNOS mRNA were significantly increased (P<0.01) in the LPS-treated group. While, the FM0807 groups exhibited significantly inhibited TNF-α, IL-6, IL-1β, and iNOS mRNA transcription (P<0.01 or P<0.05) (Figure 2), compared with the LPS-treated group,. Additionally, the reduction in TNF-α, IL-6, IL-1β, and iNOS transcription occurred in a dose-dependent manner after FM0807 treatment.
Figure 2. Effect of pretreatment with FM0807 on TNF-α, IL-6, IL-1β, and iNOS gene expression in LPS-induced RAW 264.7 cells.
(A) The expression of TNF-α mRNA. (B) The expression of IL-1β mRNA. (C) The expression of IL-6 mRNA. (D) The expression of iNOS mRNA. RAW264.7 cells were pretreated with FM0807 (10, 20, 40 μM) and hydrocortisone, respectively, for 20 h prior to LPS stimulation. After 4 h of LPS stimulation, the TNF-α, IL-6, IL-1β, and iNOS mRNA levels were accessed by quantitative-RT-PCR. Data are presented as mean ± S.D. Representation of results obtained from three independent experiments are shown. **P<0.01 compared with the control group; #P<0.01 and ##P<0.05 compared with the LPS-treated group. Abbreviation: RT-PCR, real-time PCR.
The effect of FM0807 on apoptosis in RAW 264.7 cells
The antiapoptotic effect of FM0807 was investigated by flow cytometry (Figure 3). Compared with the control group, the apoptosis rate was significantly increased in the LPS-treated group (P<0.01). In contrast, treatment of the cells with different concentrations of FM0807 reduced the percentage of apoptotic cells (P<0.01 or P<0.05).
Figure 3. Effect of pretreatment with FM0807 on apoptosis in LPS-induced RAW 264.7 cells.
Flow cytometry results are represented as (A) cells without stimulation, (B) cells treated with LPS for 4 h, (C) cells pretreated with hydrocortisone for 20 h, (D–F) cells pretreated with FM0807 (10, 20, 40 μM), respectively, for 20 h, (G) bar graph representation of apoptosis rate in each group previously described. After 4 h of LPS stimulation, apoptosis rate was detected with Annexin-V and PI for flow cytometry analysis. Data are presented as mean ± S.D. Representation of results obtained from three independent experiments are shown. **P<0.01 compared with the control group; #P<0.01 and ##P<0.05 compared with the LPS-treated group.
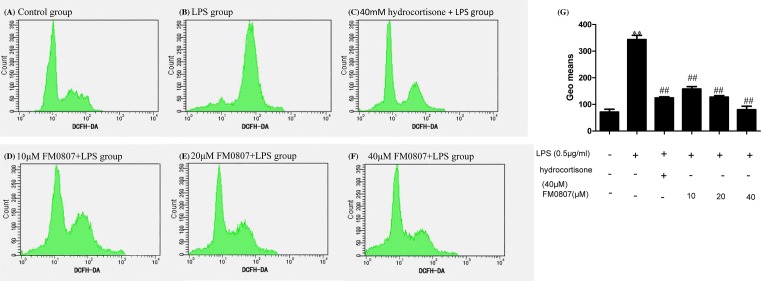
The effect of FM0807 on ROS release by LPS-induced RAW 264.7 cells
Previous studies have indicated that ROS are involved in sepsis. To determine whether FM0807 can inhibit ROS induced by LPS, RAW 264.7 cells were treated with the DCFH-DA dye after treatment with different concentrations of FM0807. The DCFH-DA fluorescence results are shown in Figure 4. The control group and the LPS-treated group had obviously different levels of fluorescence (P<0.01), suggesting that LPS can stimulate the cells to produce ROS. After pretreatment with FM0807, the DCFH-DA fluorescence in the LPS-induced cells was reduced (P<0.01). These results suggest that FM0807 had an inhibitory effect on the level of ROS in LPS-treated cells.
Figure 4. Effect of pretreatment with FM0807 on levels of ROS.
DCFH-DA fluorescence was confirmed at 488 nm excitation and 525 nm emission. (A) Cells without stimulation, (B) cells treated with LPS for 4 h, (C) cells pretreated with hydrocortisone for 20 h, (D–F) cells pretreated with FM0807 (10, 20, 40 μM) respectively for 20 h, (G) bar graph representation of geo means in each group previously described. **P<0.01 compared with the control group; ##P<0.01 compared with the LPS-treated group.
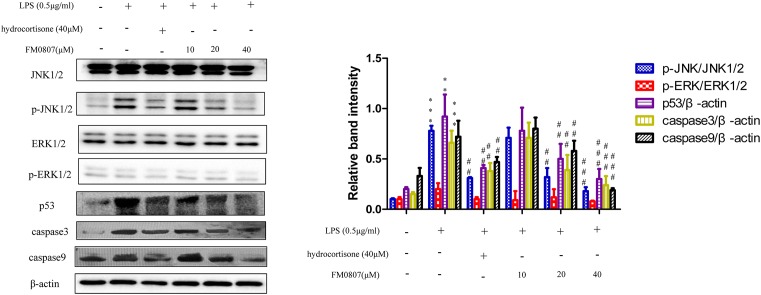
The effect of FM0807 on the apoptosis pathway after stimulation with LPS
To further investigate the molecular mechanism of the anti-inflammatory effect of FM0807, the effects of different concentrations of FM0807 on the apoptosis signaling pathway after stimulation with LPS were observed. We explored the expression of p-JNK/JNK1/2 and p-ERK/ERK1/2 in the MAPK signaling pathway. p53 is activated in response to ROS, resulting in the activation of caspase-3 and caspase-3. Hence, p53, caspase-3 and caspase-9 were also detected. The results are shown in Figure 5. p-JNK/JNK1/2, p53, caspase-9, and caspase-3 levels increased after LPS stimulation. p-ERK/ERK1/2 exhibited no changes in the present study. The changes in p-JNK/JNK1/2, p53, caspase-3, and caspase-9 were reversed by treatment with FM0807 at doses of 20 and 40 μM.
Figure 5. Inhibitory effects of FM0807 on the LPS-induced apoptosis signaling pathway.
(A) Representative Western botting analysis of p-JNK/JNK1/2, p-ERK/ERK1/2, p53, caspase-3, and caspase-9 with β-actin protein expression as an internal control. (B) Quantitation of Western blot analysis of p-JNK/JNK1/2, p-ERK/ERK1/2, p53, caspase-3, and caspase-9. **P<0.01 and ***P<0.005 compared with the control group; ##P<0.05 and ###P<0.0001 compared with the LPS-treated group.
Discussion
Sepsis is characterized as a disorder of inflammatory cytokine and ROS production. Antioxidants can inhibit or alleviate the adverse effects of free radicals on the body, playing a significant role in the prevention and treatment of sepsis [22–24]. Curcumin has a wide range of pharmacological effects, such as antitumor, anti-inflammatory, and hypolipidemic effects [16,25–27]. Recent evidence has demonstrated that curcumin can alleviate LPS-induced sepsis by suppressing oxidative stress-related inflammation [16]. The curcumin derivative FM0807 was synthesized in our laboratory, where its antioxidant and anti-inflammatory activities were evaluated. Our study focussed on the inhibition of inflammatory cytokines to explore the antioxidant and anti-inflammatory effects of FM0807 in vitro. We found that pretreatment with FM0807 inhibited the inflammatory factor TNF-α, IL-1β, IL-6, iNOS at the protein and gene levels. FM0807 also inhibited ROS and apoptosis. We further demonstrated that the activation of the ROS/JNK/p53 signaling pathway was inhibited by FM0807 in RAW 264.7 cells. Collectively, our data demonstrated that FM0807 has anti-inflammatory activity in vitro. The anti-inflammatory activity of FM0807 may be mediated by the ROS/JNK/p53 signaling pathway.
As one of the inflammatory mediators involved in sepsis, TNF-α is an important inflammatory cytokine during the early stage of inflammation and is mainly produced by monocytes and macrophages. TNF-α can activate the cytokine cascade, resulting in a ‘waterfall effect’ and triggering the synthesis of secondary inflammatory mediators, such as IL-1β, IL-6, and NO [28]. The excessive production of TNF-α, IL-1β, IL-6, and other cytokines can further activate macrophages. Then, the cells will induce the expression of iNOS, which can produce and release a large amount of NO. NO is an effector molecule by which activated macrophages and tumor cells kill pathogenic microorganisms. NO is also an important mediator of inflammation. The excessive production of NO plays a variety of roles in the development of inflammation. Cytokines also play an important role in the development of sepsis. In summary, TNF-α, IL-1β, IL-6, and NO are important indicators for evaluating inflammatory responses and therapeutic effects in clinical and basic research. The inhibition of inflammatory cytokines is a target for sepsis therapy. Gupta et al. showed that curcumin can inhibit the production of inflammatory cytokines and NO [29–31]. In this study, an in vitro inflammation model was established successfully. RAW 264.7 cells stimulated with LPS release TNF-α, IL-1β, IL-6, and NO. In this context, we found that the curcumin derivative FM0807 has inhibitory effects on TNF-α, IL-1β, IL-6, and NO at the protein and gene levels in LPS-induced RAW 264.7 cells, indicating that FM0807 has anti-inflammatory activity.
ROS are highly reactive and include superoxide anion radicals, hydroxyl radicals, and hydrogen peroxide. The mitochondrial respiratory chain is a major source of ROS. Under normal circumstances, the production and elimination of free radicals in vivo is balanced. When free radical production becomes excessive or the antioxidant system fails, the metabolism of free radicals in the body becomes unbalanced, leading to cellular damage, this process has a relationship with sepsis [32,33]. In this study, we found that FM0807 inhibited LPS-induced cells production of ROS in LPS-induced RAW 264.7 cells. ROS and TNF-α contribute to apoptosis, and apoptosis is a key process in sepsis. Apoptosis is characterized by the activation of caspases. Previous studies have indicated that mitochondria play an important role in apoptosis, which activates caspase-9 and caspase-3. We explored the effect of FM0807 on apoptosis in LPS-induced RAW 264.7 cells. Our study suggested that FM0807 has inhibitory effects on the apoptosis rate.
Furthermore, the mechanism of this inhibitory effect was investigated in the present study. The MAPK family participates in many cellular processes, such as cellular differentiation, proliferation, and apoptosis. The role of MAPKs in apoptosis has been well established in a variety of models, including mitochondrial dysfunction and oxidative stress. A previous study found that ROS accumulation led to the activation of JNK and resulted in apoptosis [34,35]. The activation of ERK is usually involved in cell survival and proliferation, while JNK and p38 are implicated in the promotion of apoptosis by activating a series of genes [36,37]. p53, which can be activated by JNK, acts as a key player in apoptosis. p53 can activate caspase-9 and contribute to the activation of caspase-3. We investigated the influence of FM0807 on the JNK/p53 signaling pathway in LPS-induced RAW 264.7 cells. Our results indicated that FM0807 inhibits JNK/p-JNK but has no effect on ERK/p-ERK. JNK can activate p53, which activates caspase-9, contributing to the activation of caspase-3. Our study proved that FM0807 can decrease p53 levels, resulting in the reduction in caspase-9 and caspase-3. The curcumin analogs, displaying the LPS-induced inflammation inhibition, have been previously reported to participate in the Toll-like receptor (TLR) signaling complex through the myeloid differentiation protein 2 (MD2) and the TLR4 [38,39]. Here, we concluded that ROS accumulation and the subsequent activation of the JNK/p53 signaling pathway play vital roles in LPS-induced apoptosis.
Our present findings suggest that the curcumin derivative FM0807 can inhibit pro-inflammatory mediators, apoptosis, and ROS. These results indicate that FM0807 has antioxidant and anti-inflammatory activities in vitro. The anti-inflammatory activity of FM0807 may be mediated by the JNK/p53 signaling pathway. FM0807 may be a promising candidate for a novel inhibitor of inflammation in sepsis. However, further studies are needed to demonstrate how FM0807 targets and affects p53.
Abbreviations
- DCF
dichlorofluorescein
- DCFH-DA
2′,7′-dichlorodihydrofluorescein diacetate
- DMEM
Dulbecco’s modified Eagle’s medium
- ERK
extracellular signal-regulated kinase
- HRP
horseradish peroxidase
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- JNK
c-jun NH2-terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- NO
nitric oxide
- PI
propidium iodide
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
Funding
This work was supported by the Fujian Medical Innovation Project [grant number 2016-CX-033]; the Fujian Natural Science Foundation [grant number 2017J01187]; the Joint Funds for the Innovation of Science and Technology, Fujian Province [grant numbers 2016Y9048, 2017Y9120]; the Medical Elite Cultivation Program of Fujian China [grant numbers 2016-ZQN-64, 2018-ZQN-61]; and the College Students’ Innovative Experiment, Fujian Medical University [grant numbers C17095, 201810392033].
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Author contribution
N.Z., J.X., L.W., and X.C. designed the study. Y.W., Z.L., W.W., H.W., and S.T. performed the experiments, analyzed the data, and wrote the draft. Y.W. and Z.L. finalized the manuscript. S.L. and P.L. contributed analysis tools.
References
- 1.Fleischmann C., Scherag A., Adhikari N.K., Hartog C.S., Tsaganos T., Schlattmann P. et al. (2016) Assessment of Global Incidence and Mortality of Hospital-treated Sepsis - current estimates and limitations. Am. J. Respir. Crit. Care Med. 193, 259–272 10.1164/rccm.201504-0781OC [DOI] [PubMed] [Google Scholar]
- 2.Oliveira Y.P., Pontes-De-Carvalho L.C., Couto R.D. and Noronha-Dutra A.A. (2017) Oxidative stress in sepsis. Possible production of free radicals through an erythrocyte-mediated positive feedback mechanism. Braz. J. Infect. Dis. 21, 19–26 10.1016/j.bjid.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prauchner C.A. (2017) Oxidative stress in sepsis: pathophysiological implications justifying antioxidant co-therapy. Burns 43, 471–485 10.1016/j.burns.2016.09.023 [DOI] [PubMed] [Google Scholar]
- 4.Montini L., DE P.S., Pennisi M.A., Rossi C., Scatena R., DE G.P. et al. (2016) Prognostic value of the reactive oxygen species in severe sepsis and septic shock patients: a pilot study. Minerva Anestesiol. 82, 1306–1313 [PubMed] [Google Scholar]
- 5.Chun T.T., Potz B.A., Young W.A. and Ayala A. (2017) Overview of the Molecular Pathways and Mediators of Sepsis, pp. 47–69, Springer [Google Scholar]
- 6.Chao W., Deng J.-S., Huang S.-S., Li P.-Y., Liang Y.-C. and Huang G.-J. (2017) 3, 4-dihydroxybenzalacetone attenuates lipopolysaccharide-induced inflammation in acute lung injury via down-regulation of MMP-2 and MMP-9 activities through suppressing ROS-mediated MAPK and PI3K/AKT signaling pathways. Int. Immunopharmacol. 50, 77–86 10.1016/j.intimp.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 7.Kim J.M., Sy K., Noh E.M., Song H.K., Lee G.S., Kwon K.B. et al. (2018) Reversine inhibits MMP‐1 and MMP‐3 expressions by suppressing of ROS/MAPK/AP‐1 activation in UV‐stimulated human keratinocytes and dermal fibroblasts. Exp. Dermatol. 27, 298–301 10.1111/exd.13494 [DOI] [PubMed] [Google Scholar]
- 8.Li C., Li L., C-f Y., Y-j Z., Wu D., Shi L. et al. (2017) Hepatoprotective effects of Methyl ferulic acid on alcohol-induced liver oxidative injury in mice by inhibiting the NOX4/ROS-MAPK pathway. Biochem. Biophys. Res. Commun. 493, 277–285 10.1016/j.bbrc.2017.09.030 [DOI] [PubMed] [Google Scholar]
- 9.Burotto M., Chiou V.L., Lee J.M. and Kohn E.C. (2015) The MAPK pathway across different malignancies: a new perspective. Cancer 120, 3446–3456 10.1002/cncr.28864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sui X., Kong N., Ye L., Han W., Zhou J., Zhang Q. et al. (2014) p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 344, 174–179 10.1016/j.canlet.2013.11.019 [DOI] [PubMed] [Google Scholar]
- 11.Wu Q., Deng J., Fan D., Duan Z., Zhu C., Fu R. et al. (2018) Ginsenoside Rh4 induces apoptosis and autophagic cell death through activation of the ROS/JNK/p53 pathway in colorectal cancer cells. Biochem. Pharmacol. 148, 64–74 10.1016/j.bcp.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 12.Jakhar R., Paul S., Bhardwaj M. and Kang S.C. (2016) Astemizole-Histamine induces Beclin-1-independent autophagy by targeting p53-dependent crosstalk between autophagy and apoptosis. Cancer Lett. 372, 89–100 10.1016/j.canlet.2015.12.024 [DOI] [PubMed] [Google Scholar]
- 13.Kim S.J., Baek K.S., Park H.J., Jung Y.H. and Lee S.M. (2016) Compound 9a, a novel synthetic histone deacetylase inhibitor, protects against septic injury in mice by suppressing MAPK signalling. Br. J. Pharmacol. 173, 1045–1057 10.1111/bph.13414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedrazza L., Cubillosrojas M., De F.M., Luft C., Cunha A.A., Rosa J.L. et al. (2017) Mesenchymal stem cells decrease lung inflammation during sepsis, acting through inhibition of the MAPK pathway. Stem Cell Res. Ther. 8, 289 10.1186/s13287-017-0734-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walshe C.M., Laffey J.G., Kevin L. and O’Toole D. (2015) Sepsis protects the myocardium and other organs from subsequent ischaemic/reperfusion injury via a MAPK-dependent mechanism. Intens. Care Med. Exp. 3, 1–11 10.1186/s40635-014-0035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong W., Qian K., Xiong J., Ma K., Wang A. and Zou Y. (2016) Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed. Pharmacother. 83, 302–313 10.1016/j.biopha.2016.06.036 [DOI] [PubMed] [Google Scholar]
- 17.Wang X.P., Wang Q.X., Lin H.P. and Chang N. (2017) Anti-tumor bioactivities of curcumin on mice loaded with gastric carcinoma. Food Funct. 8, 3319 10.1039/C7FO00555E [DOI] [PubMed] [Google Scholar]
- 18.Savcun G.Y., Özkan E., Dulundu E., Topaloğlu Ü, Şehirli AÖ, Tok O.E. et al. (2013) Antioxidant and anti-inflammatory effects of curcumin against hepatorenal oxidative injury in the experimental sepsis model created in rats. Turkish J. Trauma Emerg. Surg. 19, 507–515 10.5505/tjtes.2013.76390 [DOI] [PubMed] [Google Scholar]
- 19.] Liu W., Li Y., Yue Y., Zhang K., Chen Q., Wang H. et al. (2015) Synthesis and biological evaluation of curcumin derivatives containing NSAIDs for their anti-inflammatory activity. Bioorg. Med. Chem. Lett. 25, 3044–3051 10.1016/j.bmcl.2015.04.077 [DOI] [PubMed] [Google Scholar]
- 20.Schmölz L., Wallert M. and Lorkowski S. (2017) Optimized incubation regime for nitric oxide measurements in murine macrophages using the Griess assay. J. Immunol. Methods 449, 68–70 10.1016/j.jim.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 21.Kianiesfahani A., Bahrami S., Tavalaee M., Deemeh M.R., Mahjour A.A. and Nasresfahani M.H. (2013) Cytosolic and mitochondrial ROS: which one is associated with poor chromatin remodeling? Syst. Biol. Reprod. Med. 59, 352–359 10.3109/19396368.2013.829536 [DOI] [PubMed] [Google Scholar]
- 22.El Kamouni S., El Kebbaj R., Andreoletti P., El Ktaibi A., Rharrassi I., Essamadi A. et al. (2017) Protective effect of argan and olive oils against LPS-induced oxidative stress and inflammation in mice livers. Int. J. Mol. Sci. 18, 2181 10.3390/ijms18102181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun C., Wu Q., Zhang X., He Q. and Zhao H. (2017) Mechanistic evaluation of the protective effect of carnosine on acute lung injury in sepsis rats. Pharmacology 100, 292–300 10.1159/000479879 [DOI] [PubMed] [Google Scholar]
- 24.Chen G., Deng H., Song X., Lu M., Zhao L., Xia S. et al. (2017) Reactive oxygen species-responsive polymeric nanoparticles for alleviating sepsis-induced acute liver injury in mice. Biomaterials 144, 30 10.1016/j.biomaterials.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 25.Yılmaz G.S., Ozkan E., Dulundu E., Topaloğlu U., Sehirli A.O., Tok O.E. et al. (2013) Antioxidant and anti-inflammatory effects of curcumin against hepatorenal oxidative injury in an experimental sepsis model in rats. Turkish J. Trauma Emerg. Surg. 19, 507 10.5505/tjtes.2013.76390 [DOI] [PubMed] [Google Scholar]
- 26.Sirohi V.K., Popli P., Sankhwar P., Kaushal J.B., Gupta K., Manohar M. et al. (2017) Curcumin exhibits anti-tumor effect and attenuates cellular migration via Slit-2 mediated down-regulation of SDF-1 and CXCR4 in endometrial adenocarcinoma cells. J. Nutr. Biochem. 44, 60 10.1016/j.jnutbio.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 27.Zheng Z., Sun Y., Liu Z., Zhang M., Li C. and Cai H. (2015) The effect of curcumin and its nanoformulation on adjuvant-induced arthritis in rats. Drug Des. Dev. Ther. 9, 4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park S.H., Kang K., Giannopoulou E., Yu Q., Kang K., Kim G. et al. (2017) Type I interferons and the cytokine TNF cooperatively reprogram the macrophage epigenome to promote inflammatory activation. Nat. Immunol. 18, 1104 10.1038/ni.3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Y., Tang J.S., Hou S.X., Shi X.X., Qin J., Zhang T.S. et al. (2017) Neuroprotective effects of curcumin alleviate lumbar intervertebral disc degeneration through regulating the expression of iNOS, COX-2, TGF-β1/2, MMP-9 and BDNF in a rat model. Mol. Med. Rep. 16, 6864 10.3892/mmr.2017.7464 [DOI] [PubMed] [Google Scholar]
- 30.Min Y.A., Hwang J.S., Su B.L., Sun A.H., Hur J., Kim J.T. et al. (2017) Curcumin longaextract-loaded nanoemulsion improves the survival of endotoxemic mice by inhibiting nitric oxide-dependent HMGB1 release. Peer J. 5, e3808 10.7717/peerj.3808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han S., Xu J., Guo X. and Huang M. (2018) Curcumin ameliorates severe influenza pneumonia via attenuating lung injury and regulating macrophage cytokines production. Clin. Exp. Pharmacol. Physiol. 45, 84–93 10.1111/1440-1681.12848 [DOI] [PubMed] [Google Scholar]
- 32.Shadel G.S. and Horvath T.L. (2015) Mitochondrial ROS signaling in organismal homeostasis. Cell 163, 560–569 10.1016/j.cell.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J., Wang X., Vikash V., Ye Q., Wu D., Liu Y. et al. (2016) ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 4350965, 10.1155/2016/4350965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan X., Wang Y., Li L., Zhou W., Tian D., Lu C. et al. (2015) PM2.5 induces embryonic growth retardation: Potential involvement of ROS-MAPKs-apoptosis and G0/G1 arrest pathways. Environ. Toxicol. 31, 2028–2044 10.1002/tox.22203 [DOI] [PubMed] [Google Scholar]
- 35.Zhong W.F., Wang X.H., Pan B., Li F., Kuang L. and Su Z.X. (2016) Eupatilin induces human renal cancer cell apoptosis via ROS-mediated MAPK and PI3K/AKT signaling pathways. Oncol. Lett. 12, 2894–2899 10.3892/ol.2016.4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyun Sul K., Jae Hwa L., Hyun Soo P., Geum San L., Hyung Woo K., Ki-Tae H. et al. (2015) Schizandra chinensis extracts induce apoptosis in human gastric cancer cells via JNK/p38 MAPK activation and the ROS-mediated/mitochondria-dependent pathway. Pharm. Biol. 53, 212–219 10.3109/13880209.2014.913297 [DOI] [PubMed] [Google Scholar]
- 37.Du X., Shi Z., Peng Z., Zhao C., Zhang Y., Wang Z. et al. (2017) Acetoacetate induces hepatocytes apoptosis by the ROS‐mediated MAPKs pathway in ketotic cows. J. Cell. Physiol. 232, 3296–3308 10.1002/jcp.25773 [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y., Liu Z., Wu J., Bai B., Chen H., Xiao Z. et al. (2018) New MD2 inhibitors derived from curcumin with improved anti-inflammatory activity. Eur. J. Med. Chem. 148, 291–305 10.1016/j.ejmech.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Shan X., Dai Y., Jiang L., Chen G., Zhang Y. et al. (2015) Curcumin analog L48H37 prevents lipopolysaccharide-induced TLR4 signaling pathway activation and sepsis via targeting MD2. J. Pharmacol. Exp. Ther. 353, 539–550 10.1124/jpet.115.222570 [DOI] [PubMed] [Google Scholar]