Abstract
We conducted a retrospective study aiming to assess the risk, and associated risk factors, of developing subsequent skin cancers after having a first diagnosis of skin cancer. We included all patients with biopsy-proven skin cancer attending a dermatology clinic between July 2007 and July 2017. We assessed the frequency of new skin cancers, as well as potential demographic and clinical factors significantly associated with occurrence of such neoplasms, that were identified by means of a survival analysis. We analyzed 969 patients with a total of 1584 skin neoplasms (1122 basal cell carcinomas (BCC), 310 squamous cell carcinomas (SCC), 143 melanomas and 9 other neoplasms). 165 patients (17.0%) developed subsequent skin neoplasms. Factors identified in multivariable models to be significantly associated with development of new skin cancers included older age (adjusted HR = 1.04 per year; 95%CI = 1.02–1.05; p < 0.001), and presence of synchronous neoplasms (adjusted HR = 2.25; 95%CI = 1.61–3.14; p < 0.001). Having a history of a BCC was significantly associated with development of new BCC (adjusted HR = 1.63; 95%CI = 1.05–2.54; p = 0.030), while having a previous SCC was associated with occurrence of subsequent SCC (adjusted HR = 3.60; 95%CI = 1.93–6.72; p < 0.001). These findings point to the importance of careful follow-up (e.g., skin self-examination and full body examination) of skin cancer patients.
Introduction
The skin is the most frequent location of primary malignant neoplasms1–3. Moreover, skin cancer incidence is increasing worldwide4–6. While malignant melanoma (MM) has a higher lethality than other types of skin cancer, non-melanoma skin cancer (NMSC) - including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) – is far more common and is also associated with substantial morbidity, loss of function, disfigurement, and costs7,8.
The follow up of patients with personal history of skin cancer has been subject of debate, particularly concerning its frequency and duration9. In fact, it is known that when a patient is diagnosed for the first time with skin cancer, there is an increased risk of developing subsequent skin neoplasms10–12. However, this increased risk has been insufficiently quantified and associated epidemiological factors have not been properly identified.
Therefore, this study aims to evaluate the risk of developing a subsequent skin neoplasm after having a first diagnosis of skin cancer, as well as to identify potential factors associated with an increased risk of new neoplasms.
Results
Within the studied period, we assessed 969 different patients with a total of 1584 skin neoplasms (including 1122 BCCs, 310 SCCs, 143 MMs and 9 other neoplasms); 675 patients had a single skin neoplasm, while 294 individuals had more than one skin cancer. The median “free-of-new-neoplasms” follow-up time was of 45 months. Overall, 165 patients (17.0%; 95% CI: 14.6–19.4%) presented metachronous tumours after having been diagnosed with the first one. Moreover, a total of 178 patients had synchronous neoplasms. In most cases, the type of these new neoplasms was the same histological type as the first assessed lesion. Therefore, of all assessed patients, a total of 133 developed new BCCs (13.7%; median follow-up time until a new BCC was diagnosed: 19 months), 48 developed new SCCs (5.0%; median follow-up time: 19 months), and 7 developed new MMs (0.7%; median follow-up time: 50 months). Overall, the median follow-up time was of 17 months for patients who developed new skin neoplasms versus 11 months for the remainder. Patients’ demographic and clinical characteristics at first assessment are described in Table 1. Overall, patients’ age ranged between 17 and 102 years. Patients who developed new neoplasms were in average older than the remainder (69 versus 63 years, p < 0.001) and had a higher expression of synchronous neoplasms at first diagnosis (33.3% versus 15.3%; p < 0.001).
Table 1.
Characteristics of patients when they were first diagnosed with a skin neoplasm, and comparison between those who subsequently developed other skin neoplasms (“patients with new neoplasms”) versus those who did not (“patients with no new neoplasms”).
| All patients (n = 969) | Patients with no new neoplasms (n = 804) | Patients with new neoplasms (n = 165) | P valuea | |
|---|---|---|---|---|
| Ageb – mean (SD) | 64.0 (16.1) | 63.0 (16.6) | 69.0 (12.7) | <0.001† |
| Gender – n (%) | ||||
| Male | 458 (47.3) | 375 (46.6) | 83 (50.3) | 0.391* |
| Female | 511 (52.7) | 429 (53.4) | 82 (49.7) | |
| Histological type of neoplasmsb – n (%) | ||||
| Basal cell carcinoma | 665 (68.6) | 548 (68.2) | 117 (70.9) | 0.488* |
| Squamous cell carcinoma | 202 (20.8) | 157 (19.5) | 45 (27.3) | 0.026* |
| Melanoma | 130 (13.4) | 121 (15.0) | 9 (5.5) | 0.001* |
| Other | 8 (0.8) | 6 (0.7) | 2 (1.2) | 0.630** |
| Anatomical location of neoplasmsb – n (%) | ||||
| Head and neck | 549 (56.7) | 450 (56.0) | 99 (60.0) | 0.341* |
| Trunk | 241 (24.9) | 197 (24.5) | 44 (26.7) | 0.558* |
| Upper limb | 161 (16.6) | 126 (15.7) | 35 (21.2) | 0.082* |
| Lower limb | 121 (12.5) | 95 (11.8) | 26 (15.8) | 0.163* |
| Infiltrative neoplasmsb | 212 (21.9) | 180 (22.4) | 32 (19.4) | 0.397* |
| Presence of synchronous neoplasmsb,c | 178 (18.4) | 123 (15.3) | 55 (33.3) | <0.001* |
†Two-samples independent t-test; *Chi-square test; **Fisher’s exact test.
aP values obtained by comparing patients with no new neoplasms versus patients with new neoplasms; bVariables assessed at the time of the first skin cancer diagnosis; cIncludes 114 patients (64.0%) with synchronous BCC only, 27 patients (15.2%) with synchronous SCC only, 26 patients (14.6%) with synchronous BCC and SCC, 7 patients (3.9%) with synchronous BCC and melanoma, and 4 patients (2.2%) with synchronous melanoma only.
We performed Cox proportional hazard regression analysis to estimate variables at first assessment potentially associated with development of new skin neoplasms (there were no significant differences in follow-up time regarding any of the independent variables tested). In univariable analyses, diagnosis of an SCC, older age and presence of synchronous neoplasms were significantly associated with development of new skin neoplasms. However, in multivariable models, significant associations were only observed for age (adjusted HR = 1.04 per year; 95%CI = 1.02–1.05; p < 0.001) and presence of synchronous neoplasms (adjusted HR = 2.25; 95%CI = 1.61–3.14; p < 0.001) (Table 2).
Table 2.
Results of the univariable and multivariable Cox proportional hazard regression analyses of all new skin neoplasms survival, new basal cell carcinomas (BCC) survival, and new squamous cell carcinomas (SCC) survival.
| Univariable analysis: HR (95%CI); p value | Multivariable analysis: HR (95%CI); p value | |
|---|---|---|
| All skin neoplasms | ||
| Diagnosis of a BCCa | 1.30 (0.93–1.83); 0.117 | —c |
| Diagnosis of a SCCa | 1.67 (1.19–2.36); 0.005 | 1.03 (0.71–1.48); 0.895 |
| Agea | 1.04 (1.03–1.05); <0.001 | 1.04 (1.02–1.05); <0.001 |
| Male genderb | 1.27 (0.93–1.73); 0.128 | 1.09 (0.80–1.49); 0.590 |
| Infiltrative neoplasma | 0.78 (0.53–1.14); 0.189 | — |
| Presence of synchronous neoplasmsa | 2.92 (2.11–4.06); <0.001 | 2.25 (1.61–3.14); <0.001 |
| BCC | ||
| Diagnosis of a BCCa | 1.96 (1.30–2.97); <0.001 | 1.63 (1.05–2.54); 0.030 |
| Agea | 1.04 (1.03–1.05); <0.001 | 1.04(1.02–1.05); <0.001 |
| Male genderb | 1.36 (0.97–1.91); 0.079 | 1.18 (0.84–1.67); 0.349 |
| Infiltrative neoplasma | 0.68 (0.43–1.06); 0.076 | 0.71 (0.44–1.15); 0.161 |
| Presence of synchronous neoplasmsa | 2.98 (2.07–4.28); <0.001 | 2.27 (1.55–3.32); <0.001 |
| SCC | ||
| Diagnosis of a SCCa | 5.77(3.26–10.21); <0.001 | 3.60 (1.93–6.72); <0.001 |
| Agea | 1.07 (1.04–1.09); <0.001 | 1.04 (1.01–1.07); 0.002 |
| Male genderb | 1.40 (0.80–2.48); 0.240 | — |
| Infiltrative neoplasma | 1.55 (0.84–2.86); 0.172 | — |
| Presence of synchronous neoplasmsa | 2.64 (1.46–4.78); 0.003 | 1.93 (1.05–3.55); 0.035 |
HR = Hazard ratio; 95% CI = 95% confidence interval.
aVariables assessed at the time of the first skin cancer diagnosis; bFemale gender was defined as reference category; cDiagnosis of a BCC was not introduced in the multivariable model, as diagnosis of a SCC was already introduced.
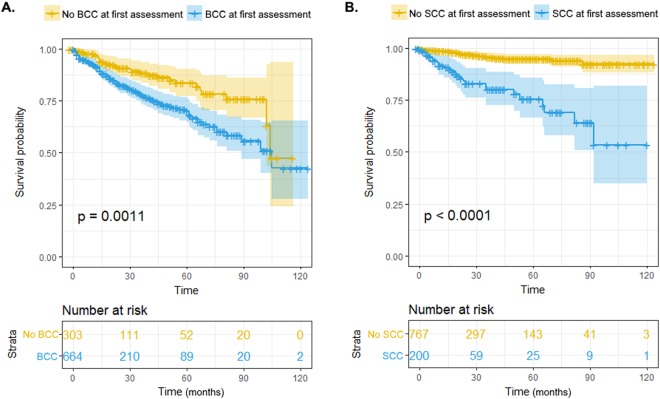
Kaplan-Meier curves for development of new BCC and SCC are depicted in Fig. 1. Patients with BCC at first assessment had lower new BCC-free survival than the remainder (p = 0.001); accordingly, patients with SCC at first assessment had lower new SCC-free survival than the remaining patients (p < 0.001) (Fig. 1). In multivariable Cox regression models, predictive factors significantly associated with development of new BCC included presence of a BCC at first assessment (adjusted HR = 1.63; 95%CI = 1.05–2.54; p = 0.030), age (adjusted HR = 1.04 per year; 95%CI = 1.02–1.05; p < 0.001) and presence of synchronous neoplasms at first assessment (adjusted HR = 2.27; 95%CI = 1.55–3.32; p < 0.001) (Table 2). Regarding SCC, significant associations were also observed for age (adjusted HR = 1.04 per year; 95%CI = 1.01–1.07; p = 0.002) and presence of synchronous neoplasms at first assessment (adjusted HR = 1.93; 95%CI = 1.05–3.55; p = 0.035), as well as for presence of an SCC at first assessment (adjusted HR = 3.60; 95%CI = 1.93–6.72; p < 0.001) (Table 2).
Figure 1.
Kaplan-Meier survival curves for diagnosis of new basal cell carcinomas (BCC) comparing patients who presented with BCC at first diagnostic assessment versus those who did not (A), and for diagnosis of new squamous cell carcinomas (SCC) comparing patients which presented with SCC at first diagnostic assessment versus those who did not (B). P values were obtained using log-rank test.
Considering only patients diagnosed with a BCC within their first assessment, having a non-superficial BCC was associated with a decreased risk of developing a new skin cancer (HR = 0.66; 95%CI = 0.46–0.96; p = 0.027), including a new BCC (HR = 0.68; 95%CI = 0.46–1.00; p = 0.048). By contrast, in those patients, having a BCC located outside the head and neck at first assessment was significantly associated with development of new skin neoplasms (HR = 1.46; 95%CI = 1.01–2.11; p = 0.041), particularly new BCC (HR = 1.54; 95%CI = 1.05–2.28; p = 0.027). These findings are inter-related, as most superficial BCC (67.8%) were found to be located outside the head and neck, while most non-superficial BCC (73.7%) were located in the head or neck.
Discussion
In this study, we assessed 969 patients with skin cancer, observing that 17.0% of them developed subsequent skin neoplasms. Advanced age and detection of synchronous neoplasms at first diagnosis were identified as risk factors for the development of new skin neoplasms. In addition, patients with a past history of BCC or SCC were found to be at an increased risk of developing skin neoplasms of the same histological type; this association, however, was stronger for SCC than for BCC.
Previous studies have reported the 3-year cumulative risk of a BCC or an SCC to be at least 10-fold increased when compared with the general population9. Our data showed a median of one year and a half for the development of a second NMSC. Meta-analysis data revealed that the 3-year cumulative risk of an SCC is 18% after a first SCC and 6% after a BCC; while the 3-year cumulative risk of BCC is 44% after a first BCC or an SCC9. In a more recent meta-analysis, the highest risk was found for the development of subsequent cutaneous neoplasms of the same type10, as reported with our data. Patients who develop BCC and SCC have lower incidence of new BCC when compared to patients who have BCC only, possibly due to sun exposure patterns favoring SCC or to innate immunity trigger13.
We observed few patients developing a MM as a second neoplasm, and therefore we were not able to explore the potential risk factors for the occurrence of a second MM. However, the median follow-up time to the diagnosis of metachronous MM was above 4 years. Previous studies have reported that among MM patients, the incidence of a second primary MM ranges from 0.2% to 8.6%14, being higher in younger people15.
This study has some limitations, particularly as it has a retrospective design and is based on a single private healthcare institution which might not be representative of the whole country. Nevertheless, the demographic and clinical characteristics of the patients assessed are mostly consistent with those described in the literature, particularly concerning their mean age, gender distribution and proportion of BCC/SCC and NMSC/MM4,16,17. Another limitation concerns the fact that the follow-up period varied according to the time of entry into the study, with participants who did not develop a new skin cancer having a lower median follow-up period than those who did, possibly resulting in an under-estimation of the incidence of metachronous neoplasms (some participants might have been identified as not developing new skin tumors simply because they were early losses to follow-up). Finally, this study did not include the analysis of SCC precursor lesions, namely actinic keratosis.
The assessment of all patients with confirmed diagnosis of skin cancer observed in a 10 year-period is an important strong point of this work. In addition, we performed univariable and multivariable Cox regression analyses, allowing adjusted results for each tested independent variable to be obtained.
Not only did we observe a high incidence of new skin neoplasms, but also a high frequency of patients presenting with more than one neoplasm at first diagnosis. In fact, almost one third of the patients that developed metachronous neoplasms had two or more skin neoplasms at first diagnosis. Skin cancer is an etiologically complex disease with ultraviolet radiation exposure, phenotype and genotype being probably involved in the risk of synchronous and metachronous lesions18,19. This fact highlights the importance of registering all skin cancers, even if having the same histological type and/or the same location. Unfortunately, NMSC has been widely disregarded in national cancer registries, possibly due to their extremely high frequency and low lethality7,8, impairing an appropriate understanding of skin cancer epidemiology and costs.
These results, along with the finding that the presence of synchronous neoplasms is associated with a higher risk of developing metachronous tumors, indicate the extreme importance of a full skin body examination20 and of a careful follow-up of skin cancer patients. Informing skin cancer patients about their risk of developing another skin cancer, together with teaching them skin self-examination every 1-2 months is mandatory, but it can cause anxiety21 if not accompanied by an easy access to physicians.
Nevertheless, the adequate follow-up frequency and duration for skin cancer patients remains debatable. An at least annual full skin examination for 3 to 5 years for NMSC, and every 3 to 6 months, depending on stage22, for 5 years for MM patients is desirable23. While follow-up surveillance of MM patients should be lifelong (although there is no international agreement for follow-up guidelines after 5 years from diagnosis)15, follow-up of NMSC for more than 3–5 years (3 years for BCC and 5 years for SCC) is currently not recomended9,24. This study’s results, however, might justify further studies to establish the best follow-up protocol for NMSC according to patients’ risk factors, while also emphasizing the importance of a comprehensive examination of these patients, assisted by dermoscopy25. This assessment is particularly important as early detection and treatment of MM and NMSC can reduce morbidity (and, particularly in the case of MM, mortality)26,27, bring a better health for the population28 and reduce the financial burden for the society29–35.
In conclusion, we observed that patients with history of skin cancer had a risk of 17.0% of developing new skin neoplasms, with advanced age and detection of synchronous neoplasms at first diagnosis identified as risk factors for the development of metachronous lesions. These findings point to the importance of total skin examination and careful follow-up of all skin cancer patients, including those with NMSC. Further studies are needed to evaluate the best follow-up protocols according to patients’ risk factors and type of neoplasms diagnosed. Meanwhile, teaching skin self-examination (including the most frequent benign lesions) and improving the general practitioners diagnosis accuracy might improve the referral to dermatology centers, which might decrease morbidity, mortality and costs.
Methods
We performed a retrospective cohort study assessing all patients diagnosed with at least one biopsy-proven skin neoplasm between July 2007 and July 2017 in a single institution (a private dermatology center in the North of Portugal). For all skin neoplasms, we recorded their histological type (BCC, SCC, MM or other), their anatomical location (categorized as “head or neck” versus “outside the head and neck”) and whether they were or not infiltrative (for BCC)/invasive (for SCC and MM). Additionally, we recorded whether each patient presented with a single or with multiple skin neoplasms simultaneously (synchronous neoplasms) at their first assessment. For BCC, we also took into account its subtype – BCC subtypes were categorized as “superficial” (including superficial and multifocal neoplasms) or “non-superficial” (including nodular, infiltrative, and ulcerated neoplasms).
The skin cancer diagnosis was clinically performed by a senior dermatologist after full body examination, and was assisted by dermoscopy followed by a biopsy for histopathological diagnostic confirmation. Full body examination is performed in the majority of patients attending our dermatology center irrespectively of their initial complaint. Patients with genetic syndromes associated with multiple skin cancers and immunosuppressed by organ transplantation were not included. Follow-up periods were established according to the skin cancer type – every 6 six months for MM in the first 5 years, and thereafter annually; every 6 months for NMSC in the first 3 years, and thereafter annually.
We assessed whether the included patients developed new cutaneous neoplasms (metachronous tumors) until July 2017 and recorded the diagnosis date for the first new neoplasm of each histological type. We calculated the skin cancer survival free time, corresponding to the time period between the date of the first diagnosed skin neoplasm(s) and either (i) the date of the next skin neoplasm diagnosed after the first one(s) or (ii), if no new skin neoplasms were detected after the first one(s), the date of the last follow-up/clinic visit. Accordingly, we also calculated BCC and SCC specific survivals, respectively based on the dates of the first BCC and of the first SCC diagnosed after the first skin neoplasm(s).
Statistical analysis
Categorical variables were described using absolute and relative frequencies, while continuous variables were described using means and standard-deviations (SD). Chi-square test and Fisher’s exact test were used to compare categorical variables, whereas two-independent samples t-test was used with continuous variables.
We performed a Cox proportional hazard regression analysis in order to identify variables (assessed at the diagnosis of the first skin cancer(s)) significantly associated with occurrence of new skin neoplasms. Tested variables included patients’ gender, age, diagnosis of a BCC, diagnosis of a SCC, neoplasm anatomical location, presentation of synchronous neoplasms, and presence of at least one infiltrative/invasive neoplasm. Firstly, we performed univariable analyses, and then we included all variables with at least marginal association (p < 0.150) with the dependent variable in a multivariable model. Similar analyses were specifically performed for the development of new BCC and new SCC. We performed a subanalysis considering only patients with initial diagnosis of BCC, in order to assess whether the BCC subtype was significantly associated with development of new neoplasms. Results were presented using hazard ratios (HR) and 95% confidence intervals (CI).
Concerning the occurrence of new BCC, we also obtained Kaplan-Meier survival curves comparing patients who presented with a BCC at first diagnosis versus those who did not. For the occurrence of new SCC, Kaplan-Meier survival curves compared patients who presented with a SCC at first diagnosis versus those who did not. Comparisons were assessed using the log-rank test.
P values lower than 0.05 were considered to be statistically significant. All statistical analyses were performed using software R (version 3.4.3); Cox regression was performed using package ‘survival’, and Kaplan-Meier curves were obtained using package ‘survminer’.
Ethical approval and informed consent
All methods were carried out in accordance with relevant guidelines and regulations. Data analysed were fully anonymised and as such the Ethics Committee of Instituto CUF waived the need for approval.
Author Contributions
A.F.D. and B.S.P. wrote the main manuscript text. B.S.P. prepared Figure 1 and tables. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers HW, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 3.Geller AC, Annas GD. Epidemiology of melanoma and nonmelanoma skin cancer. Semin Oncol Nurs. 2003;19(1):2–11. doi: 10.1053/sonu.2003.50000. [DOI] [PubMed] [Google Scholar]
- 4.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 5.Erdmann F, et al. International trends in the incidence of malignant melanoma 1953–2008–are recent generations at higher or lower risk? Int J Cancer. 2013;132(2):385–400. doi: 10.1002/ijc.27616. [DOI] [PubMed] [Google Scholar]
- 6.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015;151(10):1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 7.Stang A, et al. Malignant melanoma and nonmelanoma skin cancers in Northrhine-Westphalia, Germany: a patient- vs. diagnosis-based incidence approach. Int J Dermatol. 2007;46(6):564–570. doi: 10.1111/j.1365-4632.2006.03056.x. [DOI] [PubMed] [Google Scholar]
- 8.Rubio-Casadevall J, et al. Trends in incidence and survival analysis in non-melanoma skin cancer from 1994 to 2012 in Girona, Spain: A population-based study. Cancer Epidemiol. 2016;45:6–10. doi: 10.1016/j.canep.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136(12):1524–1530. doi: 10.1001/archderm.136.12.1524. [DOI] [PubMed] [Google Scholar]
- 10.Flohil SC, van der Leest RJ, Arends LR, de Vries E, Nijsten T. Risk of subsequent cutaneous malignancy in patients with prior keratinocyte carcinoma: a systematic review and meta-analysis. Eur J Cancer. 2013;49(10):2365–2375. doi: 10.1016/j.ejca.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 11.van der Leest RJ, Flohil SC, Arends LR, de Vries E, Nijsten T. Risk of subsequent cutaneous malignancy in patients with prior melanoma: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29(6):1053–1062. doi: 10.1111/jdv.12887. [DOI] [PubMed] [Google Scholar]
- 12.Rees JR, et al. Non melanoma skin cancer and subsequent cancer risk. PloS One. 2014;9(6):e99674. doi: 10.1371/journal.pone.0099674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran S, Rajaratnam R, Smith AG, Lear JT, Strange RC. Patients with both basal and squamous cell carcinomas are at a lower risk of further basal cell carcinomas than patients with only a basal cell carcinoma. J Am Acad Dermat. 2009;61(2):247–251. doi: 10.1016/j.jaad.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Ferrone CR, et al. Clinicopathological features of and risk factors for multiple primary melanomas. JAMA. 2005;294(13):1647–1654. doi: 10.1001/jama.294.13.1647. [DOI] [PubMed] [Google Scholar]
- 15.de Giorgi V, et al. Risk of second primary melanoma: how should be long follow-up be? Ratio of observed and expected cases. J Eur Acad Dermatol Venereol. 2012;26(11):1454–1455. doi: 10.1111/j.1468-3083.2011.04334.x. [DOI] [PubMed] [Google Scholar]
- 16.Katalinic A, Kunze U, Schafer T. Epidemiology of cutaneous melanoma and non-melanoma skin cancer in Schleswig-Holstein, Germany: incidence, clinical subtypes, tumour stages and localization (epidemiology of skin cancer) Br J Dermatol. 2003;149(6):1200–1206. doi: 10.1111/j.1365-2133.2003.05554.x. [DOI] [PubMed] [Google Scholar]
- 17.Hoey SE, et al. Skin cancer trends in Northern Ireland and consequences for provision of dermatology services. Br J Dermatol. 2007;156(6):1301–1307. doi: 10.1111/j.1365-2133.2007.07936.x. [DOI] [PubMed] [Google Scholar]
- 18.Brash DE. UV signature mutations. Photoch Photobiol. 2015;91(1):15–26. doi: 10.1111/php.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verkouteren JAC, Ramdas KHR, Wakkee M, Nijsten T. Epidemiology of basal cell carcinoma: scholarly review. Br J Dermatol. 2017;177(2):359–372. doi: 10.1111/bjd.15321. [DOI] [PubMed] [Google Scholar]
- 20.Aldridge RB, Naysmith L, Ooi ET, Murray CS, Rees JL. The importance of a full clinical examination: assessment of index lesions referred to a skin cancer clinic without a total body skin examination would miss one in three melanomas. Acta Derm Venereol. 2013;93(6):689–692. doi: 10.2340/00015555-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossman DC, et al. Behavioral Counseling to Prevent Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(11):1134–1142. doi: 10.1001/jama.2018.0161. [DOI] [PubMed] [Google Scholar]
- 22.Gershenwald JE, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancereighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheless L, Black J, Alberg AJ. Nonmelanoma skin cancer and the risk of second primary cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1686–1695. doi: 10.1158/1055-9965.EPI-10-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wehner MR, et al. Timing of subsequent new tumors in patients who present with basal cell carcinoma or cutaneous squamous cell carcinoma. JAMA Dermatol. 2015;151(4):382–388. doi: 10.1001/jamadermatol.2014.3307. [DOI] [PubMed] [Google Scholar]
- 25.Thomas L, Puig S. Dermoscopy, Digital Dermoscopy and Other Diagnostic Tools in the Early Detection of Melanoma and Follow-up of High-risk Skin Cancer Patients. Acta Derm Venereol. 2017;10:2340/00015555–2719. doi: 10.2340/00015555-2719. [DOI] [PubMed] [Google Scholar]
- 26.Helfand M, Mahon SM, Eden KB, Frame PS, Orleans CT. Screening for skin cancer. Am J Prev Med. 2001;20(3 Suppl):47–58. doi: 10.1016/S0749-3797(01)00258-6. [DOI] [PubMed] [Google Scholar]
- 27.Wei EX, et al. Trends in the diagnosis and clinical features of melanoma in situ (MIS) in US men and women: A prospective, observational study. J Am Acad Dermatol. 2016;75(4):698–705. doi: 10.1016/j.jaad.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer, J. E., Swetter, S. M., Fu, T. & Geller, A. C. Screening, early detection, education, and trends for melanoma: current status (2007–2013) and future directions: Part I. Epidemiology, high-risk groups, clinical strategies, and diagnostic technology. J Am Acad Dermatol. 71(4), 599.e591–599.e512, quiz610 (2014). [DOI] [PubMed]
- 29.Aitken JF, Youlden DR. Generational shift in melanoma incidence and mortality in Queensland, Australia, 1995-2014. Int J Cancer. 2018;142(8):1528–1535. doi: 10.1002/ijc.31141. [DOI] [PubMed] [Google Scholar]
- 30.Vallejo-Torres L, Morris S, Kinge JM, Poirier V, Verne J. Measuring current and future cost of skin cancer in England. J Public Health (Oxf). 2014;36(1):140–148. doi: 10.1093/pubmed/fdt032. [DOI] [PubMed] [Google Scholar]
- 31.Gordon LG, Rowell D. Health system costs of skin cancer and cost-effectiveness of skin cancer prevention and screening: a systematic review. Eur J Cancer Prev. 2015;24(2):141–149. doi: 10.1097/CEJ.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 32.Stratigos AJ, et al. Euromelanoma: a dermatology-led European campaign against nonmelanoma skin cancer and cutaneous melanoma. Past, present and future. Br J Dermatol. 2012;167(Suppl 2):99–104. doi: 10.1111/j.1365-2133.2012.11092.x. [DOI] [PubMed] [Google Scholar]
- 33.van der Leest RJ, et al. The Euromelanoma skin cancer prevention campaign in Europe: characteristics and results of 2009 and 2010. J Eur Acad Dermatol Venereol. 2011;25(12):1455–1465. doi: 10.1111/j.1468-3083.2011.04228.x. [DOI] [PubMed] [Google Scholar]
- 34.Breitbart EW, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66(2):201–211. doi: 10.1016/j.jaad.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Kyle JW, et al. Economic evaluation of the US Environmental Protection Agency’s SunWise program: sun protection education for young children. Pediatrics. 2008;121(5):e1074–1084. doi: 10.1542/peds.2007-1400. [DOI] [PubMed] [Google Scholar]