Abstract
Purpose
Reirradiation for recurrent glioma remains controversial without knowledge of optimal patient selection, dose, fractionation, and normal tissue tolerances. We retrospectively evaluated outcomes and toxicity after conventionally fractionated reirradiation for recurrent high-grade glioma, along with the impact of concurrent chemotherapy.
Methods and materials
We conducted a retrospective review of patients reirradiated for high-grade glioma recurrence between 2007 and 2016 (including patients with initial low-grade glioma). Outcome metrics included overall survival (OS), prognostic factors for survival, and treatment-related toxicity.
Results
Patients (n = 118; median age 47 years; median Karnofsky performance status score: 80) were re-treated at a median of 28 months (range, 5-214 months) after initial radiation therapy. The median reirradiation dose was 41.4 Gy (range, 12.6-54.0 Gy) to a median lesion volume of 202 cm3 (range, 20-901 cm3). The median cumulative (initial radiation and reirradiation combined) potential maximum brainstem dose was 76.9 Gy (range, 5.0-108.3 Gy) and optic apparatus dose was 56.0 Gy (range, 4.5-90.9 Gy). Of the patients, 56% received concurrent temozolomide, 14%, bevacizumab, and 11%, temozolomide plus bevacizumab; 19% had no chemotherapy. The planned reirradiation was completed by 90% of patients. Median OS from the completion of reirradiation was 9.6 months (95% confidence interval [CI], 7.5-11.7 months) for all patients and 14.0, 11.5, and 6.7 months for patients with initial grade 2, 3, and 4 glioma, respectively. On multivariate analysis, better OS was observed with a >24-month interval between radiation treatments (hazard ratio [HR]: 0.3; 95% CI, 0.2-0.5; P < .001), reirradiation dose >41.4 Gy (HR: 0.6; 95% CI, 0.4-0.9; P = .03), and gross total resection before reirradiation (HR: 0.6, 95% CI, 0.3-0.9; P = .02). Radiation necrosis and grade ≥3 late neurotoxicity were both minimal (<5%). No symptomatic persistent brainstem or optic nerve/chiasm injury was identified.
Conclusions
Salvage reirradiation, even at doses >41.4 Gy in conventional fractionation, along with chemotherapy, was safe and well tolerated with meaningful survival duration. These data provide information that may be useful in implementing safe reirradiation treatments for appropriately selected patients and guiding future studies to define optimal reirradiation doses, maximal safe doses to critical structures, and the role of systemic therapy.
Summary.
Repeat radiation therapy for recurrent high-grade glioma remains controversial, with limited knowledge on optimal patient selection, dose and fractionation, and normal tissue tolerances. We report outcomes and toxicities of patients treated with reirradiation, over half with concurrent temozolomide, for glioma recurrence. Our results suggest that reirradiation with higher dose conventionally fractionated radiation therapy (>41.4 Gy) and concurrent temozolomide is safe and well tolerated, even to larger volume recurrences, with meaningful survival duration.
Introduction
Recurrent high-grade glioma carries a poor prognosis, with no consensus on standard treatment regimen among the options of re-resection, additional systemic therapy, and reirradiation.1, 2 Retrospective studies suggest that cranial reirradiation is safe and potentially effective in the context of the aggressive behavior of pretreated malignant glioma, in particular for smaller recurrent tumor volumes, with survival after reirradiation of approximately 8 to 12 months.3, 4, 5, 6, 7, 8, 9, 10 Both conventionally fractionated and hypofractionated stereotactic radiation therapy techniques have been employed in the recurrent glioma setting. Stereotactic radiosurgery and hypofractionated stereotactic radiation therapy are limited to very small volume recurrences (eg, up to 30 cm3), with higher rates of toxicity reported for larger treatment volumes.11, 12, 13, 14, 15, 16
Conventionally fractionated radiation therapy (typically to 36 Gy) has been shown to be well tolerated for larger recurrent tumor volumes, but only up to a median tumor volume of 75 cm3 in published studies.3, 4, 5, 7, 8, 10, 14 In these studies, the tolerance of the brain and critical structures has not been explored; thus maximally safe doses are unknown. With regard to the use of concurrent systemic therapy, bevacizumab (BEV) has been used and studied more frequently in conjunction with conventionally fractionated reirradiation for glioma recurrence.5, 8, 17 Concurrent temozolomide (TMZ), well established in the initial treatment of high-grade glioma,18 has only been studied in a limited number of patients in the recurrent setting,3, 7 and thus, its value in this setting is still uncertain given prior TMZ use with initial treatment and potential development of resistance.19
Here, we present the outcomes of patients with recurrent or progressive high-grade glioma who were treated at our institution using a standardized approach with a median reirradiation dose of 41.4 Gy; more than half were treated with concurrent TMZ. We assessed cumulative radiation doses to critical structures of the optic pathways and brainstem. Outcome metrics included treatment-related toxicity and overall survival (OS). Prognostic factors for survival were assessed to identify patient-specific factors that may be associated with better outcomes and help optimize treatment strategies.
Methods and materials
Patient selection and treatment information collected
A retrospective review of patients treated at our institution was conducted with approval of the institutional review board. The inclusion criteria were 1) prior treatment with radiation therapy for a glioma diagnosis (World Health Organization grades 1-4); 2) development of recurrent or progressive grade 3 or 4 glioma with or without surgical resection (transformation from low grade to high-grade glioma was always confirmed by biopsy or resection) treated at our institution with repeat radiation therapy; and 3) at least 1 follow-up visit at our institution after repeat radiation therapy.
The following information was reviewed for eligible patients at the time of repeat radiation therapy: 1) patient characteristics (age, sex, Eastern Cooperative Oncology Group performance status, Karnofsky performance status [KPS], date of initial diagnosis based on initial pathology confirming glioma, initial glioma grade, O-6-methylguanine-DNA methyltransferase [MGMT] and isocitrate dehydrogenase [IDH] mutation status, histology, date of disease recurrence by magnetic resonance imaging [MRI] or surgical pathology); 2) treatment information for both initial radiation therapy and reirradiation (treatment dates, technique, dose including maximum dose to the brainstem and optic nerves/chiasm, volume, reirradiation field overlap, and steroid use within 3 months of treatment); 3) systemic therapy with initial and repeat radiation therapy; and 4) clinical outcomes (date KPS <60, neurologic side effects at last follow-up, Radiation Therapy Oncology Group [RTOG] acute and late central nervous system [CNS] toxicity, radiation necrosis, and date and cause of death).
Treatment technique
Patients were treated with conventionally fractionated external beam radiation therapy and all but 1 patient with intensity modulated radiation therapy. Patients were immobilized with a thermoplastic mask. The prescribed doses ranged from 30 to 54 Gy in 1.5 to 2.0 Gy fractions, with dose decision based on the highest dose judged likely safe and tolerable by the treating physician. The standard reirradiation volume was to the T1-weighted contrast-enhancing lesion only on MRI with a margin of 0.5 to 1.0 cm. TMZ was typically administered at a 75 mg/m2 daily dose when utilized during radiation therapy. BEV was typically administered at 10 mg/kg every 14 days.
The cumulative dose to the optic nerves, chiasm, and brainstem were reported from a composite plan of initial radiation and reirradiation treatments when available. Otherwise, the cumulative dose to these structures was reported as a maximum point dose from combined plans using a cautious assumption of complete overlap over these points on the basis of the concern that plan and image fusion technology is not accurate enough to determine overlap of individual points with precision. The maximum potential combined point dose to the optic nerves was generally used to assess risks and guide treatment planning with a bias toward safety. A typical maximum point dose in the re-treatment plan of 24 Gy was allowed (underdosing the target if needed) if the nerves had previously received a dose near the standard tolerance criteria of 50 to 54 Gy, with higher repeat-radiation doses considered allowable if the nerves had received lower doses in the initial treatment. The brainstem was limited to a maximum potential reirradiation point dose of 100 Gy.
Outcomes analyzed and statistical analysis
The following outcomes were assessed: 1) clinical outcome (date KPS <60, date and cause of death, or date of last follow-up); 2) potential radiation-related neurotoxicity (neurologic side effects at last follow-up, RTOG acute and late CNS toxicity, dexamethasone requirement, and radiation necrosis, defined as symptomatic growth of the treated lesion on conventional MRI and either strong radiographic suggestion or pathologic confirmation of radiation necrosis); 3) grade of myelosuppression within 1 month after reirradiation, as measured by Common Terminology Criteria for Adverse Events version 4.0 grade for leukopenia, lymphopenia, neutropenia, anemia, and thrombocytopenia. OS was calculated as time to death from the completion of reirradiation. Length of follow-up was defined as the time from completion of reirradiation to the last oncology visit (if the patient was still alive) or death.
Survival was evaluated using Kaplan-Meier analysis with a log-rank (Mantel-Cox) test for differences between groups. A multivariate analysis of OS was performed using Cox regression analysis. Factors evaluated on univariate analysis were included in the multivariate analysis with the exception of initial glioma grade (due to correlation with time between radiation treatments), concurrent systemic therapy (due to small numbers in some groups and selection criteria for use of different regimens), and IDH1/MGMT promoter status (given only a subset of patients with this information). The effect of treatment parameters on late CNS toxicity and radiation necrosis was analyzed using the χ2 or Fisher's exact test (for small samples, with any group having n <5). All statistical analyses were carried out using SPSS v24.0 software.
Results
Patient characteristics and treatment information
We analyzed all patients (n = 118) treated at our institution with radiation therapy for grade 3 or 4 glioma at recurrence between 2007 and 2016 (initial glioma grade 1-4) who had at least 1 follow-up evaluation. The patient characteristics are described in Table 1. At the time of initial diagnosis, the majority of patients had a high-grade glioma (76%) and received concurrent TMZ with their initial radiation treatment. The median initial radiation therapy dose received for patients with high-grade glioma was 60 Gy (range, 45-60 Gy, all but 3 patients received 60 Gy); dose for those with low-grade glioma was 54 Gy (range, 50.4-60 Gy). MGMT promoter methylation and IDH1 mutation status was known for just less than half of patients (47% and 44%, respectively). Of those with available information, 53% had MGMT promotor methylation (47% nonmethylated), and 35% were IDH1 mutated (65% wild type). For those with initial grade 2 gliomas, 69% of tumors were IDH1 mutated. Patients had a median follow-up of 7.2 months (range, 0.1-82.9 months) from reirradiation.
Table 1.
Patient demographics
| Characteristics | Number | % |
|---|---|---|
| Total number patients | 118 | |
| Sex | ||
| Male | 61 | 51.7 |
| Female | 57 | 48.3 |
| Age, y | ||
| Median: 47 (range, 14-78) | ||
| Karnofsky performance status score | ||
| Median: 80 (range, 40-100) | ||
| ≤80 | 62 | 52.5 |
| >80 | 52 | 44.1 |
| Unknown | 4 | 3.4 |
| Initial grade glioma | ||
| 1-2 | 28 | 23.7 |
| 3 | 27 | 22.9 |
| 4 | 63 | 53.4 |
| Initial radiation therapy dose | ||
| Grade 1-2 Median: 54 Gy (range, 50.4-60 Gy) | ||
| Grade 3-4 Median: 60 Gy (range 45-60 Gy) | ||
| Recurrent grade glioma | ||
| 3 | 30 | 25.4 |
| 4 | 87 | 73.7 |
| Unknown | 1 | 0.9 |
| MGMT promoter | ||
| Nonmethylated | 26 | 22.0 |
| Methylated | 29 | 24.6 |
| Unknown | 63 | 53.4 |
| IDH1 | ||
| Wild-type | 34 | 28.8 |
| Mutated | 18 | 15.3 |
| Unknown | 66 | 55.9 |
IDH1, Isocitrate dehydrogenase 1; MGMT, O6-methylguanine DNA methyltransferase.
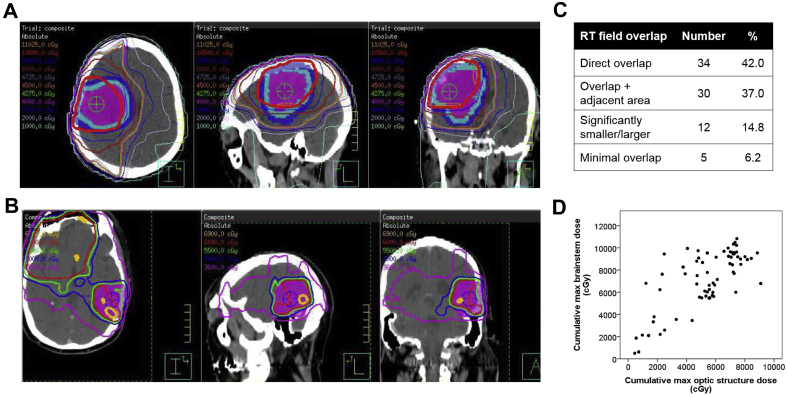
Patients were treated at a median interval of 27.6 months between the completion of initial radiation therapy and the beginning of repeat irradiation (range, 4.8-214.2 months; Table 2). The median reirradiation dose was 41.4 Gy (range, 12.6-54.0 Gy) to a median volume of 202 cm3 (range, 20-901 cm3). Of patients with evaluable initial and reirradiation treatment plans, the median cumulative maximum doses (from both treatments combined) to the brainstem and optic structure (optic chiasm and nerves) were 76.9 Gy (range, 5.0-108.3 Gy) and 56.0 Gy (range, 4.5-90.9 Gy), respectively (Table 2, Fig 1D). Nearly half of patients (42%) had direct overlap (ie, similar volume and location) between their initial and repeat radiation treatment fields, and 37% had repeat treatment fields that overlapped but also included an adjacent region not initially treated (Fig 1A-C). The remainder of patients had either significantly smaller or larger volumes at reirradiation (15%) or had fields that minimally overlapped (6%).
Table 2.
Treatment information
| Radiation therapy | Median | Min | Max |
|---|---|---|---|
| Reirradiation dose received (Gy) | 41.4 | 12.6 | 54.0 |
| Reirradiation volume (cm3) | 202 | 20 | 901 |
| Cumulative maximum brainstem dose (Gy) | |||
| All evaluable plansa | 76.9 | 5.0 | 108.3 |
| Composite onlyb | 67.9 | 5.0 | 99.5 |
| Cumulative maximum optic structure dose (Gy) | |||
| All evaluable plans | 56.0 | 4.5 | 90.9 |
| Composite only | 55.6 | 4.5 | 90.9 |
| Time from initial to repeat radiation (mos) | 27.6 | 4.8 | 214.2 |
| Concurrent systemic therapy | Number | % |
|---|---|---|
| Temozolomide | 66 | 55.9 |
| Bevacizumab | 16 | 13.6 |
| Temozolomide+bevacizumab | 13 | 11.0 |
| Other | 1 | 0.8 |
| None | 22 | 18.6 |
Cumulative maximum brainstem and optic structure doses are reported for:
All patients with plan information from initial and reirradiation treatments (summation of maximum point doses anywhere in the structure when composite plan fusion not available) (n = 69).
Patients with composite plan fusions only (n = 41).
Figure 1.
Sample composite treatment plans with (A) significant and (B) minimal overlap between initial and subsequent radiation treatment fields. Isodose lines represent composite dose of initial and repeat radiation. Shaded contours represent recurrent gross tumor volume (pink), clinical target volume (light blue), and planning target volume (dark blue). (C) Percent of reirradiation treatments with direct overlap with the initial treatment field, overlap but also inclusion of an adjacent area not previously treated to full dose, overlap but reirradiation field is significantly smaller or larger than the initial field, and minimal/<20% overlap with the prior field. (D) Cumulative maximum brainstem and optic structure doses (cGy) for patients treated with reirradiation (combined initial and repeat radiation treatments, composite plan fusion when available, summation of maximum point dose anywhere in structure otherwise).
Concurrent TMZ was given to 56% of patients, BEV to 14% (approximately 60% were BEV naïve), and TMZ+BEV for 11% (approximately 80% were BEV naïve); no systemic therapy was used for 19% of patients (Table 2). Of the patients, 90% completed their planned reirradiation course, and this was not affected by the use of concurrent systemic therapy.
Treatment toxicity
With a median follow-up of 7.2 months, minimal neurotoxicity was noted with reirradiation (Table 3). Acute grade 3+ CNS toxicity was seen in <10% of patients, and close to 70% of patients (n = 80) experienced grade 2 toxicity, primarily from the short-term requirement of steroids during or within 3 months after reirradiation. Concurrent treatment with BEV was not associated with less acute toxicity (P > .05). No late grade 3+ CNS toxicity was noted, and radiation necrosis was reported in 3.4% of all patients (5% of patients who could be evaluated for late toxicity). At the cumulative brainstem and optic structure doses described, no symptomatic persistent brainstem or optic nerve/chiasm injury was identified. Late RTOG CNS toxicity, radiation necrosis, and short-term steroid requirement were not significantly affected by reirradiation volume, maximum brainstem/optic structure dose, treatment with concurrent chemotherapy, or time interval of reirradiation (P > .05). Long-term symptoms noted in follow-up included weakness (34%), word-finding difficulties (34%), fatigue (30%), and headaches (13%), but the majority of these were present before repeat radiation or were attributable to disease progression.
Table 3.
Acute and late neurotoxicity
| Neurotoxicity | Number | % |
|---|---|---|
| RTOG acute CNS morbidity | ||
| Grade 0 | 10 | 8.5 |
| Grade 1 | 10 | 8.5 |
| Grade 2 | 80 | 67.8 |
| Grade 3 | 11 | 9.3 |
| Unknown | 7 | 5.9 |
| RTOG late CNS morbidity | ||
| Grade 0 | 37 | 31.4 |
| Grade 1 | 23 | 19.5 |
| Grade 2 | 13 | 11.0 |
| Grade 3 | 0 | 0.0 |
| N/A | 29 | 24.6 |
| Unknown | 16 | 13.6 |
| Radiation necrosis | ||
| Yes | 4 | 3.4a |
| No | 82 | 69.5 |
| N/A | 26 | 22.0 |
| Unknown | 6 | 5.1 |
| Long-term symptoms | ||
| Weakness | 40 | 33.9 |
| Word-finding | 40 | 33.9 |
| Fatigue | 35 | 29.7 |
| Headaches | 15 | 12.7 |
| Memory loss | 11 | 9.3 |
| Gait instability | 7 | 5.9 |
| Vision changes | 4 | 3.4 |
CNS, central nervous system; RTOG, Radiation Therapy Oncology Group.
Radiation necrosis in 3.4% of all patients but 5% of patients who could be evaluated for late toxicity.
Reirradiation was combined with concurrent chemotherapy in 80% of treatments, but resulted in only limited myelosuppression. Less than 10% of patients with evaluable laboratory work had grade 3+ anemia (1%), leukopenia (5%), neutropenia (6%), and thrombocytopenia (8%), but 30% of patients experienced grade 3+ lymphopenia within 1 month after completion of reirradiation.
Survival outcomes
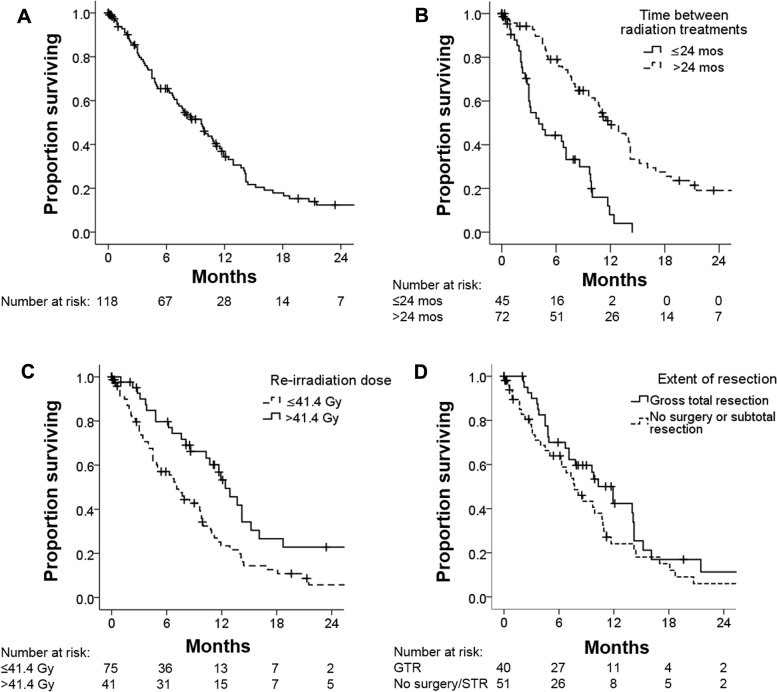
Median OS from the end of reirradiation for all patients was 9.6 months (95% confidence interval, 7.5-11.7 months). On univariate analysis, better survival was seen for patients with higher KPS, lower initial glioma grade (median OS: 14.0, 11.5, and 6.7 months for patients with initial grade 2, 3, and 4 glioma, respectively), lower recurrent glioma grade, longer time between initial and second course of radiation, and reirradiation dose >41.4 Gy (Table 4, Fig 2). Although differences in survival were observed in patients treated with different concurrent systemic therapies, we note that selection criteria played a major role in treatment with concurrent TMZ versus BEV (or both), including MGMT promoter methylation status and extent of disease and edema, and thus any direct comparisons are difficult to make. Age, MGMT promoter and IDH status, reirradiation volume, and extent of surgical resection were not significantly associated with differences in survival on univariate analysis. On multivariate analysis, longer time interval between radiation courses and reirradiation dose remained associated with better survival, but the extent of surgical resection became significantly correlated with survival outcome (Table 4).
Table 4.
Overall survival from completion of reirradiation
| Variable | Median overall survival Months (95% CI) |
P-value (log rank) | Multivariate |
|
|---|---|---|---|---|
| HR (95% CI) | P-value | |||
| All patients | 9.6 (7.5-11.7) | |||
| Age, y | ||||
| ≤50 | 9.8 (7.9-11.7) | 0.60 | Ref | .59 |
| >50 | 7.1 (5.8-8.4) | 0.9 (0.5-1.5) | ||
| KPS | ||||
| ≤80 | 6.8 (4.3-9.3) | 0.009 | Ref | |
| >80 | 11.7 (10.1-13.3) | 0.8 (0.5-1.3) | .31 | |
| Initial grade | ||||
| 2 | 14.0 (8.9-19.1) | 0.003 | ||
| 3 | 11.5 (10.1-12.9) | |||
| 4 | 6.7 (4.5-8.9) | |||
| Recurrent grade | ||||
| 3 | 11.9 (9.8-14.0) | 0.009 | Ref | |
| 4 | 7.3 (4.5-10.1) | 1.8 (1.0-3.5) | .06 | |
| MGMT promoter | ||||
| Non-methylated | 8.5 (4.2-12.8) | 0.15 | ||
| Methylated | 11.9 (5.8-18.0) | |||
| IDH1 | ||||
| Wild type | 9.6 (4.1-15.1) | 0.24 | ||
| Mutated | 14.0 (10.8-17.2) | |||
| Time between radiation treatments, mo | ||||
| ≤24 mo | 4.1 (2.1-6.1) | <0.0001 | Ref | |
| >24 mo | 11.9 (9.1-14.7) | 0.3 (0.2-0.5) | < .001 | |
| Concurrent systemic therapy | ||||
| TMZ | 10.8 (9.2-12.4) | 0.007 | ||
| BEV | 6.6 (5.7-7.5) | |||
| TMZ+BEV | 4.8 (0.8-8.8) | |||
| None | 11.5 (0.9-22.1) | |||
| Reirradiation dose received | ||||
| ≤41.4 Gy | 7.1 (5.5-8.7) | 0.006 | Ref | |
| >41.4 Gy | 12.4 (10.1-14.7) | 0.6 (0.4-0.9) | .03 | |
| Reirradiation volume | ||||
| ≤200 cm3 | 10.0 (7.7-12.3) | 0.07 | Ref | .27 |
| >200 cm3 | 7.6 (2.5-12.7) | 1.3 (0.8-2.1) | ||
| Surgical resection before reirradiation | ||||
| None or STR | 7.7 (5.6-9.8) | 0.14 | Ref | .03 |
| GTR | 11.9 (9.2-14.6) | 0.6 (0.3-0.9) | ||
BEV, bevacizumab; CI, confidence interval; GTR, gross total resection; HR, hazard ratio; IDH1, Isocitrate dehydrogenase 1; KPS, Karnofsky performance status; MGMT, O6-methylguanine DNA methyltransferase; Ref, reference; STR, subtotal resection; TMZ, temozolomide.
Figure 2.
Overall survival from completion of reirradiation for (A) all patients, (B) those with ≤24 months versus >24 months between radiation treatments, (C) those with reirradiation to <41.4 Gy versus 41.4 Gy, and (D) those who had gross total resection versus no surgery or subtotal resection prior to reirradiation.
Discussion
Repeat radiation for high-grade glioma recurrence with doses up to 45 Gy (or 54 Gy if minimal overlap with the prior field), along with concurrent daily TMZ, appears to be appropriately safe even for large reirradiation volumes, with survival outcomes that justify use as a salvage option in this poor-prognosis situation. In our data set, the median survival from reirradiation was 9.6 months for all patients (grades 3 and 4 glioma at recurrence) and 7.3 months for patients with recurrent glioblastoma. Patients with initial low-grade glioma had better survival after reirradiation, 14 months versus 7 months for those with initial glioblastoma. This outcome is similar to survival after other interventions for recurrent high-grade glioma, such as resection with placement of carmustine wafers (7.2 months),20 TMZ chemotherapy (5.4-9.4 months),21, 22 and tumor treating fields (6.6 months).23
We have identified prognostic factors for OS that may help predict which patients may benefit most from repeat radiation, although in the absence of other good salvage options, repeat radiation regimens may still be considered for patients who do not meet favorable criteria because survival outcome may still be considered meaningful by individual patients. As shown in Table 4, factors associated with better survival after reirradiation on multivariate analysis included a >24-month interval from initial radiation (which correlates with initial glioma grade; thus, this factor was not included in the multivariate analysis), reirradiation dose >41.4 Gy, and gross total resection before reirradiation. These prognostic factors are consistent with those suggested by Combs et al.24, 25, 26 Interestingly, the reirradiation volume was not associated with differences in survival such that larger volume might not be an appropriate exclusion factor for patients who may otherwise be appropriately treated with this approach.
Within a retrospective review, it is not possible to evaluate definitively the effect of treatment parameters on outcome. When judged likely to be safe, doses of 45 Gy to the recurrent tumor volume were planned. We did not attempt to identify a maximum tolerated reirradiation dose considering the likely limited clinical benefit. In addition, the maximum tolerated dose may be affected not only by maximum doses to critical structures but also by treatment volume and interval between treatments. Indeed, a small number of patients whose recurrent tumors had minimal overlap with the previously irradiated tumor volumes received reirradiation doses up to 54 Gy to the target.
With regard to concurrent systemic therapy, selection factors and small patient numbers limit our ability to draw major conclusions with regard to an optimal concurrent systemic therapy regimen (or whether concurrent systemic therapy should always be recommended). Reirradiation with concurrent TMZ appeared to be well tolerated and did not affect completion of the reirradiation course in this cohort. Given that MGMT promoter methylation status was only known in a subset of patients, we cannot comment on whether MGMT status should help guide systemic therapy decisions in the reirradiation setting. Nonetheless, based on the currently available safety data from this retrospective review and supported by the benefit of high-dose radiation and concurrent TMZ previously confirmed in the initial therapy of high-grade glioma,18 we recommend treatment with concurrent TMZ and a dose of 45 Gy when judged appropriately safe for high-grade glioma recurrence.
The standard implementation of salvage repeat radiation will require an understanding of maximum tolerated reirradiation volumes and combined dose to critical structures, such as optic nerves, chiasm, and brainstem. Generally, reirradiation volumes in this study included the T1-weighted contrast-enhancing lesion only on MRI with a margin of 0.5 to 1.0 cm and, in most cases, directly overlapped with the bulk of the original treatment volumes. In our study, the median reirradiation volume was 200 cm3 (maximum: 900 cm3), which is higher than in most reirradiation studies where median volumes have been typically 30 to 50 cm3.3, 4, 7 In some cases, volume was not possible to determine accurately because initial treatment was often received outside our institution over many years. However, overall severe injury was uncommon (Table 3) with larger volume reirradiation within the limitations of assessment in a retrospective study. Importantly, we did not pursue formal neurocognitive testing and were not able to capture side effects in a systematic fashion, which would be better done in a prospective study.
We await the results of an ongoing prospective phase 1 trial of dose escalation for reirradiation in recurrent glioblastoma, conducted by the National Cancer Institute (NCT02709226). In this study, the reirradiation dose will be increased from 3.5 Gy × 10 fractions to 3.5 Gy × 14 fractions as tolerated, with BEV allowed as concurrent therapy. In addition, the GlioCave study is evaluating gross total resection followed by observation or reirradiation to the tumor cavity to 46 Gy in 2 Gy per fraction or 39 Gy in 3 Gy per fraction for recurrent glioblastoma.27 In the meantime, we encourage the development of an understanding of tolerance by reporting injuries in the literature.
The maximum cumulative doses reported in this study are based on either composite plan fusions in which both the initial radiation and reirradiation treatments and plan fusions were completed at our institution when available, or otherwise a conservative approach of summation of maximum point dose anywhere in the critical structure. These resulted in very similar maximum cumulative doses (Table 2). With cumulative maximum brainstem and optic structure doses as described in Table 2, no persistent symptomatic injuries were encountered. Because significant injury was not encountered during the follow-up period using our approach, careful dose escalation may be feasible and requires further study. Dose escalation to the point of significant brainstem or optic nerve injury may not be warranted, and we encourage reporting of observed injuries to provide guidance about risk thresholds.
Conclusions
Repeat radiation using conventional fractionation up to a dose of 45 Gy with concurrent TMZ is a safe and potentially effective therapy for recurrent high-grade glioma. More information is needed about the tolerance of critical structures, which may limit the safe administration of 45 Gy in many circumstances but also allow for dose escalation when not at risk. Our data provide guidance for the safe implementation of repeat radiation and for the design of prospective trials needed for optimal patient selection, dose/fractionation decisions, confirmation of safe dose limits, and optimal systemic therapy.
Footnotes
Sources of support: This work was supported in part by the Nicholl Family Foundation.
Conflicts of interest: Dr. Kristin J. Redmond reports grants from Elekta AB, grants and nonfinancial support from Accuray, and personal fees from AstraZeneca and Medtronic outside the submitted work. Dr. Lawrence R. Kleinberg reports grants and personal fees from Novocure and Accuray, and personal fees from Merck outside the submitted work.
References
- 1.Birk H.S., Han S.J., Butowski N.A. Treatment options for recurrent high-grade gliomas. CNS Oncol. 2017;6:61–70. doi: 10.2217/cns-2016-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard S.P., Krauze A., Chan M.D., Tsien C., Tomé W.A. The evolving role for re-irradiation in the management of recurrent grade 4 glioma. J Neurooncol. 2017;134:523–530. doi: 10.1007/s11060-017-2392-1. [DOI] [PubMed] [Google Scholar]
- 3.Combs S.E., Bischof M., Welzel T. Radiochemotherapy with temozolomide as re-irradiation using high precision fractionated stereotactic radiotherapy (FSRT) in patients with recurrent gliomas. J Neurooncol. 2008;89:205–210. doi: 10.1007/s11060-008-9607-4. [DOI] [PubMed] [Google Scholar]
- 4.Combs S.E., Thilmann C., Edler L., Debus J., Schulz-Ertner D. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: Long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23:8863–8869. doi: 10.1200/JCO.2005.03.4157. [DOI] [PubMed] [Google Scholar]
- 5.Flieger M., Ganswindt U., Schwarz S.B. Re-irradiation and bevacizumab in recurrent high-grade glioma: An effective treatment option. J Neurooncol. 2014;117:337–345. doi: 10.1007/s11060-014-1394-5. [DOI] [PubMed] [Google Scholar]
- 6.Fogh S.E., Andrews D.W., Glass J. Hypofractionated stereotactic radiation therapy: An effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28:3048–3053. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minniti G., Armosini V., Salvati M. Fractionated stereotactic reirradiation and concurrent temozolomide in patients with recurrent glioblastoma. J Neurooncol. 2011;103:683–691. doi: 10.1007/s11060-010-0446-8. [DOI] [PubMed] [Google Scholar]
- 8.Niyazi M., Ganswindt U., Schwarz S.B. Irradiation and Bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol. 2012;82:67–76. doi: 10.1016/j.ijrobp.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Scholtyssek F., Zwiener I., Schlamann A. Reirradiation in progressive high-grade gliomas: Outcome, role of concurrent chemotherapy, prognostic factors and validation of a new prognostic score with an independent patient cohort. Radiat Oncol. 2013;8:161. doi: 10.1186/1748-717X-8-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wick W., Fricke H., Junge K. A phase II, randomized, study of weekly APG101+reirradiation versus reirradiation in progressive glioblastoma. Clin Cancer Res. 2014;20:6304–6313. doi: 10.1158/1078-0432.CCR-14-0951-T. [DOI] [PubMed] [Google Scholar]
- 11.Combs S.E., Widmer V., Thilmann C., Hof H., Debus J., Schulz-Ertner D. Stereotactic radiosurgery (SRS) Cancer. 2005;104:2168–2173. doi: 10.1002/cncr.21429. [DOI] [PubMed] [Google Scholar]
- 12.Hall W.A., Djalilian H.R., Sperduto P.W. Stereotactic radiosurgery for recurrent malignant gliomas. J Clin Oncol. 1995;13:1642–1648. doi: 10.1200/JCO.1995.13.7.1642. [DOI] [PubMed] [Google Scholar]
- 13.Shrieve D.C., Alexander E., Wen P.Y. Comparison of stereotactic radiosurgery and brachytherapy in the treatment of recurrent glioblastoma multiforme. Neurosurgery. 1995;36:275–282. doi: 10.1227/00006123-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Cho K.H., Hall W.A., Gerbi B.J., Higgins P.D., McGuire W.A., Clark H.B. Single dose versus fractionated stereotactic radiotherapy for recurrent high-grade gliomas. Int J Radiat Oncol. 1999;45:1133–1141. doi: 10.1016/s0360-3016(99)00336-3. [DOI] [PubMed] [Google Scholar]
- 15.Hudes R.S., Corn B.W., Werner-Wasik M. A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int J Radiat Oncol. 1999;43:293–298. doi: 10.1016/s0360-3016(98)00416-7. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd S.F., Laing R.W., Cosgrove V.P. Hypofractionated stereotactic radiotherapy in the management of recurrent glioma. Int J Radiat Oncol. 1997;37:393–398. doi: 10.1016/s0360-3016(96)00455-5. [DOI] [PubMed] [Google Scholar]
- 17.Schnell O., Thorsteinsdottir J., Fleischmann D.F. Re-irradiation strategies in combination with bevacizumab for recurrent malignant glioma. J Neurooncol. 2016;130:591–599. doi: 10.1007/s11060-016-2267-x. [DOI] [PubMed] [Google Scholar]
- 18.Stupp R., Mason W.P., Van Den Bent M.J. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 19.Messaoudi K., Clavreul A., Lagarce F. Toward an effective strategy in glioblastoma treatment. Part I: resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discov Today. 2015;20:899–905. doi: 10.1016/j.drudis.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Brem H., Piantadosi S. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 21.Brandes A.A., Ermani M., Basso U. Temozolomide as a second-line systemic regimen in recurrent high-grade glioma: A phase II study. Ann Oncol. 2001;12:255–257. doi: 10.1023/a:1008336732273. [DOI] [PubMed] [Google Scholar]
- 22.Chang S.M., Theodosopoulos P., Lamborn K. Temozolomide in the treatment of recurrent malignant glioma. Cancer. 2004;100:605–611. doi: 10.1002/cncr.11949. [DOI] [PubMed] [Google Scholar]
- 23.Stupp R., Wong E.T., Kanner A.A. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192–2202. doi: 10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Combs S.E., Edler L., Rausch R., Welzel T., Wick W., Debus J. Generation and validation of a prognostic score to predict outcome after re-irradiation of recurrent glioma. Acta Oncol. 2013;52:147–152. doi: 10.3109/0284186X.2012.692882. [DOI] [PubMed] [Google Scholar]
- 25.Kessel K.A., Hesse J., Straube C. Modification and optimization of an established prognostic score after re-irradiation of recurrent glioma. PLoS One. 2017;12:e0180457. doi: 10.1371/journal.pone.0180457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Combs S.E., Kessel K.A., Hesse J. Moving second courses of radiotherapy forward: Early re-irradiation after surgical resection for recurrent gliomas improves efficacy with excellent tolerability. Neurosurgery. 2018 doi: 10.1093/neuros/nyx629. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Straube C., Scherb H., Gempt J. Adjuvant stereotactic fractionated radiotherapy to the resection cavity in recurrent glioblastoma – the GlioCave study (NOA 17 – ARO 2016/3 – DKTK ROG trial) BMC Cancer. 2018;18:15. doi: 10.1186/s12885-017-3928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]