Abstract
Purpose
This study aimed to report on our institutional experience in the use of stereotactic body radiation therapy (SBRT) for the treatment of adrenal gland metastases. Specifically, we examined the outcomes and toxicity from this treatment modality on adjacent organs at risk.
Methods and Materials
Data were retrieved from patients with adrenal metastases who were treated with SBRT between 2008 and 2017. Patients with primary adrenal malignancies were excluded. Toxicities were graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. Time-to-event rates were calculated from the date of SBRT delivery.
Results
In total, 35 patients with adrenal metastases were identified. Four patients were treated for bilateral disease. The median dose was 40 Gy (range, 20-54 Gy) in 5 fractions (range, 1-6 fractions). The median follow-up time was 37 months (range, 14-451 months) from disease diagnosis and 7 months (range, 1-54 months) from the SBRT start date. With death treated as a competing risk event, the cumulative incidence of local failure was 7.6% at 1 year after SBRT and 19.2% at 3 years. The median overall survival (OS) time was 19 months (95% confidence interval, 8-54 months) and tumor size correlated with survival (P = .0006). Patients with metastases <2.9 cm had a median OS of 54 months compared with 11 months for those with adrenal metastases ≥2.9 cm (P = .01). Incidence of grade 2 toxicity was 17% with no case of grade ≥3 toxicity. SBRT did not impact renal function with a mean estimated decline in glomerular filtration rate of only 2.6 ± 8 mL/min/1.73 m2 compared with baseline. Combined kidneys V5 and combined renal cortex V17.5 did not correlate with a change in estimated glomerular filtration rate (P = .7 and P = .9, respectively).
Conclusions
SBRT offers excellent local control for the treatment of adrenal gland metastases with very low toxicity rates and no significant short-term impact on renal function.
Summary.
With the increased use of imaging for cancer diagnosis and surveillance, occult adrenal metastases are more frequently identified and many of these patients have a limited number of metastases (oligometastatic disease). Stereotactic body radiation therapy has emerged as a promising noninvasive alternative for the treatment of adrenal metastases. Using one of the largest institutional series, we demonstrated that stereotactic body radiation therapy provides excellent local control and very low toxicity rates.
Alt-text: Unlabelled box
Introduction
The recognition of an oligometastatic state between local-regional and widely metastatic disease has led to an increasing role of radical-intent definitive treatment for patients who were previously regarded as incurable, particularly as more effective systemic therapies are discovered.1, 2 Data have shown that selected patients with a low burden of metastatic disease may show long-term survival when treated with an aggressive therapy to all sites of disease and especially those with a long disease-free interval between the time of presentation of the primary tumor and the development of metastases.3, 4, 5
The adrenal gland is a common site for the metastatic spread of many types of solid malignant neoplasm, and adrenal metastases are identified during post-mortem examinations in up to 38% of patients with cancer. These metastases are commonly asymptomatic and present as incidental imaging findings.6, 7 The most common primary etiologies are non-small cell lung cancer, melanoma, and gastrointestinal tumors and often present synchronously with metastases to other sites.6 The standardization of treatment follow-up and improved imaging techniques have led to an increasing number of diagnoses of adrenal metastases, especially with the advent of positron emission tomography/computed tomography (PET/CT) scans, which show excellent sensitivity and specificity to differentiate malignant from benign adrenal tumors.8, 9, 10, 11
For clinically appropriate, oligometastatic patients with adrenal metastases, open or laparoscopic adrenalectomy remains the standard of care and offers long-term (≥5 years) overall survival (OS) rates that range between 22% and 45%.12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Percutaneous ablative procedures such as microwave and radiofrequency ablation are also frequently used, and several retrospective series have reported 3-year OS rates >50%.24, 25, 26, 27, 28, 29, 30, 31, 32 A noninvasive, ablative modality called stereotactic body radiation therapy (SBRT; also referred to as stereotactic ablative radiation therapy) has emerged as a promising alternative option for the local treatment of oligometastatic disease.33, 34, 35 Preliminary experience with the use of SBRT to treat adrenal metastases demonstrates low toxicity rates with local disease control that ranges between 55% and 95% at 1 year.36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 There have been no prospective studies to compare the efficacy of SBRT to adrenalectomy or ablative therapies for the treatment of adrenal metastases.
In this study, we aim to report our institutional experience with SBRT to treat adrenal metastases and examine the short-term impact of this treatment modality on renal function.
Methods and materials
Data collection and study population
This study is a retrospective, single-institution analysis of oligometastatic patients who were treated for adrenal metastases with SBRT between 2008 and 2017. Demographic, pathologic, radiologic, and treatment-related information were retrieved from electronic medical records after approval by an institutional review board. Patients were excluded from this analysis if they were treated for a primary malignancy of the adrenal gland or if follow-up was unavailable or inadequately documented.
Stereotactic body radiation therapy treatments
Treatment simulation was performed on a dedicated PET/CT simulator that encompassed a 4-dimensional CT scan along with a dual-phase intravenous contrast-enhanced CT and PET image. The gross tumor volume (GTV) was delineated using arterial or venous-phase CT scans with the assistance of the PET/CT images. Four-dimensional CT was used to create an internal target volume to encompass respiratory motion. The planning target volume (PTV) included the GTV or internal target volume plus a 2- to 3-mm expansion.49, 50 All doses were prescribed to the 95% isodose line. Total dose and fractionation were determined at the attending physician's discretion and dependent on the adjacent normal tissue tolerance as determined by an institutional protocol. For a 5-fraction regimen, the dosimetric liver constraints were as follows: 1) spare ≥700 cm3 of normal liver from receiving ≥15 Gy; 2) spare ≥500 cm3 of normal liver from receiving ≥7 Gy; and 3) limit the mean dose to the liver to ≤10 Gy. A V5 <50% of the combined kidneys was recommended. The spinal cord was limited to a maximum dose of 12 Gy. For the bowel, the maximum dose was limited to 40 Gy with V25 <9 cm3, V30 <5 cm3, and V35 <1 cm3.
Due to concerns about accurate targeting and experience gained from pancreatic tumor treatment, respiratory motion management was used whenever feasible.51 Typically, this technique involved the implantation of a fiducial markers for target localization. Fiducial marker placement was done under CT guidance by an interventional radiologist. If the placement of fiducials seeds was not technically feasible, a free-breathing, volume-inclusive PTV as defined by 4-dimensional CT was used. Cone beam CT and kV imaging was used for image-guidance during treatment.
Toxicity assessment and disease progression
Patients were followed at 3- to 6-month intervals with clinic visits for a physical examination and toxicity assessment. Toxicities were graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.52 The highest toxicity grade experienced by each patient during or after treatment was recorded. The main concerns with regard to toxicity were acute or late renal function decline and bowel toxicity. The kidneys were analyzed combined for whole kidney and renal cortex toxicity as well as individually for renal hilum toxicity. For whole kidney and renal cortex toxicity, the endpoint was decline of renal function which was defined as a worsening of the estimated glomerular filtration rate (eGFR) whereas for hilum toxicity, the toxicity event was an episode of malignant hypertension.53
The eGFR was calculated with the Chronic Kidney Disease-Epidemiology Collaboration equation (eGFR = 141 x min[SCr/κ, 1]α × max[SCr /κ, 1]−1.209 × 0.993Age × 1.018 [if female] × 1.159 [if Black]) and assessed at baseline as well as 3 and 6 months after SBRT.54 The lowest post-SBRT eGFR (nadir) was used to compare with baseline eGFR. The analysis of bowel toxicity was based on the contouring of the stomach, duodenum, and individual intestinal loops. Organ-at-risk dose-volume histogram data were exported from the treatment planning software for statistical analysis.
Radiation doses delivered by other than a 5-fraction SBRT regimen were converted to a 5-fraction iso-effective dose using the linear quadratic model (d2 x n2/d1 x n1 = [d1 + α/β1]/[d2 + α/β2]) with an α/β = 2 for renal and α/β = 3 for bowel toxicity assessment.55, 56 Institutional protocol dosimetric constraints were used for the correlation with treatment toxicity. Accumulated doses took into consideration the radiation doses that were delivered to the abdominal organs at risk from prior treatments within the last 24 months prior to SBRT. The choice to encompass prior courses within the past 24 months was mostly empirical but influenced by evidence that suggested that most of the occult injury induced by a certain dose of radiation is repaired within the first year, with additional recovery between 1 and 3 years from irradiation and dependent on the total prior radiation dose received.57, 58 Patients were censored for toxicity assessment at the time of disease progression.
Disease progression was assessed by CT imaging that was performed every 3 months in the first year after treatment in accordance with the Response Evaluation Criteria In Solid Tumors version 1.1 or by 18F-Fluorodeoxyglucose (FDG)-PET/CT using the PET Response Evaluation Criteria In Solid Tumors version 1.0.59, 60
Statistical analysis
Demographic, clinical, and treatment-related characteristics were summarized using means, medians, ranges, and standard deviations as appropriate. Treatment-related toxicities were coded and analyzed as categorical variables. Time-to-event outcomes were summarized using Kaplan-Meier curves and medians with 95% confidence intervals (CIs) that were calculated using Greenwood's formula. Binary outcomes were analyzed in logistical regression models. Proportions were tested using χ2 or Fisher's exact tests. Cumulative incidences of local and distant progression were estimated with death treated as a competing risk event. All tests were 2-sided with an alpha level of 0.05 and performed using the statistical software SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient and treatment characteristics
In total, 35 patients with adrenal metastases were identified. Four patients were treated for bilateral disease with a total of 39 adrenal gland metastases included in this analysis. Most of the metastases originated from non-small cell lung cancer (48%), followed by hepatocellular carcinoma (20%) and other tumors of the gastrointestinal tract (9%). For 28 patients (80%), adrenal metastases were considered metachronous (ie, identified beyond 3 months after the primary cancer diagnosis) with a median interval time of 24 months (range, 2-407 months) between the primary and adrenal metastasis diagnoses. All metastases were FDG-avid on PET/CT and the median largest dimension was 2.9 cm (range, 0.7-9 cm).
SBRT was delivered with a volumetric modulated arc therapy technique using 2 coplanar arcs in 35 metastases (90%) and in the remaining 4 metastases (10%) through coplanar or noncoplanar static intensity modulated radiation therapy fields. For 27 metastases (69%), a 5-fraction SBRT regimen was used with a median total dose of 40 Gy (range, 30-50 Gy). Four patients were treated with a single fraction of 25 Gy. The dose conformality to the target was high with a median conformity index of 1 (range, 0.9-1.2) and a median R50% of 4 (range, 3.1-9.1), which represents the intermediate dose spillage outside the PTV (ratio of the volume of the 50% isodose line/PTV volume). As per institutional practice, we limited the SBRT dose gradient inside the PTV to 130% for abdominal organs. In this study, the median homogeneity was 116% (range, 103-133%). No patient received concurrent chemotherapy or targeted therapy while treated with SBRT. Additional patient and treatment characteristics are detailed in Table 1.
Table 1.
Patient and treatment characteristics
| Characteristic | No. (%) or Median [range] |
|---|---|
| No. of patients | 35 |
| Age, years | 66 [45-85] |
| Sex, male | 21 (60) |
| Karnofsky performance status score ≥80% | 28 (80) |
| Baseline eGFR, mL/min/1.73 m2 | 78.9 ± 19.2a |
| Metastasis origin: | |
| Non-small cell lung cancer | 17 (48) |
| Hepatocellular carcinoma | 7 (20) |
| Gastrointestinal tract | 3 (9) |
| Renal cell carcinoma | 2 (6) |
| Other | 6 (17) |
| Primary tumor histology: | |
| Adenocarcinoma | 18 (51) |
| Hepatocellular carcinoma | 7 (20) |
| Squamous cell carcinoma | 4 (12) |
| Other | 6 (17) |
| Controlled/absent primary, yes | 20 (57) |
| Other sites of metastases: | |
| None | 10 (29) |
| 1 site | 11 (31) |
| >1 site | 14 (40) |
| Adrenal tumor diagnosis: | |
| Synchronous | 7 (20) |
| Metachronous | 28 (80) |
| Adrenal metastases location: | |
| Left | 17 (48) |
| Right | 14 (40) |
| Bilateral | 4 (12) |
| Adrenal metastases size, cm: | 2.9 [0.7-9] |
| Prior local therapy, yes | 3 (8) |
| Prior radiation therapy to abdomen, yes | 9 (26) |
| Stereotactic body radiation therapy treatment: | |
| Total dose, Gy | 40 [20-54] |
| No. of fractions | 5 [1-6] |
| Total BED10, Gy | 72 [30-124.8] |
| Planning technique: | |
| Volumetric modulated arc therapy | 35 (90) |
| Intensity modulated radiation therapy | 4 (10) |
| Implanted fiducials, yes | 16 (41) |
| Gross tumor volume, cm3 | 19 [1.3-213.2] |
| Planning target volume, cm3 | 50.5 [7.9-352.9] |
| Mean dose, Gy | 42.3 [21.4-57.3] |
| Maximum dose, Gy | 46.5 [23.3-61.9] |
| Minimum dose, Gy | 31.2 [17.3-47.3] |
| Conformity index | 1 [0.9-1.2] |
| R50% | 4 [3.1-9.1] |
| Homogeneity, % | 116 [103-133] |
eGFR, estimated glomerular filtration rate; BED10, biologically effective dose calculated with a α/β of 10; R50%, ratio of the 50% prescription isodose volume to PTV volume.
Mean ± standard deviation.
Survival outcomes
The median follow-up time was 37 months (range, 14-451 months) from the time of disease diagnosis and 7 months (range, 1-54 months) from the SBRT start date. Among the 39 adrenal metastases that were treated with SBRT, 3 metastases (7.6%) developed evidence of local recurrence. The first patient received 40 Gy in 5 fractions to a right-sided, 9 cm, lung squamous cell carcinoma metastasis, which recurred 6 months after SBRT. The second patient received a similar dose and fractionation to a 3.8 cm hepatocellular carcinoma metastasis, which recurred within 6 months. The third patient had a 2.2 cm lung adenocarcinoma metastasis treated with 45 Gy in 5 fractions, which recurred 3 years and 7 months after treatment. All 3 metastases were treated with motion management and target localization using implanted fiducial seeds.
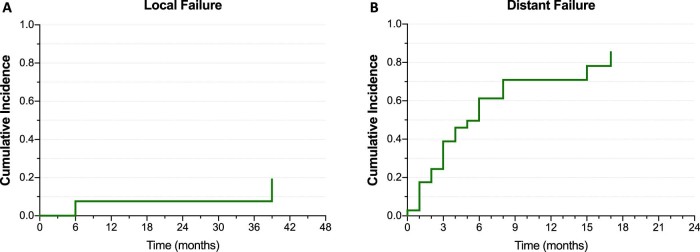
With death treated as a competing risk event, the cumulative incidence of local failure was 7.6% at 1 year after SBRT and 19.2% at 3 years. Primary tumor origin, metastasis size, GTV, SBRT doses, and motion management using fiducial seeds were tested for a correlation with local failure rates, but the analysis was limited by the small number of events and no significant correlation was found. After SBRT, 22 patients (63%) presented distant failure to sites other than the treated adrenal gland with a cumulative incidence of distant failure at 1 year of 71% (Fig 1).
Figure 1.
Cumulative incidences of (A) local failure and (B) distant failure.
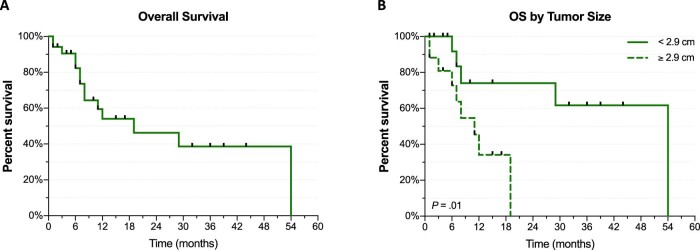
The median OS for the entire population from the date of SBRT treatment was 19 months (95% CI, 8-54 months). Tumor size was found to correlate with OS time (hazard ratio [HR]: 1.546; 95% CI, 1.206-1.982; P = .0006). When dichotomized by median size, patients with metastases <2.9 cm had a median OS of 54 months (95% CI, 7-54 months) compared with 11 months (95% CI, 6-19 months) for patients with adrenal metastases ≥2.9 cm (P = .01). Similarly, GTV also correlated with OS (HR: 1.014; 95% CI, 1.006-1.022; P = .001). Primary tumor origin did not have a significant impact on OS in this series (Fig 2).
Figure 2.
Kaplan-Meier curves of overall survival (A) for entire population and (B) by adrenal metastasis size.
Other clinical characteristics were tested for a correlation with OS such as sex, age, performance status, control of primary tumor site, number of metastatic sites, synchronous versus metachronous metastases, SBRT total dose, and tumor laterality but none of these characteristics demonstrated a statistically significant correlation.
Treatment toxicity
Overall, SBRT treatments were well tolerated with only 6 cases (17%) of acute or late grade 2 toxicity including acute grade 2 nausea (n = 3), grade 2 diarrhea (n = 1), and grade 2 fatigue (n = 2). No case of acute or late grade 3 or higher toxicity was observed. Metastasis size, GTV, SBRT doses, R50%, and fiducial seeds usage had no correlation with grades 1 and 2 toxicity occurrence. Among the 4 cases that were treated for bilateral disease, one was treated due to bilateral tumor recurrence after right and left adrenalectomy. This was the only patient with laboratorial evidence of adrenal insufficiency at the time of follow-up.
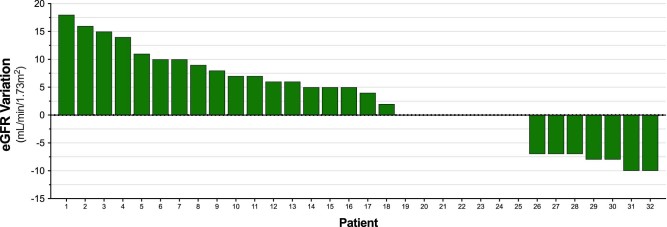
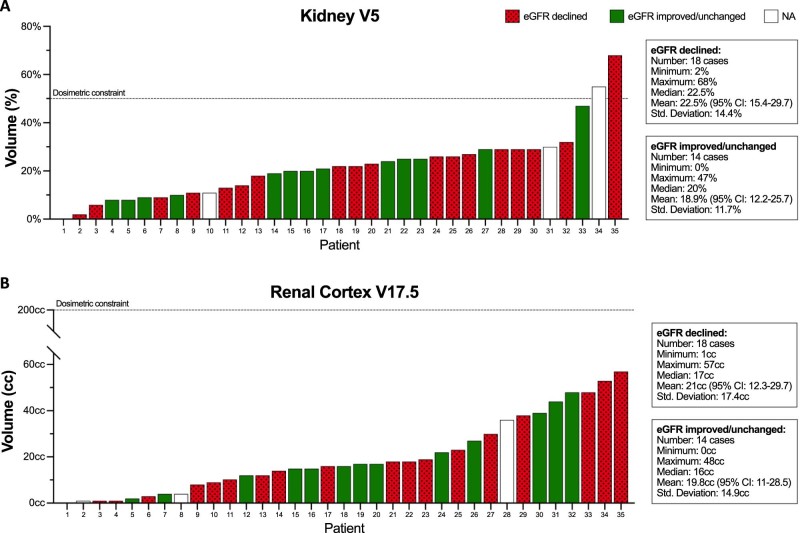
After SBRT, 18 patients (51%) presented an eGFR nadir below the baseline level with a mean drop of 9 ± 4 ml/min/1.73 m2 (range, 2-18 ml/min/1.73 m2) for this group. Seven patients (20%) had no change and 7 patients (20%) had an increase in eGFR after irradiation. The mean decline in eGFR for the entire patient population after SBRT was 2.6 ± 8 ml/min/1.73 m2. For 3 patients, post-SBRT eGFR was not available (Fig 3). Due to the small sample size, we opted to use established constraints for the dosimetric correlation with eGFR change. Only 2 patients (5.7%) had a combined kidney V5 above the dose constraint limit of 50% and no correlation with eGFR change was noted (P = .7). The combined renal cortex was also tested using the V17.5 as a reference and there was no statistically significant correlation with eGFR change (P = .9). Because no patient developed malignant hypertension after SBRT, no test for a correlation of renal hilum V23 was possible (Fig 4). Median serum creatinine did not change after SBRT with a median of 0.9 mg/dL both at baseline and post-treatment.
Figure 3.
Post-treatment variation of estimated glomerular filtration rate by patient. For 3 patients, post-stereotactic body radiation therapy estimated glomerular filtration rate was not available. Values above zero represent of decline of estimated filtration rate.
Figure 4.
Individual patient volumes of (A) combined kidneys V5 (%) and (B) combined renal cortex V17.5 (cm3). eGFR, estimated glomerular filtration rate. NA, not available.
Radiation doses to the adjacent bowel were analyzed against the incidence of acute and late grades 1 and 2 gastrointestinal toxicities but the bowel V25 (P = .4), V30 (P = .6), and V35 (P = .8) were all not correlated with gastrointestinal toxicity rates (Suppl. Fig S1; available as supplementary material online only at www.practical.radonc.org).
Discussion
Over the last 2 decades, evidence has emerged that demonstrates that a low volume of metastatic disease (i.e., oligometastatic state) may predict long-term survival when all sites of active disease are effectively treated.3, 4 Adrenal metastases are commonly part of this scenario and different treatment modalities have been employed to target adrenal gland metastases. Adrenalectomy is currently the standard of care but many patients are unable to undergo surgery due to poor performance or severe comorbidities. Moreover, in a pooled analysis of 7829 patients who were treated with adrenalectomies, the risk of surgical complications after unilateral and bilateral adrenalectomy was 15% and 23.4%, respectively, and patients who were treated for a malignant tumor showed a higher risk of complication (23% vs. 13%; P < .0001).61
Percutaneous ablative therapies such as radiofrequency, microwave, and cryoablation have also been used to treat adrenal metastatic tumors with local control rates for the primary procedure that range between 67% and 88% at 1 year.27, 32 Catecholamine surges with consequent blood pressure elevation and post-procedure pain are common with ablative procedures.24, 29
In a study of 64 adrenal metastases that were percutaneously ablated, 43% presented with hypertensive crisis (ie, acute increase in systolic blood pressure >180 mmHg or diastolic blood pressure >110 mmHg) despite the use of α-adrenergic blockade before the procedure in 14 metastases (19%). One case of ventricular tachycardia was reported, 2 cases of atrial fibrillation, and 4 cases of troponin elevation, which implies cardiac injury.62 Interestingly, no case of hypertensive crisis occurred in tumors that were previously irradiated. In another study of 58 patients with 60 adrenal metastases that were treated with thermal ablation at the Mayo Clinic (Rochester, MN), hypertensive crisis occurred in 47% of patients despite the use of α-adrenergic blockades before the procedure. Furthermore, 76% of all patients needed intravenous anti-hypertensives or vasopressors to maintain adequate blood pressure during the ablative procedure.63
SBRT is a noninvasive alternative treatment modality for adrenal metastases that has shown very low toxicity rates with grade 2 toxicity that ranges from 0% to 15%. Furthermore, due to its biologic mechanism of action, SBRT is not associated with catecholamine surges and able to preserve adrenal function in some cases of bilaterally treated adrenal tumors.64 Beyond showing low toxicity, SBRT has demonstrated equivalent or superior local control rates compared with percutaneous ablative therapies in many studies.37, 38, 39, 41, 42, 44, 46 In a series of thermal ablation of adrenal metastases that included 71 patients, 23 treatments failed at 1 year when considering only the primary ablative procedure, which resulted in a local control of 67.6%.32 A comparison of outcomes between image guided ablation and SBRT studies can be seen in Table 2.
Table 2.
Studies of percutaneous ablation and stereotactic body radiation therapy for the treatment of adrenal gland metastases
| Ablation Studies | Year | No. | Study design | Treatment technique | Local control | Toxicity |
|---|---|---|---|---|---|---|
| Mayo-Smith et al.24 | 2004 | 10 | Retrospective | Radiofrequency ablation | 84.6% | NR by grade |
| Xiao et al.25 | 2008 | 14 | Retrospective | Chemical ablation | NR | NR by grade |
| Wolf et al.26 | 2012 | 19 | Retrospective | Radiofrequency or microwave ablation | 85% | G2 10% G4 5% |
| Welch et al.27 | 2014 | 32 | Retrospective | Radiofrequency or cryoablation ablation | 1 y 88% | G3/4 8.6% |
| Hasegawa et al.28 | 2015 | 35 | Retrospective | Radiofrequency ablation | 1 y 70.5% 3y 56.4% |
G2 44% G3 6.3% |
| Men et al.29 | 2016 | 31 | Retrospective | Microwave ablation | 77.4% | NR by grade |
| Ren et al.30 | 2016 | 20 | Retrospective | Microwave ablation | 84.8% | None by SIR |
| Frenk et al.31 | 2017 | 38 | Retrospective | Radiofrequency, cryoablation, or microwave ablation | 1 y 82% 2 y 75% |
Minor: 6% Major: 12% |
| Botsa et al.32 | 2017 | 71 | Retrospective | Radiofrequency or Microwave ablation | 1 y 67.6% | Major: 0% |
| Stereotactic body radiation therapy studies | ||||||
| Chawla et al.36 | 2009 | 30 | Retrospective | 40 Gy/10 fractions | 1 y 55% 2 y 27% |
G2 0% |
| Oshiro et al.37 | 2011 | 19 | Retrospective | 45 Gy/10 fractions | 79% | G2 2% |
| Holy et al.38 | 2011 | 18 | Retrospective | 36 Gy/5 fractions | 83% | G2 5% |
| Casamassima et al.39 | 2012 | 48 | Retrospective | 36 Gy/3 fractions | 1 y 90% 2 y 90% |
G2 2% |
| Scorsetti et al.40 | 2012 | 34 | Retrospective | 32 Gy/4 fractions | 1 y 66% 2 y 32% |
G2 6% |
| Ahmed et al.41 | 2013 | 13 | Retrospective | 45 Gy/5 fractions | 100% | G2 15% |
| Li et al.42 | 2013 | 18 | Retrospective | 45 Gy/5 fractions | 77% | G3 23% |
| Rudra et al.43 | 2013 | 10 | Retrospective | 36 Gy/3 fractions | 1 y 73% | G1/2 80% |
| Gamsiz et al.44 | 2015 | 15 | Retrospective | 30 Gy/3 fractions | 86.7% | G2 0% |
| Franzese et al.45 | 2017 | 46 | Retrospective | 40 Gy/4 fractions | 1 y 65.5% 2 y 40.7% |
G1 10.9% G2 2.2% |
| Haidenberger et al.46 | 2017 | 23 | Retrospective | 22 Gy/1 fractions | 1 y 95% 2 y 81% |
G1/2 21.7% |
| Celik et al.47 | 2017 | 15 | Retrospective | 42 Gy/6 fractions | 1 y 60% 2 y 46% |
G1/2 33% |
| Chance et al.48 | 2017 | 43 | Retrospective | 60 Gy/10 fractions | 1 y 74% | G1/2 23.2% |
| Current series | 2017 | 35 | Retrospective | 40 Gy/5 fractions | 1 y 94.3% | G1 45% G2 17% |
G, grade; y, year; NR, not reported.
All local tumor control rates from the primary procedure (without retreatment). Stereotactic body radiation therapy regimens were given as median dose and number of fractions. Local control rates given at the time of the last follow-up visit unless stated otherwise.
The local control rate at 1 year in our series was 94.3%, which compares favorably with the results from recently published SBRT studies.44, 45, 47, 48 In a retrospective analysis from the MD Anderson Cancer Center of 49 adrenal metastases that were treated with hypofractionated radiation therapy with a median of 10 fractions, the 1-year local control was 74% and no case of grade ≥3 toxicity was observed. In their series, 3 of 6 cases that were treated for bilateral disease presented with adrenal insufficiency at 6 weeks, 4 months, and 7 months after treatment.48 In our series, no acute or late impairment of adrenal function was observed that could be attributed to SBRT.
Retrospective studies have reported renal functional compromise after SBRT that was directed to the kidneys and pancreatic tumors, but to our knowledge, this is the first study to address renal function decline after SBRT that targets the adrenal glands.65, 66, 67, 68 Siva et al. analyzed 21 patients with renal cell carcinoma who were treated with SBRT and assessed the eGFR through 51Cr-EDTA and 99mTc-DMSA SPECT/CT images.65 The researchers reported a mean eGFR drop of 3.2 ± 14.5 ml/min/1.73 m2 at 3 months and identified a dose-response relationship between eGFR change and SBRT doses.65 In the current series, a mean drop of 2.6 ± 8 ml/min/1.73 m2 was observed at 6 months post-SBRT, which is insignificant when considering that eGFR reference ranges are between 90 ml/min/1.73 m2 and 120 ml/min/1.73 m2 and that the physiologic variation of a patient's serum creatinine between different measurements is approximately 6%.69
This study presents some limitations including its retrospective design, single-institutional analysis, small sample size, and short median follow-up time. Also, the renal function assessment was based on clinical parameters that have considerable inter and intraindividual variability. Moreover, patients with cancer are particularly prone to fluctuations of renal function due to changes in hydration status and the use of several nephrotoxic drugs.
Conclusions
This study demonstrates that treatment of adrenal metastases with SBRT is associated with excellent local control and very low toxicity rates, which is an interesting alternative to image guided ablative procedures. Furthermore, our analysis suggests that adrenal SBRT has minimal, if any, significant impact on renal function. Further studies with a larger patient population are needed to confirm these findings.
Footnotes
Sources of support: Research support was provided by the My Blue Dots Fund.
Conflicts of interest: Dr. Diego A. S. Toesca received a research grant from Varian Medical Systems, Inc. Dr. Daniel T. Chang owns stocks in ViewRay, Inc. and received honoraria from Varian Medical Systems, Inc. The other authors declare no conflicts of interest.
Supplementary material for this article (https://doi.org/10.1016/j.adro.2018.05.006) can be found at www.practicalradonc.org.
Supplementary data
The following is the supplementary data to this article:
Individual patient volumes of (A) bowel V25 (cm3), (B) bowel V30 (cm3), and (C) bowel V35 (cm3).
References
- 1.Hellman S. Karnofsky Memorial lecture. Natural history of small breast cancers. J Clin Oncol. 1994;12:2229–2234. doi: 10.1200/JCO.1994.12.10.2229. [DOI] [PubMed] [Google Scholar]
- 2.Hellman S., Weichselbaum R.R. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Pastorino U., Buyse M., Friedel G. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 4.Wagner J.S., Adson M.A., Van Heerden J.A., Adson M.H., Ilstrup D.M. The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann Surg. 1984;199:502–508. doi: 10.1097/00000658-198405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordlinger B., Guiguet M., Vaillant J.C. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 6.Lam K.Y., Lo C.Y. Metastatic tumours of the adrenal glands: A 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 2002;56:95–101. doi: 10.1046/j.0300-0664.2001.01435.x. [DOI] [PubMed] [Google Scholar]
- 7.Abrams H.L., Spiro R., Goldstein N. Metastases in carcinoma: Analysis of 1000 autopsied cases. Cancer. 1950;3:74–85. doi: 10.1002/1097-0142(1950)3:1<74::aid-cncr2820030111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Yun M., Kim W., Alnafisi N., Lacorte L., Jang S., Alavi A. 18F-FDG PET in characterizing adrenal lesions detected on CT or MRI. J Nucl Med. 2001;42:1795–1799. [PubMed] [Google Scholar]
- 9.Kumar R., Xiu Y., Yu J.Q. 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med. 2004;45:2058–2062. [PubMed] [Google Scholar]
- 10.Ozcan Kara P., Kara T., Kara Gedik G. The role of fluorodeoxyglucose-positron emission tomography/computed tomography in differentiating between benign and malignant adrenal lesions. Nucl Med Commun. 2011;32:106–112. doi: 10.1097/MNM.0b013e32834199e7. [DOI] [PubMed] [Google Scholar]
- 11.Ardito A., Massaglia C., Pelosi E. 18F-FDG PET/CT in the post-operative monitoring of patients with adrenocortical carcinoma. Eur J Endocrinol. 2015;173:749–756. doi: 10.1530/EJE-15-0707. [DOI] [PubMed] [Google Scholar]
- 12.Lo C.Y., van Heerden J.A., Soreide J.A. Adrenalectomy for metastatic disease to the adrenal glands. Br J Surg. 1996;83:528–531. doi: 10.1002/bjs.1800830432. [DOI] [PubMed] [Google Scholar]
- 13.Wade T.P., Longo W.E., Virgo K.S., Johnson F.E. A comparison of adrenalectomy with other resections for metastatic cancers. Am J Surg. 1998;175:183–186. doi: 10.1016/s0002-9610(97)00281-x. [DOI] [PubMed] [Google Scholar]
- 14.Haigh P.I., Essner R., Wardlaw J.C., Stern S.L., Morton D.L. Long-term survival after complete resection of melanoma metastatic to the adrenal gland. Ann Surg Oncol. 1999;6:633–639. doi: 10.1007/s10434-999-0633-z. [DOI] [PubMed] [Google Scholar]
- 15.Porte H., Siat J., Guibert B. Resection of adrenal metastases from non-small cell lung cancer: A multicenter study. Ann Thorac Surg. 2001;71:981–985. doi: 10.1016/s0003-4975(00)02509-1. [DOI] [PubMed] [Google Scholar]
- 16.Mercier O., Fadel E., de Perrot M. Surgical treatment of solitary adrenal metastasis from non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;130:136–140. doi: 10.1016/j.jtcvs.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Strong V.E., D'Angelica M., Tang L. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol. 2007;14:3392–3400. doi: 10.1245/s10434-007-9520-7. [DOI] [PubMed] [Google Scholar]
- 18.Marangos I.P., Kazaryan A.M., Rosseland A.R. Should we use laparoscopic adrenalectomy for metastases? Scandinavian multicenter study. J Surg Oncol. 2009;100:43–47. doi: 10.1002/jso.21293. [DOI] [PubMed] [Google Scholar]
- 19.Muth A., Persson F., Jansson S., Johanson V., Ahlman H., Wängberg B. Prognostic factors for survival after surgery for adrenal metastasis. Eur J Surg Oncol. 2010;36:699–704. doi: 10.1016/j.ejso.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Vazquez B.J., Richards M.L., Lohse C.M. Adrenalectomy improves outcomes of selected patients with metastatic carcinoma. World J Surg. 2012;36:1400–1405. doi: 10.1007/s00268-012-1506-3. [DOI] [PubMed] [Google Scholar]
- 21.Zerrweck C., Caiazzo R., Clerquin B. Renal origin and size are independent predictors of survival after surgery for adrenal metastasis. Ann Surg Oncol. 2012;19:3621–3626. doi: 10.1245/s10434-012-2464-6. [DOI] [PubMed] [Google Scholar]
- 22.Howell G.M., Carty S.E., Armstrong M.J. Outcome and prognostic factors after adrenalectomy for patients with distant adrenal metastasis. Ann Surg Oncol. 2013;20:3491–3496. doi: 10.1245/s10434-013-3050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno P., de la Quintana Basarrate A., Musholt T.J. Adrenalectomy for solid tumor metastases: Results of a multicenter European study. Surgery. 2013;154:1215–1222. doi: 10.1016/j.surg.2013.06.021. discussion 22-23. [DOI] [PubMed] [Google Scholar]
- 24.Mayo-Smith W.W., Dupuy D.E. Adrenal neoplasms: CT-guided radiofrequency ablation–preliminary results. Radiology. 2004;231:225–230. doi: 10.1148/radiol.2311031007. [DOI] [PubMed] [Google Scholar]
- 25.Xiao Y.Y., Tian J.L., Li J.K., Yang L., Zhang J.S. CT-guided percutaneous chemical ablation of adrenal neoplasms. AJR Am J Roentgenol. 2008;190:105–110. doi: 10.2214/AJR.07.2145. [DOI] [PubMed] [Google Scholar]
- 26.Wolf F.J., Dupuy D.E., Machan J.T., Mayo-Smith W.W. Adrenal neoplasms: Effectiveness and safety of CT-guided ablation of 23 tumors in 22 patients. Eur J Radiol. 2012;81:1717–1723. doi: 10.1016/j.ejrad.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 27.Welch B.T., Callstrom M.R., Carpenter P.C. A single-institution experience in image-guided thermal ablation of adrenal gland metastases. J Vasc Interv Radiol. 2014;25:593–598. doi: 10.1016/j.jvir.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa T., Yamakado K., Nakatsuka A. Unresectable adrenal metastases: Clinical outcomes of radiofrequency ablation. Radiology. 2015;277:584–593. doi: 10.1148/radiol.2015142029. [DOI] [PubMed] [Google Scholar]
- 29.Men M., Ye X., Fan W. Short-term outcomes and safety of computed tomography-guided percutaneous microwave ablation of solitary adrenal metastasis from lung cancer: A multi-center retrospective study. Korean J Radiol. 2016;17:864–873. doi: 10.3348/kjr.2016.17.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren C., Liang P., Yu X.L., Cheng Z.G., Han Z.Y., Yu J. Percutaneous microwave ablation of adrenal tumours under ultrasound guidance in 33 patients with 35 tumours: A single-centre experience. Int J Hyperthermia. 2016;32:517–523. doi: 10.3109/02656736.2016.1164905. [DOI] [PubMed] [Google Scholar]
- 31.Frenk N.E., Daye D., Tuncali K. Local control and survival after image-guided percutaneous ablation of adrenal metastases. J Vasc Interv Radiol. 2018;29:276–284. doi: 10.1016/j.jvir.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 32.Botsa E.I., Thanou I.L., Papatheodoropoulou A.T., Thanos L.I. Thermal ablation in the management of adrenal metastasis originating from non-small cell lung cancer: A 5-year single-center experience. Chin Med J. 2017;130:2027–2032. doi: 10.4103/0366-6999.210496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palma D.A., Louie A.V., Rodrigues G.B. New strategies in stereotactic radiotherapy for oligometastases. Clin Cancer Res. 2015;21:5198–5204. doi: 10.1158/1078-0432.CCR-15-0822. [DOI] [PubMed] [Google Scholar]
- 34.Salama J.K., Hasselle M.D., Chmura S.J. Stereotactic body radiotherapy for multisite extracranial oligometastases: Final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012;118:2962–2970. doi: 10.1002/cncr.26611. [DOI] [PubMed] [Google Scholar]
- 35.Milano M.T., Katz A.W., Muhs A.G. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer. 2008;112:650–658. doi: 10.1002/cncr.23209. [DOI] [PubMed] [Google Scholar]
- 36.Chawla S., Chen Y., Katz A.W. Stereotactic body radiotherapy for treatment of adrenal metastases. Int J Radiat Oncol Biol Phys. 2009;75:71–75. doi: 10.1016/j.ijrobp.2008.10.079. [DOI] [PubMed] [Google Scholar]
- 37.Oshiro Y., Takeda Y., Hirano S., Ito H., Aruga T. Role of radiotherapy for local control of asymptomatic adrenal metastasis from lung cancer. Am J Clin Oncol. 2011;34:249–253. doi: 10.1097/COC.0b013e3181dbb727. [DOI] [PubMed] [Google Scholar]
- 38.Holy R., Piroth M., Pinkawa M., Eble M.J. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol. 2011;187:245–251. doi: 10.1007/s00066-011-2192-z. [DOI] [PubMed] [Google Scholar]
- 39.Casamassima F., Livi L., Masciullo S. Stereotactic radiotherapy for adrenal gland metastases: University of Florence experience. Int J Radiat Oncol Biol Phys. 2012;82:919–923. doi: 10.1016/j.ijrobp.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 40.Scorsetti M., Alongi F., Filippi A.R. Long-term local control achieved after hypofractionated stereotactic body radiotherapy for adrenal gland metastases: A retrospective analysis of 34 patients. Acta Oncol. 2012;51:618–623. doi: 10.3109/0284186X.2011.652738. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed K.A., Barney B.M., Macdonald O.K. Stereotactic body radiotherapy in the treatment of adrenal metastases. Am J Clin Oncol. 2013;36:509–513. doi: 10.1097/COC.0b013e3182569189. [DOI] [PubMed] [Google Scholar]
- 42.Li J., Shi Z., Wang Z. Treating adrenal tumors in 26 patients with CyberKnife: A mono-institutional experience. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0080654. e80654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudra S., Malik R., Ranck M.C. Stereotactic body radiation therapy for curative treatment of adrenal metastases. Technol Cancer Res Treat. 2013;12:217–224. doi: 10.7785/tcrt.2012.500320. [DOI] [PubMed] [Google Scholar]
- 44.Gamsiz H., Beyzadeoglu M., Sager O. Evaluation of stereotactic body radiation therapy in the management of adrenal metastases from non-small cell lung cancer. Tumori. 2015;101:98–103. doi: 10.5301/tj.5000222. [DOI] [PubMed] [Google Scholar]
- 45.Franzese C., Franceschini D., Cozzi L. Minimally invasive stereotactical radio-ablation of adrenal metastases as an alternative to surgery. Cancer Res Treat. 2017;49:20–28. doi: 10.4143/crt.2016.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haidenberger A., Heidorn S.C., Kremer N., Muacevic A., Furweger C. Robotic radiosurgery for adrenal gland metastases. Cureus. 2017;9:e1120. doi: 10.7759/cureus.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Celik E., Semrau R., Baues C., Trommer-Nestler M., Baus W., Marnitz S. Robot-assisted extracranial stereotactic radiotherapy of adrenal metastases in oligometastatic non-small cell lung cancer. Anticancer Res. 2017;37:5285–5291. doi: 10.21873/anticanres.11954. [DOI] [PubMed] [Google Scholar]
- 48.Chance W.W., Nguyen Q.N., Mehran R. Stereotactic ablative radiotherapy for adrenal gland metastases: Factors influencing outcomes, patterns of failure, and dosimetric thresholds for toxicity. Pract Radiat Oncol. 2017;7:e195–e203. doi: 10.1016/j.prro.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Li R., Mok E., Chang D.T. Intrafraction verification of gated RapidArc by using beam-level kilovoltage X-ray images. Int J Radiat Oncol Biol Phys. 2012;83:e709–e715. doi: 10.1016/j.ijrobp.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy M.J., Martin D., Whyte R., Hai J., Ozhasoglu C., Le Q.T. The effectiveness of breath-holding to stabilize lung and pancreas tumors during radiosurgery. Int J Radiat Oncol Biol Phys. 2002;53:475–482. doi: 10.1016/s0360-3016(01)02822-x. [DOI] [PubMed] [Google Scholar]
- 51.Minn A.Y., Schellenberg D., Maxim P. Pancreatic tumor motion on a single planning 4D-CT does not correlate with intrafraction tumor motion during treatment. Am J Clin Oncol. 2009;32:364–368. doi: 10.1097/COC.0b013e31818da9e0. [DOI] [PubMed] [Google Scholar]
- 52.National Institutes of Health, National Cancer Institute . National Institutes of Health; Bethesda, MD: 2010. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. [Google Scholar]
- 53.Benedict S.H., Yenice K.M., Followill D. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 54.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart F.A., Oussoren Y., Luts A. Repair of sublethal radiation injury after multiple small doses in mouse kidney: An estimate of flexure dose. Int J Radiat Oncol Biol Phys. 1987;13:765–772. doi: 10.1016/0360-3016(87)90297-5. [DOI] [PubMed] [Google Scholar]
- 56.Joiner M.C., Bentzen S.M. Fractionation: The linear-quadratic approach. In: Joiner M.V.K.A., editor. Basic Clinical Radiobiology. 4th ed. Hodder Arnold; London, UK: 2009. pp. 102–119. [Google Scholar]
- 57.Ang K.K., Jiang G.L., Feng Y., Stephens L.C., Tucker S.L., Price R.E. Extent and kinetics of recovery of occult spinal cord injury. Int J Radiat Oncol Biol Phys. 2001;50:1013–1020. doi: 10.1016/s0360-3016(01)01599-1. [DOI] [PubMed] [Google Scholar]
- 58.Wong C.S., Hao Y. Long-term recovery kinetics of radiation damage in rat spinal cord. Int J Radiat Oncol Biol Phys. 1997;37:171–179. doi: 10.1016/s0360-3016(96)00453-1. [DOI] [PubMed] [Google Scholar]
- 59.Therasse P., Arbuck S.G., Eisenhauer E.A. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 60.Wahl R.L., Jacene H., Kasamon Y., Lodge M.A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hauch A., Al-Qurayshi Z., Kandil E. Factors associated with higher risk of complications after adrenal surgery. Ann Surg Oncol. 2015;22:103–110. doi: 10.1245/s10434-014-3750-2. [DOI] [PubMed] [Google Scholar]
- 62.Fintelmann F.J., Tuncali K., Puchner S. Catecholamine surge during image-guided ablation of adrenal gland metastases: Predictors, consequences, and recommendations for management. J Vasc Interv Radiol. 2016;27:395–402. doi: 10.1016/j.jvir.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 63.Espinosa de Ycaza A.E., Welch T.L., Ospina N.S. Image-guided thermal ablation of adrenal metastases: Hemodynamic and endocrine outcomes. Endocr Pract. 2017;23:132–140. doi: 10.4158/EP161498.OR. [DOI] [PubMed] [Google Scholar]
- 64.Eldaya R.W., Paulino A.C., Blanco A.I. Preservation of adrenal function after successful stereotactic body radiation therapy of metastatic renal cell carcinoma involving the remaining contralateral adrenal gland. Pract Radiat Oncol. 2012;2:270–273. doi: 10.1016/j.prro.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Siva S., Jackson P., Kron T. Impact of stereotactic radiotherapy on kidney function in primary renal cell carcinoma: Establishing a dose-response relationship. Radiother Oncol. 2016;118:540–546. doi: 10.1016/j.radonc.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 66.Verma V., Bhirud A.R., Denniston K.A., Bennion N.R., Lin C. Quantification of renal function following stereotactic body radiotherapy for pancreatic cancer: Secondary dosimetric analysis of a prospective clinical trial. Radiat Oncol. 2017;12:71. doi: 10.1186/s13014-017-0798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jackson P., Foroudi F., Pham D. Short communication: Timeline of radiation-induced kidney function loss after stereotactic ablative body radiotherapy of renal cell carcinoma as evaluated by serial (99m)Tc-DMSA SPECT/CT. Radiat Oncol. 2014;9:253. doi: 10.1186/s13014-014-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamamoto T., Kadoya N., Takeda K. Renal atrophy after stereotactic body radiotherapy for renal cell carcinoma. Radiat Oncol. 2016;11:72. doi: 10.1186/s13014-016-0651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delanaye P., Cavalier E., Pottel H. Serum creatinine: Not so simple! Nephron. 2017;136:302–308. doi: 10.1159/000469669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual patient volumes of (A) bowel V25 (cm3), (B) bowel V30 (cm3), and (C) bowel V35 (cm3).