Abstract
Background
Higher body mass index (BMI) and low physical activity have been associated with increased prevalence of fecal incontinence (FI) in cross-sectional studies, but prospective studies examining the role of these factors are lacking. We sought to determine whether BMI and/or physical activity are associated with risk of FI among older women.
Methods
We prospectively examined the association between BMI and physical activity and risk of FI in the Nurses’ Health Study among 51,708 women who were free of FI in 2008. We defined FI as at ≥1 liquid or solid FI episode/month during the past year reported in 2010 or 2012. We used Cox proportional hazards models to calculate multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for FI according to physical activity and BMI, adjusting for potential confounding factors.
Results
During more than 175,000 person-years of follow-up, we documented 5954 cases of incident FI. Compared with women in the lowest activity category (<3 metabolic equivalent of task (MET)-hrs/week), multivariable-adjusted HRs for FI were 0.86 (95% CI 0.80–0.93) for women doing 3–8 MET-hrs/week, 0.78 (95% CI 0.72–0.84) for 9–17 MET-hrs/week, 0.76 (95% CI 0.69–0.83) for 18–26 MET-hrs/week, and 0.75 (95% CI 0.70–0.81) for 27 + MET-hrs/week (Ptrend = <0.0001). There was no association between BMI and risk of FI.
Conclusions
Higher levels of physical activity were associated with a modest reduction (25%) in risk of incident FI among older women. These results support a potential role of ongoing physical activity in the neuromuscular health of the anorectal continence mechanism with aging.
Translational impact
These results support a potential role of ongoing physical activity in the neuromuscular health of the anorectal continence mechanism with aging.
Introduction
Fecal incontinence (FI) affects an estimated 7–15% of women in the community and exerts a significant impact on quality of life mediated primarily through social stigma, embarrassment, and impaired hygiene1. Despite this considerable burden, our knowledge about risk factors for FI—and thus potential populations for targeted prevention strategies—is limited2,3. Of particular interest are risk factors that are potentially modifiable, such as body weight and physical activity. Previous cross-sectional analyses from our group and others observed increased risk of FI among women with higher body mass index (BMI) and lower levels of physical activity4–7. Conversely, others have found no association between BMI and FI in a prospective study8 and increased risk in a cross-sectional study of FI in young women engaged in intensive sports9. However, results of cross-sectional analyses may be biased due to reverse causation, as individuals with FI may reduce their physical activity in response to FI symptoms and subsequently gain weight. Additionally, several conditions associated with obesity (diabetes mellitus and hypertension) are known to be associated with FI4, making casual inference more difficult.
We therefore sought to prospectively examine the associations of BMI and physical activity with FI in the Nurses’ Health Study, a large, ongoing study of older the US women. This cohort includes regularly updated information on lifestyle factors (including physical activity), BMI, and medical diagnoses to enable a comprehensive examination of potentially modifiable lifestyle characteristics and the incidence of FI.
Methods
Study population
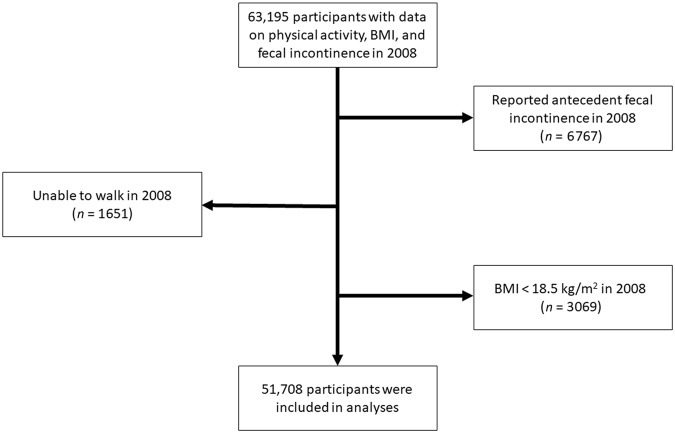
The Nurses’ Health Study is a prospective cohort that began in 1976 when 121,701 US female registered nurses, aged 30–55, completed a self-administered, mailed questionnaire about their health and lifestyle10. Participants have provided updated information on biennial questionnaires, which are mailed in June of even-numbered years. Repeated mailings are sent to non-responders during the 2-year period before the next biennial questionnaire is mailed. To increase response rates, shorter versions of the questionnaire are sent to participants who do not respond to the standard version. Questions about FI were first included on the standard version of the 2008 questionnaire. For this analysis, we included 68,890 women who returned the 2008 standard questionnaire and were not missing physical activity data (n = 5695), leaving 63,195 women. We subsequently excluded 6767 women with prevalent FI in 2008 and 3069 women with a BMI < 18.5 kg/m2 because of concerns about unmeasured confounding related to both FI and low BMI. To address potential confounding by functional limitations, we excluded 1651 women who reported they were unable to walk11. Thus, the analytic population included 51,708 women (Fig. 1). The study was approved by the Institutional Review Board at Brigham and Women’s Hospital.
Fig. 1.
Flow of eligible participants into the study
Assessment of physical activity and BMI
On the 2008 questionnaire, participants were asked about their average time per week during the preceding year spent doing any of the following activities: walking or hiking outdoors; jogging; running; bicycling; swimming laps; tennis; calisthenics, aerobics, aerobic dance, or rowing machine; squash or racquet ball; and other vigorous activities (i.e., mowing the lawn). Responses ranged from 0 to ≥11 h/week. We also obtained information on usual outdoor walking pace and number of flights of stairs climbed daily. Each activity was assigned a value for metabolic equivalent task (MET) according to established criteria12. Each participant’s MET score for walking was assigned on the basis of her reported walking pace. We calculated MET hours per week (MET-h/wk) for each activity by multiplying the MET score by the participant’s reported number of hours of physical activity per week. Total physical activity was computed as the sum of MET-h/wk from all individual activities. In a previous validation study among NHS participants, scores from the physical activity questionnaire administered 2 years apart were moderately reproducible (r = 0.59), and physical activity recalled for the previous year was highly correlated with past week recalls (r = 0.79)13. Because strenuous, high-impact activity may cause pelvic floor muscle fatigue and thus increase risk of FI3,9,14,15, we summed MET-h/wk from all high-impact recreational activities (jogging, running, tennis, and aerobics) and created two categories for participants with high and low rates of high-impact physical activity based on the group median. We did the same for all other physical activity that was not high impact—considered low-impact physical activity.
BMI was calculated using participants’ self-reported body weight and height, which has been previously validated in this cohort16. Height was reported at enrollment on the 1976 questionnaire while weight has been updated on each biennial questionnaire.
Ascertainment of fecal incontinence
On the 2008, 2010, and 2012 questionnaires, participants were asked “On average, how often in the past year have you experience any amount of accidental bowel leakage?” Response categories included “Never,” “Less than 1/month,” “1–3×/month,” “About once/week,” “Several times/week,” or “Nearly daily.” Women were considered to have FI if they reported incontinence of liquid or solid stool at least monthly.
Assessment of covariates
On each biennial questionnaire, participants were asked about several factors pertinent to the risk of FI, including smoking status, parity, history of cholecystectomy (asked in 1982–2002), use of menopausal hormone therapy (MHT), and diagnosis with diabetes mellitus, high blood pressure, and neurologic disease—defined as amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), or Parkinson’s disease.
Statistical analysis
We calculated person-time for each participant from the return date of the 2008 questionnaire to the return date of the questionnaire on which FI was reported minus 1 year, date of the last questionnaire returned, date of death from any cause, or June 1, 2012, whichever came first. We used Cox proportional hazards models to calculate multivariable-adjusted hazard ratios (HR) and 95% confidence intervals (CI) for incident FI according to physical activity category (<3, 3–8, 9–17, 18–26, 27+ MET-h/wk). Also, to address the possibility that the association between physical activity and risk of FI varies by BMI, we conducted additional analyses within BMI strata: normal weight (18.5–24.9 kg/m2), overweight (25–29 kg/m2), and obese (30+ kg/m2). Because developing FI may lead to decreased physical activity, we performed a lagged analysis examining risk of FI according to physical activity derived from the 2004 questionnaire, 4 years prior to the first assessment of FI. Since bowel disturbances may impact the relationship between physical activity and FI, we conducted separate analyses examining the association of physical activity with risk of liquid or solid stool FI alone. Covariates were selected a priori based on previously reported risk factors for FI1,2,4,17. BMI, MHT use, smoking, and diagnoses of diabetes mellitus, hypertension, or neurologic disease were included as time-varying covariates to account for changes in these exposures over time. Data on physical activity (which is only available every 4 years) and parity were not updated. For any covariates with missing data on an interval questionnaire (usually only a small percentage of women), we carried forward values from the previous questionnaire. Statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC). All authors had access to the study data and reviewed and approved the final manuscript.
Results
From 2008 to 2012, we documented 5954 incident cases of FI among 51,708 older women who contributed 175,447 person-years of follow-up. Compared with women in the lowest category of physical activity (<3 MET-h/wk), women in the highest category of physical activity (27+ MET-h/wk) were younger and less likely to be obese, have diabetes or hypertension, or have undergone a cholecystectomy (Table 1). Race, smoking, parity, and presence of neurologic disease were similar across MET-h/wk categories. Compared with normal weight women (BMI 18.5–24.9 kg/m2), women who were overweight or obese (BMI ≥ 25 kg/m2) were less physically active and more likely to have diabetes or hypertension and have undergone a cholecystectomy (Supplementary Table 1)
Table 1.
Baseline characteristics of women in the Nurses’ Health Study according to physical activity category in 2008
| MET hours/week category | |||||
|---|---|---|---|---|---|
| <3 (N = 11,706) | 3–8 (N = 10,816) | 9–17 (N = 10,175) | 18–26 (N = 6647) | 27+ (N = 12,364) | |
| Age (y), mean (SD) | 74.4 (7.0) | 73.3 (6.7) | 72.5 (6.6) | 72.1 (6.3) | 71.7 (6.0) |
| Race (% non-white) | 6 | 6 | 6 | 5 | 6 |
| BMI (%), kg/m 2 | |||||
| 18.5–24.9 | 33 | 40 | 44 | 49 | 55 |
| 25–29.9 | 35 | 37 | 35 | 35 | 33 |
| 30–34.9 | 20 | 17 | 15 | 12 | 9 |
| 35–39.9 | 8 | 5 | 5 | 3 | 2 |
| ≥40 | 4 | 5 | 2 | 1 | 1 |
| Smoking (%) | |||||
| Never | 44 | 47 | 47 | 48 | 46 |
| Past | 49 | 48 | 49 | 49 | 50 |
| Current | 7 | 5 | 5 | 4 | 4 |
| Parity (%) | |||||
| 0 | 6 | 5 | 5 | 5 | 5 |
| 1 | 7 | 7 | 6 | 6 | 7 |
| 2 | 28 | 28 | 30 | 31 | 31 |
| ≥3 | 59 | 60 | 58 | 57 | 57 |
| Diabetes mellitus (%) | 14 | 10 | 9 | 7 | 6 |
| Hypertension (%) | 58 | 54 | 49 | 46 | 43 |
| Neurologic diseasea (%) | 3 | 2 | 2 | 1 | 1 |
| Cholecystectomy (%) | 22 | 19 | 17 | 15 | 14 |
MET metabolic equivalent of task
aDefined as a confirmed diagnosis of amyotrophic lateral sclerosis, multiple sclerosis, or Parkinson’s disease
In age-adjusted analyses of physical activity and risk of incident FI, risk of FI decreased with increasing physical activity (Table 2). Results were similar after adjusting for known risk factors for FI. Compared with women in the lowest category of physical activity, multivariable-adjusted hazard ratios for FI were 0.86 (95% CI 0.80–0.93) for 3–8 MET-h/wk, 0.78 (95% CI 0.72–0.84) for 9–17 MET-h/wk, 0.76 (95% CI 0.69–0.83) for 18–26 MET-h/wk, and 0.75 (95% CI 0.70–0.81) for ≥27 MET-h/wk (Ptrend < 0.0001).
Table 2.
Physical activity, BMI and, risk of fecal incontinence
| Person-years of follow-up | No. of cases | Age-adjusted HR (95% CI) | Multivariable-adjusted HR without potential mediators (95% CI)a | Multivariable-adjusted HR (95% CI)b | |
|---|---|---|---|---|---|
| MET hours/week category | — | — | — | — | — |
| <3 | 37,440 (3%) | 1679 | 1.00 | 1.00 | 1.00 |
| 3–8 | 36,486 (14%) | 1325 | 0.84 (0.78–0.90) | 0.84 (0.79–0.91) | 0.86 (0.80–0.93) |
| 9–17 | 35,053 (20%) | 1081 | 0.75 (0.69–0.81) | 0.76 (0.70–0.82) | 0.78 (0.72–0.84) |
| 18–26 | 23,142 (12%) | 670 | 0.72 (0.66–0.78) | 0.73 (0.67–0.80) | 0.76 (0.69–0.83) |
| 27+ | 43,326 (50%) | 1199 | 0.70 (0.65–0.76) | 0.72 (0.66–0.77) | 0.75 (0.70–0.81) |
| Ptrendc | — | — | <0.0001 | <0.0001 | <0.0001 |
| BMI category, kg/m2 | — | — | — | — | — |
|---|---|---|---|---|---|
| 18.5–24.9 | 77,494 (44%) | 2662 | 1.00 | 1.00 | 1.00 |
| 25–29.9 | 61,307 (35%) | 2017 | 1.03 (0.97–1.09) | 1.02 (0.97–1.09) | 0.97 (0.91–1.03) |
| 30–34.9 | 25,503 (15%) | 868 | 1.16 (1.07–1.25) | 1.14 (1.06–1.23) | 1.02 (0.94–1.11) |
| 35–39.9 | 7893 (4%) | 288 | 1.35 (1.19–1.52) | 1.32 (1.16–1.49) | 1.12 (0.99–1.27) |
| 40+ | 3249 (2%) | 119 | 1.47 (1.22–1.77) | 1.46 (1.21–1.75) | 1.18 (0.97–1.42) |
| Ptrendc | — | — | <0.0001 | <0.0001 | 0.71 |
aModel as below with potential mediators between physical activity and fecal incontinence (BMI, hypertension, diabetes mellitus) excluded for physical activity model and with potential mediators of BMI and fecal incontinence (physical activity, hypertension, diabetes mellitus) excluded for BMI model
bModels adjusted for age (months), race, smoking (never, past, current), BMI (<18.5, 18.5–24.9, 25–29.9, 30–34.9, ≥35 kg/m2) (for physical activity model), physical activity (<3, 3–8, 9–17, 18–26, 27+ MET hours/week) (for BMI model), menopausal hormone therapy use (never, past, current), parity (number of live births), hypertension (yes/no), diabetes mellitus (yes/no), neurologic disease (yes/no), and history of cholecystectomy (yes/no)
cLinear trend estimated by entering the medians of each MET hours/week quintile or BMI as a continuous variable in the model
In age-adjusted models for BMI and risk of incident FI, we observed an increasing risk of FI as BMI category increased (Ptrend < 0.0001). This association persisted in model 2, which did not include covariates that are potential mediators of the relation between BMI and FI (physical activity, hypertension, and diabetes) (Ptrend < 0.0001) (Table 2). However, after additionally adjusting for these potential mediating variables, the association between increasing BMI category and risk of FI significantly attenuated (Ptrend = 0.71). This attenuation was primarily due to adjustment for physical activity, which may be an important confounding variable in addition to a potential mediator. To address the possibility that recent weight gain may be a more important factor in FI development than current BMI, we evaluated the association of weight gain over 2 years and subsequent risk of FI. Compared to women who lost or gained 2 kg from 2008 to 2012, the multivariable-adjusted hazard ratios for FI were 1.00 (95% CI 0.97–1.12) for women who gained 2.1–5 kg and 1.09 (95% CI 0.97–1.22) for women who gained ≥5 kg.
Because the association between physical activity and FI may vary according to BMI, we repeated the analysis within strata of BMI. Associations were similar across strata (comparing the lowest versus highest category of physical activity, multivariable-adjusted HRs were 0.75 for normal weight women, 0.76 for overweight women, and 0.76 for obese women) (Table 3).
Table 3.
Physical activity and risk of fecal incontinence according to strata of BMI
| MET hours/week category | ||||||
|---|---|---|---|---|---|---|
| <3 | 3–8 | 9–17 | 18–26 | 27+ | P trend a | |
| Normal weight (BMI 18.5–24.9 kg/m2) | ||||||
| Person-years of follow-up | 12,369 | 14,395 | 15,556 | 11,425 | 23,750 | |
| No. of cases | 599 | 565 | 490 | 348 | 660 | |
| Age-adjusted HR (95% CI) | 1.00 | 0.87 (0.77–0.97) | 0.75 (0.66–0.84) | 0.76 (0.66–0.87) | 0.72 (0.64–0.80) | <0.0001 |
| Multivariate-adjusted HR (95% CI)b | 1.00 | 0.87 (0.78–0.98) | 0.76 (0.67–0.89) | 0.78 (0.68–0.89) | 0.75 (0.66–0.84) | <0.0001 |
| Overweight (BMI 25–29.9 kg/m2) | ||||||
| Person-years of follow-up | 13,114 | 13,560 | 12,375 | 7962 | 14,296 | |
| No. of cases | 560 | 459 | 385 | 234 | 379 | |
| Age-adjusted HR (95% CI) | 1.00 | 0.84 (0.74–0.95) | 0.82 (0.71–0.93) | 0.79 (0.68–0.92) | 0.73 (0.64–0.84) | <0.0001 |
| Multivariate-adjusted HR (95% CI)b | 1.00 | 0.85 (0.75–0.96) | 0.84 (0.74–0.96) | 0.82 (0.70–0.96) | 0.76 (0.67–0.87) | 0.0006 |
| Obese (BMI ≥ 30 kg/m2) | ||||||
| Person-years of follow-up | 11,957 | 8531 | 7122 | 3755 | 5280 | |
| No. of cases | 520 | 301 | 206 | 88 | 160 | |
| Age-adjusted HR (95% CI) | 1.00 | 0.84 (0.73–0.97) | 0.70 (0.60–0.83) | 0.58 (0.46–0.72) | 0.74 (0.62–0.89) | <0.0001 |
| Multivariate-adjusted HR (95% CI)b | 1.00 | 0.84 (0.73–0.97) | 0.72 (0.61–0.84) | 0.61 (0.49–0.77) | 0.76 (0.64–0.91) | 0.0004 |
aLinear trend estimated by entering the medians of each METS hours/week quintile as a continuous variable in the model
bModels adjusted for age (months), race, smoking (never, past, current), BMI (kg/m2, continuous), menopausal hormone therapy use (never, past, current), parity (number of live births), hypertension (yes/no), diabetes mellitus (yes/no), neurologic disease (yes/no), and history of cholecystectomy (yes/no)
To address the possibility that high-impact activity places higher stress on the continence mechanism and thereby increases risk of FI, we conducted separate analyses of high- and low-impact physical activity in relation to risk of FI. Since relatively few women participated in high-impact activities, we categorized participants into two groups based on those above and below the group median for each type of activity. For high-impact activity, risk of FI was similar among women above and below the median (multivariable-adjusted HR 1.01, 95% CI 0.95–1.08) (Table 4). However, higher levels of low-impact activity were associated with lower risk of developing FI (multivariable-adjusted HR 0.84, 95% CI 0.79–0.88 comparing low-impact MET-h/wk above versus below the median).
Table 4.
Impact level of physical activity and risk of fecal incontinence
| MET hours/week categorya | ||
|---|---|---|
| Lowest | Highest | |
| High-impact activity b | ||
| Person-years of follow-up | 132,185 | 43,262 |
| No. of cases | 4652 | 1302 |
| Age-adjusted HR (95% CI) | 1.00 | 0.99 (0.93–01.06) |
| Multivariate-adjusted HR without mediators (95% CI)c | 1.00 | 1.00 (0.94–1.06) |
| Multivariate-adjusted HR (95% CI)d | 1.00 | 1.01 (0.95–1.08) |
| Low-impact activity b | ||
| Person-years of follow-up | 87,145 | 88,298 |
| No. of cases | 3420 | 2534 |
| Age-adjusted HR (95% CI) | 1.00 | 0.80 (0.76–0.84) |
| Multivariate-adjusted HR without mediators (95% CI)c | 1.00 | 0.81 (0.77–0.85) |
| Multivariate-adjusted HR (95% CI)d | 1.00 | 0.84 (0.79–0.88) |
High-impact activities sum of MET hours/week from high-impact activities (jogging, running, tennis, aerobics), low-impact activities all other MET hours/week that were not high-impact
aLowest category is below the 50th percentile (0 MET hours/week for high-impact activities, <10.1 MET hours/week for high impact activities), highest category above the 50th percentile (>0 METs/week for high-impact activities, >10 for low-impact activities)
bHigh-impact activity and low-impact activity are adjusted for each other in all models
cModel as below with potential mediators between physical activity and fecal incontinence (BMI, hypertension, diabetes mellitus) excluded
dModels adjusted for age (months), race, smoking (never, past, current), BMI (<18.5, 18.5–24.9, 25–29.9, 30–34.9, ≥35 kg/m2), menopausal hormone therapy use (never, past, current), parity (number of live births), hypertension (yes/no), diabetes mellitus (yes/no), neurologic disease (yes/no), and history of cholecystectomy (yes/no)
In secondary analyses, we examined whether the association of physical activity with risk of FI might differ according to the consistency of the stool. We found a similar lower relative risk of solid stool FI and liquid stool FI among women in the highest versus lowest category of physical activity. Compared to women in the lowest category of physical activity, multivariable-adjusted hazard ratios were 0.77 (95% CI 0.72–0.84, Ptrend < 0.0001) for solid stool FI and 0.76 (95% CI 0.70–0.82, Ptrend < 0.0001) for liquid stool FI among women with the highest physical activity levels (Supplementary Table 2).
Finally, we explored the possibility that the association we observed between higher physical activity and lower FI risk might be partially explained by reverse causation—i.e., some women with very early symptoms of FI may have limited their physical activity, leading to bias in the observed associations. Thus, we performed a lagged analysis using physical activity data collected on the 2004 questionnaire, 4 years prior to the start of the main analysis timeframe, and observed associations between physical activity and risk of incident FI after 2008 of similar direction and magnitude. For example, compared with women in the lowest category of physical activity, women in the highest category of physical activity had a multivariable-adjusted hazard ratio for FI of 0.78 (95% CI 0.72–0.85, Ptrend < 0.0001) (Supplementary Table 3).
Discussion
In this large prospective cohort of older women, higher levels of physical activity were associated with a modest reduction in risk of FI, which persisted even after adjustment for suspected mediators between physical activity and FI, including BMI, diabetes, and hypertension. We observed a 25% lower risk of FI among women who engaged in physical activity of 27+ MET-h/wk versus <3 MET-h/wk. The inverse relationship between physical activity level and FI risk FI was restricted to women engaged in high levels of low-impact physical activity and not those engaged in high levels of high-impact physical activity. Conversely, we found no association between higher BMI and FI risk after adjustment for physical activity.
Our finding of an inverse relation between physical activity and FI is consistent with previous, cross-sectional studies5,6,11 and an analysis in this cohort which found higher odds of FI among women with lower levels of physical activity (OR 1.46 comparing women in the lowest vs. highest quartiles of MET-h/week)4. However, in the same analysis higher BMI (≥27.5 kg/m2) was associated with an increased risk of FI, consistent with the cross-sectional findings of other groups as well7,18. Still others did not observe a consistent association between BMI and FI after multivariable adjustment5,19,20. In the setting of these conflicting data, our study provides an important contribution through a prospective analysis of a large cohort of older women with well-characterized data on potential confounders related to physical activity, BMI, and FI.
Although prospective assessments of the relationship between physical activity, BMI, and FI are lacking, there is some precedent examining the relationship between weight loss and risk of both FI and urinary incontinence. A randomized clinical trial among 338 overweight and obese women with urinary incontinence demonstrated that a behavioral intervention targeting weight loss resulted in reduced frequency of urinary incontinence episodes21 and a subgroup analysis among patients with baseline FI in this population demonstrated improved FI severity as well22. The weight loss intervention included a gradual increase in moderate physical activity (brisk walking or activities of similar intensity) until participants were active for at least 200 min per week. Taken together with our findings, this suggests that increased physical activity may be a more important factor in the prevention/improvement of FI than modifying one’s body weight.
Although our understanding of the pathogenesis of FI may be incomplete, our findings have biologic plausibility. Multiple studies have linked pelvic floor activation with concomitant activity of the abdominal musculature23–25 with pelvic floor activity known to be associated with lifting tasks, spinal stabilization26, and functional tasks such as head and shoulder raising as well23. This suggests that the pelvic floor musculature and the muscles of the rest of the body function as an integrated unit such that overall physical activity may benefit the pelvic floor as well. In that aging is associated with significant changes in the female pelvic floor, including decreased sphincter strength, perineal laxity, and reduced rectal sensation and compliance27, ongoing physical activity could mitigate the risk of FI among those at increased risk.
However, our data suggest that not all physical activity provides the same degree of protection against FI. Indeed, moderate, low-impact physical activity may be enough to reduce the risk of FI, an encouragement for preventative interventions among older adults. Conversely, women participating in higher amounts of high-impact physical activity had similar risk of FI to those with less high-impact physical activity. Thus, low-impact physical activity may result in strengthened pelvic floor musculature while high-impact activity may stress the pelvic floor and increase the likelihood of FI among women already at risk28. In a cross-sectional analysis of women less than 40 years old without conventional risk factors for FI, intensive exercise was associated with an almost threefold increased risk of anal incontinence (defined as incontinence of feces and flatus)9. Among studies examining risk factors for urinary incontinence, there is ample evidence of increased risk of urinary leakage among female athletes3. However, there is evidence, including data from this cohort, that moderate-intensity physical activity is associated with reduced risk of developing urinary incontinence in older women 29.
Our analysis has several notable strengths. Most importantly, as we conducted a prospective rather than cross-sectional analysis, we could assess the relationship between physical activity, BMI, and incident FI while minimizing potential bias due to reverse causality. Additionally, we were able to control for several potential confounding variables, including lifestyle and medical factors.
We acknowledge several limitations. As our study is observational, we cannot exclude the possibility of residual confounding; however, multivariable adjustment for many known risk factors for FI did not significantly alter our age-adjusted effect estimates, except for physical activity, which greatly attenuated the BMI-FI association. Additionally, data were not available on bowel disturbances such as diarrhea and constipation, important risk factors for FI30. However, we were able to separately consider incidence of solid versus liquid stool FI, with the latter a reasonable surrogate for chronic bowel disturbances. Finally, the Nurses’ Health Study represents a health-literate group of primarily white women, and thus our results may not be representative of the population at large. However, the incidence rate of FI in our cohort is consistent with other reports of FI in the US 31.
In summary, in this large prospective cohort of older women, higher physical activity was associated with a modestly decreased risk of FI, with high-impact activities appearing less protective against FI than low-impact activities. Whether these associations with new-onset FI imply a benefit to those with existing incontinence is unclear and merits further study. Additionally, further studies are needed on the mechanism by which increased low-impact physical activity provides-specific benefit to the pelvic floor continence mechanism. If these findings are coroborated by others, our study may inform lifestyle interventions among women with established non-modifiable risk factors for FI to modify the risk of disease or limit its progression among women with preexisiting incontinence.
Study Highlights
WHAT IS CURRENT KNOWLEDGE
Despite affecting an estimated 7–15% of women, knowledge about risk factors for fecal incontinence is limited.
Studies exploring the association between body mass index (BMI) and physical activity and risk of fecal incontinence have produced conflicting results.
WHAT IS NEW HERE
In a large, ongoing prospective cohort study, higher levels of physical activity were associated with lower risk of fecal incontinence. Higher BMI was not associated with an increased risk of fecal incontinence.
TRANSLATIONAL IMPACT
Ongoing physical activity may help maintain the neuromuscular health of the anorectal continence mechanism in aging women.
Higher BMI alone may not be an independent risk factor for fecal incontinence in older women.
Electronic supplementary material
Acknowledgements
We thank the participants and staff of the Nurses’ Health Study for their valuable contributions.
Financial support
The infrastructure of the Nurses’ Health Study cohort is supported by the NIH UM1 CA186107. K.S. is supported by an American Gastroenterological Association career development award. M.S. is supported by a grant from the American Association for Cancer Research. B.K. is supported by NIH 1U01DK112193. A.T.C. is supported by NIH DK098311.
Conflict of interest
Guarantor of the article: K.S.
Specific author contributions: K.S., F.G., A.T.C., and M.K.T. planned and designed the study; K.S., M.S., and M.K.T. analyzed the data; K.S. drafted the manuscript; all authors interpreted the results and contributed to critical review of the manuscript; K.S. had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. All authors approved the final manuscript.
Potential competing interests
K.S. has received clinical trial support from Astra-Zeneca, Gelesis, and Pathway Genomics. M.S., F.G., and M.K.T. report no disclosures. W.E.W. has received clinical trial support from Salix. C.A.M. has received research support from Boston Scientific and Pelvalon and has served as a consultant for Pelvalon. B.K. has received research funding from Astra-Zeneca, Gelesis, and Medtronic and served as a consultant to Medtronic and Ironwood. A.T.C. has served as a consultant to Bayer AG and Pfizer Inc.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information: The online version of this article (10.1038/s41424-018-0068-6) contains supplementary material, which is available to authorized users.
References
- 1.Rao Satish S.C., Bharucha Adil E., Chiarioni Giuseppe, Felt-Bersma Richelle, Knowles Charles, Malcolm Allison, Wald Arnold. Anorectal Disorders. Gastroenterology. 2016;150(6):1430-1442.e4. doi: 10.1053/j.gastro.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharucha AE, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am. J. Gastroenterol. 2015;110:127–136. doi: 10.1038/ajg.2014.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nygaard IE, Shaw JM. Physical activity and the pelvic floor. Am. J. Obstet. Gynecol. 2016;214:164–171. doi: 10.1016/j.ajog.2015.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townsend MK, Matthews CA, Whitehead WE, Grodstein F. Risk factors for fecal incontinence in older women. Am. J. Gastroenterol. 2013;108:113–119. doi: 10.1038/ajg.2012.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitehead WE, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512–517. doi: 10.1053/j.gastro.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rømmen Kathrine, Schei Berit, Rydning Astrid, H Sultan Abdul, Mørkved Siv. Prevalence of anal incontinence among Norwegian women: a cross-sectional study. BMJ Open. 2012;2(4):e001257. doi: 10.1136/bmjopen-2012-001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varma MG, et al. Fecal incontinence in females older than aged 40 years: who is at risk? Dis. Colon Rectum. 2006;49:841–851. doi: 10.1007/s10350-006-0535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markland AD, et al. Incidence and risk factors for fecal incontinence in black and white older adults: a population-based study. J. Am. Geriatr. Soc. 2010;58:1341–1346. doi: 10.1111/j.1532-5415.2010.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitton V, et al. Impact of high-level sport practice on anal incontinence in a healthy young female population. J. Women’s Health. 2011;20:757–763. doi: 10.1089/jwh.2010.2454. [DOI] [PubMed] [Google Scholar]
- 10.Bao Y, et al. Origin, methods, and evolution of the three Nurses’ Health Studies. Am. J. Public Health. 2016;106:1573–1581. doi: 10.2105/AJPH.2016.303338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson R, Norton N, Cautley E, Furner S. Community-based prevalence of anal incontinence. JAMA. 1995;274:559–561. doi: 10.1001/jama.1995.03530070057030. [DOI] [PubMed] [Google Scholar]
- 12.Ainsworth BE, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Wolf AM, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int. J. Epidemiol. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 14.Almeida MB, et al. Urinary incontinence and other pelvic floor dysfunctions in female athletes in Brazil: a cross-sectional study. Scand. J. Med. Sci. Sports. 2016;26:1109–1116. doi: 10.1111/sms.12546. [DOI] [PubMed] [Google Scholar]
- 15.Loprinzi PD, Rao SS. Association between fecal incontinence and objectively measured physical activity in U.S. adults. N. Am. J. Med. Sci. 2014;6:575–579. doi: 10.4103/1947-2714.145473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rimm EB, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Staller K, et al. Menopausal hormone therapy is associated with increased risk of fecal incontinence in women after menopause. Gastroenterology. 2017;152:1915–1921. doi: 10.1053/j.gastro.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bharucha AE, Zinsmeister AR, Schleck CD, Melton LJ., 3rd Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559–1566. doi: 10.1053/j.gastro.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melville JL, Fan MY, Newton K, Fenner D. Fecal incontinence in US women: a population-based study. Am. J. Obstet. Gynecol. 2005;193:2071–2076. doi: 10.1016/j.ajog.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Matthews CA, Whitehead WE, Townsend MK, Grodstein F. Risk factors for urinary, fecal, or dual incontinence in the Nurses’ Health Study. Obstet. Gynecol. 2013;122:539–545. doi: 10.1097/AOG.0b013e31829efbff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subak LL, et al. Weight loss to treat urinary incontinence in overweight and obese women. N. Engl. J. Med. 2009;360:481–490. doi: 10.1056/NEJMoa0806375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markland AD, et al. Weight loss improves fecal incontinence severity in overweight and obese women with urinary incontinence. Int. Urogynecol J. 2011;22:1151–1157. doi: 10.1007/s00192-011-1444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bo K, Stien R. Needle EMG registration of striated urethral wall and pelvic floor muscle activity patterns during cough, Valsalva, abdominal, hip adductor, and gluteal muscle contractions in nulliparous healthy females. Neurourol. Urodyn. 1994;13:35–41. doi: 10.1002/nau.1930130106. [DOI] [PubMed] [Google Scholar]
- 24.Sapsford RR, et al. Co-activation of the abdominal and pelvic floor muscles during voluntary exercises. Neurourol. Urodyn. 2001;20:31–42. doi: 10.1002/1520-6777(2001)20:1<31::AID-NAU5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 25.Neumann P, Gill V. Pelvic floor and abdominal muscle interaction: EMG activity and intra-abdominal pressure. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2002;13:125–132. doi: 10.1007/s001920200027. [DOI] [PubMed] [Google Scholar]
- 26.Hemborg B, et al. Intra-abdominal pressure and trunk muscle activity during lifting. III. Effect of abdominal muscle training in chronic low-back patients. Scand. J. Rehabil. Med. 1985;17:15–24. [PubMed] [Google Scholar]
- 27.Fox JC, et al. Effect of aging on anorectal and pelvic floor functions in females. Dis. Colon Rectum. 2006;49:1726–1735. doi: 10.1007/s10350-006-0657-4. [DOI] [PubMed] [Google Scholar]
- 28.Bo K. Urinary incontinence, pelvic floor dysfunction, exercise and sport. Sports Med. 2004;34:451–464. doi: 10.2165/00007256-200434070-00004. [DOI] [PubMed] [Google Scholar]
- 29.Danforth KN, et al. Physical activity and urinary incontinence among healthy, older women. Obstet. Gynecol. 2007;109:721–727. doi: 10.1097/01.AOG.0000255973.92450.24. [DOI] [PubMed] [Google Scholar]
- 30.Whitehead WE, et al. Treatment of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases workshop. Am. J. Gastroenterol. 2015;110:138–146. doi: 10.1038/ajg.2014.303. [DOI] [PubMed] [Google Scholar]
- 31.Rey E, et al. Onset and risk factors for fecal incontinence in a US community. Am. J. Gastroenterol. 2010;105:412–419. doi: 10.1038/ajg.2009.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.