Abstract
Purpose
There are limited treatment options for locally advanced, unresectable pancreatic cancer (LAPC) and no likelihood of cure without surgery. Radiation offers an option for local control, but radiation dose has previously been limited by nearby bowel toxicity. Advances in on-board imaging and treatment planning may allow for dose escalation not previously feasible and improve local control. In preparation for development of clinical trials of dose escalation in LAPC, we undertook a dosimetric study to determine the maximum possible dose escalation while maintaining known normal tissue constraints.
Methods and Materials
Twenty patients treated at our institution with either SBRT or dose-escalated hypofractionated IMRT (DE-IMRT) were re-planned using dose escalated SBRT to 70 Gy in 5 fractions to the GTV and 40 Gy in 5 fractions to the PTV. Standard accepted organ at risk (OAR) constraints were used for planning. Descriptive statistics were generated for homogeneity, conformality, OAR's and GTV/PTV.
Results
Mean iGTV coverage by 50 Gy was 91% (±0.07%), by 60 Gy was 61.3% (±0.08%) and by 70 Gy was 24.4% (±0.05%). Maximum PTV coverage by 70 Gy was 33%. Maximum PTV coverage by 60 Gy was 77.5%. The following organ at risk (OAR) constraints were achieved for 90% of generated plans: Duodenum V20 < 30 cc, V30 < 3 cc, V35 < 1 cc; Small Bowel V20 < 15 cc, V30 < 1 cc, V35 < 0.1 cc; Stomach V20 < 20 cc, V30 < 2 cc, V35 < 1 cc. V40 < 0.5 cc was achieved for all OAR.
Conclusions
Dose escalation to 60 Gy is dosimetrically feasible with adequate GTV coverage. The identified constraints for OAR's will be used in ongoing clinical trials.
Summary.
Locally advanced pancreatic cancer (LAPC) is challenging to treat since it often responds poorly to chemotherapy, abuts critical sensitive organs, and is by definition unresectable. Dose escalated radiation techniques may improve local control and thus decrease morbidity and mortality related to local progression. Here, we performed an in silico dose escalation study and produced practical dose constraints that could be adapted for clinical practice as part of a protocol.
Introduction
Locally advanced pancreatic cancer (LAPC) is particularly difficult to treat because of its poor response to chemotherapy and unresectable nature. A large proportion of patients experience significant morbidity and mortality due to local progression. As systemic therapy improves with the advent of gemcitabine/abraxane1 and FOLFIRINOX,2 the burden of local disease will only increase. Standard radiation therapy doses have failed to improve survival, which is not unexpected given the anatomical and technical limitations. The LAP-07 trial3 failed to show an overall survival benefit to radiation at standard doses, but did show benefits in terms of local control and time off of chemotherapy. However, at higher radiation doses, our institutional data suggest improved overall survival (17.8 vs 15 months)4 and recurrence-free survival (10.2 vs 6.2 months). These studies also demonstrated <1% Grade 3+ gastrointestinal (GI) toxicity5 with a high dose (biological effective dose [BED] >70 Gy) by using advanced radiation delivery techniques, including 4-dimensional computed tomography, breath-hold technique, and image guided radiation therapy (IMRT).
Given these initial retrospective data, we initiated a phase 1/2 adaptive dose escalation trial using image-guided stereotactic body radiation therapy (SBRT) for LAPC at our institution (ClinicalTrials.gov Identifier: NCT03340974) to determine the clinical maximum tolerated dose using SBRT. In preparation for the activation of this protocol, we undertook a dosimetric feasibility study to determine the following: (1) whether all patients treated with IMRT could also be planned with SBRT, and (2) the maximum feasible delivered BED using dose-escalated SBRT (DE-SBRT) while maintaining standard organ-at-risk (OAR) constraints to GI mucosa with daily imaging, motion management, and treatment planning techniques that would be available at most academic centers. Our goal was to evaluate the feasibility of DE-SBRT in 2 separate cohorts of patients: Patients treated with DE-IMRT and patients treated with SD-SBRT.
Methods and materials
Patient selection
The first 10 sequential patients who were treated at our institution with SBRT at a dose of 40 Gy in 5 fractions or 36 Gy in 5 fractions (SD-SBRT) were selected for this study. Ten patients who were originally treated with dose-escalated hypofractionated IMRT (DE-IMRT; 67.5 Gy in 15 fractions) were randomly selected from our previously published cohort4, 5 to obtain a fair distribution of patients. All patients received 4 to 6 months of standard induction chemotherapy and on restaging exhibited unresectable disease on the basis of a multidisciplinary review of computed tomography (CT) images using standard criteria.6 Patients who were previously treated with DE-IMRT generally had tumors located >5 mm from GI mucosa (OARs) with no predefined size limit, but SD-SBRT patients had tumors <4 cm in maximal dimension with no evidence of duodenal invasion on imaging or endoscopy.
Immobilization and simulation
SD-SBRT patients had multiple fiducial markers implanted before simulation. All patients were immobilized using upper-body vac-lock cradles. All patients were instructed to have nothing to eat or drink by mouth (NPO) for 3 hours prior to simulation. All patients were then given intravenous (IV) contrast prior to 5 to 6 inspiration breath hold (IBH) scans for reproducibility. A real-time position management system (Varian Medical Systems, Palo Alto, CA) was used to provide respiratory feedback to the patients. Patients were instructed to take an IBH at a comfortable level by using the phrase “hold your breath.” This approach was found to maximize the breath-hold reproducibility during each treatment delivery and throughout the entire course of the treatment compared with requesting patients to take a deep or light IBH, because deep IBH previously led to patient fatigue during the last 2 to 3 treatment fields during treatment delivery.
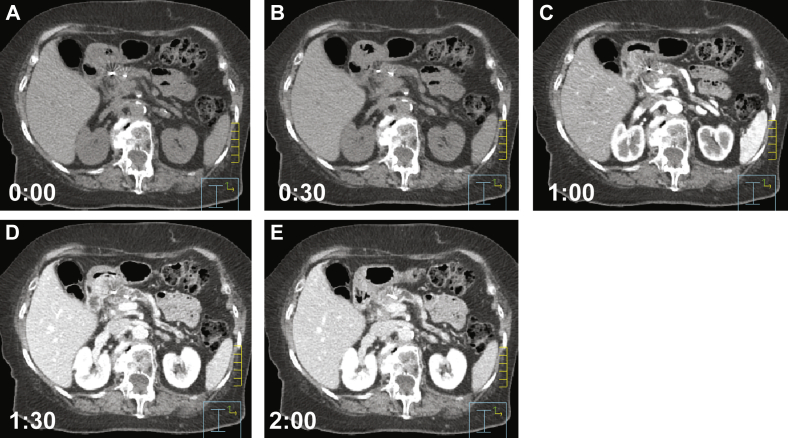
Typically 2 IBH CT scans were acquired without IV contrast, followed by 3 to 4 IBH CT scans acquired after IV contrast injection that was performed in intervals of approximately 30 seconds between scans and beginning 30 seconds after IV contrast administration. The simulation technique and example of IBH CT scans are described in Figure 1.
Figure. 1.
Selected images from a typical simulation using intravenous contrast and without oral contrast. Patients are immobilized with an upper-body vac-lock cradle and instructed to be NPO for 3 hours to reduce gastric and duodenal filling. Patients were instructed to perform a comfortable inspiration breath hold with respiratory feedback provided by a real-time management system (Varian Medical Systems, Palo Alto, CA). One or 2 noncontrast scans are taken (A) before the administration of a 150 cc bolus of intravenous contrast, infused at a rate of 3 to 5 cc/second, followed by 4 to 6 scans at 30-second intervals after contrast administration (B-E).
Target delineation and creation of simultaneous integrated boost
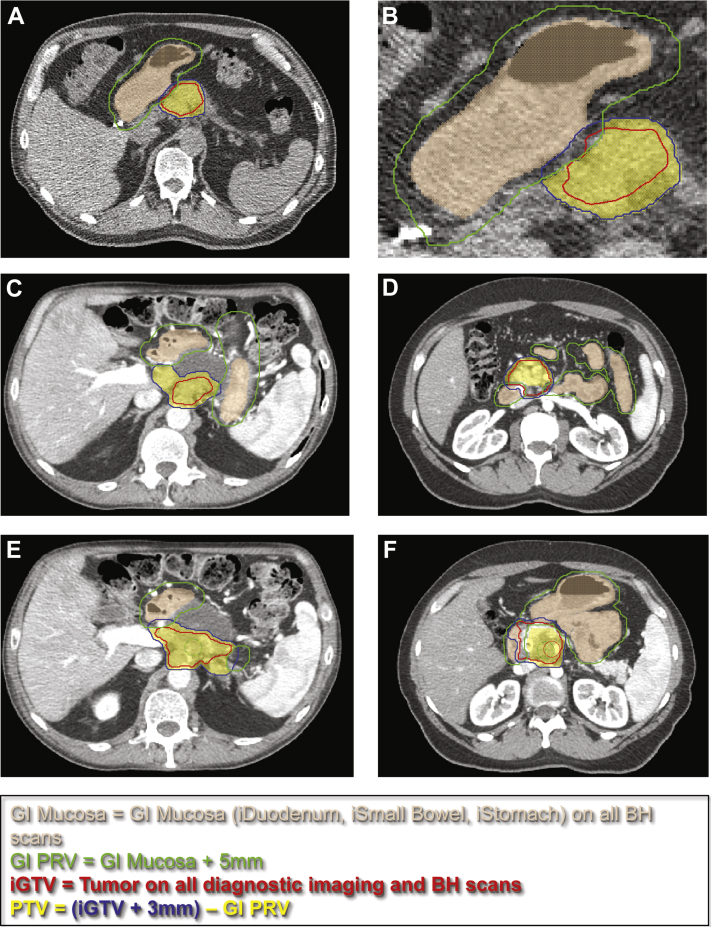
All tumors from DE-IMRT and SD-SBRT patients were recontoured and validated by 2 separate physicians (LE and CT) with identical targets using commercial treatment planning software (Phillips Pinnacle, version 9.10). The IBH CT images were used to contour an integrated gross target volume (iGTV) and integrated OAR structures (iDuodenum, iStomach, and iSmallBowel; Fig 2A). This is similar in concept to a respiratory internal target volume, created by accounting for physiologic movement of a target. iGTV was also delineated using all available pretreatment imaging fused to simulation imaging, including pancreatic protocol (multiphasic CT) and abdominal MRI, where available. The integrated OAR structures were uniformly expanded by 5 mm to create a GI mucosa planning risk volume (GI_PRV). Example contours are provided in Figure 2.
Figure. 2.
Target and organ-at-risk contouring technique using breath-hold scans. The gastrointestinal planning risk volume and high-dose prescription target volumes are defined in panels A-F. Target and organs at risk were delineated using an internal target volume based on all inspiration breath-hold scans to account for motion. An integrated gross target volume was also delineated using all available pretreatment imaging fused to simulation imaging, including pancreatic protocol (multiphasic computed tomography) and abdominal magnetic resonance imaging, where available.
For each DE-IMRT and SD-SBRT, we attempted to create a dose-escalated SBRT (DE-SBRT) plan with a simultaneous integrated boost (SIB) to 40 Gy (8 Gy/fx) and 70 Gy (14 Gy/fx) in 5 fractions. The prescription target volume (PTV)-40 was created by adding 3 mm to the iGTV and subtracting the GI-PRV. A PTV-70 was created from the iGTV with 3 mm contraction in 3 dimensions. DE-IMRT patients were replanned for a 40 Gy in 5 fractions SBRT plan and a 40 Gy in 5 fractions plan with an increased SIB dose until the highest possible dose was reached (up to 70 Gy) while maintaining prestudy OAR constraints Table 1. The 10 SD-SBRT patients were replanned with a DE-IMRT plan and DE-SBRT plan with increased SIB. A tumor-vessel interface has been included in recent trials to escalate dose to the SMA and tumor interface. This was not used in our study to simplify plan comparisons, but areas of increased dose were pushed posteriorly to vessel interface rather than anteriorly whenever possible.
Table 1.
Pre- and poststudy dose constraints for dose escalated SBRT
| Target | Constraint used for planning | Adapted constraint |
|---|---|---|
| Duodenum | V20 < 20 cc V35 < 1 cc∗ Dmax < 40 Gy |
V20 < 30 cc V30 < 3 cc V35 < 1 cc V40 < 0.5 cc |
| Small bowel | V20 < 20 cc V35 < 1 cc∗ Dmax < 40 Gy |
V20 < 15 cc V30 < 1 cc V35 < 0.1 cc V40 < 0.5 cc |
| Stomach | V20 < 20 cc V35 < 1 cc∗ Dmax < 40 Gy∗ |
V20 < 20 cc V30 < 2 cc V35 < 1 cc V40 < 0.5 cc |
| Kidneys | V12 < 25%∗ | V12 < 25% |
| Liver | V12 < 50%∗ | V12 < 50% |
| Spinal cord | V20 < 1 cc∗ | V20 < 1 cc |
SBRT, stereotactic body radiation therapy
Mandatory constraints.
Treatment planning and evaluation
Planning was performed in one of the IV contrast IBH CT scans without density override for the IV contrast. A dose of 40 Gy was prescribed to the PTV-40 with a SIB technique prescribing 70 Gy to the PTV-70. There was no minimum coverage requirement for the iGTV, but >95% coverage was requested for the PTVs. OARs were prioritized during IMRT planning over target coverage. Successful plans typically had between 7 and 12 coplanar beam angles. Volumetric modulated arc therapy was acceptable if available for treatment delivery with breath hold. Example treatment plans are presented in Figure 3. The patient is first set up using skin marks at the linear particle accelerator (LINAC) side. The patient couch is then rotated to the CT side, and a CT scan is acquired. Subsequently, the couch is rotated back to the LINAC side where the patient is repositioned for treatment using a commercial, ultrasound-based localization technique (BAT, NO- MOS Corp., Sewickley, PA). The CT images were not used to guide the actual patient treatment in this protocol scanner, and LINAC share the same patient couch.9 Based on toxicities observed in previous SBRT trials and associated constraints Table 2 the dose constraints defined for planning purposes (Table 1) were Duodenum V20 < 20 cc, V35 < 1 cc, Dmax < 40 Gy; Small Bowel V20 < 20 cc, V35 < 1 cc, Dmax < 40 Gy; Stomach V20 < 20 cc, V35 < 1 cc, Dmax < 40 Gy.
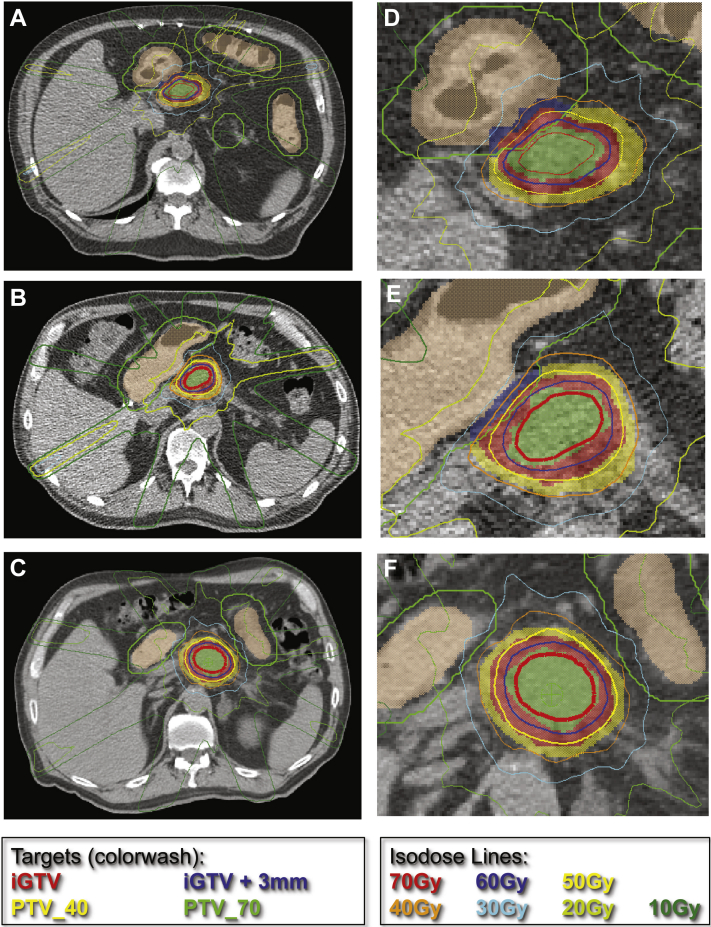
Figure. 3.
Sample plans for 3 individual dose-escalation stereotactic body radiation therapy patients. Representative targets and isodose lines for (A-C) patients 1 to 3, with the critical regions magnified for emphasis in (D-F). These examples demonstrate excellent coverage of prescription target volume (PTV)-70 by the 70 Gy isodose line. The PTV-40 (yellow color wash) was created by adding 3 mm to the integrated gross target volume structure and subtracting the gastrointestinal planning risk volume (green). The PTV-70 structure (green color wash) was created by a 3 mm contraction in 3 dimensions from the integrated gross target volume structure. For all plans, a high level of conformality was maintained, and careful attention was paid that the 40 Gy isodose line (orange) did not cross the internal target volume for gastrointestinal mucosa (beige).
Table 2.
Previously reported dose constraints and associated toxicities
| Target | Overall dose | Constraint | Toxicity observed | Study |
|---|---|---|---|---|
| Duodenum | 45 Gy in 6 fx | D1 < 36 Gy | None >G3 | Comito et al., 20179 |
| Duodenum | 25 Gy in 1 fx | <5% of volume <22.5 Gy; <50% of volume <12.5 Gy | 1% G3 (duodenal stricture), 1% G4 (perforation) | Chang et al., 200910 |
| Stomach | 25 Gy in 1 fx | <4% of volume <22.5 Gy | 4% G3 (gastric ulcers) | Chang et al., 200910 |
| All GI mucosa | V38 < 5 cc; V32.5 < 15 cc; V20 < 30 cc; Maximum dose 42 Gy | No Acute G3+; No late G3+ | Barney et al., 201211 | |
| Duodenum/stomach/small bowel | 35-50 Gy in 5 fx | Max 35 Gy; Mean <20 Gy, V30 < 5 cc, V35 < 1 cc | No acute G3+; 5% Late G3 in 4 patients (GI bleed) | Chuong et al., 201312 |
| Duodenum/Stomach | 33 Gy in 5 fx | V15 Gy < 9 cc; V20 Gy < 3 cc;V33 Gy < 1 cc | 2% acute G3+ (ulcer); 8.5% Late G3+ | Herman et al., 201413 |
| Duodenum | 45 Gy in 6 fx | V36 < 1 cc | None >G2 | Tozzi et al., 20137 |
| Duodenum | 25 Gy in 5 fx | V25 < 1 cc | None >G2 | Gurka et al., 201315 |
| Duodenum/stomach/small bowel | 20-60 Gy in 3-5 fx | Mean <20 Gy; V30 < 2 cc, V35 < 0.5 cc | 7% G3+ (GI bleed) | Mellon et al., 201516 |
| Stomach | 45 Gy in 3 fx | V36 < 10% | None Acute/Late >G3 | Shaib et al., 201617 |
| Duodenum | 45 Gy in 3 fx | <3 cm3 to receive >1.5 Gy/fx | None Acute/Late >G3 | Shaib et al., 201617 |
fx, fraction; G, Grade; GI, gastrointestinal
The conformality index was calculated using prescription isodose volume (PIV; 40 Gy)/PTV, and the homogeneity index was calculated using both D95/D5 and Dmax/Dmin formulas. The gradient index was calculated using 50%/100% PIV.
Statistical analysis
Descriptive statistics were generated for all targets and OARs. The 90th percentile was used as a minimum threshold for reasonably achievable OAR constraints. A minimum coverage of 60% was defined as acceptable for a dose escalation level.
Results
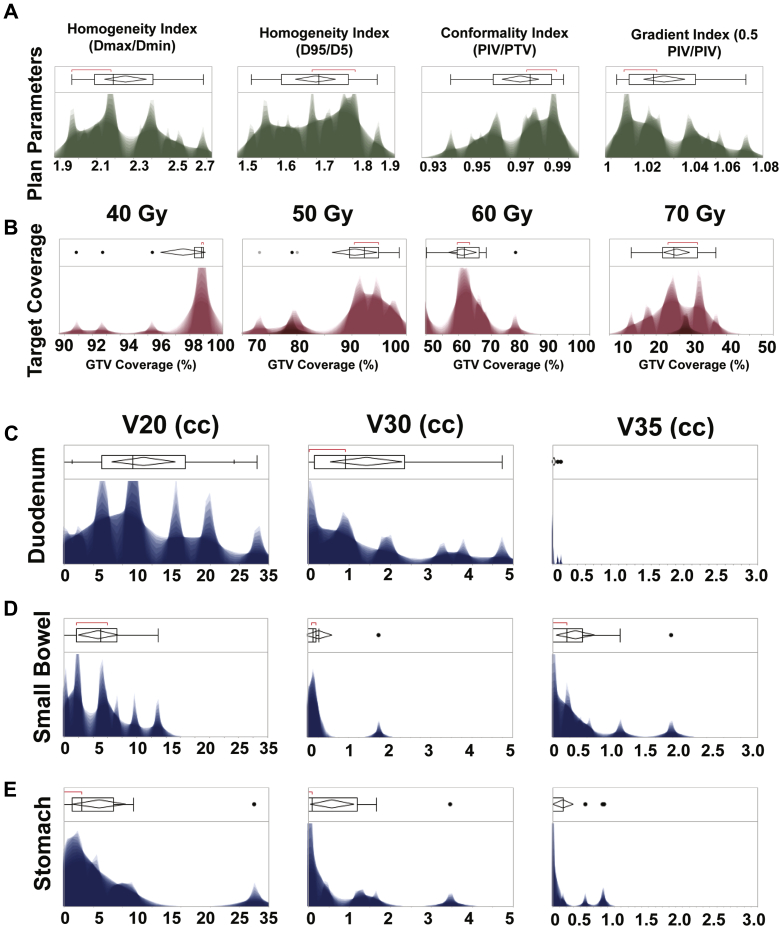
Clinically acceptable DE-SBRT plans on the basis of iGTV and PTV coverage and OAR constraints were generated for 100% of patients who were originally treated with DE-IMRT. Acceptable DE-IMRT plans were also generated for 100% of patients originally planned with SD-SBRT. For the maximum dose-escalated SBRT plans (Fig 4), mean PTV volume was 58.8 cc (range, 8.7-171.91 cc; ±41.3 cc). Mean iGTV volume was 40.4 cc (range, 3.3-119.06 cc; ±31.9 cc). The mean conformality index was quite high at 0.98 (±0.24). The mean homogeneity index using Dmax/Dmin was 2.26 (±0.21), and using D95/D5 it was 1.77 (±0.05). Mean PTV coverage with the 40 Gy line was 97.6% (±0.02%). Mean iGTV coverage by 50 Gy was 91% (±0.07%), by 60 Gy was 61.3% (±0.08%), and by 70 Gy was 24.4% (±0.05%).
Figure. 4.
Distribution of organ-at-risk constraints and gross/planning target volume coverage for all dose-escalation stereotactic body radiation therapy plans. Plan parameters (A) included the homogeneity index (Dmax/Dmin and D95/Dmin), conformality index (prescription isodose volume/planning target volume), and gradient index (0.5 prescription iodose volume/planning target volume). Conformality was overall very high (mean: confidence interval, 0.98 ± 24). Integrated gross target volume coverage (B) was evaluated for percent coverage by the 40 Gy, 50 Gy, 60 Gy, and 70 Gy isodose lines. Mean integrated gross target volume coverage by 50 Gy was 91% (±0.07%), by 60 Gy 61.3% (±0.08%), and by 70 Gy 24.4% (±0.05%). Distributions for V20, V35, and V35 are given for the duodenum (C), small bowel (D), and stomach (E).
Maximum PTV coverage by 70 Gy was 33%. Maximum PTV coverage by 60 Gy was 77.5%. The distributions for conformality and homogeneity and gradient indices and target coverage are provided in Figure 3. The distributions for V20, V30, and V35 for all OARs are provided in Figure 3. The following OAR constraints were achieved for ≥ 90% of the generated plans: Duodenum V20 < 30 cc, V30 < 3 cc, V35 < 1 cc; Small Bowel V20 < 15 cc, V30 < 1 cc, V35 < 0.1 cc; Stomach V20 < 20 cc, V30 < 2 cc, V35 < 1 cc. V40 < 0.5 cc was achieved for all OAR. These achieved dose constraints are listed in Table 1.
Discussion
Dosimetrically, all patients treated with IMRT could also have been treated with SBRT with similar (or improved) BED delivery. Given this, SBRT techniques are worth investigating for patient convenience and continuity of systemic therapy. Based on the 90th percentile used in this analysis as a minimum threshold for achievable dose constraints, dose escalation with an SIB technique to 60 Gy in 5 fractions is achievable while maintaining acceptable target coverage and standard OAR constraints. Although the original goal was 70 Gy in 5 fractions, the overall GTV coverage was low using this goal.
Of note, our study was enriched in patients with a more favorable anatomy (ie, uncinate and body tumors), but we believe that our approach and dose constraints would also apply to any patient who is eligible for pancreatic SBRT. Our SIB approach covered the GTV between 60% and 80% by the highest doses while still maintaining >98% PTV coverage by the 40 Gy line. Pancreatic tumors are particularly hypoxic at their core,7 and delivering high doses to this hypoxic core may have a radiobiologic advantage despite not achieving full target coverage. This concept is similar to the acceptance of dose heterogeneity within the GTV in other forms of SBRT.8, 14 Past SBRT trials, even with 3 and 1 fraction regimens have shown that duodenum, small bowel, and stomach constraints of V20 < 30 cc, V35 < 1 cc, and maximum dose <40 Gy are safe and well tolerated Table 2.
Conclusions
Using these planning techniques, these same dose constraints are achievable in 90% of cases, which supports the idea that dose escalation for LAPC is feasible and should be investigated in clinical trials. Our data provide a roadmap for other clinicians looking to achieve dose escalation up to 60 Gy in 5 fractions for pancreatic cancer in the appropriate setting.
Acknowledgments
L.E.C. acknowledges Radiological Society of North. America (RSNA). C.M.T. acknowledges funding from the Cancer Prevention & Research Institute of Texas (CPRIT) grant RR140012, V Foundation (V2015-22), Kimmel Foundation, Sabin Family Foundation Fellowship and the McNair Foundation.
Footnotes
Conflicts of interest: None.
References
- 1.Von Hoff D.D., Ervin T., Arena F.P. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conroy T., Desseigne F., Ychou M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Hammel P., Huguet F., van Laethem J.L. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: The LAP07 randomized clinical trial. JAMA. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan S., Chadha A.S., Suh Y. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94:755–765. doi: 10.1016/j.ijrobp.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colbert L.E., Moningi S., Chadha A. Dose escalation with an IMRT technique in 15 to 28 fractions is better tolerated than standard doses of 3DCRT for LAPC. Adv Radiat Oncol. 2017;2:403–415. doi: 10.1016/j.adro.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isaji S., Mizuno S., Windsor J.A. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18:2–11. doi: 10.1016/j.pan.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Tozzi A., Comito T., Alongi F. SBRT in unresectable advanced pancreatic cancer: preliminary results of a mono-institutional experience. Radiat Oncol. 2013;8:148. doi: 10.1186/1748-717X-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaparpalvi R., Garg M.K., Shen J. Evaluating which plan quality metrics are appropriate for use in lung SBRT. Br J Radiol. 2018;91:20170393. doi: 10.1259/bjr.20170393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comito T., Cozzi L., Zerbi A. Clinical results of stereotactic body radiotherapy (SBRT) in the treatment of isolated local recurrence of pancreatic cancer after R0 surgery: A retrospective study. Eur J Surg Oncol. 2017;43:735–742. doi: 10.1016/j.ejso.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Chang D.T., Schellenberg D., Shen J. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 11.Barney B.M., Olivier K.R., Macdonald O.K., Fong de Los Santos L.E., Miller R.C., Haddock M.G. Clinical outcomes and dosimetric considerations using stereotactic body radiotherapy for abdominopelvic tumors. Am J Clin Oncol. 2012;35:537–542. doi: 10.1097/COC.0b013e31821f876a. [DOI] [PubMed] [Google Scholar]
- 12.Chuong M.D., Springett G.M., Freilich J.M. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86:516–522. doi: 10.1016/j.ijrobp.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Herman J.M., Chang D.T., Goodman K.A. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarria P., Ascolese A.M., Mancosu P. Volumetric modulated arc therapy with flattening filter free (FFF) beams for stereotactic body radiation therapy (SBRT) in patients with medically inoperable early stage non small cell lung cancer (NSCLC) Radiother Oncol. 2013;107:414–418. doi: 10.1016/j.radonc.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Gurka M.K., Collins S.P., Slack R. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: A pilot trial demonstrating safety. Radiat Oncol. 2013;8:44. doi: 10.1186/1748-717X-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellon E.A., Hoffe S.E., Springett G.M. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. 2015;54:979–985. doi: 10.3109/0284186X.2015.1004367. [DOI] [PubMed] [Google Scholar]
- 17.Shaib W.L., Hawk N., Cassidy R.J. A phase 1 study of stereotactic body radiation therapy dose escalation for borderline resectable pancreatic cancer after modified FOLFIRINOX ( NCT01446458) Int J Radiat Oncol Biol Phys. 2016;96:296–303. doi: 10.1016/j.ijrobp.2016.05.010. [DOI] [PubMed] [Google Scholar]