Abstract
Ubiquitination is a prevalent post-translational modification involved in all aspects of cell physiology. It is mediated by an enzymatic cascade and the E2 ubiquitin–conjugating enzymes (UBCs) lie at its heart. Even though E3 ubiquitin ligases determine the specificity of the reaction, E2s catalyze the attachment of ubiquitin and have emerged as key mediators of chain assembly. They are largely responsible for the type of linkage between ubiquitin moieties and thus, the fate endowed onto the modified substrate. However, in vivo E2–E3 pairing remains largely unexplored. We therefore interrogated the interaction selectivity between 37 Arabidopsis E2s and PUB22, a U-box type E3 ubiquitin ligase that is involved in the dampening of immune signaling. We show that whereas the U-box domain, which mediates E2 docking, is able to interact with 18 of 37 tested E2s, the substrate interacting armadillo (ARM) repeats impose a second layer of specificity, allowing the interaction with 11 E2s. In vitro activity assayed by autoubiquitination only partially recapitulated the in vivo selectivity. Moreover, in vivo pairing was modulated during the immune response; pairing with group VI UBC30 was inhibited, whereas interaction with the K63 chain-building UBC35 was increased. Functional analysis of ubc35 ubc36 mutants shows that they partially mimic pub22 pub23 pub24 enhanced activation of immune responses. Together, our work provides a framework to interrogate in vivo E2–E3 pairing and reveals a multi-tiered and dynamic E2–E3 network.
Keywords: ubiquitin, ubiquitin-conjugating enzyme (E2 enzyme), E3 ubiquitin ligase, innate immunity, cell signaling
Introduction
To date no physiological E2 ubiquitin–conjugating enzyme–E3 ubiquitin ligase pair has been reported for plants. Most research has focused on E3 ligases because they are the specificity determinants of the ubiquitination process. However, E2s mediate the attachment of ubiquitin mainly onto lysine residues of a substrate, in most cases guided by one of the more than 1400 E3s encoded in the Arabidopsis genome. The surfacing complexity of the different functions carried out by E2s contradicts their early image as simple carriers of activated ubiquitin. They are first loaded with ubiquitin by the ubiquitin-activating enzyme E1 via transthiolation forming a thioester bond with their catalytic cysteine and subsequently are recognized by an E3 ligase. The E2 then discharges ubiquitin onto either the E3, a process commonly used to test E3 activity termed autoubiquitination, or onto a substrate, in which case the E3 acts as an adaptor.
E2s have emerged as key mediators of ubiquitin chain assembly and have been demonstrated to be able to govern the switch from ubiquitin chain initiation to elongation, as well as regulate the processivity of chain formation (1). Substrates can be modified by attachment of a ubiquitin polymer (polyubiquitination), in which single moieties are linked to one another through one of seven lysine residues present in ubiquitin, or its initial methionine (2).
Importantly, E2s to a large extent dictate the Lys residue within ubiquitin used to link the moieties in a chain (1). The resulting different linkage types lead to alternative topologies of the polyubiquitin chains, which in turn are responsible for the different fates endowed onto the modified proteins (2). These can include the degradation of proteins tagged with Lys48-linked ubiquitin chains by the 26S proteasome, or regulate the transit of integral membrane proteins through the endomembrane system.
The Arabidopsis genome encodes 37 ubiquitin E2 enzymes, all of which display a highly conserved cysteine residue at the predicted catalytic site and are classified into 16 groups based on the similarity to each other (3). Compared with ubiquitin ligases, relatively little is known about the biological processes in which specific E2s participate. However, we recently were able to show intrinsic biochemical activity of 31 E2s in an orthogonal system using synthetic biology (3, 4), confirming that most of the predicted, but largely unexplored E2s, display activity.
Previous studies spearheaded the analysis of E2–E3 pairing by assaying the ubiquitination activity of E2s in combination with a set of RING E3 ligases employing in vitro autoubiquitination assays (3, 5). However, whether in vitro autoubiquitination activity faithfully recapitulates all aspects of E2–E3 pairing in vivo remains open. Therefore, data interpretation pertaining to its relevance in vivo must be made with caution for several reasons. (i) The E2s usually employed to perform in vitro ubiquitination assay display high processivity and promiscuity and are therefore active with a wide range of E3 ligases. These include the commonly used UBC8 and UBC11 from group VI, which are homologues of the human UBE2D group of E2s shown to prime ubiquitination (6), but are unlikely to confer chain-linkage specificity (7). (ii) Factors that may be required to determine the specificity of an E2–E3 pairing, such as additional interacting proteins or post-translational modifications, are absent. (iii) High concentrations of recombinant proteins employed in these assays can lead to unspecific E2–E3 interactions. (iv) Spatio-temporal resolution that restricts the encounter of E2–E3 pairs within the cell is absent.
Therefore, to fully understand the potentially distinct cellular roles played by any E3, it is of central importance to identify its physiological E2 counterparts, as they define to a large extent its biochemical properties. Here we interrogated the in vivo E2–E3 pairing employing the well-characterized plant U-box protein 22 (PUB22),3 which contributes to the regulation of immune signaling (8–12), as well as drought responses (13–16). Mutants of PUB22 and its homologues PUB23 to PUB26, display additive hyper-activation of signaling mediated by plasma membrane-localized pattern recognition receptors (PRRs) that perceive pathogen-derived molecules (10, 16, 17). These include the bacterial protein flagellin, or its derived peptide flg22, which is recognized by the FLS2 receptor (18). Similar to animals, plant PRRs activate innate immune responses to fend off pathogens (19). PUB22 mediates the degradation of components of the exocyst complex, which are required for full activation of PRR signaling, during the immune response (8).
PUB22 is composed of an N-terminal U-box that mediates E2 pairing and four armadillo (ARM) repeats that interact with its substrates (Fig. 1A). The U-box is a domain of ∼70 amino acids present in proteins from yeast to human. It consists of a typical fold stabilized by a network of hydrogen bonds that is structurally similar to RING domains (20).
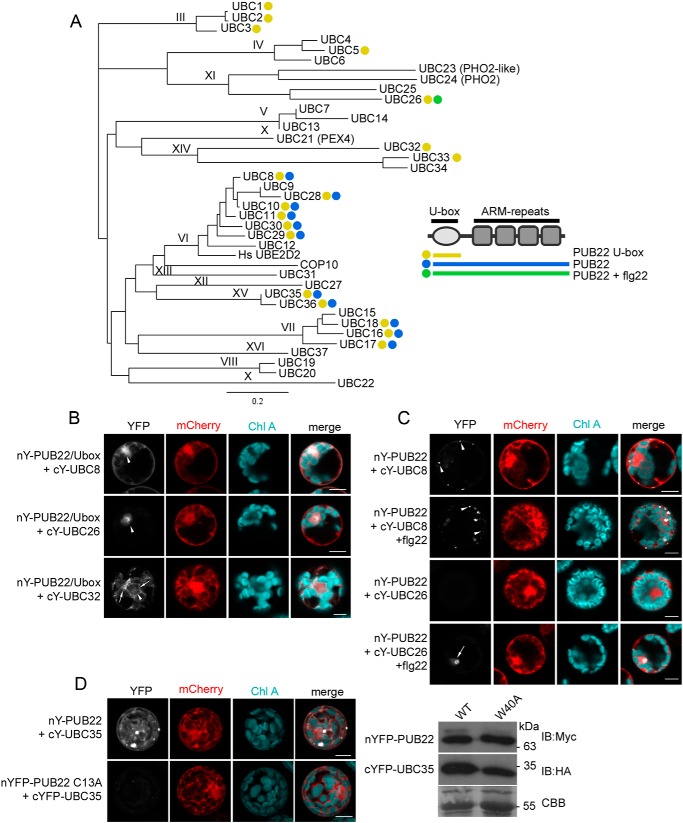
Figure 1.
PUB22–E2 pairing is determined by both the U-box domain and ARM repeats and can be induced by flg22. A, PUB22–E2 pairing as determined by BiFC in Arabidopsis protoplasts. Detected interactions with cYFP-UBCs are denoted with a yellow dot for nYFP-PUB22 U-box, blue for nYFP-PUB22 full-length, and green for interactions of full-length nYFP-PUB22 after treatment with 1 μm flg22 for 1 h. The experiment was repeated with similar results. Shown are the phylogenetic relationships between A. thaliana E2 UBCs, and roman numbers indicate subgroups. The well-characterized human HsUBE2D2 enzyme was included for comparison. The phylogenetic tree was generated using the neighbor-joining method. B, BiFC of the nYFP-PUB22 U-box with cYFP-UBC8, cYFP-UBC26, or cYFP-UBC32, as indicated. Arrowheads show the nucleus and arrows indicate perinuclear mesh-like structure. C, BiFC of full-length nYFP-PUB22 with cYFP-UBC8 or cYFP-UBC26, as indicated. Protoplasts were treated with 1 μm flg22 for 1 h. Arrowheads indicate the nucleus and arrows show punctate structures. D, BiFC of nYFP-PUB22 or the inactive nYFP-PUB22 W40A mutant variant with cYFP-UBC35. Protein expression was confirmed by immunoblot of total protein samples. Coomassie Brilliant Blue (CBB) shows equal loading. B–D, interactions were assayed using transient expression in A. thaliana Col-0 protoplasts. Free mCherry was coexpressed to label the cytoplasm and nucleus. Pictures are representative of three independent experiments with similar results. Y, YFP. Scale bar = 50 μm. IB, immunoblot.
Employing cell-based assays we determined in vivo E2–PUB22 pairing and uncover different layers of specificity. The U-box, which provides the docking surface, mediates interaction with a subset of 18 E2s. However, the ARM repeats impose higher specificity allowing interaction with 11 E2s. Of note, activation of the immune response-modulated interaction with E2s from different subgroups known to have distinct catalytic properties. This suggests that PUB22 mediates distinct catalytic activities. The observed pairing specificities in vivo were only partially recapitulated by in vitro autoubiquitination. Our work therefore reveals various factors that regulate E2–E3 pairing, whereas in addition providing a framework for their study.
Results
PUB22–E2 pairing is specified by different layers
To reveal the cellular function of the E3 ligase PUB22 we screened for PUB22–E2 pairs using a cell-based assay employing bimolecular fluorescence complementation (BiFC) that allows the in vivo visualization of protein–protein interactions. We subcloned all 37 E2 genes encoded by the Arabidopsis genome (3) to generate N-terminal HA-cYFP and Myc-nYFP E2 or E3 fusion proteins, respectively.
We first assayed the interaction between the E2s and a truncated PUB22 carrying the U-box only to test the specificity conferred by this domain on its own. The UBC domain of E2s, which mediates pairing, is highly conserved. We therefore anticipated that the U-box of PUB22 would be able to interact with various E2s. Indeed, BiFC was detected for 18 out 37 tested E2s, including UBCs 1, 2, 3, 5, 8, 10, 11, 16, 17, 18, 26, 28, 29, 30, 32, 33, 35, and 36 (Fig. 1A, and Figs. S1 and S2). Therefore, the U-box domain on its own conferred only a low degree of specificity.
U-box PUB22–E2 pairs displayed distinct subcellular localizations. Most pairs, including UBC5, UBC8, and UBC36, showed a dual nuclear and cytoplasmic localization (Fig. 1B and Table S1). By contrast, the interaction with UBC26 was localized exclusively in the nucleus and was more pronounced in the nucleolus (Fig. 1B). UBC32 was shown to localize in the endoplasmic reticulum where it participates in endoplasmic reticulum-associated degradation (21). Accordingly, the interaction with UBC32 displayed reticulate and perinuclear localization, reminiscent of the endoplasmic reticulum (Fig. 1B).
We next tested whether the ARM repeats, which mediate the interaction with substrates, influence the specificity of the PUB22–E2 pairing. The presence of the ARM domains had a dramatic impact on both the localization and the specificity of the PUB22–E2 pairing. Full-length PUB22 interaction was restricted to 10 E2s: UBCs 8, 10, 16, 17, 18, 28, 29, 30, 35, and 36 (Table S1, Fig. 1A, and Figs. S1 and S2). In most cases the ARM repeats shifted the subcellular localization of PUB22–E2 pairs from the nucleus and cytoplasm to punctae in the cytoplasm (Fig. 1C and Table S1). This localization is reminiscent of that observed for PUB22 and its cognate substrate Exo70B2 in BiFC (9). By contrast, interaction with UBC35 or UBC36 was also cytoplasmic (Fig. 1D), similar to the subcellular localization detected for the PUB22-MPK3 interaction in BiFC (8). On the other hand, interactions between full-length PUB22 and UBCs 1, 2, 3, 5, 11, 26, 32, 33, which interacted with the U-box only, were not detected (Fig. 1A and Table S1).
PUB22 is regulated by the activation of PRRs, including FLS2 (8, 9). We therefore tested whether treatment with flg22 influenced PUB22–E2 pairing. Elicitation with flg22 induced the interaction of the full-length PUB22 with UBC26, which was detected in the nucleus and nucleolus (Fig. 1C). However, in all other cases no major effects could be observed.
Finally, we tested the specificity of PUB22–E2 pairing by including the W40A PUB22 point mutant variant, which is impaired in its autoubiquitination activity (4, 10). Without exception, interaction of all PUB22-interacting E2s with the W40A mutant variant was inhibited (Fig. 1D and Table S1). This additionally confirms that the PUB22–E2 pairing occurs via the canonical U-box and UBC surfaces (22).
Together, we identified 11 PUB22-interacting E2s and we show that both U-box, as well as the ARM repeats, contribute to the pairing specificity. Moreover, the data revealed that activation of the immune response can induce interaction between PUB22 and UBC26.
Autoubiquitination activity only partially reflects in vivo interaction specificity
PUB22 interacted with the majority of the eight E2s belonging to group VI (Fig. 1A). Group VI are prototypical E2s, which are almost exclusively composed of the UBC domain (Fig. 2A and Fig. S3).
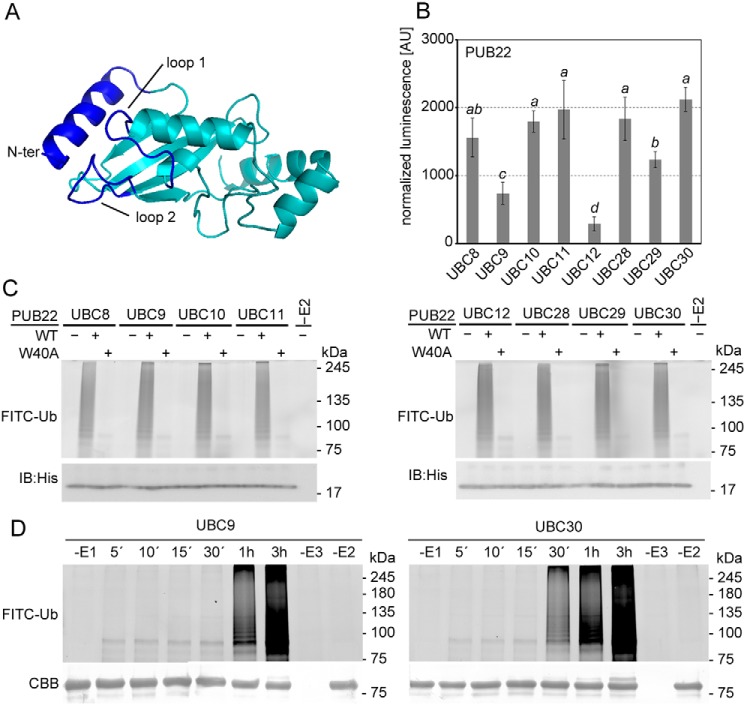
Figure 2.
PUB22 pairing selectivity with the highly homologous group VI E2s. A, structural model of UBC30 from group VI. U-box/RING interacting surfaces are highlighted in blue. B, SLCA of cLUC-PUB22 with nLUC-fused group VI E2s: UBC8, UBC9, UBC10, UBC11, UBC12, UBC28, UBC29, and UBC30, as indicated. Arabidopsis mesophyll protoplasts were transiently co-transformed with constructs containing the indicated genes. Transformation efficiencies were normalized by Renilla luciferase in the vector harboring the E2. Values indicate the average value of three independent biological experiments ± S.D. Statistically significant differences indicated by different letters were determined by one-way analysis of variance (ANOVA) and Tukey post hoc test (p < 0.05). C, in vitro autoubiquitination assay using MBP-tagged wildtype (WT) or W40A mutant variant of PUB22 (E3), His-UBA1 (E1), His-UBCs (E2s) from group VI, and fluorescein-tagged ubiquitin (FITC-Ub). D, in vitro time course autoubiquitination assay using MBP-PUB22, His-UBA1, His-UBC9, or His-UBC30, and FITC-Ub. Coomassie brilliant blue (CBB) shows equal loading. IB, immunoblot.
E2s dock onto U-box/RING domain of E3s via the L1 and L2 loops and the N-terminal α-helix (Fig. 2A, dark blue) with low affinity, which is thought to be a requirement for the rapid exchange between binding to the ubiquitin-E2 conjugate, the aminolysis reaction and the subsequent undocking of the unloaded E2 (1, 23). As a consequence, commonly used techniques such as co-immunoprecipitations are unsuitable to confirm the interaction. We therefore opted to use a split luciferase complementation assay (SLCA), which allows the detection of weak interactions, and in contrast to BiFC in which complementation is irreversible, maintains association-dissociation dynamics (24). Moreover, normalization of the transformation efficiency was performed via Renilla luciferase (Luc) harbored in the vector containing the UBCs by dual-luciferase detection.
We validated the specificity of the interaction detected via SLCA by using two different variants of PUB22 carrying point mutations to alanine in the U-box known to interfere with autoubiquitination (10). Trp40 is predicted to be located on an α-helix distant from the E2 docking surface and not to be required to maintain the core structure of the U-box (25). Cys13 is located on one of the two loops that make contact with the E2 (22). When compared with the WT PUB22, the W40A variant was still able to partially interact, whereas the C13A mutation abrogated the interactions with UBC30 as well as UBC10 and UBC11 (Fig. S4). These results show the high sensitivity and specificity of the SLCA to investigate E2–E3 interactions.
Analysis of PUB22 and group VI E2 pairing by SLCA confirmed that PUB22 interacted with UBC8, UBC10, UBC11, UBC28, UBC29, and UBC30 (Fig. 2B and Fig. S5). Signal intensities for PUB22 interactions with UBC9 and UBC12, which were not detected by BiFC, were also significantly weaker in SLCA. Hence, we were able to confirm our initial results and show in vivo specificity in the interaction of PUB22 and group VI E2s.
Previous reports show that the U-box domain binds with higher affinity to the loaded E2 (26), which can be mediated by binding of the E2 and the loaded ubiquitin to the RING domain (27, 28). To investigate a possible effect of ubiquitin in binding of the E2 with the U-box of PUB22 we replaced the catalytic cysteine residue at position 85 of UBC30 with alanine, which impairs ubiquitin loading, or a lysine, which is able to receive ubiquitin by forming an isopeptide bond (27). Compared with the WT UBC30, the inactive C85A variant displayed reduced, whereas the C85K variant displayed enhanced, Luc activity (Fig. S6). This suggests that ubiquitin loading may enhance UBC30 binding to PUB22 U-box.
To test whether interaction specificity is maintained in vitro, we carried out autoubiquitination assays. In contrast to the differences observed between the interactions in vivo, ubiquitin chain formation activities of PUB22 in combination with group VI E2s did not show any major changes under the used conditions (Fig. 2C). Nevertheless, in all instances ubiquitination was inhibited in the case of the W40A mutant, indicating that interaction with the E3 was required for activity (Fig. 2C). However, detailed time course analyses of PUB22 autoubiquitination revealed differences in the processivity between the in vivo noninteracting UBC9 and the in vivo interacting UBC30 (Fig. 2D).
E2s are activated by PUB22 but do not always mediate autoubiquitination
We next selected representative E2s that were identified in the BiFC screen and compared the interaction using SLCA. Selected E2s included UBC17 (group VII), UBC26 (group XI), UBC28 (group VI), UBC30 (group VI), and UBC35 (group XV). In addition, we included as negative controls UBC9 and UBC12 (group VI), which did not interact with PUB22 in BiFC, as well as UBC1 (group III) and UBC5 (group IV), which interacted in BiFC only with PUB22 U-box, but not the full-length protein. Furthermore, we also included constitutive photomorphogenic 10 (COP10), a repressor of photomorphogenesis (29). COP10 is a UBC variant that lacks the catalytic cysteine residue but is able to enhance E2 activity (30, 31). SLCA confirmed the pairing between PUB22 and E2s that had been identified by BiFC, as well as COP10. By contrast, noninteracting PUB22–E2 pairs displayed low Luc activity, suggesting that they do not interact in vivo with PUB22 (Fig. 3A and Fig. S7A).
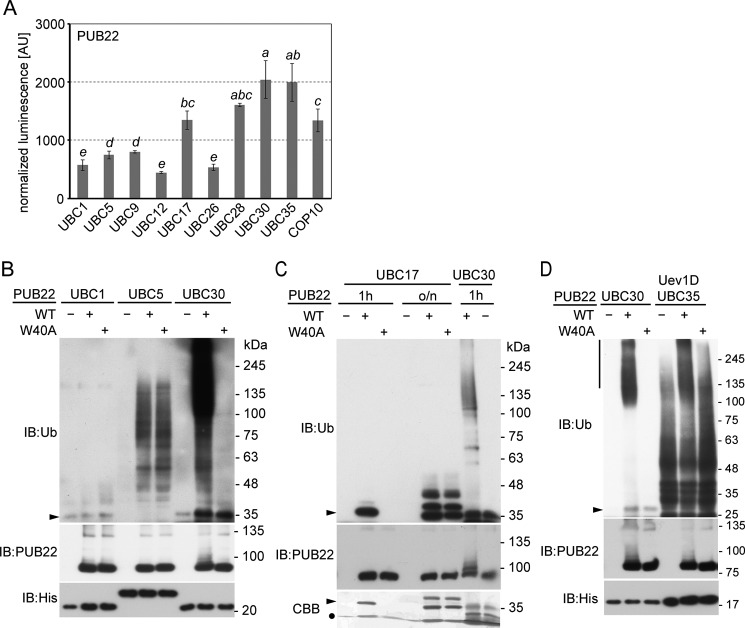
Figure 3.
Characterization of PUB22-interacting E2s. A, SLCA of cLUC-PUB22 with nLUC-fused UBC1 (group III), UBC5 (group IV), UBC9 (group VI), UBC12 (group VI), UBC17 (group VII), UBC26 (group XI), UBC28 (group VI), UBC30 (group VI), COP10, and UBC35 (group XV) fused to cLuc with nLuc-PUB22 transiently co-transformed in Arabidopsis mesophyll protoplasts. Transformation efficiencies were normalized by Renilla luciferase harbored in the vector containing the E2. Values indicate the average value of three independent biological experiments ± S.D. Statistically significant differences indicated by different letters were determined by one-way ANOVA and Tukey post hoc test (p < 0.05). B, in vitro autoubiquitination assay with MBP-PUB22 in the presence of ubiquitin, Arabidopsis His-UBA1, and His-tagged E2s: UBC1 (group III), UBC5 (group IV), or UBC30 (group VI), as a positive control. Ubiquitination reactions were stopped after 2 h. C, in vitro autoubiquitination of MBP-PUB22 in the presence of ubiquitin, His-UBA1, and His-tagged UBC17 (group VII) or UBC30 (group VI), as a positive control. Ubiquitination reaction was incubated for 1 h or overnight (o/n). D, in vitro autoubiquitination of MBP-PUB22 in the presence of ubiquitin, His-UBA1 and His-tagged UBC35 (group XV) with His-tagged Uev1D, or UBC30 (group VI), as a positive control. Ubiquitination reaction was stopped after 2 h. Vertical line indicates high molecular weight species of poly-ubiquitinated PUB22. B–D, ubiquitination reactions were resolved in a 7 and 12% Tris glycine PAGE. Arrowhead indicates the expected size of the ubiquitin-E2 conjugate. CBB, Coomassie brilliant blue; Ub, ubiquitin; IB, immunoblot.
In vitro autoubiquitination assays showed that UBC1, which did not interact with PUB22, was unable to mediate ubiquitination in vitro under conditions that resulted in strong UBC30-mediated autoubiquitination of PUB22 (Fig. 3B). UBC5 did not display any intrinsic activity in the control reaction without the E3. However, the presence of PUB22 resulted in the formation of free ubiquitin chains, but not autoubiquitination of PUB22. This effect was also observed with the W40A variant, which abrogates group VI E2-dependent activity of PUB22 (Fig. 3B). This may suggest that the W40A mutation still allows interaction of PUB22 with UBC5 and therefore, a distinct mode of interaction than with group VI E2s.
Despite interacting with PUB22 in vivo, UBC17 did not mediate in vitro autoubiquitination under the used conditions. However, the presence of PUB22 enhanced the ubiquitin-bound form of UBC17 after 1 h in support of interaction taking place in vitro (Fig. 3C). Overnight incubation resulted in similar levels of ubiquitin-bound forms due to the inherent reactivity of UBC17 in the presence of the W40A variant (Fig. 3C).
Finally, we assayed the activity of UBC35, which is the homologue of the yeast UBC13 (32) and the human UBE2N that mediates the formation of K63-linked chains (32–34). Similar to its homologues, UBC35 requires a ubiquitin-conjugating enzyme variant (Uev) as a cofactor for activity (34). In the presence of Uev1D, UBC35 generated free ubiquitin chains (Fig. 3D), as previously reported (34). However, in the presence of PUB22, UBC35 activity was stimulated resulting in the increased generation of high molecular chains (Fig. 3D).
Varying pairing specificities between different types of ubiquitin ligases
We next interrogated the pairing specificities between the selection of E2s and additional E3s. For this purpose, we included the closely related PUB20 and PUB24, which are also composed of an N-terminal U-box and a four ARM repeats domain (Fig. 4, A and B). PUB24 also contributes to the dampening of the immune response (10), whereas the function of PUB20 remains unknown.
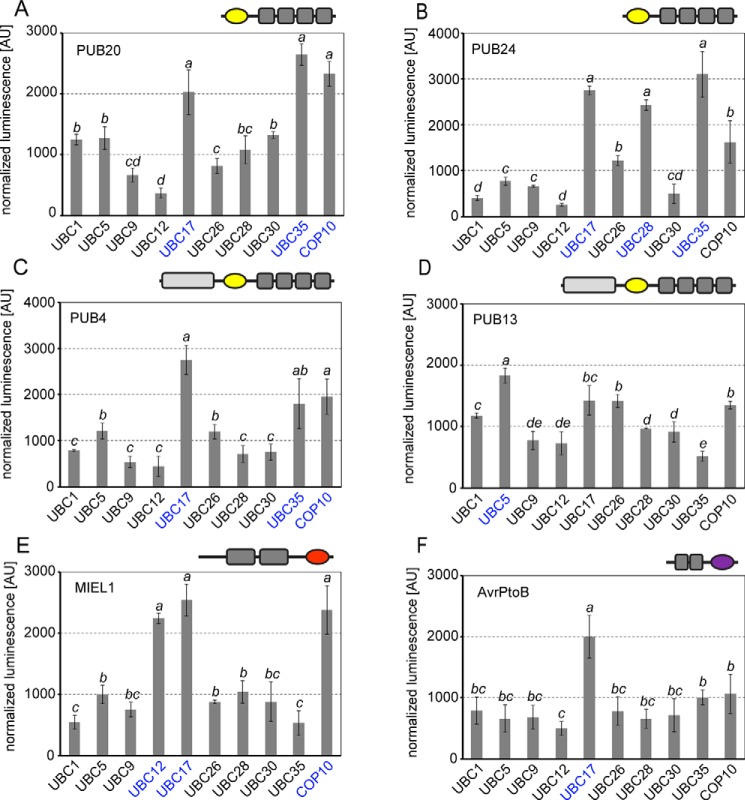
Figure 4.
Pairing of selected E2s with different types of E3s. A–F, SLCA of PUB20 composed of U-box (yellow) and ARM repeats (A), PUB24 composed of U-box (yellow) and ARM repeats (B), PUB4 composed of UND domain, U-box (yellow) and ARM repeats (C), PUB13 composed of UND domain, U-box (yellow) and ARM repeats (D), MIEL1 composed of two zinc fingers and a RING domain (red) (E), and AvrPtoB composed of two kinase-interacting domains and a U-box-like domain (purple) (F). E3s were assayed against UBC1 (group III), UBC5 (group IV), UBC9 (group VI), UBC12 (group VI), UBC17 (group VII), UBC26 (group XI), UBC28 (group VI), UBC30 (group VI), UBC35 (group XV), and COP10 transiently co-transformed in Arabidopsis mesophyll protoplasts. Transformation efficiencies were normalized by Renilla Luc harbored in the E2-containing vector. Strongest signal intensities are shown in blue. Values indicate the average of three independent biological experiments ± S.D. Statistically significant differences indicated by different letters were determined by one-way ANOVA and Tukey post hoc test (p < 0.05).
Similar to PUB22, its homologues PUB20 and PUB24 displayed strong Luc complementation with UBC35 (Fig. 4, A and B, and Fig. S8, A and B), suggesting this group of PUBs is able to mediate K63-linked polyubiquitination. Interaction between PUB20 and COP10 or UBC35 resulted in comparably high Luc signals (Fig. 4A and Fig. S8). Similar high signal levels were also observed for the pairing of PUB24 with UBC28, UBC35, and UBC17 (Fig. 4B). In contrast to PUB22 (Fig. 3A), PUB20 only displayed a weak signal with the tested group VI E2s UBC9, UBC12, UBC28, and UBC30 (Fig. 4A).
We additionally tested PUB4, implicated in the regulation of cell division, cytokinin signaling, and chloroplast homeostasis (35–38), as well as PUB13, which regulates the levels of various receptor kinases, and plays a role in hormone signaling (39–43). Both PUB4 and PUB13 contain an additional U-box N-terminal domain (UND) of unknown function and 5 to 6 predicted ARM repeats. Both PUB4 and PUB13 showed low Luc complementation with group VI E2s (Fig. 4, C and D, and Fig. S8, C and D). PUB4, however, showed the strongest Luc signal in combination with UBC35, UBC17, and COP10 (Fig. 4C and Fig. S8C). In contrast to most tested PUBs, the strongest Luc signal for PUB13 was observed in the presence of UBC5 and the lowest with UBC35, suggesting that PUB13 represents a special case, when compared with the tested E3s (Fig. 4D and Fig. S8).
We also included the RING-type E3 MIEL1, which was shown to target Myb transcription factors to modulate ABA signaling and the immune response (44, 45). In contrast to all other E3s, MIEL1 showed a strong Luc complementation with UBC12, which did not interact with any of the tested PUB E3s and additionally interacted with UBC17 and COP10 (Fig. 4E and Fig. S8).
Finally, we analyzed E2 pairing with the bacterial effector AvrPtoB, which is a structural mimic of the eukaryotic U-box (46). AvrPtoB was shown to inhibit immune signaling by targeting both receptor kinases (47, 48) as well as cytosolic kinases involved in the immune response (49, 50). AvrPtoB showed a comparably low Luc signal with all tested E2, except for UBC17 (Fig. 4F and Fig. S8F). Together, the tested E3s show distinct predilections for E2 potentially reflecting differences in chain-building activities.
E2–E3 pairing is modulated during the immune response
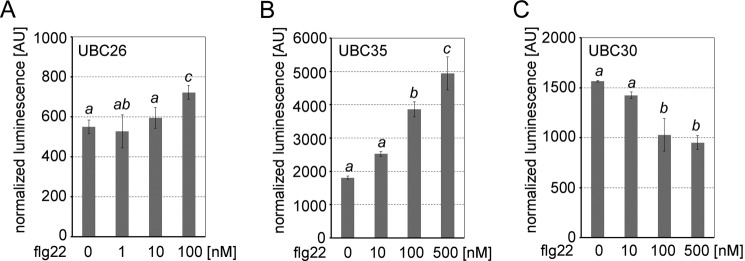
It remains unknown whether E2–E3 pairing is regulated in response to specific cellular cues. Our BiFC analysis already suggested that interaction between PUB22 and UBC26 is induced by treatment with the immunogenic peptide flg22 (Fig. 1C and Table S1). Indeed, SLCA confirmed that activation of the immune response by flg22 resulted in enhanced interaction (Fig. 5A and Fig. S7B). We also tested whether interactions with UBC35 (group XV) and UBC30 (group VI) were affected. As observed for UBC26, interaction with UBC35 was dramatically increased after immunostimulation in a dose-dependent manner (Fig. 5B and Fig. S7C). By contrast, association of the group VI UBC30 to PUB22 was decreased (Fig. 5C and Fig. S7C). These results show that beyond a static interaction specificity, changes in the cellular status can modulate the pairing between PUB22 and various E2s.
Figure 5.
Activation of the immune response by flg22 treatment modulates E2–PUB22 pairing. A, SLCA of cLuc-PUB22 with nLuc-UBC26 transiently co-transformed in Arabidopsis mesophyll protoplasts and treated with the indicated concentrations of flg22 for 1 h. B, SLCA of nLuc-PUB22 with cLuc-UBC35 transiently co-transformed in Arabidopsis mesophyll protoplasts and treated with the indicated concentrations of flg22 for 1 h. C, SLCA of nLuc-PUB22 with cLuc-UBC30 transiently co-transformed in Arabidopsis mesophyll protoplasts and treated with the indicated concentrations of flg22 for 1 h. A–C, transformation efficiencies were normalized by Renilla Luc harbored in the E2-containing vector. Values indicate the average of three independent biological experiments ± S.D. Statistically significant differences indicated by different letters were determined by one-way ANOVA and Tukey post hoc test (p < 0.05).
UBC35 and UBC36 participate in the immune response
PUB22 is involved in the regulation of immune responses (8–12). Of note, the pairing between PUB22 and UBC35 increased upon immunostimulation (Fig. 5B). In addition, the closely related PUB20 and PUB24 also interacted with UBC35 (Fig. 4, A and B). We therefore hypothesized that UBC35, and its close homologue UBC36, may mediate the activity of PUB22, as well as that of its homologues, to dampen immune responses.
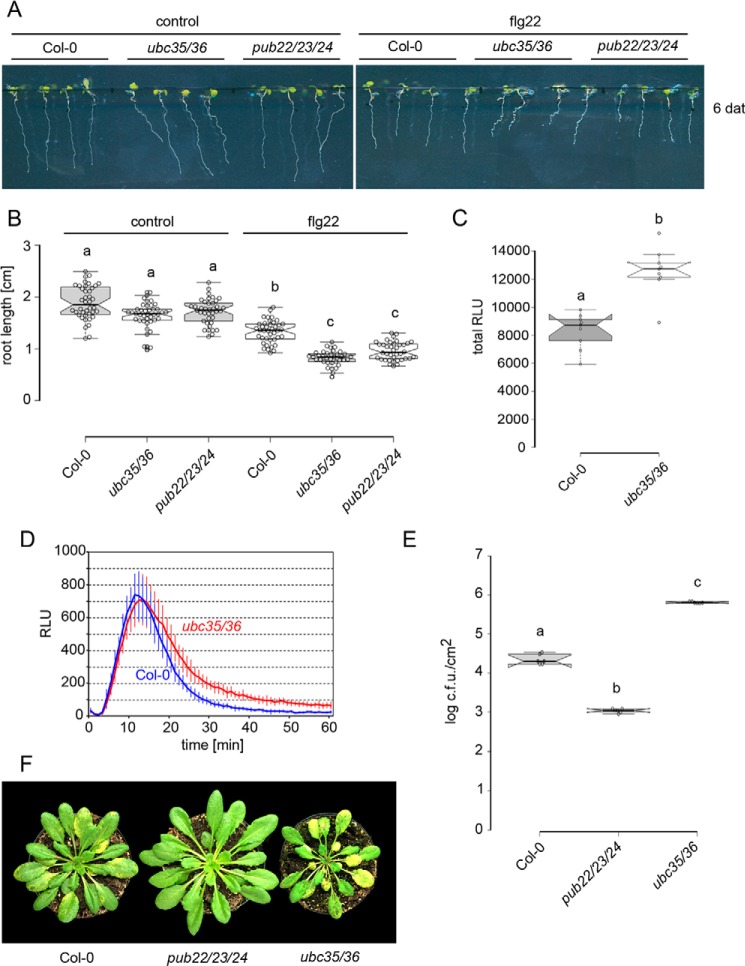
We first tested responses related to FLS2 activation. The triggering of immune responses results in a trade-off in growth, which is proportional to the intensity of the response. Growth inhibition of the main root by flg22 in the ubc35 ubc36 double mutant (51) was significantly higher, compared with Col-0 WT, but comparable with the pub22 pub23 pub24 triple mutant (9, 10) (Fig. 6, A and B). We also assayed the production of reactive oxygen species (ROS), which is an early reaction triggered by activation of immune receptors and reflects downstream signaling. Indeed, ubc35 ubc36 displayed a delayed down-regulation of ROS production, resulting in higher total levels (Fig. 6, C and D) reminiscent of the pub22 pub23 pub24 response (10).
Figure 6.
UBC35 and UBC36 participate in immunity. A, root growth inhibition in Col-0 WT, pub22 pub23 pub24, and ubc35 ubc36 seedlings 6 days after transplanting (dat) onto media containing 1 μm flg22. B, length of the main root of seedlings in A. Data shown as mean of three independent experiments ± S.D., n ≥ 38. Statistically significant differences indicated by different letters were determined by one-way ANOVA and Tukey post hoc test (p < 0.05). C, total production of ROS during 60 min after treatment with 100 nm flg22. Data are shown as median ± S.D. Statistical significance compared with Col-0 plants is indicated with asterisks (Student's t test, **, p < 0.01). ROS production was evaluated in three independent experiments with similar results. D, oxidative burst generated during 1 h post-treatment with 100 nm flg22. Data are shown as mean ± S.D., n = 9. ROS production was evaluated in three independent experiments with similar results. E, infection assays with the virulent bacterial pathogen Pst DC3000. Col-0, pub22 pub23 pub24, and ubc35 ubc36 plants were spray-inoculated with a bacterial suspension of 5 × 108 colony forming units/ml. Infection and bacterial growth were assessed at 3 days after inoculation (dai). Data shown as median ± S.D., n = 6. Similar results were obtained in four independent experiments. F, representative Col-0, pub22 pub23 pub24, and ubc35 ubc36 seedlings 3 days after inoculation with the virulent bacterial pathogen Pst DC3000.
We additionally tested the role of UBC35 and UBC36 in the resistance against pathogens by inoculating plants with the virulent Pseudomonas syringae pv tomato (Pst). Surprisingly, ubc35 ubc36 plants were more susceptible to Pst compared with Col-0 WT plants (Fig. 6, E and F). By contrast, the pub22 pub23 pub24 mutant was more resistant in agreement with previous results (10). This indicates that although ubc35 ubc36 PRR-mediated signaling is increased, they are unable to mount effective defense responses.
Discussion
E2s are responsible for the catalysis of ubiquitin attachment and to a large extent the type of ubiquitination. As such, it is necessary to determine the E2s that pair with a specific E3 to elucidate the biochemical function(s) mediated by the E3. Using two complementary cell-based assays we identified physiological PUB22–E2 pairs. We reveal that the full-length protein displays increased selectivity compared with the U-box on its own. This indicates that the U-box is not the sole determinant of E2–E3 pairing and that adjoining domains, such as the ARM repeats in the case of PUB22, are able to impose a second layer of specificity, in addition to mediating substrate recognition. Neighboring domains may restrict E2 interaction by sequestering PUB22 to its determined subcellular localization. Indeed, localization of the BiFC signal was distinct between the U-box only and the full-length PUB22 (Fig. 1, B and C). However, many of the E2s that interacted only with the truncated form were cytoplasmic and should in principle have access to the U-box domain of the full-length protein. It is conceivable that the ARM repeats, and/or their interacting proteins, including substrates, prevent the binding to some E2s by steric hindrance. In fact, most E2s that exclusively interacted with the U-box contain extended C-terminal tails in their amino acid sequence (Fig. S3).
All E2s characterized so far recognize E3s through the L1 and L2 loops, as well as the N-terminal α-helix 1 on the E2 surface (Fig. 2A and Fig. S3) (1). Slight sequence variations in these motifs contribute to the specificity of E3 binding. The SPA motif in loop 2 was shown to be required for binding of E2s to the U-box type E3 CHIP (53). PUB22-interacting E2s from groups VI, VII, XI, and XV, contain the SPA motif (Fig. S3). However, E2s that were unable to interact with PUB22 (e.g. UBC9 and UBC12; Fig. 1A) also contained the motif (Fig. S3), suggesting that this feature is not critical for specificity, but generally required for interaction.
Within group VI, PUB22 interacted with the majority of E2s in vivo, except UBC9 and UBC12. The overall sequence conservation of the E2s belonging to group VI is very high, especially within the surfaces responsible for the interaction with the E3. The main difference in UBC9 is a 30-amino acid extension at its N terminus (Fig. S3). In the case of UBC12 the only striking feature is the replacement at position 16 of a conserved aspartic acid present in members of group VI by a histidine (3).
Of note, the PUB22–E2 pairing specificity observed in vivo was only partially reflected by the in vitro autoubiquitination activity (Fig. 2, B and C). Analyses of end point ubiquitination failed to identify relevant differences in processivity between group VI E2s, which are commonly used for their high activity and promiscuity. However, a time course analysis revealed that indeed the PUB22-interacting UBC30 displayed higher processivity when compared with UBC9 (Fig. 2D), which did not interact in vivo (Fig. 2B). Whether the distinct pairing preferences displayed by PUB22 in vivo between UBC9 and UBC30 (Fig. 2B) are faithfully recapitulated by the differences in processivity is unclear. Additional factors, such as competing E2s, are likely to play a role in E2–E3 pairing specificity in vivo and will be a subject of future studies. Pairing with a particular E2 does not necessarily result in E3 autoubiquitination. Some E2–E3 combinations may only support modification of the substrate. This may be the case for UBC17, which interacted with most of tested E3s. Its closest homologue in humans is UBE2W, which was shown to monoubiquitinate the N terminus of substrates priming them for Lys63-linked polyubiquitination by UBE2N (54–56). Accordingly, UBC17 did not support autoubiquitination of PUB22 in vitro (Fig. 3C). Nevertheless, the presence of PUB22 enhanced the levels of ubiquitin-bound UBC17, which depended on an intact U-box.
There is evidence that substrate ubiquitination is in some cases not the result of a single E2–E3 pair. Although UBC17, like its human homologue, may only monoubiquitinate substrates, several chain-building E2s, including Ube2N and UbeR1, are only capable of transferring their conjugated ubiquitin moiety to another ubiquitin molecule (57–59). This indicates that there can be a “division of labor” among E2s in which one E2 initiates (or primes) chain synthesis and a second E2 builds and extends the polyubiquitin chain on a substrate (1). The fact that PUB22 pairs both with E2s that are able to prime chain formation, such as group VI UBC30 or group VII UBC17, as well as the chain-elongating UBC35 and UBC36 (group XV), suggests that such a division of labor is in principle also possible for E2s interacting with PUB22.
Of note, our observations reveal that some PUB22–E2 pairings are dynamic and change in response to cellular status. Interaction with UBC30 was reduced, whereas PUB22 association to UBC26 and UBC35 was induced after activation of the immune response by flg22 (Figs. 1C and 5, A–C). These results therefore open the possibility that in addition to a division of labor, the catalytic properties of PUB22, as conveyed by the paired E2, change in response to altered cellular status, such as the activation of the immune response. In line with these results, under basal conditions PUB22 autoubiquitinates and mediates its degradation by the proteasome (8). However, during the immune response phosphorylation by MAPK3 inhibits PUB22 oligomerization and autoubiquitination in trans. By contrast its substrate Exo70B2 is degraded via the vacuole, which commonly involves Lys63-linked chains (60–62).
Within the tested U-box ligases most interacted with UBC35 (Figs. 3A and 4, A–C), suggesting a general role in the modification of substrates with Lys63 chains. The exception was PUB13 that was reported to be involved in the regulation of receptor kinases (42, 63). PUB13 interacted strongest with UBC5 (Fig. 4D). Although the function of UBC5 in plants is unknown, its closest homologue in humans, UBE2H, is active with MARCH E3s, which in analogy to PUB13, are involved in the regulation of plasma membrane proteins (64, 65).
Although the requirements of Lys63 chains for endocytosis in plants seems to vary between membrane proteins, they play a general function in sorting of endocytosed cargos into multi-vesicular bodies by the endosomal sorting complex required for transport (66). The pairing of PUB22 with UBC35 and the enhanced signaling of PRRs observed in the pub22 pub23 pub24 mutant suggest a conserved function in the regulation of the endocytic degradatory pathway. Analyses of PRR-mediated responses in the ubc35 ubc36 double mutants showed that they partially phenocopied the pub22 pub23 pub24 triple mutant ROS burst (Fig. 6, A–D). Although this response was prolonged and the total production of ROS in ubc35 ubc36 increased, the initial peak was not higher than in WT plants, as shown for pub22 pub23 pub24 (10), indicating differences between their functions. By contrast, ubc35 ubc36 mutants were more susceptible to bacterial infection (Fig. 6, E and F). Immune responses triggered by PRRs include the production and secretion of toxic compounds, cell wall reinforcement or programmed cell death (67). Enhanced susceptibility of ubc35 ubc36 mutants may therefore reflect impaired deployment of immune responses as compared with immune signaling.
It is important to appreciate that 37 E2s in Arabidopsis potentially cater for more than 1400 E3s. Hence, the inactivation of UBC35 and UBC36 is most likely to affect the function of a wide group of E3 ligases. This is supported by the fact that Lys63 chains are the second most abundant (68). As a matter of fact, ubc35 ubc36 display defects in iron homeostasis, auxin responses, and DNA repair, whereas additionally Lys63-linked chains are implicated in endocytosis and late transport into the vacuole via the endosomal sorting complex required for transport (66). Moreover, as we have shown here, PUB22 interacts with various E2s that possess distinct catalytic properties and are located in different subcellular compartments. Therefore, from the perspective of the E3, inactivation of two of its E2 interactors can only result in the partial inhibition of its function. Finally, PUB22 and its homologues have different targets, which play distinct functions during the immune response and it is unlikely that all of them are modified by the same PUB22-E2 pair (16). Together, our study illustrates the intricate in vivo E2–E3 interaction network while at the same time highlighting the biochemical complexity of ubiquitination.
Experimental procedures
Plant materials and growth conditions
Arabidopsis thaliana ecotype Col-0 was used in the BiFC and SLCA assays and as a control in oxidative burst measurement, disease resistance, and root growth inhibition assay. The pub22 pub23 pub24 triple mutant plants (SALK_07261, SALK_151594, and SALK_041046, respectively) and ubc35 ubc36 double mutant plants (CS851269 and SALK_047381) were described previously (10, 51). Plants for protoplasts isolations, oxidative burst measurement, and disease resistance assay were grown for 4–5 weeks in a phytochamber at 21 °C in 8 h light (combination of cool white fluorescent and incandescent lamps, 250 μmol/m2/s irradiance) and 16 h dark at 60% humidity, upon stratification for 3 days at 4 °C in the dark. Protoplasts were isolated as described previously (69).
BiFC assay
Constructs of WT PUB22 and PUB22 U-box in pE-SPYNE-GW (Myc-nYFP) were previously published (9), whereas C13A and W40A variants were cloned into pE-SPYNE-GW (70) via LR reaction (Invitrogen). E2s were cloned into pE-SPYCE-GW (HA-cYFP) via LR reaction (Invitrogen). Plasmids encoding either E2 or E3 were used for co-transfection with mCherry-expressing vector at final concentrations of 40 and 20 μg ml−1, respectively. Transformed protoplasts were incubated at 18 °C for 16 h in the dark before the specified treatments were performed. For flg22 treatment (1 μm), confocal microscopy analysis was conducted 1 h post-treatment, respectively. Confocal laser scanning microscope (CLSM) imaging with LSM780 (Zeiss) was performed with the following settings: YFP excitation, 488 nm, emission, 510–560 nm; mCherry excitation, 594 nm, emission, 600–640 nm. To analyze protein levels, protoplasts were pelleted (200 × g, 2 min), flash-frozen in liquid nitrogen, and re-suspended in Laemmli sample loading buffer (125 mm Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, and 0.004% bromphenol blue). After denaturation (65 °C, 20 min), samples were centrifuged (6000 × g, 1 min) prior to protein gel blot analysis.
Dual reporter split luciferase assay
Plasmids carrying genes encoding selected E2s (UBC: 1, 5, 8, 9, 10, 11, 12, 17, 26, 28, 29, 30, and 35) or COP10 and E3s (PUB20, PUB22, PUB24, PUB4, PUB13, MIEL1, and AvrPtoB) were generated with the use of Golden Gate cloning (71). Briefly, the expression vector encoding E2 protein with N-terminal cLuc or nLuc portions of Firefly Luc and HA- or Myc-tag also carried a gene-encoded Renilla Luc, and the expression of the E2 fusion protein and Renilla Luc protein was driven by separate 35S promoters. Genes encoding each of the selected E3 proteins were subcloned downstream to the N-terminal cLuc or nLuc portions of Firefly Luc and HA- or Myc-tag and expression of the E3 fusion protein was driven by the 35S promoter. Protoplasts were isolated from 5-week-old Col-0 plants and transformed with 50 μg ml−1 of each construct. Transformed protoplasts were incubated at 18 °C for 16 h in the dark before the specified treatments were performed. Treatment with flg22 (1-0.5 μm) was conducted for 1 h. SLCA analyses were performed 1 day after transfection. Firefly Luc activity was assessed by adding 1 mm d-luciferin (Promega) substrate to the protoplasts solution and measuring the luminescence with a Tecan Spark multimode microplate reader (Tecan) at 550–575 nm wavelength with a signal integration time of 2 s after 3 min incubation of the plate in the dark. Renilla Luc activity was assessed by adding 10 μm ViviRen Live Cell substrate (Promega) to the protoplasts solution and measuring the luminescence with the microplate reader at 460–500 nm wavelength with signal integration time of 2 s after 2 min incubation of the plate in the dark. The signal obtained from Firefly Luc activity was normalized to Renilla Luc readout to compensate for differences in the E2 expression levels resulting from uneven transfection rates between different samples. Protoplasts were subsequently pelleted and protein expression levels were analyzed as described above.
Site-directed mutagenesis and recombinant protein expression and purification
Generation of the substitutions at position 85 (C85A and C85K) of UBC30 was performed with the use of Phusion site-directed mutagenesis kit (Thermo Scientific). The cloning of PUB22 into pMal-c2X vector (New England Biolabs), expression of the MBP-PUB22 in Escherichia coli Rosetta 2(DE3) pLysS and its purification by affinity chromatography using amylose resin (New England Biolabs) was previously described (8). UBA1 and genes encoded the selected E2s (UBC: 1, 5, 8, 9, 10, 11, 12, 17, 26, 28, 29, 30, 35) or MMZ4 were cloned into pENTR/D-TOPO (Invitrogen) entry clone and used for cloning into pDEST17 Gateway vector (Thermo Fisher Scientific) for His-tagged protein expression via LR reaction (Invitrogen). Recombinant His-tagged proteins were expressed in E. coli BL21(DE3) and purified using Ni-Ted resin (Macherey-Nagel).
In vitro autoubiquitination assay
In vitro autoubiquitination assay of freshly purified MBP-PUB22 was performed with the use of purified E1 and E2 proteins stored at −80 °C. Each 30-μl reaction mixture contained 0.02 μg of His-UBA1, 0.03–0.09 μg of His-E2, 0.04 μg of His-MMZ4 (in case of His-UBC35 reaction), and 0.15 μg of MBP-PUB22 in ubiquitination buffer (40 mm Tris-HCl, pH 7.4, 5 mm MgCl2, 50 mm KCl, 2 mm ATP, 1 mm DTT, and 60 μg of ubiquitin from bovine erythrocytes (Sigma) or 0.6 μg of fluorescein (FITC)-labeled ubiquitin (UBPBio)). The reactions were incubated at 30 °C for the indicated time and terminated by addition of Laemmli sample loading buffer and incubating for 20 min at 65 °C. The proteins were resolved by 7% SDS-PAGE followed by detection of the ubiquitinated PUB22 by immunoblotting.
Immunoblot analysis and antibodies used in this study
Protein samples were analyzed on 7 or 12% bis-acrylamide Tris glycine SDS-PAGE. Following the separation on SDS-PAGE and blotting on polyvinylidene difluoride membranes (GE Healthcare), immunoblot analyses were performed with the following antibodies: anti-c-Myc 1:5000 (Sigma; catalog number C3956), anti-GFP 1:3000 (Santa Cruz Biotechnology; catalog number Sc-8334), anti-ubiquitin P4D1 1:5000 (Santa Cruz Biotechnology; catalog number Sc-8017), anti-MBP 1:1000 (Sigma; catalog number M1321), anti-His 1:10,000 (MACS; catalog number 120-003-811), and anti-luciferase 1:5000 (Sigma; catalog number L0159). The anti-PUB22 antibody was developed against the C-terminal peptide RVWRESPCVPRNLYDSYPA (Thermo Fisher Scientific) and used at 1:1000 dilution.
Oxidative burst measurement
Leaf discs (0.125 cm2) of 4-week-old plants were incubated overnight in water in a 96-well titer plate, with one leaf disc per well. ROS produced by leaf discs were measured with a luminol-based assay (20 μg ml−1 of horseradish peroxidase and 30 μg ml−1 of luminol). Luminescence is shown in relative light units and was measured with a Tecan Spark multimode microplate reader after a 1-min incubation of the plate in the dark, with signal integration time of 1 s over 1 min.
Bacterial infection assay
Six plants per each genotype were spray-inoculated with a solution of P. syringae pv tomato as described (52). Briefly, Pst were streaked out on Kings B media, grown for 2 days at 28 °C, and subsequently resuspended in water to a final concentration of 5 × 108 colony-forming units ml−1 followed by addition of 0.04% (v/v) Silwet L-77 (Lehle Seeds). Bacterial growth in leaf discs excised from leaves (1 leaf per each plant) collected from 6 plants per each genotype was measured 3 days after inoculation. Upon grinding of the tissue, the samples were vortex-mixed, diluted serially, and plated on LB agar supplemented with 50 μg ml−1 of rifampicin and 50 μg ml−1 of kanamycin, and incubated for 2 days at 28 °C, after which the colony-forming units were counted.
Root growth inhibition assay
T3 generation homozygous seeds were surface-sterilized and stratified for 3 days at 4 °C on 0.5 × MS agar (Duchefa) supplemented with 0.1% sucrose. Seedlings were grown vertically for 5 days under short-day conditions (8 h light/16 h dark). Seedlings were then transferred onto solid 0.5 × MS medium containing 0.1% sucrose supplemented with 1 μm flg22 or the vehicle (DMSO) and grown vertically for another 7 days. Length of the main root was then scored and measured using ImageJ software.
Author contributions
I. T., N. T., R. L., and M. T. formal analysis; I. T., N. T., and R. L. validation; I. T., N. T., and R. L. investigation; I. T., R. L., and M. T. visualization; I. T. and M. T. methodology; I. T. and M. T. writing-original draft; M. T. conceptualization; M. T. supervision; M. T. funding acquisition; M. T. project administration; M. T. writing-review and editing.
Supplementary Material
Acknowledgments
We thank Giulia Furlan and members of the Trujillo laboratory for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) and the Leibniz Gemeinschaft. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Table S1 and Figs. S1–S8.
- PUB22
- plant U-box protein 22
- PRR
- pattern recognition receptor
- ARM
- armadillo
- BiFC
- bimolecular fluorescence complementation
- HA
- hemagglutinin
- UBC
- ubiquitin-conjugating enzyme
- COP10
- constitutive photomorphogenic 10
- UND
- U-box N-terminal domain
- ROS
- reactive oxygen species
- ANOVA
- analysis of variance
- MBP
- maltose-binding protein
- SLCA
- split luciferase complementation assay
- Luc
- luciferase
- Uev
- ubiquitin-conjugating enzyme variant
- YFP
- yellow fluorescent protein.
References
- 1. Stewart M. D., Ritterhoff T., Klevit R. E., and Brzovic P. S. (2016) E2 enzymes: more than just middle men. Cell Res. 26, 423–440 10.1038/cr.2016.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kulathu Y., and Komander D. (2012) Atypical ubiquitylation: the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat. Rev. Mol. Cell Biol. 13, 508–523 10.1038/nrm3394 [DOI] [PubMed] [Google Scholar]
- 3. Kraft E., Stone S. L., Ma L., Su N., Gao Y., Lau O. S., Deng X. W., and Callis J. (2005) Genome analysis and functional characterization of the E2 and RING-type E3 ligase ubiquitination enzymes of Arabidopsis. Plant Physiol. 139, 1597–1611 10.1104/pp.105.067983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kowarschik K., Hoehenwarter W., Marillonnet S., and Trujillo M. (2018) UbiGate: a synthetic biology toolbox to analyse ubiquitination. New Phytol. 217, 1749–1763 10.1111/nph.14900 [DOI] [PubMed] [Google Scholar]
- 5. Wiborg J., O'Shea C., and Skriver K. (2008) Biochemical function of typical and variant Arabidopsis thaliana U-box E3 ubiquitin-protein ligases. Biochem. J. 413, 447–457 10.1042/BJ20071568 [DOI] [PubMed] [Google Scholar]
- 6. Jin L., Williamson A., Banerjee S., Philipp I., and Rape M. (2008) Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell 133, 653–665 10.1016/j.cell.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brzovic P. S., Lissounov A., Christensen D. E., Hoyt D. W., and Klevit R. E. (2006) A UbcH5/ubiquitin noncovalent complex is required for processive BRCA1-directed ubiquitination. Mol. Cell 21, 873–880 10.1016/j.molcel.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 8. Furlan G., Nakagami H., Eschen-Lippold L., Jiang X., Majovsky P., Kowarschik K., Hoehenwarter W., Lee J., and Trujillo M. (2017) Changes in PUB22 ubiquitination modes triggered by mitogen-activated protein kinase3 dampen the immune response. Plant Cell 29, 726–745 10.1105/tpc.16.00654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stegmann M., Anderson R. G., Ichimura K., Pecenkova T., Reuter P., Žársky V., McDowell J. M., Shirasu K., and Trujillo M. (2012) The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in Arabidopsis. Plant Cell 24, 4703–4716 10.1105/tpc.112.104463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trujillo M., Ichimura K., Casais C., and Shirasu K. (2008) Negative regulation of PAMP-triggered immunity by an E3 ubiquitin ligase triplet in Arabidopsis. Curr. Biol. 18, 1396–1401 10.1016/j.cub.2008.07.085 [DOI] [PubMed] [Google Scholar]
- 11. Chen Y. C., Wong C. L., Muzzi F., Vlaardingerbroek I., Kidd B. N., and Schenk P. M. (2014) Root defense analysis against Fusarium oxysporum reveals new regulators to confer resistance. Sci. Rep. 4, 5584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobs S., Zechmann B., Molitor A., Trujillo M., Petutschnig E., Lipka V., Lipka V., Kogel K. H., and Schäfer P. (2011) Broad-spectrum suppression of innate immunity is required for colonization of Arabidopsis roots by the fungus Piriformospora indica. Plant Physiol. 156, 726–740 10.1104/pp.111.176446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cho S. K., Ryu M. Y., Song C., Kwak J. M., and Kim W. T. (2008) Arabidopsis PUB22 and PUB23 are homologous U-Box E3 ubiquitin ligases that play combinatory roles in response to drought stress. Plant Cell 20, 1899–1914 10.1105/tpc.108.060699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seo D. H., Ahn M. Y., Park K. Y., Kim E. Y., and Kim W. T. (2016) The N-terminal UND motif of the Arabidopsis U-Box E3 ligase PUB18 is critical for the negative regulation of ABA-mediated stomatal movement and determines its ubiquitination specificity for exocyst subunit Exo70B1. Plant Cell 28, 2952–2973 10.1105/tpc.16.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seo D. H., Ryu M. Y., Jammes F., Hwang J. H., Turek M., Kang B. G., Kwak J. M., and Kim W. T. (2012) Roles of four Arabidopsis U-box E3 ubiquitin ligases in negative regulation of abscisic acid-mediated drought stress responses. Plant Physiol. 160, 556–568 10.1104/pp.112.202143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trujillo M. (2018) News from the PUB: plant U-box type E3 ubiquitin ligases. J. Exp. Bot. 69, 371–384 10.1093/jxb/erx411 [DOI] [PubMed] [Google Scholar]
- 17. Wang J., Grubb L. E., Wang J., Liang X., Li L., Gao C., Ma M., Feng F., Li M., Li L., Zhang X., Yu F., Xie Q., Chen S., Zipfel C., Monaghan J., and Zhou J. M. (2018) A regulatory module controlling homeostasis of a plant immune kinase. Mol. Cell 69, 493–504.e496 [DOI] [PubMed] [Google Scholar]
- 18. Chinchilla D., Bauer Z., Regenass M., Boller T., and Felix G. (2006) The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 18, 465–476 10.1105/tpc.105.036574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Couto D., and Zipfel C. (2016) Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16, 537–552 10.1038/nri.2016.77 [DOI] [PubMed] [Google Scholar]
- 20. Ohi M. D., Vander Kooi C. W., Rosenberg J. A., Ren L., Hirsch J. P., Chazin W. J., Walz T., and Gould K. L. (2005) Structural and functional analysis of essential pre-mRNA splicing factor Prp19p. Mol. Cell. Biol. 25, 451–460 10.1128/MCB.25.1.451-460.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cui F., Liu L., Zhao Q., Zhang Z., Li Q., Lin B., Wu Y., Tang S., and Xie Q. (2012) Arabidopsis ubiquitin conjugase UBC32 is an ERAD component that functions in brassinosteroid-mediated salt stress tolerance. Plant Cell 24, 233–244 10.1105/tpc.111.093062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang M., Windheim M., Roe S. M., Peggie M., Cohen P., Prodromou C., and Pearl L. H. (2005) Chaperoned ubiquitylation: crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP-Ubc13-Uev1a complex. Mol. Cell 20, 525–538 10.1016/j.molcel.2005.09.023 [DOI] [PubMed] [Google Scholar]
- 23. Eletr Z. M., and Kuhlman B. (2007) Sequence determinants of E2-E6AP binding affinity and specificity. J. Mol. Biol. 369, 419–428 10.1016/j.jmb.2007.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stefan E., Aquin S., Berger N., Landry C. R., Nyfeler B., Bouvier M., and Michnick S. W. (2007) Quantification of dynamic protein complexes using Renilla luciferase fragment complementation applied to protein kinase A activities in vivo. Proc. Natl. Acad. Sci. U.S.A. 104, 16916–16921 10.1073/pnas.0704257104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohi M. D., Vander Kooi C. W., Rosenberg J. A., Chazin W. J., and Gould K. L. (2003) Structural insights into the U-box, a domain associated with multi-ubiquitination. Nat. Struct. Biol. 10, 250–255 10.1038/nsb906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soss S. E., Klevit R. E., and Chazin W. J. (2013) Activation of UbcH5c∼Ub is the result of a shift in interdomain motions of the conjugate bound to U-box E3 ligase E4B. Biochemistry 52, 2991–2999 10.1021/bi3015949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plechanovova A., Jaffray E. G., McMahon S. A., Johnson K. A., Navratilova I., Naismith J. H., and Hay R. T. (2011) Mechanism of ubiquitylation by dimeric RING ligase RNF4. Nat. Struct. Mol. Biol. 18, 1052–1059 10.1038/nsmb.2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dou H., Buetow L., Sibbet G. J., Cameron K., and Huang D. T. (2012) BIRC7-E2 ubiquitin conjugate structure reveals the mechanism of ubiquitin transfer by a RING dimer. Nat. Struct. Mol. Biol. 19, 876–883 10.1038/nsmb.2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suzuki G., Yanagawa Y., Kwok S. F., Matsui M., and Deng X. W. (2002) Arabidopsis COP10 is a ubiquitin-conjugating enzyme variant that acts together with COP1 and the COP9 signalosome in repressing photomorphogenesis. Genes Dev. 16, 554–559 10.1101/gad.964602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lau O. S., and Deng X. W. (2009) Effect of Arabidopsis COP10 ubiquitin E2 enhancement activity across E2 families and functional conservation among its canonical homologues. Biochem. J. 418, 683–690 10.1042/BJ20081943 [DOI] [PubMed] [Google Scholar]
- 31. Yanagawa Y., Sullivan J. A., Komatsu S., Gusmaroli G., Suzuki G., Yin J., Ishibashi T., Saijo Y., Rubio V., Kimura S., Wang J., and Deng X. W. (2004) Arabidopsis COP10 forms a complex with DDB1 and DET1 in vivo and enhances the activity of ubiquitin conjugating enzymes. Genes Dev. 18, 2172–2181 10.1101/gad.1229504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hofmann R. M., and Pickart C. M. (1999) Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 96, 645–653 10.1016/S0092-8674(00)80575-9 [DOI] [PubMed] [Google Scholar]
- 33. VanDemark A. P., Hofmann R. M., Tsui C., Pickart C. M., and Wolberger C. (2001) Molecular insights into polyubiquitin chain assembly: crystal structure of the Mms2/Ubc13 heterodimer. Cell 105, 711–720 10.1016/S0092-8674(01)00387-7 [DOI] [PubMed] [Google Scholar]
- 34. Yin X. J., Volk S., Ljung K., Mehlmer N., Dolezal K., Ditengou F., Hanano S., Davis S. J., Schmelzer E., Sandberg G., Teige M., Palme K., Pickart C., and Bachmair A. (2007) Ubiquitin lysine 63 chain forming ligases regulate apical dominance in Arabidopsis. Plant Cell 19, 1898–1911 10.1105/tpc.107.052035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kinoshita A., Seo M., Kamiya Y., and Sawa S. (2015) Mystery in genetics: PUB4 gives a clue to the complex mechanism of CLV signaling pathway in the shoot apical meristem. Plant Signal. Behav. 10, e1028707 10.1080/15592324.2015.1028707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kinoshita A., ten Hove C. A., Tabata R., Yamada M., Shimizu N., Ishida T., Yamaguchi K., Shigenobu S., Takebayashi Y., Iuchi S., Kobayashi M., Kurata T., Wada T., Seo M., Hasebe M., et al. (2015) A plant U-box protein, PUB4, regulates asymmetric cell division and cell proliferation in the root meristem. Development 142, 444–453 10.1242/dev.113167 [DOI] [PubMed] [Google Scholar]
- 37. Wang Y., Wu Y., Yu B., Yin Z., and Xia Y. (2017) Extra-large G proteins interact with E3 ligases PUB4 and PUB2 and function in cytokinin and developmental processes. Plant Physiol. 173, 1235–1246 10.1104/pp.16.00816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Woodson J. D., Joens M. S., Sinson A. B., Gilkerson J., Salomé P. A., Weigel D., Fitzpatrick J. A., and Chory J. (2015) Ubiquitin facilitates a quality-control pathway that removes damaged chloroplasts. Science 350, 450–454 10.1126/science.aac7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kong L., Cheng J., Zhu Y., Ding Y., Meng J., Chen Z., Xie Q., Guo Y., Li J., Yang S., and Gong Z. (2015) Degradation of the ABA co-receptor ABI1 by PUB12/13 U-box E3 ligases. Nat. Commun. 6, 8630 10.1038/ncomms9630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li W., Ahn I. P., Ning Y., Park C. H., Zeng L., Whitehill J. G., Lu H., Zhao Q., Ding B., Xie Q., Zhou J. M., Dai L., and Wang G. L. (2012) The U-Box/ARM E3 ligase PUB13 regulates cell death, defense, and flowering time in Arabidopsis. Plant Physiol. 159, 239–250 10.1104/pp.111.192617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liao D., Cao Y., Sun X., Espinoza C., Nguyen C. T., Liang Y., and Stacey G. (2017) Arabidopsis E3 ubiquitin ligase PLANT U-BOX13 (PUB13) regulates chitin receptor lysin motif receptor kinase5 (LYK5) protein abundance. New Phytol. 214, 1646–1656 10.1111/nph.14472 [DOI] [PubMed] [Google Scholar]
- 42. Lu D., Lin W., Gao X., Wu S., Cheng C., Avila J., Heese A., Devarenne T. P., He P., and Shan L. (2011) Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332, 1439–1442 10.1126/science.1204903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou J., Liu D., Wang P., Ma X., Lin W., Chen S., Mishev K., Lu D., Kumar R., Vanhoutte I., Meng X., He P., Russinova E., and Shan L. (2018) Regulation of Arabidopsis brassinosteroid receptor BRI1 endocytosis and degradation by plant U-box PUB12/PUB13-mediated ubiquitination. Proc. Natl. Acad. Sci. U.S.A. 115, E1906–E1915 10.1073/pnas.1712251115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee H. G., and Seo P. J. (2016) The Arabidopsis MIEL1 E3 ligase negatively regulates ABA signalling by promoting protein turnover of MYB96. Nat. Commun. 7, 12525 10.1038/ncomms12525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marino D., Froidure S., Canonne J., Ben Khaled S., Khafif M., Pouzet C., Jauneau A., Roby D., and Rivas S. (2013) Arabidopsis ubiquitin ligase MIEL1 mediates degradation of the transcription factor MYB30 weakening plant defence. Nat. Commun. 4, 1476 10.1038/ncomms2479 [DOI] [PubMed] [Google Scholar]
- 46. Janjusevic R., Abramovitch R. B., Martin G. B., and Stebbins C. E. (2006) A bacterial inhibitor of host programmed cell death defenses is an E3 ubiquitin ligase. Science 311, 222–226 10.1126/science.1120131 [DOI] [PubMed] [Google Scholar]
- 47. Göhre V., and Robatzek S. (2008) Breaking the barriers: microbial effector molecules subvert plant immunity. Annu. Rev. Phytopathol. 46, 189–215 10.1146/annurev.phyto.46.120407.110050 [DOI] [PubMed] [Google Scholar]
- 48. Gimenez-Ibanez S., Hann D. R., Ntoukakis V., Petutschnig E., Lipka V., and Rathjen J. P. (2009) AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr. Biol. 19, 423–429 10.1016/j.cub.2009.01.054 [DOI] [PubMed] [Google Scholar]
- 49. Ntoukakis V., Mucyn T. S., Gimenez-Ibanez S., Chapman H. C., Gutierrez J. R., Balmuth A. L., Jones A. M., and Rathjen J. P. (2009) Host inhibition of a bacterial virulence effector triggers immunity to infection. Science 324, 784–787 10.1126/science.1169430 [DOI] [PubMed] [Google Scholar]
- 50. Rosebrock T. R., Zeng L., Brady J. J., Abramovitch R. B., Xiao F., and Martin G. B. (2007) A bacterial E3 ubiquitin ligase targets a host protein kinase to disrupt plant immunity. Nature 448, 370–374 10.1038/nature05966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li W., and Schmidt W. (2010) A lysine-63-linked ubiquitin chain-forming conjugase, UBC13, promotes the developmental responses to iron deficiency in Arabidopsis roots. Plant J. 62, 330–343 10.1111/j.1365-313X.2010.04150.x [DOI] [PubMed] [Google Scholar]
- 52. Zipfel C., Robatzek S., Navarro L., Oakeley E. J., Jones J. D., Felix G., and Boller T. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428, 764–767 10.1038/nature02485 [DOI] [PubMed] [Google Scholar]
- 53. Soss S. E., Yue Y., Dhe-Paganon S., and Chazin W. J. (2011) E2 conjugating enzyme selectivity and requirements for function of the E3 ubiquitin ligase CHIP. J. Biol. Chem. 286, 21277–21286 10.1074/jbc.M111.224006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Scaglione K. M., Basrur V., Ashraf N. S., Konen J. R., Elenitoba-Johnson K. S., Todi S. V., and Paulson H. L. (2013) The ubiquitin-conjugating enzyme (E2) Ube2w ubiquitinates the N terminus of substrates. J. Biol. Chem. 288, 18784–18788 10.1074/jbc.C113.477596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tatham M. H., Plechanovová A., Jaffray E. G., Salmen H., and Hay R. T. (2013) Ube2W conjugates ubiquitin to α-amino groups of protein N-termini. Biochem. J. 453, 137–145 10.1042/BJ20130244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fletcher A. J., Christensen D. E., Nelson C., Tan C. P., Schaller T., Lehner P. J., Sundquist W. I., and Towers G. J. (2015) TRIM5α requires Ube2W to anchor Lys63-linked ubiquitin chains and restrict reverse transcription. EMBO J. 34, 2078–2095 10.15252/embj.201490361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu K., Kovacev J., and Pan Z. Q. (2010) Priming and extending: a UbcH5/Cdc34 E2 handoff mechanism for polyubiquitination on a SCF substrate. Mol. Cell 37, 784–796 10.1016/j.molcel.2010.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Christensen D. E., Brzovic P. S., and Klevit R. E. (2007) E2-BRCA1 RING interactions dictate synthesis of mono- or specific polyubiquitin chain linkages. Nat. Struct. Mol. Biol. 14, 941–948 10.1038/nsmb1295 [DOI] [PubMed] [Google Scholar]
- 59. Windheim M., Peggie M., and Cohen P. (2008) Two different classes of E2 ubiquitin-conjugating enzymes are required for the mono-ubiquitination of proteins and elongation by polyubiquitin chains with a specific topology. Biochem. J. 409, 723–729 10.1042/BJ20071338 [DOI] [PubMed] [Google Scholar]
- 60. Katsiarimpa A., Anzenberger F., Schlager N., Neubert S., Hauser M. T., Schwechheimer C., and Isono E. (2011) The Arabidopsis deubiquitinating enzyme AMSH3 interacts with ESCRT-III subunits and regulates their localization. Plant Cell 23, 3026–3040 10.1105/tpc.111.087254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Katsiarimpa A., Muñoz A., Kalinowska K., Uemura T., Rojo E., and Isono E. (2014) The ESCRT-III-interacting deubiquitinating enzyme AMSH3 is essential for degradation of ubiquitinated membrane proteins in Arabidopsis thaliana. Plant Cell Physiol. 55, 727–736 10.1093/pcp/pcu019 [DOI] [PubMed] [Google Scholar]
- 62. Teh O-k., Lee C.-W., Ditengou F. A., Klecker T., Furlan G., Zietz M., Hause G., Eschen-Lippold L., Hoehenwarter W., Lee J., Ott T., and Trujillo M. (2018) Phosphorylation of the exocyst subunit Exo70B2 contributes to the regulation of its function. bioRxiv 10.1101/266171 10.1101/266171 [DOI] [Google Scholar]
- 63. Zhou J., Liu D., Wang P., Ma X., Lin W., Chen S., Mishev K., Lu D., Kumar R., Vanhoutte I., Meng X., He P., Russinova E., and Shan L. (2018) Regulation of Arabidopsis brassinosteroid receptor BRI1 endocytosis and degradation by plant U-box PUB12/PUB13-mediated ubiquitination. Proc. Natl. Acad. Sci. U.S.A. 115, E1906–E1915 10.1073/pnas.1712251115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. De Gassart A., Camosseto V., Thibodeau J., Ceppi M., Catalan N., Pierre P., and Gatti E. (2008) MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc. Natl. Acad. Sci. U.S.A. 105, 3491–3496 10.1073/pnas.0708874105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bartee E., Mansouri M., Hovey Nerenberg B. T., Gouveia K., and Früh K. (2004) Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J. Virol. 78, 1109–1120 10.1128/JVI.78.3.1109-1120.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Romero-Barrios N., and Vert G. (2018) Proteasome-independent functions of lysine-63 polyubiquitination in plants. New Phytol. 217, 995–1011 10.1111/nph.14915 [DOI] [PubMed] [Google Scholar]
- 67. Bednarek P., Kwon C., and Schulze-Lefert P. (2010) Not a peripheral issue: secretion in plant-microbe interactions. Curr. Opin. Plant Biol. 13, 378–387 10.1016/j.pbi.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 68. Kim D. Y., Scalf M., Smith L. M., and Vierstra R. D. (2013) Advanced proteomic analyses yield a deep catalog of ubiquitylation targets in Arabidopsis. Plant Cell 25, 1523–1540 10.1105/tpc.112.108613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu F. H., Shen S. C., Lee L. Y., Lee S. H., Chan M. T., and Lin C. S. (2009) Tape-Arabidopsis sandwich: a simpler Arabidopsis protoplast isolation method. Plant Methods 5, 16 10.1186/1746-4811-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ehlert A., Weltmeier F., Wang X., Mayer C. S., Smeekens S., Vicente-Carbajosa J., and Droge-Laser W. (2006) Two-hybrid protein-protein interaction analysis in Arabidopsis protoplasts: establishment of a heterodimerization map of group C and group S bZIP transcription factors. Plant J. 46, 890–900 10.1111/j.1365-313X.2006.02731.x [DOI] [PubMed] [Google Scholar]
- 71. Engler C., and Marillonnet S. (2014) Golden Gate cloning. Methods Mol. Biol. 1116, 119–131 10.1007/978-1-62703-764-8_9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.