Abstract
Background
We compared the efficacy between trifocal and bifocal diffractive intraocular lens (IOL) implantation.
Methods
Through PubMed, MEDLINE, EMBASE, and CENTRAL, we searched potentially relevant articles published from 1990 to 2018. Defocus curves, visual acuities (VAs) were measured as primary outcomes. Spectacle dependence, postoperative refraction, contrast sensitivity (CS), glare, and higher-order aberrations (HOAs) were measured as secondary outcomes. Effects were pooled using random-effects method.
Results
We included 11 clinical trials, with a total of 787 eyes (395 subjects). The trifocal IOL group showed better binocular distance VA corrected with defocus levels of −0.5, −1.0, −1.5, and −2.5 diopter than the bifocal IOL group (All P ≤ 0.004). The trifocal IOL group showed better monocular uncorrected distance and intermediate VAs (mean difference [MD], −0.04 logarithm of the minimum angle of resolution [logMAR]; 95% confidence interval [CI], −0.07, −0.01; P = 0.006 and MD, −0.07 logMAR; 95% CI, −0.13, −0.01; P = 0.03, respectively). Postoperative refraction, glare, CS, and HOAs were not significantly different from each other.
Conclusion
The overall findings indicate that trifocal diffractive IOL implantation is better than the bifocal diffractive IOL in intermediate VA, and provides similar or better in distance and near VAs without any major deterioration in the visual quality.
Keywords: Bifocal, Diffractive, Intraocular Lens, Meta-analysis, Trifocal
Graphical Abstract
INTRODUCTION
Cataract extraction with intraocular lens (IOL) implantation is an effective method for restoring visual acuity (VA). The traditional IOL design was monofocal, which targeted distance vision in most patients. One of the main factors for dissatisfaction after monofocal IOL implantation is loss of accommodative ability. The bifocal diffractive IOL was later introduced,1 and has been widely used in patients who want to achieve spectacle independence after cataract surgery.
The bifocal diffractive IOL provides better near VA than the monofocal IOL and similar distance VA.2 More subjects achieve spectacle independence with the bifocal diffractive IOL than with the monofocal IOL.2,3 The bifocal diffractive IOL creates two focal points for near and far distances, so intermediate VA is less than near or far VA.4 Intermediate vision is increasingly important for the younger generation who view the computer screen or dashboard. To overcome the weakness in intermediate vision associated with the bifocal diffractive IOL, the trifocal diffractive IOL was recently introduced.5
The trifocal diffractive IOL theoretically achieves improvements in intermediate vision without impairing near and far vision.6 However, experimental studies have reported that bifocal diffractive IOL shows better modulation transfer function than trifocal diffractive IOL at far focal points, but shows varying results at near and intermediate focal points.7,8,9 Clinical studies also showed inconsistent results in distance, intermediate, and near VAs.10,11,12,13 Furthermore, the most of the clinical studies were limited by the small sample size. Thus, we conducted a meta-analysis to compare visual outcomes, between the trifocal and bifocal diffractive IOLs.
METHODS
Literature search
Two independent investigators conducted an extensive literature search on February 08, 2018, in the PubMed, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov. We restricted all searches to articles published from 1990 to 2018. The main search keywords were ‘trifocal’ and ‘intraocular lens.’ Detailed search strategy is provided in the Supplementary Table 1.
Eligibility criteria
This meta-analytic study followed the tenets of the Declaration of Helsinki. The inclusion criteria for the selection of studies were as follows: 1) studies conducted in adults (18 years and older) who were underwent trifocal or bifocal diffractive IOL implantation because of cataract or refractive lens exchange; 2) randomized controlled trials (RCTs) or non-randomized comparative studies (NRCSs) that compared between trifocal and bifocal diffractive IOLs; 3) studies that reported postoperative VA or defocus curves; 4) studies that included more than 10 eyes to each study arms; and 5) postoperative follow up were more than 1 month. The exclusion criteria for the selection of studies were as follows: 1) nonpublished articles (e.g., conference proceedings and abstracts); 2) studies not published in English; 3) studies with overlapping cases; 4) use of a refractive procedure (e.g., LASIK); and 5) in vitro studies. As this meta-analysis based on existing studies in the published literature, Institutional Review Board approval was not necessary.
Data extraction
Two independent investigators conducted data extraction using pre-defined data fields. A third investigator reviewed the results and a consensus was reached. Binocular corrected defocus curves and distance, intermediate, and near VAs were measured as primary outcome parameters. If near or intermediate VA was assessed at more than one distance, we selected the data associated with a distance closest to 66 cm and 40 cm for intermediate VA and near VA, respectively. Spectacle dependence, postoperative refractive error, glare, contrast sensitivity (CS), and higher-order aberrations (HOAs) were measured as secondary outcome parameters. For meta-analysis of continuous variables, data were converted to mean ± standard deviation. When standard deviation was reported as zero, we replaced zero value with the largest number expected before rounding (e.g., 0.00 to 0.0049). For studies that presented median, maximum, minimum, and interquartile range values, they were converted to mean and standard deviation values using the method outlined by Wan et al.14 When only graphs were available in a study, we measured the values directly from the figures in ImageJ 1.50i (National Institutes of Health, Bethesda, MD, USA) using the method outlined by Sistrom and Mergo.15 For studies with more than two intervention groups (multi-arm studies), we combined groups to create a single pair-wise comparison by using the methods set out in the Cochrane Handbook for Systematic Reviews of Interventions to avoid double-counting study participants.16 We tried to contact the original authors if the detailed information needed.
Quality assessment
The selected study was independently reviewed by 2 investigators. Discrepancies between reviewers were resolved by consensus or adjudication by the third reviewer. The authors independently assessed the sources of the bias in the studies according to the methods set out in the Cochrane Handbook for Systematic Reviews of Interventions for RCTs and the Newcastle-Ottawa Scale for NRCSs.16,17
Statistical analysis
A random-effects model was used for the data analysis, because the designs of studies were heterogeneous and we wished to generalize results to a large population. The inverse variance method was used for continuous data and the Mantel-Haenszel method was used for dichotomous outcomes. For continuous outcome, we used mean difference (MD) because the studies reported similar outcomes and reported them with same scales (e.g., logarithm of the minimum angle of resolution [logMAR]). For dichotomous outcomes, we calculated a risk ratio (RR). When we combine dichotomous and continuous studies together, we used the method described by Chinn18 to convert continuous data to odds ratio and pooled using the generic inverse-variance method.16 Review Manager software version 5.2 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) was used for meta-analyses. Statistical heterogeneity was tested using the χ2 and I2 statistic. A P value less than 0.05 was considered statistically significant.
RESULTS
Search results
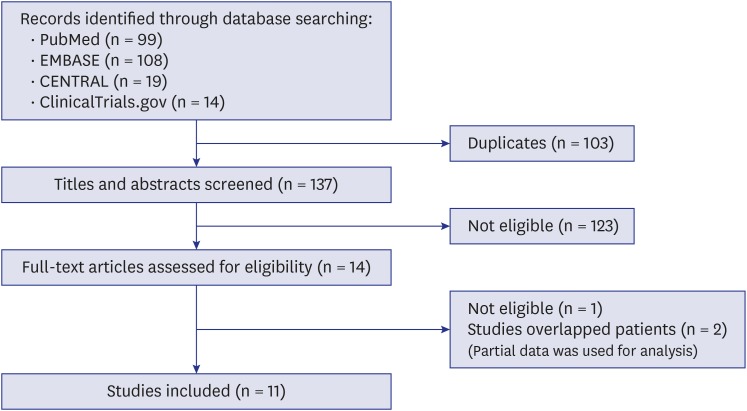
Fig. 1 shows the search process. We found 240 articles, 99 from PubMed, 108 from EMBASE, 19 from CENTRAL, and 14 from ClinicalTrials.gov. One hundred three studies were duplicated. Of 137 articles, 123 articles were excluded after titles and abstracts evaluation. After 14 articles were reviewed in full text, 1 article was excluded because it represented only mean values19 and two articles13,20 were excluded because their patients were duplicated with the other two studies.21,22 In those two overlapped cases, only the most recent results were used for analysis and some non-duplicated results of excluded studies were used for analysis. Of 11 selected studies, 6 were RCTs11,12,21,22,23,24 and 5 were NRCSs10,25,26,27,28 with a total of 395 subjects and 787 eyes (trifocal: 401 eyes of 201 subjects; bifocal: 386 eyes of 194 subjects). All studies were published between 2014 and 2018. Two NRCSs25,28 which were similar study design and written by same author were included because we personally received reply from corresponding author that patients of those studies were not overlapped. The follow up times varied from 1 to 14.43 and all exams were conducted after 1 month. Table 1 shows the characteristics of the included studies. Mix-and-match method (blended implantation) was performed for bifocal IOL implantation in 3 studies. Quality of the studies was summarized in Supplementary Fig. 1.
Fig. 1. Flow chart showing searching strategy used in selection of publications for inclusion in this meta-analysis.
CENTRAL = Cochrane Central Register of Controlled Trials.
Table 1. Characteristics of included studies.
| Study, yr | Design | IOL | Add power | Patients, No. | Eyes, No. | Age (Mean ± SD or Median [IQR]) | Follow up, mon |
|---|---|---|---|---|---|---|---|
| Bilbao-Calabuig et al. (2016)23 | RCT | FineVision | +1.75 and +3.50 D | 12 | 24 | 56.3 ± 6.9 | 3 |
| ReSTOR SN6AD2 (dominant distance eye) and SN6AD1 (contralateral eye) | +2.50 D (SN6AD2), +3.00 D (SN6AD1) | 11 | 22 | ||||
| Brito et al. (2015)10 | NRCS | AT LISA tri 839 M | +1.66 and +3.33 D | 17 | 33 | 57.9 ± 6.6 | 10.27 ± 4.16 |
| AT LISA toric 909 MP | +3.75 D | 8 | 15 | 57.1 ± 11.3 | 7.13 ± 3.48 | ||
| Cochener (2016)24 | RCT | FineVision | +1.75 and +3.50 D | 15 | 30 | 58.7 ± 6.4 | 5.07 ± 1.4 |
| ZMB00 | +4.00 D | 12 | 24 | 60.6 ± 9.1 | 3.42 ± 1.16 | ||
| Gundersen and Potvin (2016)12 | RCT | FineVision toric | +1.75 and +3.50 D | 11 | 22 | 62.1 ± 7.5 | 3 |
| ReSTOR SND1T | +3.00 D | 11 | 22 | 70.2 ± 7.8 | |||
| Gundersen and Potvin (2016)27 | NRCS | AT LISA tri 839 MP | +1.66 and +3.33 D | 25 | 50 | 53 ± 8 | 14.43 ± 7.23 |
| ReSTOR SV25T0 (dominant distance eye) and SN6AD1 (contralateral eye) | +2.50 D (SV25T0), +3.00 D (SN6AD1) | 30 | 60 | 65 ± 9 | 13.7 ± 8.6 | ||
| Jonker et al. (2015)11 | RCT | FineVision | +1.75 and +3.50 D | 15 | 30 | 62.6 ± 8.7 | 6 |
| ReSTOR SN6AD1 | +3.00 D | 13 | 26 | 64.0 ± 8.8 | |||
| Kaymak et al. (2017)21 | RCT | AT LISA tri 839 MP | +1.66 and +3.33 D | 16 | 32 | 62.5 ± 6.9 | 12 |
| AT LISA 809 M, | +3.75 D | 19 | 38 | 64.4 ± 7.5 | |||
| ReSTOR SN6AD1 | +3.00 D | 17 | 34 | 42.4 ± 8.9 | |||
| Mojzis et al. (2017)22 | RCT | AT LISA tri 839 MP | +1.66 and +3.33 D | 20 | 40 | 44–70 | 12 |
| AT LISA 801 | +3.75 D | 18 | 35 | ||||
| Plaza-Puche and Alio (2016)25 | NRCS | AT LISA tri 839 MP | +1.66 and +3.33 D, | 15 | 30 | 63.07 ± 9.66 | 3 |
| FineVision | +1.75 and +3.50 D | 15 | 30 | 66.78 ± 6.20 | |||
| ReSTOR SN6AD1 | +3.00 D | 15 | 30 | 62.15 ± 10.27 | |||
| Plaza-Puche et al. (2016)28 | NRCS | AT LISA tri 839 MP | +1.66 and +3.33 D, | 15 | 30 | 63 (19.00) | 3 |
| FineVision | +1.75 and +3.50 D | 15 | 30 | 68 (7.50) | |||
| Acri.Lisa 366D | +3.75 D, | 15 | 30 | 61 (14.50) | |||
| ReSTOR SN6AD1 | +3.00 D | 15 | 30 | 62 (17.75) | |||
| Vilar et al. (2017)26 | NRCS | PanOptix | +2.17 and +3.25 D | 10 | 20 | 64.2 ± 8.34 | 1 |
| ReSTOR SV25T0 (dominant distance eye) and SN6AD1 (contralateral eye) | +2.50 D (SV25T0), +3.00 D (SN6AD1) | 10 | 20 | 61.9 ± 4.45 |
IOL = intraocular lens, SD = standard deviation, IQR = interquartile range, RCT = randomized controlled trial, NRCS = non-randomised controlled study, D = diopter.
Primary outcomes
Defocus curves
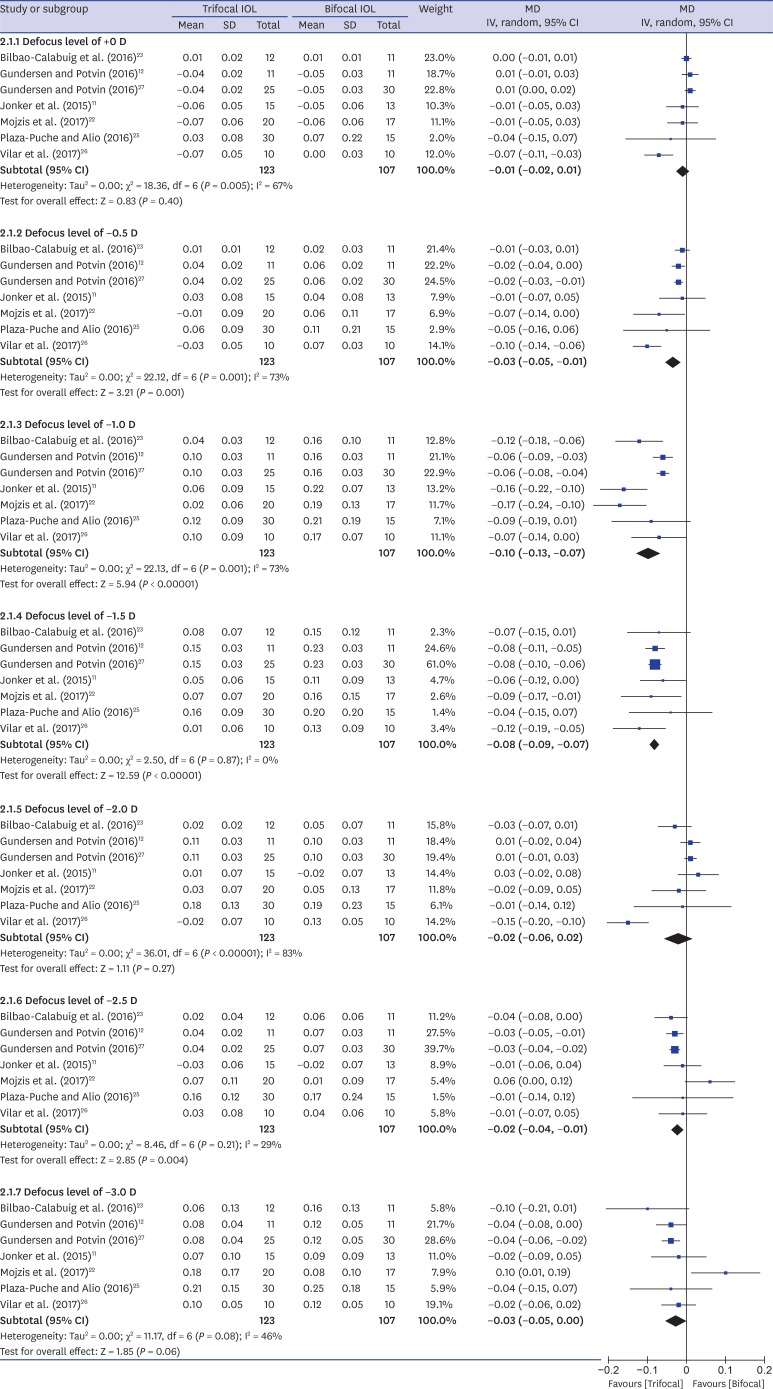
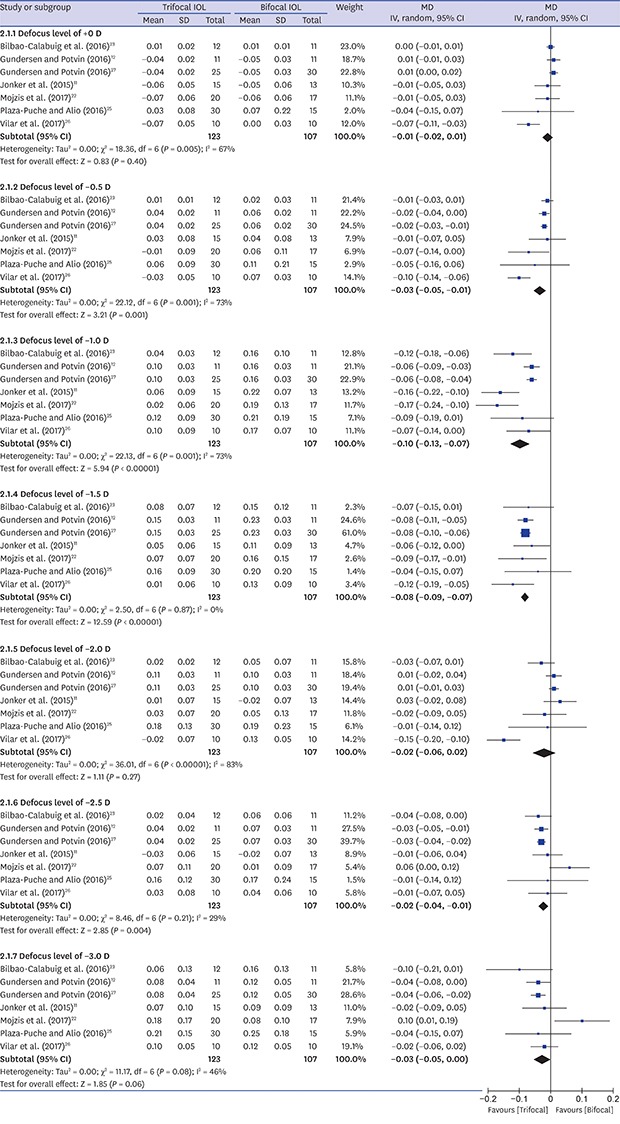
Seven studies11,12,22,23,25,26,27 reported postoperative binocular distance corrected defocus curves (Fig. 2). One study24 that did not describe the condition of examination (binocular/monocular and corrected/uncorrected) was excluded from the analysis. We tried to contact the corresponding author but did not receive a reply. The trifocal IOL group showed better VA in defocus levels of −0.5 (MD, −0.03 logMAR; 95% confidence interval [CI], −0.05, −0.01; P = 0.001), −1.0 (MD, −0.10 logMAR; 95% CI, −0.13, −0.07; P < 0.001), −1.5 (MD, −0.08 logMAR; 95% CI, −0.09, −0.07; P < 0.001), and −2.5 diopter (D) (MD, −0.02 logMAR; 95% CI, −0.04, −0.01; P = 0.004) than the bifocal IOL group.
Fig. 2. Forest plot of trifocal versus bifocal diffractive IOL: binocular corrected defocus curves.
IOL = intraocular lens, MD = mean difference, SD = standard deviation, IV = inverse variance, CI = confidence interval, D = diopter.
Postoperative VA
Monocular VA
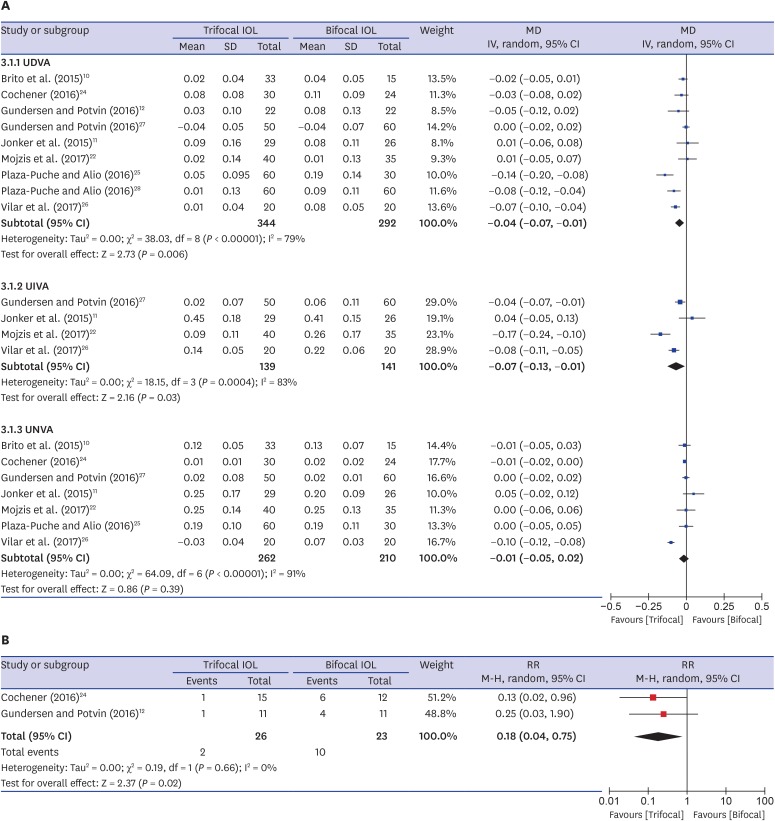
The meta-analysis results for monocular VA outcomes are summarized in Fig. 3A. Nine10,11,12,22,24,25,26,27,28 and four11,22,26,27 studies reported monocular uncorrected distance VA and intermediate VA (UDVA and UIVA), respectively. Meta-analysis showed that the trifocal IOL group was better VA than the bifocal IOL group in monocular UDVA and UIVA (MD, −0.04 logMAR; 95% CI, −0.07, −0.01; P = 0.006 and MD, −0.07 logMAR; 95% CI, −0.13, −0.01; P = 0.03, respectively). Seven10,11,22,24,25,26,27 studies reported monocular uncorrected near VA (UNVA). Meta-analysis showed no significant differences in monocular UNVA. Ten,10,11,12,22,23,24,25,26,27,28 three,11,22,27 and seven10,11,22,24,25,26,27 studies reported monocular corrected distance VA (CDVA), distance corrected intermediate and near VA (DCIVA and DCNVA), respectively. Meta-analysis showed no significant differences in monocular CDVA (MD, −0.01 D; 95% CI, −0.02–0.00; P = 0.05), DCIVA (MD, −0.06 D; 95% CI, −0.18–0.05; P = 0.29), and DCNVA (MD, 0.00 D; 95% CI, −0.00–0.00; P = 0.90) between the two groups.
Fig. 3. Forest plot of trifocal versus bifocal diffractive IOL: (A) monocular VAs and (B) the number of patients who did not achieve binocular UIVA of 0.1 logMAR.
IOL = intraocular lens, VA = visual acuity, UDVA = uncorrected distance visual acuity, UIVA = uncorrected intermediate visual acuity, UNVA = uncorrected near visual acuity, logMAR = logarithm of the minimum angle of resolution, SD = standard deviation, MD = mean difference, IV = inverse variance, CI = confidence interval, M-H = Mantele-Haenszel, RR = risk ratio.
Binocular VA
Four studies11,12,21,24 reported binocular UDVA, UIVA, and UNVA. Meta-analysis showed no significant differences in binocular UDVA (MD, −0.02 logMAR; 95% CI, −0.04–0.01; P = 0.21), UIVA (MD, −0.03 logMAR; 95% CI, −0.07–0.01; P = 0.15), and UNVA (MD, 0.00 logMAR; 95% CI, −0.00–0.00; P = 0.97). Three,11,12,21 two,11,21 and two11,21 studies reported binocular CDVA, DCIVA, and DCNVA, respectively. Meta-analysis showed no significant differences in binocular CDVA (MD, −0.01 logMAR; 95% CI, −0.03–0.01; P = 0.57), DCIVA (MD, 0.01 logMAR; 95% CI, −0.11–0.14; P = 0.83) and DCNVA (MD, 0.01 logMAR; 95% CI, −0.07–0.08; P = 0.84) between the two groups. Two studies12,24 reported the prevalence of binocular UIVA of 0.1 logMAR or better as a good visual outcome. The proportion of patients who did not achieve binocular UIVA of 0.1 logMAR was lower in the trifocal IOL (7.7%) than in the bifocal IOL (43.5%) (RR, 0.18; 95% CI, 0.04–0.75; P = 0.02) (Fig. 3B).
Secondary outcomes
Spectacle dependence
Two studies12,24 reported spectacle dependence. The number of patients who required spectacles was not different between the two groups (trifocal IOL group [10.0%]; bifocal IOL group [29.2%]; RR, 0.38; 95% CI, 0.13–−1.13; P = 0.08).
Postoperative refractive error
Five,10,13,24,25,28 eight,10,11,12,13,24,25,27,28 and six11,12,13,23,26,27 studies reported postoperative sphere, cylinder, and spherical equivalent results, respectively. The meta-analysis results showed that sphere (MD, 0.09 D; 95% CI, −0.06–0.24; P = 0.22), cylinder (MD, 0.07 D; 95% CI, 0.00–0.14; P = 0.05), and spherical equivalent (MD, 0.05 D; 95% CI, −0.08–0.17; P = 0.47) were not significantly different between the two groups.
CS
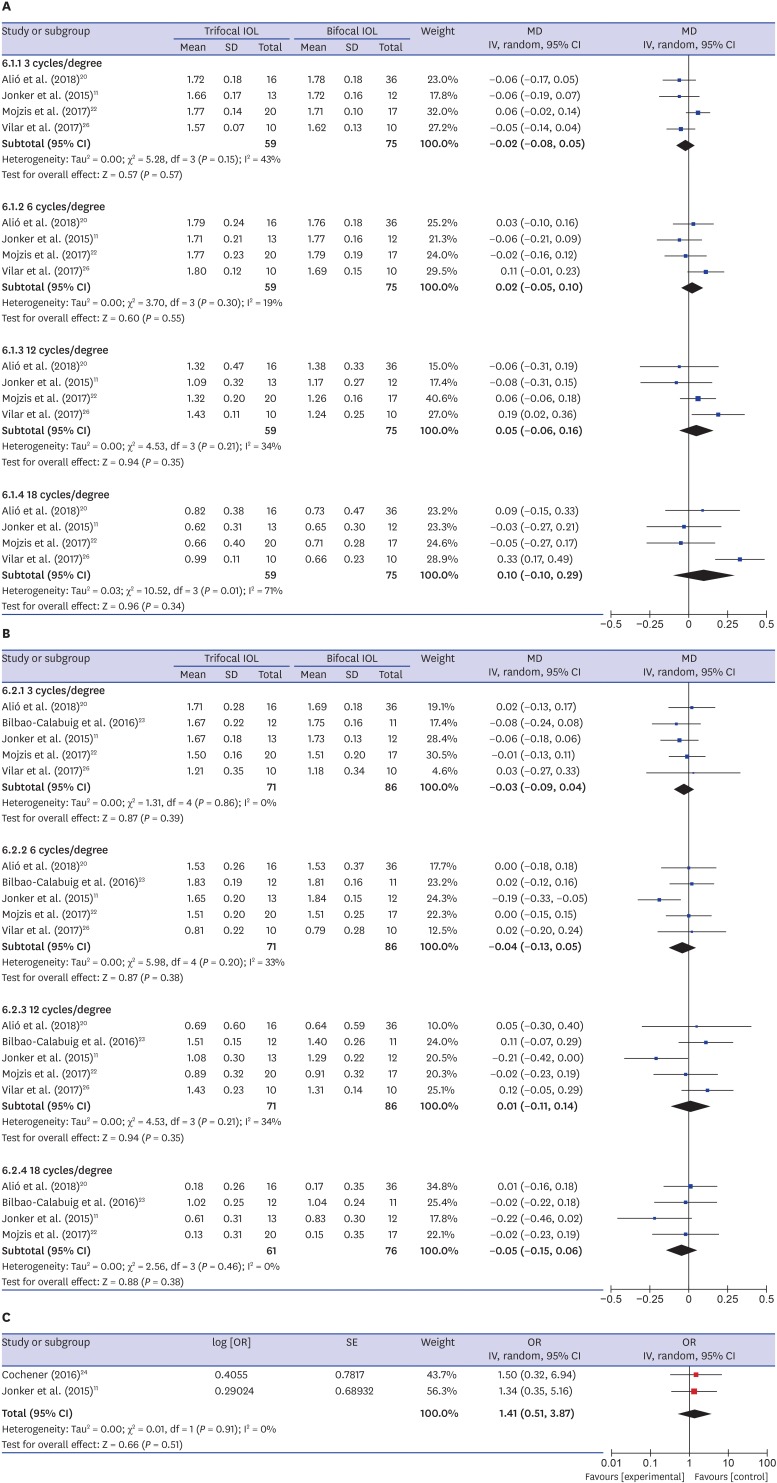
Binocular CS under photopic and mesopic condition reported in four11,20,22,26 and five11,20,22,23,26 studies. Meta-analysis showed no significant differences in 3, 6, 12, and 18 cycles per degree between the two groups (Fig. 4A and B).
Fig. 4. Forest plot of trifocal versus bifocal diffractive IOL: binocular CS under photopic (A) and mesopic (B) conditions; and glare (C).
IOL = intraocular lens, MD = mean difference, CS = contrast sensitivity, SD = standard deviation, IV = inverse variance, CI = confidence interval, SE = standard error, OR = odds ratio.
Glare
Two studies11,24 reported glare symptom. The pooled RR was not significantly different between the two groups (RR, 1.41; 95% CI, 0.51–3.87; P = 0.51) (Fig. 4C).
HOAs
Two studies13,24 reported coma, trefoil, and spherical aberration. Meta-analysis showed no significant differences in coma (MD, −0.02; 95% CI, −0.04–0.01; P = 0.16), trefoil (MD, −0.00; 95% CI, −0.04–0.04; P = 0.98), and spherical aberration (MD, 0.04; 95% CI, −0.04–0.12; P = 0.32) between the two groups.
DISCUSSION
The initial bifocal diffractive IOL was designed with +4.0 D add power. Afterwards, bifocal diffractive IOL with +3.0 D add power was introduced in order to improve intermediate VA.29,30 However, the bifocal diffractive IOL was intrinsically designed to focus on near and far distances and had limitations in intermediate VA.31,32 The trifocal diffractive IOL was developed to overcome the poor intermediate vision of the bifocal diffractive IOL by using 3 different focuses (far, intermediate, and near).6
In this analysis, the trifocal IOL group had better visual outcomes in binocular corrected defocus levels of −0.5, −1.0, −1.5, and −2.5 D. As the trifocal diffractive IOL has an inherent IOL design to improve intermediate distance vision, it would be expected that the trifocal IOL group showed better results in defocus levels of −0.5, −1.0, and −1.5 D, which correspond to distances of 2.0 m, 1.0 m, and 67 cm of VAs, respectively.25 Interestingly, the trifocal IOL group showed better or comparable outcomes compared to the bifocal IOL group for defocus levels of −2.5 D and −3.0 D, respectively. Although we do not know the exact mechanism of this phenomenon, we suggested some plausible explanations and added relevant sentences in the Discussion section as follows: 1) There were 3 studies that used the mix-and-match method (blended implantation) for the bifocal IOL implantation. The add power (the focal point) was different in each eye of the bifocal IOL groups. Therefore, it may affect the binocular near vision. When those 3 mix-and-match studies were excluded, the trifocal IOL group showed no statistical difference in defocus levels of −2.5 D and −3.0 D, when compared to the bifocal IOL group. We added this result as one of the explanations in the Discussion section and in Supplementary Fig. 2. 2) Binocular testing using defocus curve may affect near vision in a better way than trifocal IOL, although bifocal IOL has more strength in monocular near vision. 3) Another possible reason is that longitudinal chromatic aberration (LCA). LCA for near of trifocal IOL in one study33 may be smaller than that of bifocal IOL in other studies.34,35 Nevertheless, given that MD in defocus level of −2.5 D was −0.02 logMAR, indicates less than a line difference in decimal scale of the Snellen chart at near vision, which seems to be clinically irrelevant. This outcome in our study corresponds to a previous study that showed comparable outcome in near VA between trifocal and bifocal IOLs.36
The trifocal IOL group showed a better outcome in monocular UDVA and UIVA. More patients achieved binocular UIVA < 0.1 logMAR in trifocal IOL group. However, there were no significant differences in monocular UNVA, CDVA, DCIVA and DCNVA, and all binocular VAs. Monocular UDVA was better in the trifocal group than in the bifocal group. However, this difference was small (MD, −0.04 logMAR) and most studies accomplished mean monocular UDVA less than 0.1 logMAR. It might be due to less astigmatism in the trifocal group than in bifocal group, although it was not significant (MD, 0.07 D; 95% CI, 0.00–0.14; P = 0.05). Together with the results of defocus curves and VA, the trifocal diffractive IOL is better than the bifocal diffractive IOL in terms of intermediate VA and is comparable in near and distance VAs.
Regarding spectacle independence, bifocal diffractive IOLs have resulted in less spectacle dependence than monofocal IOLs.37 The trifocal IOL group showed better results than the bifocal IOL groups but this was not significant. We pooled data from only two studies with a total of 54 subjects. Further study might be needed to confirm this result.
Regarding the quality of vision, glare, a positive dysphotopsia, is one of the factors related with dissatisfaction after multifocal IOL implantation. Glare has been reported more in diffractive IOLs than in monofocal IOLs38 and some patients wanted to IOL exchange procedure after multifocal IOL implantation because of this phenomenon.39 In this analysis, the trifocal IOL group was not different from the bifocal IOL group in glare phenomenon. Reduction of CS is also the cause of a decrease in quality of vision and has been reported with the diffractive IOL37 because it divides light into several focuses and forms several images of an object at a certain distance.40 Bifocal IOLs included in this study have an asymmetrical light distribution of 50% to 70% for distance focus and 18% to 50% for near focus.23 Trifocal IOLs included in this study have light distribution of 42% to 50% for distance focus, 14% to 20% for intermediate focus, and 29% to 30% for near focus.23,32 Although the trifocal diffractive IOL has more number of focuses and less light energy, the trifocal IOL group was not different from the bifocal IOL group in CS.
There were several limitations in this analysis. First, our study was based on 11 studies restricted to articles published in English. We did not search unpublished studies or abstracts of relevant conference meetings. Second, the studies included in this study evaluated VA at various intermediate (60, 66, 70, and 80 cm) and near (33 and 40 cm) distances. Heterogeneity was high in monocular and binocular VAs. Third, the conditions of VA varied (e.g., corrected/uncorrected or monocular/binocular) so that most meta-analyses did not include all 11 studies. Fourth, given that 3 studies of the bifocal IOL group, with blended implantation to strengthen the binocular intermediate vision, were included, it may affect the outcome of near vision.
Previously, two meta-analysis were reported favorable aspect of trifocal diffractive IOL.36,41 After then, two RCTs21,22 and one NRCS26 have been reported, and we analyzed various outcomes, including the results of these studies. To the best of our knowledge, this is the largest evidence-based meta-analysis comparing trifocal and bifocal diffractive IOLs. In summary, this meta-analysis found that the trifocal diffractive IOL showed better intermediate VA than the bifocal diffractive IOL. Near and distance VAs of the trifocal diffractive IOL are comparable with those of the bifocal diffractive IOL. The quality of vision like glare, CS, and HOAs were also similar between the two groups.
ACKNOWLEDGMENTS
We thank Dr. Soraya M.R. Jonker, University Eye Clinic Maastricht, Maastricht University Medical Center, the Netherlands, for providing data of glare.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Kim MK. Data curation: Yoon CH, Shin I. Formal analysis: Yoon CH, Shin I. Methodology: Yoon CH, Shin I, Kim MK. Software: Yoon CH, Shin I. Validation: Shin I, Kim MK. Writing - original draft: Yoon CH. Writing - review & editing: Shin I, Kim MK.
SUPPLEMENTARY MATERIALS
Detailed search strategy
Summary of quality of the studies: (A) randomized controlled studies by Cochrane risk of bias tool, (B) non-randomized controlled studies by Newcastle-Ottawa Scale.
Forest plot of the binocular corrected defocus curves: subgroup analysis after exclusion of the 3 studies that used the mix-and-match method.
References
- 1.Keates RH, Pearce JL, Schneider RT. Clinical results of the multifocal lens. J Cataract Refract Surg. 1987;13(5):557–560. doi: 10.1016/s0886-3350(87)80114-1. [DOI] [PubMed] [Google Scholar]
- 2.Calladine D, Evans JR, Shah S, Leyland M. Multifocal versus monofocal intraocular lenses after cataract extraction. Sao Paulo Med J. 2015;133(1):68. doi: 10.1590/1516-3180.20151331T2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah S, Peris-Martinez C, Reinhard T, Vinciguerra P. Visual outcomes after cataract surgery: multifocal versus monofocal intraocular lenses. J Refract Surg. 2015;31(10):658–666. doi: 10.3928/1081597X-20150611-01. [DOI] [PubMed] [Google Scholar]
- 4.Lane SS, Morris M, Nordan L, Packer M, Tarantino N, Wallace RB., 3rd Multifocal intraocular lenses. Ophthalmol Clin North Am. 2006;19(1):89–105. doi: 10.1016/j.ohc.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Voskresenskaya A, Pozdeyeva N, Pashtaev N, Batkov Y, Treushnicov V, Cherednik V. Initial results of trifocal diffractive IOL implantation. Graefes Arch Clin Exp Ophthalmol. 2010;248(9):1299–1306. doi: 10.1007/s00417-010-1424-8. [DOI] [PubMed] [Google Scholar]
- 6.Gatinel D, Pagnoulle C, Houbrechts Y, Gobin L. Design and qualification of a diffractive trifocal optical profile for intraocular lenses. J Cataract Refract Surg. 2011;37(11):2060–2067. doi: 10.1016/j.jcrs.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 7.Montés-Micó R, Madrid-Costa D, Ruiz-Alcocer J, Ferrer-Blasco T, Pons AM. In vitro optical quality differences between multifocal apodized diffractive intraocular lenses. J Cataract Refract Surg. 2013;39(6):928–936. doi: 10.1016/j.jcrs.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 8.Madrid-Costa D, Ruiz-Alcocer J, Ferrer-Blasco T, García-Lázaro S, Montés-Micó R. Optical quality differences between three multifocal intraocular lenses: bifocal low add, bifocal moderate add, and trifocal. J Refract Surg. 2013;29(11):749–754. doi: 10.3928/1081597X-20131021-04. [DOI] [PubMed] [Google Scholar]
- 9.Papadatou E, Del Águila-Carrasco AJ, Esteve-Taboada JJ, Madrid-Costa D, Montés-Micó R. Assessing the in vitro optical quality of presbyopic solutions based on the axial modulation transfer function. J Cataract Refract Surg. 2016;42(5):780–787. doi: 10.1016/j.jcrs.2015.11.049. [DOI] [PubMed] [Google Scholar]
- 10.Brito P, Salgado-Borges J, Neves H, Gonzalez-Meijome J, Monteiro M. Light-distortion analysis as a possible indicator of visual quality after refractive lens exchange with diffractive multifocal intraocular lenses. J Cataract Refract Surg. 2015;41(3):613–622. doi: 10.1016/j.jcrs.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 11.Jonker SM, Bauer NJ, Makhotkina NY, Berendschot TT, van den Biggelaar FJ, Nuijts RM. Comparison of a trifocal intraocular lens with a +3.0 D bifocal IOL: results of a prospective randomized clinical trial. J Cataract Refract Surg. 2015;41(8):1631–1640. doi: 10.1016/j.jcrs.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Gundersen KG, Potvin R. Comparison of visual outcomes after implantation of diffractive trifocal toric intraocular lens and a diffractive apodized bifocal toric intraocular lens. Clin Ophthalmol. 2016;10:455–461. doi: 10.2147/OPTH.S103375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mojzis P, Kukuckova L, Majerova K, Liehneova K, Piñero DP. Comparative analysis of the visual performance after cataract surgery with implantation of a bifocal or trifocal diffractive IOL. J Refract Surg. 2014;30(10):666–672. doi: 10.3928/1081597X-20140903-06. [DOI] [PubMed] [Google Scholar]
- 14.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sistrom CL, Mergo PJ. A simple method for obtaining original data from published graphs and plots. AJR Am J Roentgenol. 2000;174(5):1241–1244. doi: 10.2214/ajr.174.5.1741241. [DOI] [PubMed] [Google Scholar]
- 16.Shinichi A. Cochrane handbook for systematic reviews of interventions. Online Kensaku. 2014;35(3):154–155. [Google Scholar]
- 17.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. http://www.ohri.ca/Programs/clinical_epidemiology/oxford.asp. [Accessed Oct 19, 2016].
- 18.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19(22):3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Postolache C, Postolache O. Comparation of refractive results with bifocal implants at Lisa 809 and trifocal at Lisa tri839. Rom J Ophthalmol. 2015;59(2):100–102. [PMC free article] [PubMed] [Google Scholar]
- 20.Alió JL, Kaymak H, Breyer D, Cochener B, Plaza-Puche AB. Quality of life related variables measured for three multifocal diffractive intraocular lenses: a prospective randomised clinical trial. Clin Exp Ophthalmol. 2018;46(4):380–388. doi: 10.1111/ceo.13084. [DOI] [PubMed] [Google Scholar]
- 21.Kaymak H, Breyer D, Alió JL, Cochener B. Visual performance with bifocal and trifocal diffractive intraocular lenses: a prospective three-armed randomized multicenter clinical trial. J Refract Surg. 2017;33(10):655–662. doi: 10.3928/1081597X-20170504-04. [DOI] [PubMed] [Google Scholar]
- 22.Mojzis P, Kukuckova L, Majerova K, Ziak P, Piñero DP. Postoperative visual performance with a bifocal and trifocal diffractive intraocular lens during a 1-year follow-up. Int J Ophthalmol. 2017;10(10):1528–1533. doi: 10.18240/ijo.2017.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bilbao-Calabuig R, González-López F, Amparo F, Alvarez G, Patel SR, Llovet-Osuna F. Comparison between mix-and-match implantation of bifocal intraocular lenses and bilateral implantation of trifocal intraocular lenses. J Refract Surg. 2016;32(10):659–663. doi: 10.3928/1081597X-20160630-01. [DOI] [PubMed] [Google Scholar]
- 24.Cochener B. Prospective clinical comparison of patient outcomes following implantation of trifocal or bifocal intraocular lenses. J Refract Surg. 2016;32(3):146–151. doi: 10.3928/1081597X-20160114-01. [DOI] [PubMed] [Google Scholar]
- 25.Plaza-Puche AB, Alio JL. Analysis of defocus curves of different modern multifocal intraocular lenses. Eur J Ophthalmol. 2016;26(5):412–417. doi: 10.5301/ejo.5000780. [DOI] [PubMed] [Google Scholar]
- 26.Vilar C, Hida WT, de Medeiros AL, Magalhães KRP, de Moraes Tzelikis PF, Chaves MAPD, et al. Comparison between bilateral implantation of a trifocal intraocular lens and blended implantation of two bifocal intraocular lenses. Clin Ophthalmol. 2017;11:1393–1397. doi: 10.2147/OPTH.S139909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gundersen KG, Potvin R. Comparison of visual outcomes and subjective visual quality after bilateral implantation of a diffractive trifocal intraocular lens and blended implantation of apodized diffractive bifocal intraocular lenses. Clin Ophthalmol. 2016;10:805–811. doi: 10.2147/OPTH.S107162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plaza-Puche AB, Alio JL, Sala E, Mojzis P. Impact of low mesopic contrast sensitivity outcomes in different types of modern multifocal intraocular lenses. Eur J Ophthalmol. 2016;26(6):612–617. doi: 10.5301/ejo.5000777. [DOI] [PubMed] [Google Scholar]
- 29.Liu JW, Haw WW. Optimizing outcomes of multifocal intraocular lenses. Curr Opin Ophthalmol. 2014;25(1):44–48. doi: 10.1097/ICU.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 30.de Vries NE, Webers CA, Montés-Micó R, Ferrer-Blasco T, Nuijts RM. Visual outcomes after cataract surgery with implantation of a +3.00 D or +4.00 D aspheric diffractive multifocal intraocular lens: comparative study. J Cataract Refract Surg. 2010;36(8):1316–1322. doi: 10.1016/j.jcrs.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 31.Cochener B, Vryghem J, Rozot P, Lesieur G, Heireman S, Blanckaert JA, et al. Visual and refractive outcomes after implantation of a fully diffractive trifocal lens. Clin Ophthalmol. 2012;6:1421–1427. doi: 10.2147/OPTH.S32343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marques EF, Ferreira TB. Comparison of visual outcomes of 2 diffractive trifocal intraocular lenses. J Cataract Refract Surg. 2015;41(2):354–363. doi: 10.1016/j.jcrs.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 33.Vinas M, Gonzalez-Ramos A, Dorronsoro C, Akondi V, Garzon N, Poyales F, et al. In vivo measurement of longitudinal chromatic aberration in patients implanted with trifocal diffractive intraocular lenses. J Refract Surg. 2017;33(11):736–742. doi: 10.3928/1081597X-20170814-01. [DOI] [PubMed] [Google Scholar]
- 34.Millán MS, Vega F, Ríos-López I. Polychromatic image performance of diffractive bifocal intraocular lenses: longitudinal chromatic aberration and energy efficiency. Invest Ophthalmol Vis Sci. 2016;57(4):2021–2028. doi: 10.1167/iovs.15-18861. [DOI] [PubMed] [Google Scholar]
- 35.Millán MS, Vega F. Extended depth of focus intraocular lens: chromatic performance. Biomed Opt Express. 2017;8(9):4294–4309. doi: 10.1364/BOE.8.004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen Z, Lin Y, Zhu Y, Liu X, Yan J, Yao K. Clinical comparison of patient outcomes following implantation of trifocal or bifocal intraocular lenses: a systematic review and meta-analysis. Sci Rep. 2017;7(1):45337. doi: 10.1038/srep45337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Silva SR, Evans JR, Kirthi V, Ziaei M, Leyland M. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst Rev. 2016;12:CD003169. doi: 10.1002/14651858.CD003169.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiam PJ, Chan JH, Aggarwal RK, Kasaby S. ReSTOR intraocular lens implantation in cataract surgery: quality of vision. J Cataract Refract Surg. 2006;32(9):1459–1463. doi: 10.1016/j.jcrs.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 39.de Vries NE, Webers CA, Touwslager WR, Bauer NJ, de Brabander J, Berendschot TT, et al. Dissatisfaction after implantation of multifocal intraocular lenses. J Cataract Refract Surg. 2011;37(5):859–865. doi: 10.1016/j.jcrs.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 40.Cetinkaya S, Dadaci Z, Acir NO, Cetinkaya YF, Yener HI, Ozcimen M. Visual outcomes of multifocal intraocular lens implantation in patients with cataract and high hyperopia and patient selection. Int J Ophthalmol. 2015;8(6):1258–1260. doi: 10.3980/j.issn.2222-3959.2015.06.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Z, Cao D, Chen X, Wu S, Wang X, Wu Q. Comparison of clinical performance between trifocal and bifocal intraocular lenses: a meta-analysis. PLoS One. 2017;12(10):e0186522. doi: 10.1371/journal.pone.0186522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed search strategy
Summary of quality of the studies: (A) randomized controlled studies by Cochrane risk of bias tool, (B) non-randomized controlled studies by Newcastle-Ottawa Scale.
Forest plot of the binocular corrected defocus curves: subgroup analysis after exclusion of the 3 studies that used the mix-and-match method.