Abstract
Immunotherapy is reshaping cancer treatment paradigms; however, response rates to immune therapies are low and depend on the host's pre-existing antitumor immunity. The tumor microenvironment is comprised of malignant cells, stroma, and extracellular molecules and can hinder immune control of tumors. Herein, we review how anti-tumor immune responses are formed and how tumors avoid immune destruction. We also outline potential therapeutic targets in the immunosuppressive tumor microenvironment to promote immune control of tumors.
Introduction
Avoidance of immune control of tumor growth and spread is a hallmark of cancer.1 Most human tumors express antigens, which are identified by the host immune system.2 Clinical success with adoptive cell transfer demonstrates the potential for immune-mediated destruction of tumors.3 Clinically evident tumors do not typically regress in the absence of immune therapy. Thus, in these patients, tumors have escaped immune control.
Immune therapy is based on the stimulation of the immune system to regain control of tumor growth, and its clinical practice predates modern chemotherapy and radiation. Immune therapy has evolved from Coley's toxin to systemic cytokines such as interleukin (IL) 2, to the more recently Food and Drug Administration—approved immune checkpoint inhibitors (ICIs) anti-CTLA-4 and anti-PD-1/PD-L1.4, 5, 6, 7
Clinical successes with ICIs have led to new indications for immune therapy, with many active clinical trials underway looking to expand the role of immune therapy in modern cancer care.8 Despite the potential for dramatic responses, initial response rates with ICIs are limited and depend on the host's pre-existing immunity to the cancer.9, 10, 11 Methods to increase immune therapy response rates are under investigation and include identifying novel immune therapy targets and combinations with established therapies.
Tumors are composed of cancer cells and stromal features such as vasculature, fibroblasts, and infiltrating immune cells, which collectively form the tumor microenvironment (TME). The TME is highly variable between tumors and is vital for tumor growth, spread, and escape from immune-mediated destruction.12, 13 After clinical successes with ICI, preclinical models targeting other TME-mediated immunosuppressive pathways have identified novel targets.14, 15
Combining immune therapy with conventional treatment modalities can improve response rates. Targeted ionizing radiation is a logical choice for combination with immune therapy because of its ability to cause focused cancer-cell death and release cancer-antigen and immune-activating molecules to prime T cell responses.16, 17, 18 Preclinical models have demonstrated T cell priming after radiation19 and increased homing of effector T cells into radiated tumors that display increased sensitivity to immune destruction.20, 21 Thus, tumor control after radiation therapy depends on T cell responses in the host.19 However, radiation also induces changes in the TME, that inhibit immune control of tumors.22 Understanding and targeting these radiation-induced changes will lead to rational radioimmune therapy treatments and are under active investigation.
In this review, the role of the TME in controlling antitumor immune responses is described. The basics of priming antitumor T cell responses and the mechanisms of T cell–mediated cell killing are discussed, and the impact of individual components of the TME on both processes will be examined.
Immune system and cancer
The immune system can detect and eliminate cancers before they manifest clinically. Mice that have been genetically engineered to have defective immune systems exhibit increased incidences of carcinogen-induced and spontaneous tumors.23 Clinically, increased incidence of a variety of cancers is observed in immunosuppressed patients.24 However, most patients with clinically apparent cancers are immune competent and harbor tumors that have escaped immune control. Regaining immune control of tumor growth by promoting robust, adaptive immune responses can lead to tumor regression and is the primary goal of immune therapy.
Antitumor adaptive immune responses are predicated on the processing of tumor antigens by antigen-presenting cells, which, when subjected to appropriate activation signals, migrate to secondary lymphoid tissue where they prime naïve T cells. Antigen-presenting cells in the tumor include macrophages, B cells, and dendritic cells (DCs). Of these, DCs express both major histocompatibility complexes (MHC) I and II for activation of cluster of differentiation (CD) 8 and CD4 T cells, respectively, and can also produce costimulatory molecules that overcome the activation threshold of naïve T cells. DCs uniquely possess the ability to access phagocytosed antigen in the cytosol for presentation on MHC I, a requisite to cross-present tumor antigen to prime cytotoxic CD8 T cells. Increased DC infiltration correlates with favorable outcomes.25 Of note, there are multiple subsets of DCs with different impacts on tumor immunology.26, 27 Of these, CD8+CD103+Batf3+ DCs are critical to priming CD8 T-cell responses in preclinical studies.28, 29, 30
Naïve T cells recognize antigen in complex with major histocompatibility via their T cell receptor. In the presence of a second, costimulatory signal such as B7 ligands binding to CD28, the naïve T cell is stimulated to proliferate and differentiate into effector T cells. Subsequently, effector CD8 T cells can recirculate back into the tumor, where they induce caspase-dependent apoptosis in cancer cells expressing the recognized antigen on MHC I. Durable immune control of tumors may depend on continuous priming of robust, adaptive immune responses to tumors that evolve under selective immune pressure.31
Tumor microenvironment is a barrier to effector immune cells
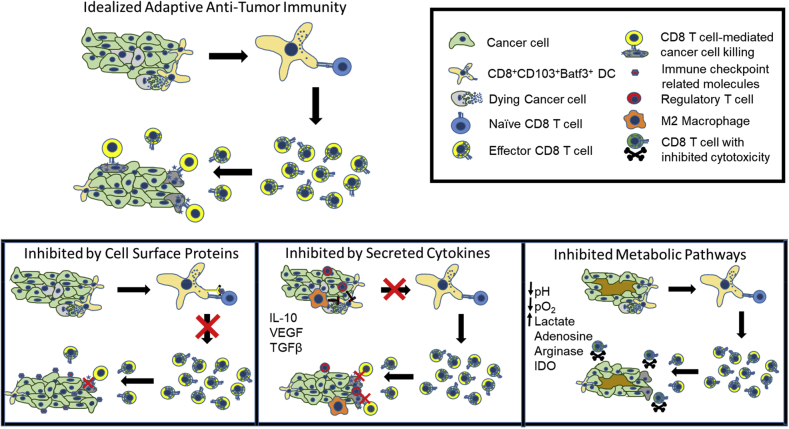
Clinically evident tumors have, by default, escaped host immune control despite evidence for tumor-reactive T cells.32, 33 Administration of ICIs may regain immune control of tumor growth by boosting the host's own pre-existing tumor immunity. In addition to expression of immune checkpoint–related molecules, mechanisms by which the TME may suppress efficacy of effector T cells are listed in Table 1 and displayed in Figure 1. Dysregulated cancer cell growth can lead to tumor intrinsic immunosuppressive features, such as regions of hypoxia and elevated levels of lactate that can inhibit effector T cell function.34 However, the majority of immune suppression is due to the presence of normal immune regulatory cells and molecules within the tumor that inhibit T-cell priming or suppress cytotoxic T cell function.35, 36 Of these, immune checkpoint–related molecules have garnered the most attention, with proven clinical success of ICIs. Accordingly, expression of immune checkpoint–related molecules by both tumor and stromal cells has been reviewed extensively elsewhere.37
Table 1.
Mechanisms of immunosuppression in the tumor microenvironment
| Mediator | Mechanism of immunosuppression | References |
|---|---|---|
| Cell surface proteins | ||
| Programmed death-ligand 1 | Induce T-cell tolerance/anergy after ligation with programmed cell death protein 1 on T cells | 37 |
| CTLA-4 | Inhibit activation of naïve T cells | 37 |
| Enhance regulatory T cell function | 74 | |
| ↓Major histocompatibility complex I | Avoid detection by effector CD8 T cells | 75 |
| ↓FAS | Avoid FAS ligand–mediated cell killing | |
| ↓TRAIL | Avoid TRAIL-mediated cell killing | |
| CD39/CD73 | Convert extracellular immunostimulatory adenosine triphosphate to immunosuppressive adenosine | 76 |
| Secreted cytokines | ||
| Transforming growth factor beta | Inhibit T cell priming and infiltration | 77 |
| Suppress effector cell cytotoxicity | 47 | |
| Vascular endothelial growth factor | Inhibit dendritic cell maturation | 78 |
| Enhance programmed cell death protein 1/programmed death-ligand 1/2 expression | 79 | |
| Enhance interleukin-10 secretion | ||
| Interleukin-10 | Inhibit major histocompatibility complex II expression on antigen presenting cells | 80 |
| Suppress M1 cytokine secretion | 81 | |
| Suppress iNOS (inducible Nitric Oxide Synthase) | 82 | |
| Induce T cell anergy | 83 | |
| Metabolic pathways | ||
| Indoleamine-2,3 dioxygenase | Convert tryptophan to kynurenine | 55 |
| Inhibit T cell proliferation | 84 | |
| Adenosine | Inhibit T cell proliferation and activation | 85, 86 |
| Hypoxia | Inhibit effector T cell function | 87 |
| Promote prostaglandin E2 synthesis | 88 | |
| Lactate | Inhibit effector T cell function | 89 |
| Arginase | Degrades L-arginine needed for cytotoxic iNOS production | 90 |
| Prostaglandin E2 | Inhibit effector T cell function | 91, 92 |
| Suppress M1 cytokine secretion | 93 | |
| Recruit myeloid-derived suppressor cells | 94 |
Abbreviations: CD = cluster of differentiation; TRAIL = tumor necrosis factor-related apoptosis-inducing ligand.
Figure. 1.
Adaptive antitumor immunity and mechanisms of inhibition in the tumor microenvironment.
A highly variable component of the TME with significant impact on the immune control of tumors is tumor-infiltrating lymphocytes (TILs). TILs provide evidence for an antitumor immune response but do not always correlate with favorable prognosis.38 Advances in analytical tools have identified diverse subsets of infiltrating immune cells with either directly cytotoxic, immune-promoting or immunosuppressive functions. Thus, infiltrating lymphocytes may lead to an immune supporting or suppressing TME depending on which cell subsets dominate.
Of the immunosuppressive infiltrates, regulatory T cells (Tregs) are identified as CD4+CD25+FOXP3+ and play an important role in normal physiology by moderating immune destruction and preventing autoimmune disease.39 Tregs are commonly found in solid tumors and promote immunosuppression by several mechanisms including secretion of immunosuppressive cytokines, competing for activating cyokines with effector cells, and after direct cellular contact with infiltrating effector cells.40, 41 However, elevated levels of Treg infiltrates in tumors can accompany elevated levels of effector T cell infiltrates and thus are not absolute indicators of an overall immunosuppressive TME.42 Recent data using advanced image analysis techniques demonstrated that the proximity of effector CD8 T cells to Tregs correlated with worse prognosis in patients with oral cancer,43 suggesting that spatial distribution of subsets within the tumor, rather than mere presence or prevalence, may determine their immunosuppressive effect.
Tregs contribute to the level of transforming growth factor beta (TGFβ) in the tumor, which is a mediator of immunosuppression and subverts both adaptive immune priming and effector responses. TGFβ can disrupt T cell activation by limiting the mobility and longevity of DCs and separately may preferentially promote activation of Tregs.44, 45 TGFβ promotes alternatively polarized macrophages (discussed in the next section), which may compete with DCs for tumor antigen and further inhibit T cell priming.46 TGFβ also inhibits the cytotoxicity of CD8 T cells.47 Thus, inhibiting TGFβ has the potential to disrupt the immunosuppressive TME on multiple fronts, and preclinical studies have shown improved tumor responses to radiation when combined with TGFβ inhibition.48, 49
The most prevalent antigen-presenting cells within the TME are macrophages. Macrophages can be differentiated into opposing phenotypes, M1 and M2 (Fig 2). M1 phenotypes can be induced by Toll-like receptor stimulation in the presence of interferon gamma and express proinflammatory cytokines, such as IL-12. M2 phenotypes are induced by IL-4 and IL-13 produced by CD4+ T helper 2 cells,50 and are associated with the production of arginase I and anti-inflammatory IL-10.51 Arginase converts arginine to ornithine and urea, making arginine unavailable for nitric oxide–mediated cell killing. Arginase also downregulates the ζ chain of T cell receptor, which inhibits T cell activation.52 Preclinical models demonstrate a role for arginase expression by macrophages in limiting T cell control of irradiated tumors.53
Figure. 2.
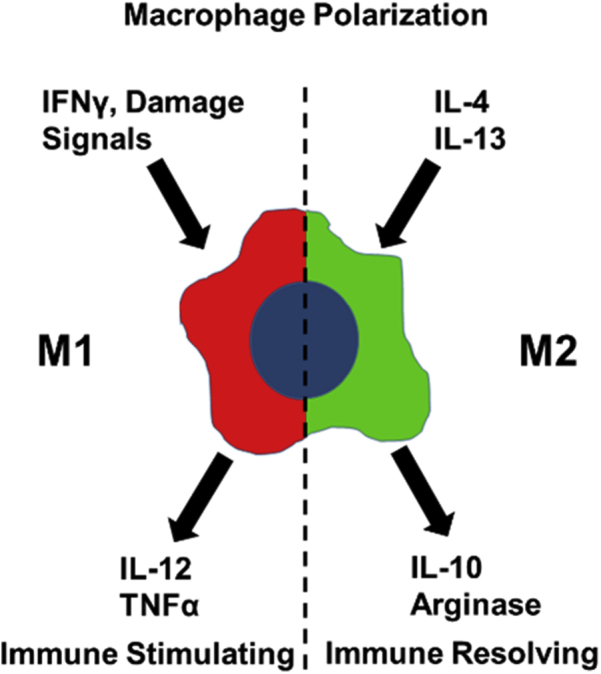
Polarization states of macrophages.
A range of other metabolic features of tumors limit immune activation, including the enzyme indoleamine 2,3-dioxygenase (IDO). IDO plays a role in immune tolerance to apoptotic cells in normal physiology54 and is readily induced in the tumor. IDO converts tryptophan to kynurenine, which suppresses effector T cell activation and promotes the generation of Tregs and infiltration of myeloid-derived suppressor cells.55, 56, 57 In preclinical models, inhibiting IDO improves the efficacy of conventional cancer treatments and the immune control of tumors.58 Moreover, IDO-/- mice showed increased responses to ICIs in a melanoma model, suggesting better immune therapy outcomes for ICIs in combination with IDO inhibitors.59
Although macrophages can promote or suppress adaptive immunity depending on phenotype, the presence of macrophages in human tumors is associated with poor prognosis.60 In preclinical studies, macrophages are the most abundant infiltrating cell in the tumor stroma after tumor irradiation and exhibit M2 properties that suppress subsequent immune responses.53, 61, 62 Depleting macrophages with anti–colony stimulating factor 1 antibodies or chemokine ligand 2 blockade slowed growth and improved responses to immune therapy and ionizing radiation in a number of tumor models.63, 64 Apart from depletion, several strategies have been demonstrated to repolarize macrophages to an M1 phenotype, which restores adaptive immune control of tumors.65 The prevalence of macrophages in tumors and the potential for repolarizing to M1 phenotypes makes them an attractive target for establishing an immune-promoting TME.
Another critical component of tumors and a prerequisite for antigen presentation is the presence of dead cancer cells. In tumors, cell death occurs from dysregulated growth and cytotoxic therapies. Identification and phagocytosis of apoptotic cells is a tightly regulated process and critical for normal physiology and cell turnover. Phagocytosis of apoptotic tumor cells could comprise a source for processing and presenting cancer antigens on MHC II. However, mounting evidence indicates that phagocytosis of apoptotic cell leads to immune-tolerizing rather than immune-activating antigen presentation.66
Blocking phagocytic pathways can improve tumor control after therapeutic radiation.61, 67 Invoking non-apoptotic cell death with release of inflammatory mediators may be key to promoting synergy between ionizing radiation and immune therapy.68, 69, 70 Thus, in this scenario, the goal of radiation is not only to induce cancer cell killing, but to cause immunogenic cell death while controlling how the TME handles the dead cells to promote an adaptive immune response.71
Clinical trials combining radiation with immune therapy targeting the TME
Targeting the immunosuppressive TME can promote adaptive immune priming and improve the efficacy of effector cell killing and cytotoxic therapies. Radiation is a logical choice to synergize with immune therapies because it may induce immunogenic cell death intratumorally to prime an adaptive immune response and increase the homing of effector cells into tumors. Clinical success with ICIs has demonstrated the potential for targeting the TME to promote immune control of tumors. Hundreds of active phase 2 and 3 clinical trials are evaluating novel immune therapies and concurrent immune therapy combinations targeting the immunosuppressive TME. A comprehensive listing of active trials is beyond the scope of this review, and readers are directed to serial publications aimed at reviewing such trials for further edification.72, 73 The results from these trials will guide future studies of novel targeted immune therapies and immune therapy combinations.
Footnotes
Sources of support: This work was funded by National Cancer Institute grants R01CA182311 (Dr Michael J. Gough) and R01CA208644 (Dr Marka R. Crittenden). Dr Gough receives research funding from Bristol-Meyers Squibb for research that is not directly related to this manuscript.
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Boon T., Old L.J. Cancer tumor antigens. Curr Opin Immunol. 1997;9:681–683. doi: 10.1016/s0952-7915(97)80049-0. [DOI] [PubMed] [Google Scholar]
- 3.Dudley M.E., Rosenberg S.A. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarthy E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154–158. [PMC free article] [PubMed] [Google Scholar]
- 5.Atkins M.B., Lotze M.T., Dutcher J.P. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 6.Hodi F.S., O'Day S.J., McDermott D.F. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamid O., Robert C., Daud A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanpouille-Box C., Lhuillier C., Bezu L. Trial watch: Immune checkpoint blockers for cancer therapy. Oncoimmunology. 2017;6:e1373237. doi: 10.1080/2162402X.2017.1373237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbst R.S., Soria J.C., Kowanetz M. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumeh P.C., Harview C.L., Yearley J.H. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crittenden M.R., Zebertavage L., Kramer G. Tumor cure by radiation therapy and checkpoint inhibitors depends on pre-existing immunity. Sci Rep. 2018;8:7012. doi: 10.1038/s41598-018-25482-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiteside T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 14.Vonderheide R.H. The immune revolution: A case for priming, not checkpoint. Cancer Cell. 2018;33:563–569. doi: 10.1016/j.ccell.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thommen D.S., Schumacher T.N. T cell dysfunction in cancer. Cancer Cell. 2018;33:547–562. doi: 10.1016/j.ccell.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gough M.J., Crittenden M.R. Combination approaches to immunotherapy: The radiotherapy example. Immunotherapy. 2009;1:1025–1037. doi: 10.2217/imt.09.64. [DOI] [PubMed] [Google Scholar]
- 17.Gough M.J., Crittenden M.R. Immune system plays an important role in the success and failure of conventional cancer therapy. Immunotherapy. 2012;4:125–128. doi: 10.2217/imt.11.157. [DOI] [PubMed] [Google Scholar]
- 18.Apetoh L., Ghiringhelli F., Tesniere A. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y., Auh S.L., Wang Y. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lugade A.A., Moran J.P., Gerber S.A., Rose R.C., Frelinger J.G., Lord E.M. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 21.Chakraborty M., Abrams S.I., Coleman C.N., Camphausen K., Schlom J., Hodge J.W. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 22.Wennerberg E., Lhuillier C., Vanpouille-Box C. Barriers to radiation-induced in situ tumor vaccination. Front Immunol. 2017;8:229. doi: 10.3389/fimmu.2017.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vesely M.D., Kershaw M.H., Schreiber R.D., Smyth M.J. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 24.Vajdic C.M., McDonald S.P., McCredie M.R. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 25.Broz M.L., Binnewies M., Boldajipour B. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demoulin S., Herfs M., Delvenne P., Hubert P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: Insight into the molecular mechanisms. J Leukoc Biol. 2013;93:343–352. doi: 10.1189/jlb.0812397. [DOI] [PubMed] [Google Scholar]
- 27.Chen W., Liang X., Peterson A.J., Munn D.H., Blazar B.R. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.den Haan J.M., Lehar S.M., Bevan M.J. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuertes M.B., Kacha A.K., Kline J. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hildner K., Edelson B.T., Purtha W.E. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim R., Emi M., Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mihm M.C., Jr., Clemente C.G., Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: A histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74:43–47. [PubMed] [Google Scholar]
- 33.Miescher S., Whiteside T.L., Moretta L., von Fliedner V. Clonal and frequency analyses of tumor-infiltrating T lymphocytes from human solid tumors. J Immunol. 1987;138:4004–4011. [PubMed] [Google Scholar]
- 34.Fischer K., Hoffmann P., Voelkl S. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 35.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Ertl H.C. Starved and asphyxiated: How can CD8(+) T cells within a tumor microenvironment prevent tumor progression. Front Immunol. 2016;7:32. doi: 10.3389/fimmu.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topalian S.L., Drake C.G., Pardoll D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tosolini M., Kirilovsky A., Mlecnik B. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1267. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 39.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 40.Budhu S., Schaer D.A., Li Y. Blockade of surface-bound TGF-beta on regulatory T cells abrogates suppression of effector T cell function in the tumor microenvironment. Sci Signal. 2017;10:494. doi: 10.1126/scisignal.aak9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishikawa H., Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 42.Shang B., Liu Y., Jiang S.J., Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Z., Bethmann D., Kappler M. Multiparametric immune profiling in HPV- oral squamous cell cancer. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito M., Minamiya Y., Kawai H. Tumor-derived TGFbeta-1 induces dendritic cell apoptosis in the sentinel lymph node. J Immunol. 2006;176:5637–5643. doi: 10.4049/jimmunol.176.9.5637. [DOI] [PubMed] [Google Scholar]
- 45.Weber F., Byrne S.N., Le S. Transforming growth factor-beta1 immobilises dendritic cells within skin tumours and facilitates tumour escape from the immune system. Cancer Immunol Immunother. 2005;54:898–906. doi: 10.1007/s00262-004-0652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byrne S.N., Knox M.C., Halliday G.M. TGFbeta is responsible for skin tumour infiltration by macrophages enabling the tumours to escape immune destruction. Immunol Cell Biol. 2008;86:92–97. doi: 10.1038/sj.icb.7100116. [DOI] [PubMed] [Google Scholar]
- 47.Thomas D.A., Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Young K.H., Newell P., Cottam B. TGFbeta inhibition prior to hypofractionated radiation enhances efficacy in preclinical models. Cancer Immunol Res. 2014;2:1011–1022. doi: 10.1158/2326-6066.CIR-13-0207. [DOI] [PubMed] [Google Scholar]
- 49.Vanpouille-Box C., Diamond J.M., Pilones K.A. TGFbeta is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 2015;75:2232–2242. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeNardo D.G., Barreto J.B., Andreu P. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuang D.M., Zhao Q., Peng C. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodriguez P.C., Zea A.H., Culotta K.S., Zabaleta J., Ochoa J.B., Ochoa A.C. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J Biol Chem. 2002;277:21123–21129. doi: 10.1074/jbc.M110675200. [DOI] [PubMed] [Google Scholar]
- 53.Crittenden M.R., Savage T., Cottam B. Expression of arginase I in myeloid cells limits control of residual disease after radiation therapy of tumors in mice. Radiat Res. 2014;182:182–190. doi: 10.1667/RR13493.1. [DOI] [PubMed] [Google Scholar]
- 54.Ravishankar B., Liu H., Shinde R. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2012;109:3909–3914. doi: 10.1073/pnas.1117736109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munn D.H., Sharma M.D., Baban B. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 56.Mezrich J.D., Fechner J.H., Zhang X., Johnson B.P., Burlingham W.J., Bradfield C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holmgaard R.B., Zamarin D., Li Y. Tumor-expressed IDO recruits and activates MDSCs in a Treg-dependent manner. Cell Rep. 2015;13:412–424. doi: 10.1016/j.celrep.2015.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lob S., Konigsrainer A., Rammensee H.G., Opelz G., Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. 2009;9:445–452. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 59.Holmgaard R.B., Zamarin D., Munn D.H., Wolchok J.D., Allison J.P. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J.J., Lin Y.C., Yao P.L. Tumor-associated macrophages: The double-edged sword in cancer progression. J Clin Oncol. 2005;23:953–964. doi: 10.1200/JCO.2005.12.172. [DOI] [PubMed] [Google Scholar]
- 61.Crittenden M.R., Baird J., Friedman D. Mertk on tumor macrophages is a therapeutic target to prevent tumor recurrence following radiation therapy. Oncotarget. 2016;7:78653–78666. doi: 10.18632/oncotarget.11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gough M.J., Young K., Crittenden M. The impact of the myeloid response to radiation therapy. Clin Dev Immunol. 2013;2013:281958. doi: 10.1155/2013/281958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu J., Escamilla J., Mok S. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalbasi A., Komar C., Tooker G.M. Tumor-derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2017;23:137–148. doi: 10.1158/1078-0432.CCR-16-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruffell B., Affara N.I., Coussens L.M. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poon I.K., Lucas C.D., Rossi A.G., Ravichandran K.S. Apoptotic cell clearance: Basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jinushi M., Sato M., Kanamoto A. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J Exp Med. 2009;206:1317–1326. doi: 10.1084/jem.20082614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Apetoh L., Tesniere A., Ghiringhelli F., Kroemer G., Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. 2008;68:4026–4030. doi: 10.1158/0008-5472.CAN-08-0427. [DOI] [PubMed] [Google Scholar]
- 69.Green D.R., Ferguson T., Zitvogel L., Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vanpouille-Box C., Alard A., Aryankalayil M.J. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tormoen G.W., Crittenden M.R., Gough M.J. The TAM family as a therapeutic target in combination with radiation therapy. Emerging Topics Life Sci. 2017;1:493–500. doi: 10.1042/ETLS20170066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buque A., Bloy N., Aranda F. Trial watch-small molecules targeting the immunological tumor microenvironment for cancer therapy. Oncoimmunology. 2016;5:e1149674. doi: 10.1080/2162402X.2016.1149674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vacchelli E., Bloy N., Aranda F. Trial watch: Immunotherapy plus radiation therapy for oncological indications. Oncoimmunology. 2016;5:e1214790. doi: 10.1080/2162402X.2016.1214790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wing K., Onishi Y., Prieto-Martin P. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 75.Reeves E., James E. Antigen processing and immune regulation in the response to tumours. Immunology. 2017;150:16–24. doi: 10.1111/imm.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Antonioli L., Blandizzi C., Pacher P., Hasko G. Immunity, inflammation and cancer: A leading role for adenosine. Nat Rev Cancer. 2013;13:842–857. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 77.Mariathasan S., Turley S.J., Nickles D. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fukumura D., Kloepper J., Amoozgar Z., Duda D.G., Jain R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khan K.A., Kerbel R.S. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. 2018;15:310–324. doi: 10.1038/nrclinonc.2018.9. [DOI] [PubMed] [Google Scholar]
- 80.Moore K.W., de Waal Malefyt R., Coffman R.L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Ann Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 81.Fiorentino D.F., Zlotnik A., Mosmann T.R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 82.Dokka S., Shi X., Leonard S., Wang L., Castranova V., Rojanasakul Y. Interleukin-10-mediated inhibition of free radical generation in macrophages. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1196–L1202. doi: 10.1152/ajplung.2001.280.6.L1196. [DOI] [PubMed] [Google Scholar]
- 83.Steinbrink K., Graulich E., Kubsch S., Knop J., Enk A.H. CD4(+) and CD8(+) anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–2476. doi: 10.1182/blood.v99.7.2468. [DOI] [PubMed] [Google Scholar]
- 84.Uyttenhove C., Pilotte L., Theate I. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 85.Kobie J.J., Shah P.R., Yang L. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5'-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 86.Saze Z., Schuler P.J., Hong C.S., Cheng D., Jackson E.K., Whiteside T.L. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood. 2013;122:9–18. doi: 10.1182/blood-2013-02-482406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang C.H., Curtis J.D., Maggi L.B., Jr. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee J.J., Natsuizaka M., Ohashi S. Hypoxia activates the cyclooxygenase-2-prostaglandin E synthase axis. Carcinogenesis. 2010;31:427–434. doi: 10.1093/carcin/bgp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singer K., Gottfried E., Kreutz M., Mackensen A. Suppression of T-cell responses by tumor metabolites. Cancer Immunol Immunother. 2011;60:425–431. doi: 10.1007/s00262-010-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodriguez P.C., Ochoa A.C., Al-Khami A.A. Arginine metabolism in myeloid cells shapes innate and adaptive immunity. Front Immunol. 2017;8:93. doi: 10.3389/fimmu.2017.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holt D., Ma X., Kundu N., Fulton A. Prostaglandin E(2) (PGE (2)) suppresses natural killer cell function primarily through the PGE(2) receptor EP4. Cancer Immunol Immunother. 2011;60:1577–1586. doi: 10.1007/s00262-011-1064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinet L., Jean C., Dietrich G., Fournie J.J., Poupot R. PGE2 inhibits natural killer and gamma delta T cell cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA type I-dependent signaling. Biochem Pharmacol. 2010;80:838–845. doi: 10.1016/j.bcp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 93.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mao Y., Sarhan D., Steven A., Seliger B., Kiessling R., Lundqvist A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res. 2014;20:4096–4106. doi: 10.1158/1078-0432.CCR-14-0635. [DOI] [PubMed] [Google Scholar]