Abstract
Solute carrier family 30 member 8 (SLC30A8), encoding the pancreatic zinc transporter ZnT8, is a susceptibility gene for type 2 diabetes (T2D). Reducing ZnT8 transport activity or down-regulating its cellular expression is hypothesized to be an antidiabetogenic strategy mimicking the protective effect of SLC30A8 haploinsufficiency in humans. However, research tools to inhibit ZnT8 activity and measure cellular ZnT8 levels are not available. Here, we report the identification of two anti-ZnT8 mAbs applicable to addressing these unmet needs. Both mAbs exhibited subnanomolar affinities for human ZnT8 and were selective against homologous zinc transporters with distinct cross-species reactivities and epitope recognition. We showed that antigen-binding fragments (Fabs) protected ZnT8 from unfolding and inhibited ZnT8-mediated zinc transport in proteoliposomes. Negative-stain EM revealed a ternary binding complex of a ZnT8 monomer and two different Fabs at a 1:1:1 stoichiometry. Moreover, dual bindings of two different mAbs to a single ZnT8 protein multiplied the individual anti-ZnT8 specificities, enabling quantification of cellular ZnT8 levels by homogeneous time-resolved fluorescence (HTRF). Our results demonstrate the utilities of the two generated mAbs as allosteric inhibitors and highly specific biosensors of human ZnT8.
Keywords: zinc, transport, monoclonal antibody, inhibitor, pancreatic islet, diabetes, drug discovery, ZnT8, mAb, drug screening, pancreatic beta-cells, HTRF, therapeutic target
Introduction
The human genome encodes a multitude of zinc transporters involved in regulations of cellular zinc homeostasis and signaling (1). Among all zinc transporters, zinc transporter-8 (ZnT8) is unique in its tissue-specific expression (2). Microarray profiling of mouse tissues (3) and single-cell transcriptome profiling of human pancreatic islets (4) showed that SCL30A8 transcription is mostly restricted to the endocrine cells of pancreatic islets. The transcriptional profile of SLC30A8 has a degree of islet specificity similar to that of well-known β-cell markers such as PDX1 and NKX6.1 (4). Single-cell RNA-Seq showed that these β-cell markers also had “spill-over” transcription to non-β-endocrine cells, but the spill-over did not extend to exocrine or ductal cells (4). In islet β-cells, ZnT8 is primarily expressed in insulin secretory granules (2, 5), where ZnT8 is required for granular zinc enrichment and zinc-insulin crystalline packaging in the form of hexameric insulins (6, 7). The zinc content within insulin granules is over 10 mm (8). This high zinc level is implicated in insulin synthesis, storage, regulation of insulin secretion, and hepatic insulin clearance following glucose-stimulated insulin secretion (GSIS)3 (9–11).
Islet β-cells are the sole source in the human body for providing insulin. Insufficient insulin production is a major pathogenic component of T2D (12). The common T2D is a complex polygenic disease associated with more than 150 T2D risk genes (13). Until now, SCL30A8 is the only one known for harboring protective loss-of-function (LOF) mutations (14). Genotyping ∼150,000 individuals across multiple population cohorts revealed that carriers of LOF mutations had a 65% lower risk for T2D (15). This unique position of SLC30A8 in the genetic landscape of T2D susceptibility makes ZnT8 an attractive therapeutic target (16). Notably, a missense SNP in SCL30A8 (rs13266634) is associated with increased susceptibility to T2D (17–19). Zinc transport activity of the higher-risk Arg-325 variant is hyperactive compared with the lower-risk Trp-325 variant (20), corresponding to a higher zinc level in human pancreatic islets from donors carrying the Arg-325 variant (21). Moreover, a transgenic mouse model overexpressing the Arg-325 variant increases islet zinc level and decreases glucose tolerance after a high-fat diet (22). A knock-in mouse model carrying a human LOF mutation increases GSIS under hyperglycemic conditions (23), although GSIS phenotypes of ZnT8-KO mouse models are heterogeneous (6, 7, 24, 25). Emerging evidence from ZnT8 biochemistry, animal models, and human genetics has coalesced to suggest a causal relationship linking the gain-of-function Arg-235 variant and increased T2D risk, supporting the case for ZnT8 inhibition as a potential antidiabetogenic strategy (16, 26).
Two alternative approaches may be used to down-regulate ZnT8 activity in β-cells: inhibiting zinc transport or reducing ZnT8 expression. The mechanism driving zinc transport is conserved from bacteria to humans (27, 28). In a bacterial homolog YiiP (29), zinc transport is susceptible to allosteric regulation by zinc binding to a cytosolic C-terminal domain (CTD) (30). Likewise, allostery may be targeted for ZnT8 inhibition using mAb to trap ZnT8 in a fixed conformation, but a proof-of-principle allosteric inhibitor is still lacking. Furthermore, functional regulation of ZnT8 expression in β-cells has yet to be investigated due to a lack of ZnT8-specific reagents for tracking the cellular ZnT8 level over a heterogeneous background. Discovering specific and high-affinity mAbs would permit testing the allosteric inhibition hypothesis while developing assays to quantify the cellular ZnT8 level.
In the present work, we identified two mAbs (mAb20 and mAb39) through a multitier screening against purified human ZnT8 in its native conformations. Detailed mAb characterization revealed distinct binding properties and specificities with a common ability to stabilize ZnT8 structural folding and, consequently, inhibit its transport activity by slowing down the zinc turnover rate. These findings demonstrate the utility of mAbs as allosteric inhibitors of human ZnT8. We also identified a stable ternary complex of a ZnT8 monomer with two distinct Fabs. The ZnT8-mediated proximity of two mAbs allowed for ZnT8 quantification based on a FRET signal between a pair of donor and acceptor fluorophores covalently linked to bound mAbs. In this assay, we used a caged lanthanide donor (terbium cryptate) that is characterized by a long fluorescence emission half-life. The FRET signal derived from the ternary complex can be obtained using a delayed fluorescence measurement after the decay of the short-lived background fluorescence. Thus, the assay can be performed in a homogeneous format without the need to separate the binding complex from unbound mAbs and other nonspecific fluorophores in the binding mix (31). This simple add-and-read HTRF assay highlights another utility of mAbs as a ZnT8-specific biosensor.
Results
Identifying distinct anti-ZnT8 mAbs
Human ZnT8 (B-form) is a homodimer of two 35.1-kDa protomers, each consisting of a 23.8-kDa transmembrane domain (TMD) followed by a 11.3-kDa CTD. A ZnT8 homodimer is referred to as ZnT8 hereafter. Immunization of ZnT8-KO mice with purified human ZnT8 in proteoliposomes yielded serum antibodies that exhibited a ∼100-fold increase in antibody titers compared with titers of two commercial antibodies directed to linear peptide sequences from the N- and C-terminal domain (NTD and CTD), respectively (32). Splenocytes of immunized mice were isolated and fused with myeloma cells to establish immortalized hybridomas. Preliminary screening by ELISA identified anti-ZnT8 antibodies that showed different reactivities toward a full-length ZnT8 antigen in proteoliposomes and a monomeric CTD antigen (amino acids 275–369). These hybridoma cells were subjected to single cell sorting and clonal expansion, and then two secreted monoclonal antibodies (mAb20 and mAb39) were purified for further characterization. Commercial anti-NTD and anti-CTD antibodies (NTDA and CTDA) or purified mouse serum antibodies (MSA) from nonimmunized ZnT8-KO mice were used as a positive or negative control. mAb20, mAb39, CTDA, and NTDA all yielded positive readouts against a ZnT8 proteoliposome antigen (Fig. 1A). In contrast, only mAb20 and the positive control CTDA recognized a monomeric CTD antigen. mAb39 and the negative control NTDA gave negative results in ELISA probed with CTD (Fig. 1B), suggesting that mAb20 recognized an epitope distinct from that of mAb39.
Figure 1.
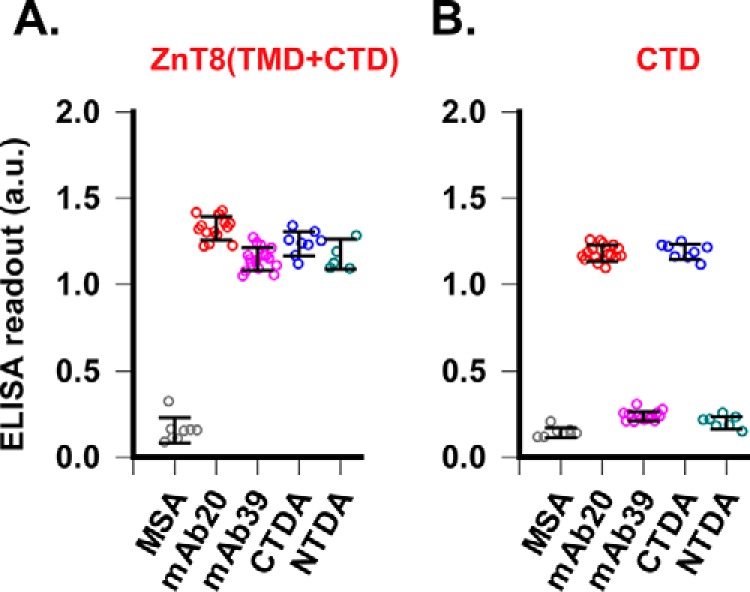
Mapping mAb-binding sites. A, ELISA against ZnT8 (TMD + CTD) in proteoliposomes immobilized by passive coating. Bound antibodies were detected by HRP-conjugated secondary antibodies. The assay was calibrated by CTDA and NTDA as positive controls and MSA as a negative control with a Z′ factor > 0.5. Error bars, S.D. B, ELISA against a monomeric CTD antigen immobilized to a Ni-NTA plate via a C-terminal polyhistidine tag. CTDA and NTDA were used as a positive and negative control, respectively (Z′ > 0.5).
Stable antibody–ZnT8 complexes
The formation of stable mAb- or Fab–ZnT8 complexes was examined by analytical size-exclusion HPLC. mAbs in excess to ZnT8 tended to form cross-linked protein aggregates. By adjusting the mAb/ZnT8 stoichiometry, we detected a series of eight distinct monodisperse peaks, labeled 1–8 in Fig. 2. mAb20 and Fab20 were labeled with a green fluorophore and mAb39 with a red fluorophore. mAb20/Fab20 and mAb39 in complex with ZnT8 were monitored in separate channels of a fluorescence detector (Fig. 2). The apparent molecular weight (MW) of each peak was calculated, and its predicted complex composition consistent with the molecular weight is summarized in Table 1. For binary binding, Fab20 formed a single (Fab20)2–ZnT8 complex (peak 3), and mAb20 formed a major mAb20–ZnT8 complex (peak 2). By comparison, mAb39 predominantly formed a (mAb39)2–ZnT8 complex (peak 1) and a secondary mAb39–ZnT8 complex aligning with peak 2. For ternary binding among ZnT8, mAb20/Fab20, and mAb39, both green and red channels recorded similar (mAb39)2–ZnT8–mAb20 (peaks 5 and 7) and mAb39–ZnT8–(Fab20)2 complexes (peaks 6 and 8), indicating that mAb20/Fab20 and mAb39 bound independently to a single ZnT8 (peak 4).
Figure 2.
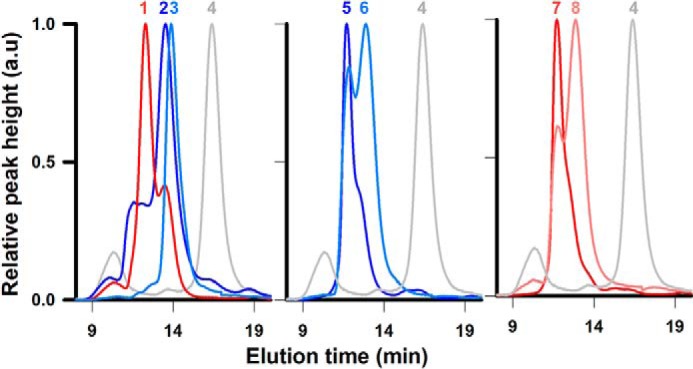
ZnT8–antibody complexes Size-exclusion HPLC chromatograms of ZnT8 in complex with mAb20, Fab20, and/or mAb39 in various combinations and binding stoichiometries are shown. Eight distinct peaks were resolved, labeled 1–8. Corresponding complex molecular weights and predicted complex compositions are summarized in Table 1. mAb20/Fab20 and mAb39 were fluorescently labeled and detected either in the green (shown as blue traces) or red (red traces) channel of a multichannel fluorescence detector. Gray traces, unbound ZnT8 detected by UV absorption. a.u., arbitrary units.
Table 1.
Summary of the binary and ternary complex peaks
| Peak number | Elution time | Apparent molecular mass | Complex composition |
|---|---|---|---|
| min | kDa | ||
| 1 | 12.3 | 490 | (mAb39)2 + ZnT8 |
| 2 | 13.5 | 307 | mAb20 + ZnT8 |
| 3 | 13.9 | 260 | (Fab20)2 + ZnT8 |
| 4 | 16.4 | 118 | ZnT8 |
| 5 | 11.7 | 618 | (mAb39)2 + mAb20 + ZnT8 |
| 6 | 12.9 | 388 | mAb39 + (Fab20)2 + ZnT8 |
| 7 | 11.7 | 618 | (mAb39)2 + mAb20 + ZnT8 |
| 8 | 12.9 | 388 | mAb39 + (Fab20)2 + ZnT8 |
Single-particle EM analysis
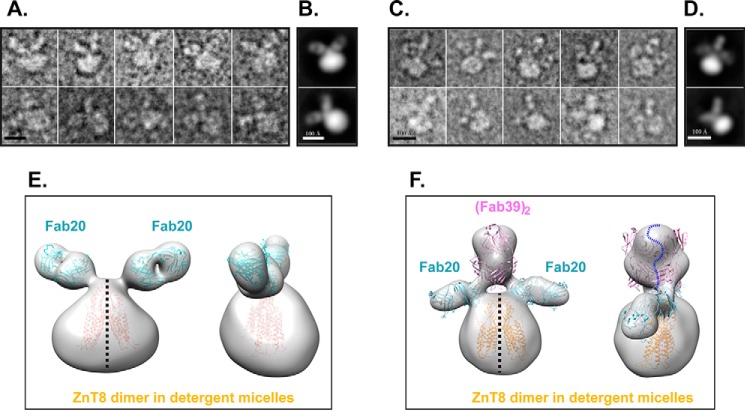
To further characterize antibody binding, we purified the binary (Fab20)2–ZnT8 and ternary (Fab20)2–ZnT8–(Fab39)2 complexes and directly visualized Fab bindings by negative staining EM. The raw images of the binary complex (Fig. 3A) and corresponding 2D reference-free averages revealed two Fabs in one intact complex, each with a characteristic Fab groove that was clearly visible between the variable and constant region (Fig. 3B). Thus, 2-fold symmetry was applied to single-particle 3D reconstruction, yielding a low-resolution (∼3-nm) 3D map. The 3D envelope was fitted with the crystal structures of two Fab molecules (PDB code 1M71) and one ZnT8 homolog, YiiP (PDB code 3H90). The ZnT8 envelop was larger than the crystal structure, likely due to the detergent micelles surrounding the transmembrane region (Fig. 3E). For the ternary complex, the raw images revealed bindings of multiple Fab molecules to a single ZnT8 dimer (Fig. 3C). The representative 2D averages also showed multiple Fab bindings (Fig. 3D). 3D reconstructions revealed three discrete protrusions on the CTD surface (Fig. 3F). The two outer protrusions were in positions similar to but slightly lower than those observed in ZnT8-(Fab20)2; thus, they were assigned as a pair of Fab20 molecules (Fig. 3F). The middle protrusion was larger and could be fitted by two Fab molecules straddling the 2-fold axis at the ZnT8 dimer interface, and therefore assigned as two Fab39 molecules (Fig. 3F). Although the map resolution was insufficient to distinguish different types of Fabs in the ternary complex, homologue structure docking of the 3D model suggested that CTD dimerization was required for mAb39 binding. This finding was supported by a negative mAb39 readout in CTD-ELISA against a monomeric CTD antigen (Fig. 1B). By comparison, each Fab20 bound to a separate CTD monomer in the 3D model (Fig. 3, E and F), in agreement with a positive mAb20 readout in CTD-ELISA.
Figure 3.
EM analysis of ZnT8–Fab complexes. A, representative raw particles of the binary complex ZnT8–(Fab20)2 in negative stain. Scale bar, 100 Å. B, representative reference-free 2D class averages of the binary complexes. C, representative raw particles of ternary complex (Fab20)2–ZnT8–(Fab39)2. D, representative reference-free 2D class averages of the ternary complexes. E, 3D map of ZnT8–(Fab20)2 in two views related by a 90° rotation around a 2-fold axis perpendicular to the membrane plane (black dashed line). Atomic models of Fab20 (PDB code 1M71) and ZnT8 homolog (PDB code 3H90) were fitted into the low-resolution envelope and colored in cyan and yellow, respectively. F, 3D model of (Fab20)2–ZnT8–(Fab39)2 in two views docked with four Fab structures, representing two Fab20 (cyan) and two Fab39 (magenta), respectively. The ZnT8 dimer model is shown in yellow. A blue dashed curve marks the boundary between two Fab39 molecules on top in close proximity.
Folding dependence
To compare the difference in mAb bindings to native and denatured ZnT8, we used 2% SDS to unfold immobilized ZnT8-His on a Ni-NTA plate, and then compared denatured ELISA readouts with nondenatured replicates. The native readout of mAb20 was about 2-fold higher than the denatured readout (Fig. 4A), indicating a moderate selectivity for folded conformation over unfolded ZnT8. As a control, CTDA reacted with native and denatured ZnT8 indiscriminately. Unexpectedly, mAb39, despite its binding to the dimer interface and anti-CTD negativity (Fig. 1B), was ELISA-positive for denatured ZnT8 immobilized to the Ni-NTA plate (Fig. 4A). One possibility was that mAb39 epitope was close to the C terminus, where a pair of His tags from a ZnT8 dimer might stabilize local dimeric interaction by Ni-NTA binding. To test this hypothesis, we added a bulkier GFP to the C terminus and examined its potential steric hindrance to mAb binding. Size-exclusion HPLC showed that mAb20 shifted the ZnT8-GFP peak leftward as predicted for a mAb20–ZnT8-GFP binary complex (Fig. 4B). In contrast, the C-terminal GFP completely blocked the formation of mAb39–ZnT8-GFP binary complex. The steric clash between GFP and mAb39 binding localized the mAb39 binding site to the C terminus. This result further validated the mAb39 docking to a distal CTD surface straddling the dimer interface (Fig. 3F).
Figure 4.
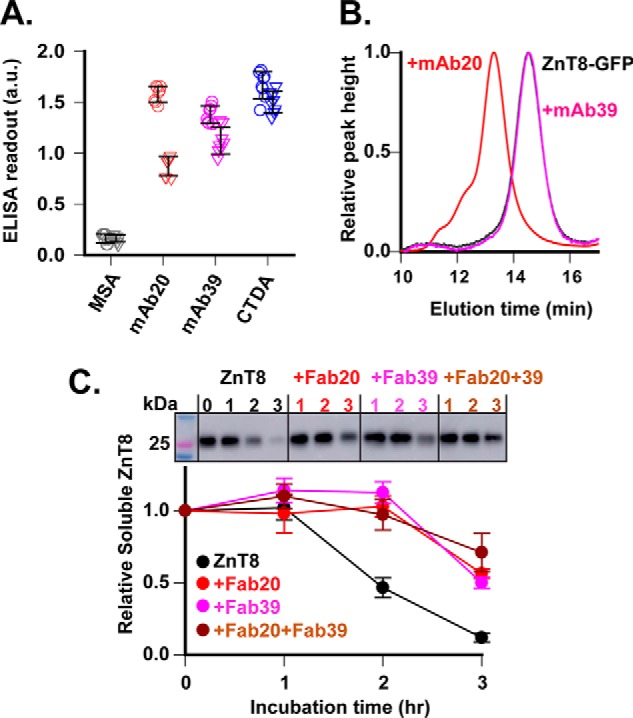
Stabilizing ZnT8 folding by mAb binding. A, mAb binding to folded or denatured ZnT8. ZnT8-His in detergent micelles was immobilized to a Ni-NTA plate in replication without SDS denaturation. ELISAs against denatured (triangle) and nondenatured ZnT8 (circle) were compared for mAb20 and mAb39 as indicated. CTDA and MSA were used as a positive and negative control, respectively. Error bars, S.D. B, mAb binding to folded ZnT8-GFP in detergent micelles. mAb20 or mAb39 was mixed with ZnT8-GFP, and then the binding mixture was analyzed by size-exclusion HPLC with GFP fluorescence detection. Note that mAb20 shifted the unbound ZnT8-GFP peak (black) leftward to a mAb20–ZnT8-GFP position (red), whereas mAb39 did not cause peak shift (magenta). C, protection of ZnT8 unfolding. Aliquots of ZnT8 in detergent micelles were incubated with Fab20 (red), Fab39 (magenta), or Fab20 + Fab39 (brown). Free ZnT8 was used as a control (black). After a designated time of heat denaturation as indicated, protein aggregates were removed by ultracentrifugation, and the amount of remaining ZnT8 in the supernatant was detected by mAb20 immunoblotting (top). Error bars, S.E. of six independent densitometric measurements of one representative experiment using ImageJ. a.u., arbitrary units.
Enhancing ZnT8 stability
mAb binding to a conformational epitope is expected to stabilize the protein folding. Unfolded ZnT8 could be precipitated as protein aggregates by ultracentrifugation. To evaluate the effect of antibody binding on ZnT8 stability, we incubated detergent-solubilized ZnT8 with or without Fab binding and then exposed the protein sample to heat denaturation at 37 °C and monitored the folded ZnT8 in the supernatant post-ultracentrifugation. Quantitative immunoblotting showed that ZnT8 was not stable and began to precipitate after 1 h of heat exposure (Fig. 4C). Fab20 or Fab39 binding delayed the onset of ZnT8 precipitation to 2 h, and this protective effect was further augmented by concurrent bindings of Fab20 and Fab39. At 3 h, only 12% of initial ZnT8 remained in the supernatant, whereas more than 70% of (Fab20)2–ZnT8–(Fab39)2 was still soluble.
Inhibiting zinc transport
The stabilizing effects of Fab20 and Fab39 suggested that Fab binding trapped ZnT8 in more stable conformations. We further examined the effect of conformational constraints on kinetics of ZnT8-mediated zinc transport. Purified human ZnT8 was reconstituted into proteoliposomes encapsulated with a zinc fluorescent indicator, FluoZin-3, and then exposed to a sequence of extravesicular zinc concentrations on a stopped-flow apparatus (Fig. 5A). A fixed 1 mm citrate was added to assay buffers with increasing zinc concentrations, yielding free zinc concentrations from 1.6 to 116 μm. This ensured a constant supply of free zinc to satisfy the steady-state condition. The transmembrane orientations of the reconstituted ZnT8 were estimated to expose 50–75% of the CTD on the extravesicular side of proteoliposomes based on the difference in mAb immunofluorescence staining between sealed and detergent-perforated proteoliposomes. Fab20 or Fab39 was loaded to proteoliposomes at an estimated Fab/ZnT8 molar ratio of 2.8. Sizing HPLC of resolubilized, Fab-treated proteoliposomes confirmed the full occupancy of Fab binding. A rapid mixing of proteoliposomes with a zinc buffer triggered a quasi-linear increase of FluoZin-3 fluorescence within the initial 10 s (Fig. 5A). The rate of fluorescence rise was calculated and fitted to the Michaelis–Menten equation (Fig. 5B). The Vmax and Km values of the steady-state kinetics are summarized in Table 2, showing a modest reduction (30–41%) of Vmax by Fab20 or Fab39 as compared with the unbound proteoliposome control. The inhibitory effects of individual Fab bindings were additive, giving a 57% Vmax reduction when ZnT8 was doubly bound with Fab20 and Fab39. The effect size of Fab inhibition was similar to that of a lower-risk Trp-325 variant, which is ∼50% of the higher-risk Arg-325 variant (20). No significant difference was observed for the Km value, indicating that Fab inhibited ZnT8 by reducing its transport rate.
Figure 5.
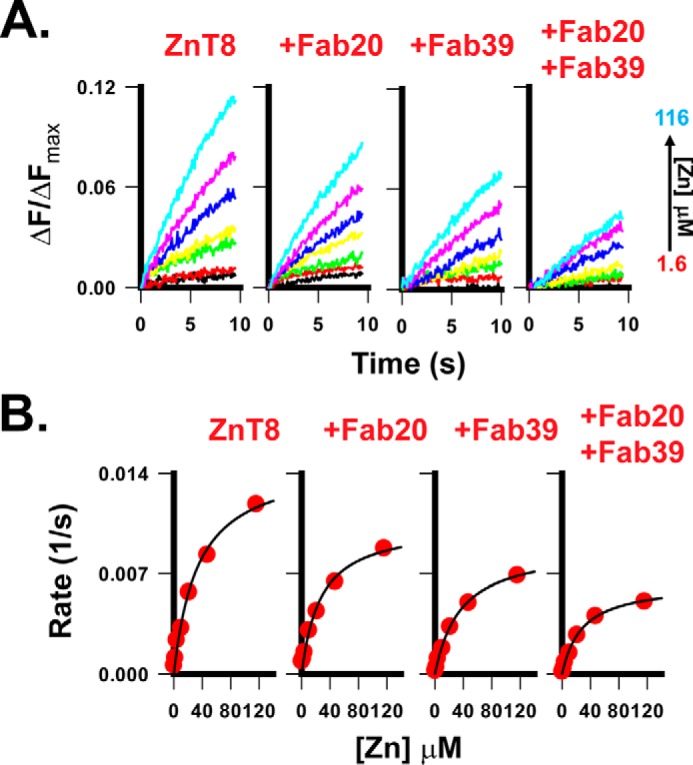
Inhibiting zinc transport by Fab binding. A, FluoZin-3 fluorescence traces in response to a rapid mixing of ZnT8 proteoliposomes with an extravesicular zinc buffer. The free zinc concentration in the buffer was increased from 1.6 to 116 μm, as indicated by an arrow. B, steady-state kinetics for zinc transport mediated by ZnT8 or by ZnT8 bound with Fab20, Fab39, or Fab20 + Fab39, as indicated. Solid lines, least-squares fits to the Michaelis–Menten equation. Km and Vmax values are summarized in Table 2.
Table 2.
Parameters of steady-state zinc transport kinetics
| Vmax | Km | |
|---|---|---|
| ×0.001 s−1 | μm | |
| ZnT8 | 15.0 ± 1.3 | 33.7 ± 7.0 |
| ZnT8 + Fab20 | 10.5 ± 1.0 | 26.6 ± 6.4 |
| ZnT8 + Fab39 | 8.9 ± 0.4 | 36.1 ± 4.1 |
| ZnT8 + Fab20 + Fab39 | 6.4 ± 0.2 | 28.3 ± 2.9 |
Binding affinity
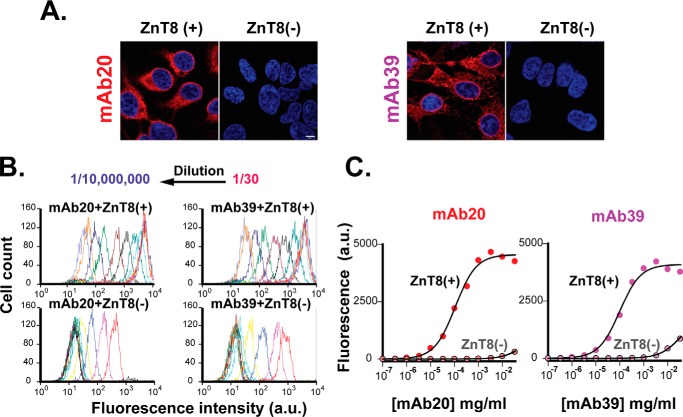
We examined mAb binding to HEK293 cells stably expressing human ZnT8. Confocal microscopy imaging of immunofluorescence staining with mAb20 and mAb39 revealed strong intracellular signals (Fig. 6A). No mAb staining was detected in parental HEK293 cells without ZnT8 overexpression. Next, we extended the imaging analysis to bulk cell populations using flow cytometry to analyze stably transfected or parental cells labeled with mAb20 or mAb39 in serial dilutions. For each mAb concentration, about 3000 cell-counting events were recorded to form a fluorescence intensity histogram (Fig. 6B). mAb dilutions progressively shifted the peak leftward, yielding a quantitative measure of concentration-dependent mAb labeling. mAb20 and mAb39 showed a similar binding profile with an affinity of 0.8 ± 0.1 and 0.7 ± 0.1 nm, respectively (Fig. 6C).
Figure 6.
mAb binding affinity. A, representative confocal microscopy images of immunofluorescence staining of fixed and saponin-permeabilized HEK293 cells with mAb20 or mAb39 staining as indicated. ZnT8(+) or ZnT8(−), HEK293 cells with or without ZnT8 overexpression. Red, mAb20 or mAb39 staining; blue, 4′,6-diamidino-2-phenylindole; scale bar, 5 μm. B, histograms of flow cytometry counting events. Arrows, leftward shifts of the histogram as a result of mAb serial dilutions. C, mAb binding to HEK293 cells with or without ZnT8 overexpression as indicated. Immunofluorescence intensities of mAb20 or mAb39 labeling in serial dilutions were obtained from B. Solid lines, least-squares fits of the binding data to a one-component binding process. a.u., arbitrary units.
Binding specificities
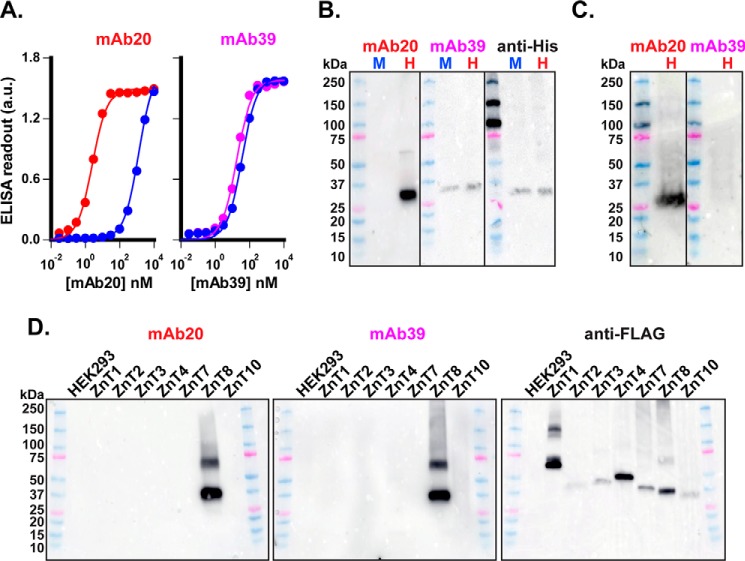
We first examined cross-species specificities by comparing mAb bindings to human and mouse ZnT8; both were overexpressed in HEK293 cells and then solubilized by detergent (DDM) and immobilized to a Ni-NTA plate via a C-terminal polyhistidine tag. mAb20 titrations revealed a sharp human–mouse difference, shifting the mouse binding curve rightward with a 566.8-fold difference in apparent binding affinities (2.5 ± 0.2 versus 1422.0 ± 72.6 nm) (Fig. 7A). In contrast, mAb39 binding to human ZnT8 was similar to that of mouse ZnT8 with a marginal 2.0-fold difference (17.6 ± 1.7 versus 35.8 ± 3.0 nm) (Fig. 7A). These results indicated that mAb20 is specific to human ZnT8, whereas mAb39 recognizes both human and mouse ZnT8. Of note, the epitope-masking effect of ZnT8 immobilization weakened the apparent binding affinities of both mAbs as compared with the affinities determined by flow cytometry, and such a weakening effect was more pronounced for mAb39, probably due to a closer proximity of the mAb39 epitope to the C-terminal His tag. Next, we compared mAb immunoreactivities to denatured ZnT8. Immunoblotting lysates of HEK293 transfectants showed that mAb20 only detected human and not mouse ZnT8 (Fig. 7B). On the other hand, mAb39 recognized both denatured human and mouse ZnT8, consistent with the ELISA results (Fig. 7A). However, ZnT8 denaturation on SDS-PAGE significantly reduced the immunoreactivities of mAb39, attributable to the loss of ZnT8 dimeric association. Nevertheless, mAb39 reactivities seemed comparable with that of commercial antibody to His-tagged ZnT8 on a duplicated blot (Fig. 7B). Next, we used mAbs to probe endogenous ZnT8 expression in EndoC-βH, an insulinoma cell line derived from human pancreatic β-cells (33). Immunoblotting EndoC-βH lysates with mAb20 revealed a single ZnT8 protein band over a low background. mAb39 detected neither ZnT8 nor nonspecific endogenous protein (Fig. 7C). Finally, we examined mAb20 and mAb39 specificities to ZnT homologs using lysates of HEK293 cells overexpressing ZnT1, ZnT2, ZnT3, ZnT4, ZnT7, ZnT8, or ZnT10, each tagged with a C-terminal FLAG octapeptide. With similar total protein loading in each lane, both mAb20 and mAb39 detected ZnT8 only (Fig. 7D), demonstrating superb selectivities for human ZnT8 against other ZnT homologs. Anti-FLAG signal intensities varied among ZnT homologs due to an inherent difference in their expression levels.
Figure 7.
mAb binding specificities. A, mAb binding to ZnT8-His immobilized to Ni-NTA plate. Bound mAb20 (red) or mAb39 (magenta) was detected by an HRP-conjugated secondary antibody. Solid lines, least-squares fits of binding data points to a one-component binding process. Error bars, S.E. (n = 8). B, mouse ZnT8-His (M) and human ZnT8-His (H) were SDS-denatured on immunoblots and detected by mAb20 or mAb39, as indicated. A replicated immunoblot was probed by an anti-His tag antibody to show that approximately the same amounts of HEK293 crude lysates were loaded to each lane. All immunoblots were recorded using an identical exposure time on Amersham Biosciences Imager 600. C, detection of endogenous human ZnT8 in EndoC-βH cells by mAb20 or mAb39 immunoblotting. An equal amount of EndoC-βH lysate (∼50 μg of total protein) was loaded to each lane. D, immunoblotting of HEK lysates with overexpression of a ZnT homolog as indicated. An approximately equal amount of total lysate protein (5 μg) was located to each lane, probed by either mAb20, mAb39, or anti-FLAG antibody, as indicated. Note that the mAb39 immunoblot was recorded with an over 100-fold longer exposure time as compared with that of the mAb20 immunoblot. a.u., arbitrary units.
Quantifying the cellular ZnT8 level
Co-binding of the mAb20–mAb39 pair to a single ZnT8 protein allowed for quantification of the ZnT8 level by measuring FRET signal derived from labeled fluorescence donor and acceptor in close proximity in a ternary binding complex. mAb20 was labeled with long-emitting terbium cryptate as an energy donor, whereas mAb39, a near-IR energy acceptor, was labeled with d2. A fixed amount of donor and acceptor at a 1:1 ratio was added to lysates of HEK293 cells overexpressing human ZnT8, mouse ZnT8, or human ZnT3. HTRF calibration over a range of lysate dilutions revealed a linear HTRF response up to a human ZnT8 concentration of 50 ng/ml, which was then inflected horizontally due to binding saturation (Fig. 8). Mouse ZnT8 or human ZnT3, the closest homolog of human ZnT8, showed negligible or no HTRF response even at the highest ZnT concentration of 500 ng/ml. Accordingly, the mouse ZnT8 and human ZnT3 readouts were taken as the assay background. The limit of detection (LoD) for human ZnT8 was 0.51 ng/ml at 3 × S.D. above the background, and the limit of quantification (LoQ) was 1.71 ng/ml at 10 × S.D. (Fig. 8, inset). The coefficient of variance at LoQ was 9.8%.
Figure 8.
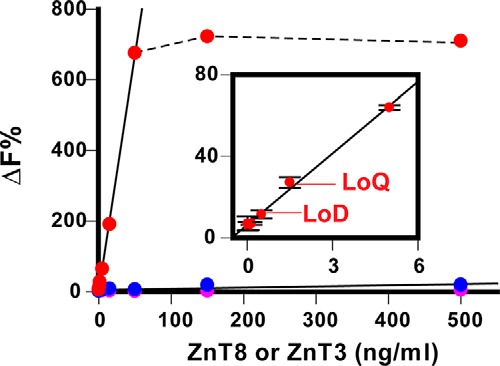
HTRF responses to human ZnT8, mouse ZnT8, and human ZnT3. Serial dilutions of human ZnT8 (red), human ZnT3 (magenta), or mouse ZnT8 (blue) were detected by a pair of mAb20 and mAb39 labeled with terbium cryptate and d2, respectively. ΔF%, normalized ratiometric readout of HTRF fluorescence at 620 and 665 nm. Error bars, S.D. of four experiments, each with triplicated measurements. Solid lines, linear regression analysis with data points within the linear range. A dashed line indicates saturation binding of human ZnT8 above 50 ng/ml.
Discussion
Unusual biochemical properties of ZnT8 pose significant challenges to developing ZnT8-specific reagents. Until now, all anti-ZnT8 antibodies have been raised against linear epitopes derived from ZnT8 protein sequence, consequently lacking sufficient specificity for immunodetection of cellular ZnT8. In the present work, we characterize two anti-ZnT8 mAbs raised against full-length human ZnT8 in reconstituted proteoliposomes. Both mAbs recognize folded ZnT8 with subnanomolar binding affinities and are capable of protecting ZnT8 from unfolding and partially inhibiting ZnT8 transport activity. The folding dependence does not preclude mAbs from recognizing denatured ZnT8 on SDS-PAGE, although the reactivity of mAb39 is greatly reduced. Both mAbs are highly specific for human ZnT8, showing no detectable reactivity to a panel of human ZnT homologs on immunoblots. The specific immunodetection of human ZnT8 is further augmented by simultaneous bindings of two ZnT8-specific mAbs to a single ZnT8 protein. The mAb20–mAb39 pair did not yield a detectable FRET readout for mouse ZnT8 because mAb20 lacks cross-species reactivity (Fig. 7B). Binding of mAb39 alone to mouse ZnT8 (Fig. 7A) failed to elicit a FRET response above the assay LoD, indicating that the specificity of the HTRF assay is a product of multiplying two individual mAb specificities. The reliance on co-binding of two different mAbs for a positive HTRF readout would effectively filter out nonspecific bindings of individual mAbs to cellular proteins.
The inhibitory effect of Fab binding mirrored the effect of a polymorphic Arg → Trp substitution at position 325 of human ZnT8. An earlier comparison of steady-state kinetics between the Arg-325 and Trp-325 variants showed that Trp-325 reduced Vmax by ∼50% with no significant effect on Km (20). The R325W polymorphic variations were localized to a distal dimer interface near the C terminus based on homology modeling of human ZnT8 (20). Coincidentally, Fab39 appears to bind to the same region by our EM analysis. Functional characterization of polymorphic CTD variants revealed that CTD-Trp-325 had a higher dimerization affinity than CTD-Arg-325 (34), whereas removal of zinc binding to CTD was shown to trigger a hinge-like motion to yield two splayed CTDs (30, 35). The Trp-325 variant and Fab39 binding both resulted in stabilization of dimeric interactions, leading to a partial inhibition of the ZnT8 transport activity. Thus, Fab39 binding may mimic the inhibitory effect of the Trp-325 variant by acting on the same allosteric pathway. Our results demonstrate the utility of mAbs as an allosteric inhibitor.
Emerging data suggest that human ZnT8 activity in pancreatic β-cells may be inversely related to the risk of developing T2D (15, 20, 22). At present, the molecular mechanism underlying the causality between LOF mutations in human ZnT8 and lower T2D risk is still unclear. A mechanistic understanding of this natural protective effect can facilitate translation to novel therapies. The allele frequency for the risk Arg-325 variant is about 72% in the general population (36), but its expression level in human pancreatic islets has yet to be quantified and correlated with the T2D risk. The HTRF assay is highly specific to human ZnT8, accurate, and adaptable for high-throughput screening. This assay may be used to measure the abundance of human ZnT8 in healthy and diseased pancreatic islets. A possible alteration of the expression level of the Arg-325 variant is expected to either exacerbate or alleviate hyperactivity of zinc transport. The effect size of SLC30A8 polymorphisms may be small. A reliable measurement of islet ZnT8 expression is critically important to establish a correlation between the islet ZnT8 expression profile and T2D-related clinical metrics. The application of mAbs as a specific ZnT8 biosensor to clinical research may yield new insights into ZnT8 pathophysiology.
Experimental procedures
Generating hybridoma
Four pairs of male/female homozygous Slc30a8−/− mice were immunized by weekly intraperitoneal injections of a human ZnT8 antigen (the Arg-325 variant) in reconstituted proteoliposomes. All experiments with mice were approved by the institutional animal care and use committee of the Johns Hopkins University School of Medicine. Serum antibody titering and validation of anti-ZnT8 specificity by CRISPR/Cas9 ZnT8 knockout were described previously (32). Following a final immunization boost, spleens were isolated. Splenocytes were fused with an immortalized myeloma cell line Sp2/0-Ag14 (CRL-1581, ATCC) at a 4:1 splenocyte/myeloma ratio by adding 1 ml of 50% (w/v) PEG 1500 (Roche Applied Science) to the cell mixture drop-by-drop over 1 min (38). The cells stayed in the PEG solution for an additional 1 min with gentle mixing, and then 20 ml of serum-free DMEM was added in a stepwise fashion over 4–5 min. Cells were pelleted and then resuspended in 15 ml of Dulbecco's modified Eagle's medium with 10% FBS supplemented with hybridoma fusion and cloning supplement and hypoxanthine, aminopterin, and thymidine (HAT). Eight days after fusion, dead Sp2/0 cells were removed, and hybridoma cells were plated in 96-well plates at a density of ∼5 cells/well and maintained under HAT selection.
Screening hybridoma
Multitier ELISAs were developed to evaluate hybridoma supernatants. First screening used native human ZnT8 antigen in reconstituted proteoliposome by passive coating (200 ng of ZnT8/well). The coated plates were preblocked with 5% BSA in an assay buffer (20 mm HEPES, 100 mm NaCl, pH 7.0), and then 20 μl of hybridoma supernatant was added to each well, incubated at room temperature for 2 h, and then washed three times with assay buffer plus 1% BSA. Bound antibodies were detected by an HRP-conjugated goat anti-mouse secondary antibody (Invitrogen, catalog no. 62-6500) with 1:3000 dilutions in assay buffer, followed by ABTS colorimetric reaction (Life Technologies, Inc.) and signal readout at 415 nm on a plate reader (Molecular Devices). Second screening used a native CTD-His monomer by affinity binding to Ni-NTA plates (100 ng of CTD/well). ELISA procedures were the same as above. Third screening used native ZnT8 in detergent micelles. The antigen was bound between two layers of antibodies. The capture antibody was mAb20 immobilized to protein A/G plates (800 ng/well). Only a detection antibody bound to a nonoverlapping epitope gave a positive ELISA readout. Purified MSA and commercial NTDA (Proteintech, catalog no. 16169-I-AP) and CTDA (Amgen) were used as negative and positive controls to calibrate ELISA dynamic range and sensitivity.
Validating mAbs
Monoclonal hybridoma cell lines were established by flow cytometry single-cell sorting to ensure clonal purity. After clonal expansion, secreted mAbs were purified by protein A/G affinity chromatography (Thermo Scientific, catalog no. 20423) and subtyped (Thermo Scientific, catalog no. 37503), and then Fab fragments were prepared by papain digestion (Thermo Scientific, catalog no. 44985). Purified mAbs or Fabs were tested for (i) formation of binding complexes with purified ZnT8 or ZnT8-GFP on size-exclusion HPLC, (ii) immunofluorescence staining of HEK293 cells with or without ZnT8 expression followed by confocal microscopy imaging, (iii) immunofluorescence staining of bulk cell population followed by flow cytometry quantitation, and (iv) formation of Fab–ZnT8 complexes by negative staining EM and single-particle analysis.
Fluorescence size-exclusion HPLC
Purified mAb20/Fab20 or mAb39 was mixed with Alexa Fluor 488 or Alexa Fluor 555 carboxylic acid succinimidyl ester in a 1:20 molar ratio in PBS buffer for 60 min at room temperature, and then the labeling reaction was terminated by 100 mm Tris-HCl quenching. The fluorescently labeled mAb or Fab was HPLC-purified to remove trace amounts of free fluorescent dyes and concentrated to 1 mg/ml for storage in PBS buffer at 4 °C. Purified human ZnT8 in detergent micelles was incubated with mAb20/Fab20 or mAb30 for 2 h, centrifuged at 258,000 × g for 30 min to precipitate cross-linked ZnT8 aggregates, and then injected into a TSKgel G3000SWXL size-exclusion HPLC column (Tosoh Biosciences) preequilibrated with the assay buffer plus 0.02% DDM. Alternatively, ZnT8-GFP in detergent crude extract of INS-1E cells stably expressing human ZnT8 was purified by GFP-fluorescence sizing-HPLC. Next, the peak fraction of ZnT8-GFP was incubated with mAb20 or mAb39 for 3 h at 4 °C, centrifuged to remove cross-linked aggregates, and then injected into the TSK column as above. The elution profile was monitored using a multichannel fluorescence detector with the following excitation/emission pairs: 480/520, 485/555, and 480/520 nm for Alexa Fluor 488, Alexa Fluor 555, and GFP fluorescence, respectively. ZnT8 peak alone was monitored by a UV detector in line with the fluorescence detector.
Negative staining EM
Human ZnT8 was purified as described previously (20). Fab20 or Fab20 + Fab39 was added to ZnT8 in a 10:1 molar ratio. The binding complexes were repurified by size-exclusion HPLC and concentrated to 1–2 mg/ml. The protein samples were diluted to 20 μg/ml, and aliquots of 3 μl of diluted sample were applied on glow-discharged EM grids covered with a continuous thin carbon film and stained by 2% uranyl acetate aqueous solution for 1.5 min. Grids were loaded onto a Tecnai Spirit electron microscope operated at a high tension of 120 kV. Raw images were collected in low-dose mode (10 e/Å) using a Gatan Orius CCD camera with an underfocus value ranging from 1 to 2.5 μm and at a magnification of 30,000, corresponding to 2.1 Å/pixel at the specimen level. We recorded 221 and 71 micrographs for the binary (Fab20)2–ZnT8 and ternary (Fab20)2–ZnT8–(Fab39)2 complexes, respectively. The contrast transfer function parameters of each image were determined by CTFFIND version 4.1.5 (39). 76,274 binary and 6,107 ternary particles were picked from the micrographs. After 2D classification in RELION version 2.1.0 (40) and 3D classification in cryoSPARC (41), 15,745 binary particles and 2369 ternary particles were retained for 3D reconstruction. 3D refinement was performed using cryoSPARC. Both binary and ternary complex maps had an estimated resolution of 3 nm. Crystal structures of a Fab targeting Shigella flexneri Y lipopolysaccharide (PDB code 1M71) (42) and an E. coli zinc transporter, YiiP (PDB code 3H90) (30), were used to dock the 3D maps in UCSF Chimera (43).
Immunofluorescence staining and cell imaging
Flp-In, T-REx-HEK293 cells stably expressing human ZnT8 variants were grown and induced on coverslips at 50% confluence as described previously (20). Cells were fixed (R&D Systems, catalog no. FC004) and permeabilized (R&D Systems, catalog no. FC005). HEK293 cells were stained with mAb20 or mAb39 at a 1:3000 dilution from a 1 mg/ml mAb stock. Parental Flp-In, T-REx-HEK293 cells without ZnT8 expression were used as a negative control following the identical procedure. A goat-anti-mouse secondary antibody conjugated with Alexa Fluor 594 (Thermo Scientific, catalog no. A11005; dilution 1:1000) and 4′,6-diamidino-2-phenylindole were used for fluorescence imaging on a Zeiss LSM 700 inverted confocal microscope with a ×63 oil objective as described previously (20).
Flow cytometry analysis
HEK293 cells with or without ZnT8 overexpression were fixed, permeabilized, and resuspended in PBS plus 5% BSA at a density of 1 × 106/ml as described previously (37). Aliquots of 100 μl of cell suspension were stained with mAb20 or mAb39 in 3-fold serial dilutions for 2 h at room temperature and then stained with Alexa Fluor 647–conjugated donkey anti-mouse secondary antibody in a 1:1000 dilution with 5% BSA (Thermo Fisher Scientific, catalog no. A31571). The labeled cells were analyzed immediately by flow cytometry. Cells gated on forward and side scatter revealed a single population represented in a histogram of ∼3000 cell-counting events. The average immunofluorescence intensity and S.E. were obtained using the flow cytometry analysis software Summit version 4.3 (Beckman Coulter).
Immunoblotting
SDS-solubilized cell lysate of EndoC-βH cells or HEK293 cells overexpressing ZnT8 was subjected to SDS-PAGE and probed with a 1:3000 mAb20 or mAb39, followed by 1:3000 anti-mouse HRP-conjugated secondary antibody (GE Healthcare, catalog no. NA931V). To compare mAb reactivities to different ZnT homologs, we used lysates of HEK293 cells overexpressing ZnT1, ZnT2, ZnT3, ZnT4, ZnT7, ZnT8, or ZnT10, each tagged by a FLAG tag at the C terminus (Origene, catalog nos. LY500001, LC412033, LC423751, LC401170, LY415673, LC428544, LC406320, and LC412886). Approximately equal amounts of total protein (5 μg) were loaded to each lane and then probed with mAb20, mAb39, or anti-FLAG antibody (Origene, catalog no. TA50011-100) as above.
Stopped-flow kinetics
As described previously (20), human ZnT8 (the Arg-325 variant) was purified and reconstituted into proteoliposomes with a defined lipid composition of DOPC/DOPG/DOPE (2:1:1) at a protein/lipid ratio of 1:50 (w/w). Preformed proteoliposomes mixed with 200 μm FluoZin-3, without 100 μg of Fab in 0.2 ml of assay buffer underwent three cycles of freeze–thaw, followed by a 10-s sonication to complete encapsulation of dye and Fab. The extravesicular FluoZin-3 was removed by washing vesicles with 3× 25 ml of assay buffer, and then an additional 100 μg of Fab was added to ensure saturation of Fab binding. The estimated Fab/ZnT8 molar ratio was 2.8:1. ZnT8-mediated zinc transport into proteoliposomes was triggered by a rapid mixing of proteoliposomes and assay buffer on an SFM-3000 stopped-flow apparatus (Bio-logic) as described previously (20). Increasing amounts of ZnSO4 were added to the assay buffer with 1 mm sodium citrate that buffered the free zinc concertation from 6 to 116 μm according to Maxchelator. All kinetic traces were cumulative averages of nine successive recordings. Liposome traces were collected as baselines and subtracted from proteoliposome traces to yield net fluorescence changes ΔF. ΔF/ΔFmax was obtained by normalizing ΔF to the maximum proteoliposome response elicited by an assay buffer containing 2 mm ZnSO4 plus 2% β-octyl glucoside. The initial rate of zinc influx was obtained by linear regression of data points (t < 10 s) in the quasilinear phase of the initial fluorescence rise. Concentration dependence data were analyzed by least squares fits of the initial transport rate to a hyperbola defined by v = Vmax M/(M + Km), where M represents the free zinc ion concentration, Vmax is the maximum transport rate when the rate of transport approaches the quasi-stationary state, and Km is the Michaelis-Menten constant. Fits of experimental data were performed using the data analysis software SIGMAPLOT (SPSS Inc.).
HTRF assay
Purified mAb20 and mAb39 were labeled with terbium cryptate and d2, respectively, following the manufacturer's protocol (Cisbio Bioassays, Bedford, MA). 293-F cells transiently expressing human ZnT8, human ZnT3, or mouse ZnT8 in suspension culture were harvested. Detergent crude extracts were prepared by 1% DDM solubilization of cell pellets in 20 mm HEPES, 100 mm NaCl, pH 7.0, with protease mixture and centrifuged at 16,000 × g for 15 min to remove debris. The supernatants were collected for ZnT8 detection. The HTRF assays were performed in 384-well plates (Greiner Bio-one, catalog no. 784075) by adding labeled mAb20 and mAb39 in a 1:1 ratio and detergent extract of 293-F cells in proper dilutions to a total volume of 20 μl. After mAb binding for 2 h at room temperature, fluorescence emissions were measured using a microplate reader (Molecular Devices) equipped with a flash lamp for a 40-μs delayed fluorophore excitation at 314 nm, a 620-nm filter for terbium cryptate fluorescence reading, and a 665-nm filter for the d2 fluorescence detection. Data were reduced as a ratio of fluorescence measured at 665 nm (d2) and 620 nm (terbium cryptate). The ratiometric measurement was corrected for well-to-well variability and signal quenching from potential interfering components in the binding mix. Background signal was measured and used to calculate the relative ZnT8 readout ΔF for interassay comparisons: ΔF = (Ratiosample − Ratiobackground)/Ratiobackground. The actual ZnT8 concentrations in the assay were estimated based on the ZnT8 yield of 293-F cell transient expression, correlated to the HTRF readouts by linear regression (r2 = 0.99) to yield a standard curve. LoD and LoQ values were obtained by interpolation of measured data points on the standard curve.
Author contributions
D. F. conceived the idea and developed assays with C. M., Hua Li, and Huilin Li. C. M. performed the biochemistry and cell biology experiments, and Hua Li performed the EM experiments and imaging processing. All authors contributed to data analysis. D. F. wrote the paper with C. M. and Hua Li.
Acknowledgments
We thank Hao Zhang from the Flow Cytometry and Immunology Core Facility at the Johns Hopkins Bloomberg School of Public Health for assistance in data acquisition and analysis. We also thank Barbara Smith from the microscopy facility of the Johns Hopkins University School of Medicine for assistance in cell imaging. The Zeiss confocal microscope was funded through National Institutes of Health Shared Instrumentation Grant S10OD016374. The MoFlo XDP cell sorter was funded through National Institutes of Health Grants S10 OD016315 and S10 RR13777001.
This work was supported by National Institutes of Health Grant R01 GM065137 (to D. F.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- GSIS
- glucose-stimulated insulin secretion
- T2D
- type 2 diabetes
- CTD
- C-terminal domain
- NTD
- N-terminal domain
- CTDA
- CTD antibody
- NTDA
- NTD antibody
- KO
- knockout
- TMD
- transmembrane domain
- MSA
- mouse serum antibodies
- 2D and 3D
- two- and three-dimensional, respectively
- PDB
- Protein Data Bank
- Ni-NTA
- nickel-nitrilotriacetic acid
- DDM
- dodecyl maltoside
- LoD
- limit of detection
- LoQ
- limit of quantification
- HTRF
- homogeneous time-resolved fluorescence
- HAT
- hypoxanthine, aminopterin, and thymidine
- DOPC
- 1,2-dioleoyl-sn-glycero-3-phosphocholine
- DOPG
- 1,2-dioleoyl-sn-glycero-3-phosphoglycerol
- DOPE
- 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- HRP
- horseradish peroxidase
- LOF
- loss-of-function.
References
- 1. Kambe T., Hashimoto A., and Fujimoto S. (2014) Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol. Life Sci. 71, 3281–3295 10.1007/s00018-014-1617-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chimienti F., Devergnas S., Favier A., and Seve M. (2004) Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 53, 2330–2337 10.2337/diabetes.53.9.2330 [DOI] [PubMed] [Google Scholar]
- 3. Lemaire K., Chimienti F., and Schuit F. (2012) Zinc transporters and their role in the pancreatic beta-cell. J. Diabetes Investig. 3, 202–211 10.1111/j.2040-1124.2012.00199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Segerstolpe Ă., Palasantza A., Eliasson P., Andersson E. M., Andréasson A. C., Sun X., Picelli S., Sabirsh A., Clausen M., Bjursell M. K., Smith D. M., Kasper M., Ämmälä C., and Sandberg R. (2016) Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 24, 593–607 10.1016/j.cmet.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chimienti F., Devergnas S., Pattou F., Schuit F., Garcia-Cuenca R., Vandewalle B., Kerr-Conte J., Van Lommel L., Grunwald D., Favier A., and Seve M. (2006) In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J. Cell Sci. 119, 4199–4206 10.1242/jcs.03164 [DOI] [PubMed] [Google Scholar]
- 6. Lemaire K., Ravier M. A., Schraenen A., Creemers J. W., Van de Plas R., Granvik M., Van Lommel L., Waelkens E., Chimienti F., Rutter G. A., Gilon P., in't Veld P. A., and Schuit F. C. (2009) Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc. Natl. Acad. Sci. U.S.A. 106, 14872–14877 10.1073/pnas.0906587106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nicolson T. J., Bellomo E. A., Wijesekara N., Loder M. K., Baldwin J. M., Gyulkhandanyan A. V., Koshkin V., Tarasov A. I., Carzaniga R., Kronenberger K., Taneja T. K., da Silva Xavier G., Libert S., Froguel P., Scharfmann R., et al. (2009) Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 58, 2070–2083 10.2337/db09-0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foster M. C., Leapman R. D., Li M. X., and Atwater I. (1993) Elemental composition of secretory granules in pancreatic islets of Langerhans. Biophys. J. 64, 525–532 10.1016/S0006-3495(93)81397-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dunn M. F. (2005) Zinc-ligand interactions modulate assembly and stability of the insulin hexamer–a review. Biometals 18, 295–303 10.1007/s10534-005-3685-y [DOI] [PubMed] [Google Scholar]
- 10. Rutter G. A. (2010) Think zinc: new roles for zinc in the control of insulin secretion. Islets 2, 49–50 10.4161/isl.2.1.10259 [DOI] [PubMed] [Google Scholar]
- 11. Tamaki M., Fujitani Y., Hara A., Uchida T., Tamura Y., Takeno K., Kawaguchi M., Watanabe T., Ogihara T., Fukunaka A., Shimizu T., Mita T., Kanazawa A., Imaizumi M. O., Abe T., Kiyonari H., Hojyo S., Fukada T., Kawauchi T., Nagamatsu S., Hirano T., Kawamori R., and Watada H. (2013) The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J. Clin. Invest. 123, 4513–4524 10.1172/JCI68807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ashcroft F. M., and Rorsman P. (2012) Diabetes mellitus and the beta cell: the last ten years. Cell 148, 1160–1171 10.1016/j.cell.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prasad R. B., and Groop L. (2015) Genetics of type 2 diabetes-pitfalls and possibilities. Genes 6, 87–123 10.3390/genes6010087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonnefond A., and Froguel P. (2015) Rare and common genetic events in type 2 diabetes: what should biologists know? Cell Metab. 21, 357–368 10.1016/j.cmet.2014.12.020 [DOI] [PubMed] [Google Scholar]
- 15. Flannick J., Thorleifsson G., Beer N. L., Jacobs S. B., Grarup N., Burtt N. P., Mahajan A., Fuchsberger C., Atzmon G., Benediktsson R., Blangero J., Bowden D. W., Brandslund I., Brosnan J., Burslem F., et al. (2014) Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 46, 357–363 10.1038/ng.2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pearson E. (2014) Zinc transport and diabetes risk. Nat. Genet. 46, 323–324 10.1038/ng.2934 [DOI] [PubMed] [Google Scholar]
- 17. Sladek R., Rocheleau G., Rung J., Dina C., Shen L., Serre D., Boutin P., Vincent D., Belisle A., Hadjadj S., Balkau B., Heude B., Charpentier G., Hudson T. J., Montpetit A., et al. (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445, 881–885 10.1038/nature05616 [DOI] [PubMed] [Google Scholar]
- 18. Scott L. J., Mohlke K. L., Bonnycastle L. L., Willer C. J., Li Y., Duren W. L., Erdos M. R., Stringham H. M., Chines P. S., Jackson A. U., Prokunina-Olsson L., Ding C. J., Swift A. J., et al. (2007) A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316, 1341–1345 10.1126/science.1142382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeggini E., Weedon M. N., Lindgren C. M., Frayling T. M., Elliott K. S., Lango H., Timpson N. J., Perry J. R., Rayner N. W., Freathy R. M., Barrett J. C., et al. (2007) Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316, 1336–1341 10.1126/science.1142364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merriman C., Huang Q., Rutter G. A., and Fu D. (2016) Lipid-tuned zinc transport activity of human ZnT8 protein correlates with risk for type-2 diabetes. J. Biol. Chem. 291, 26950–26957 10.1074/jbc.M116.764605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wong W. P., Allen N. B., Meyers M. S., Link E. O., Zhang X., MacRenaris K. W., and El Muayed M. (2017) Exploring the association between demographics, SLC30A8 genotype, and human islet content of zinc, cadmium, copper, iron, manganese and nickel. Sci. Rep. 7, 473 10.1038/s41598-017-00394-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li L., Bai S., and Sheline C. T. (2017) hZnT8 (Slc30a8) Transgenic mice that overexpress the R325W polymorph have reduced islet Zn2+ and proinsulin levels, increased glucose tolerance after a high-fat diet, and altered levels of pancreatic zinc binding proteins. Diabetes 66, 551–559 10.2337/db16-0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kleiner S., Gomez D., Megra B., Na E., Bhavsar R., Cavino K., Xin Y., Rojas J., Dominguez-Gutierrez G., Zambrowicz B., Carrat G., Chabosseau P., Hu M., Murphy A. J., Yancopoulos G. D., et al. (2018) Mice harboring the human SLC30A8 R138X loss-of-function mutation have increased insulin secretory capacity. Proc. Natl. Acad. Sci. U.S.A. 115, E7642–E7649 10.1073/pnas.1721418115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pound L. D., Sarkar S. A., Benninger R. K., Wang Y., Suwanichkul A., Shadoan M. K., Printz R. L., Oeser J. K., Lee C. E., Piston D. W., McGuinness O. P., Hutton J. C., Powell D. R., and O'Brien R. M. (2009) Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem. J. 421, 371–376 10.1042/BJ20090530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pound L. D., Sarkar S. A., Ustione A., Dadi P. K., Shadoan M. K., Lee C. E., Walters J. A., Shiota M., McGuinness O. P., Jacobson D. A., Piston D. W., Hutton J. C., Powell D. R., and O'Brien R. M. (2012) The physiological effects of deleting the mouse SLC30A8 gene encoding zinc transporter-8 are influenced by gender and genetic background. PLoS One 7, e40972 10.1371/journal.pone.0040972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Friend S. H., and Schadt E. E. (2014) Translational genomics. Clues from the resilient. Science 344, 970–972 10.1126/science.1255648 [DOI] [PubMed] [Google Scholar]
- 27. Ohana E., Hoch E., Keasar C., Kambe T., Yifrach O., Hershfinkel M., and Sekler I. (2009) Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J. Biol. Chem. 284, 17677–17686 10.1074/jbc.M109.007203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoch E., Lin W., Chai J., Hershfinkel M., Fu D., and Sekler I. (2012) Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proc. Natl. Acad. Sci. U.S.A. 109, 7202–7207 10.1073/pnas.1200362109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu M., and Fu D. (2007) Structure of the zinc transporter YiiP. Science 317, 1746–1748 10.1126/science.1143748 [DOI] [PubMed] [Google Scholar]
- 30. Lu M., Chai J., and Fu D. (2009) Structural basis for autoregulation of the zinc transporter YiiP. Nat. Struct. Mol. Biol. 16, 1063–1067 10.1038/nsmb.1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Degorce F., Card A., Soh S., Trinquet E., Knapik G. P., and Xie B. (2009) HTRF: a technology tailored for drug discovery–a review of theoretical aspects and recent applications. Curr. Chem. Genomics 3, 22–32 10.2174/1875397300903010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merriman C., Huang Q., Gu W., Yu L., and Fu D. (2018) A subclass of serum anti-ZnT8 antibodies directed to the surface of live pancreatic beta-cells. J. Biol. Chem. 293, 579–587 10.1074/jbc.RA117.000195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ravassard P., Hazhouz Y., Pechberty S., Bricout-Neveu E., Armanet M., Czernichow P., and Scharfmann R. (2011) A genetically engineered human pancreatic beta cell line exhibiting glucose-inducible insulin secretion. J. Clin. Invest. 121, 3589–3597 10.1172/JCI58447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parsons D. S., Hogstrand C., and Maret W. (2018) The C-terminal cytosolic domain of the human zinc transporter ZnT8 and its diabetes risk variant. FEBS J. 285, 1237–1250 10.1111/febs.14402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cherezov V., Höfer N., Szebenyi D. M., Kolaj O., Wall J. G., Gillilan R., Srinivasan V., Jaroniec C. P., and Caffrey M. (2008) Insights into the mode of action of a putative zinc transporter CzrB in Thermus thermophilus. Structure 16, 1378–1388 10.1016/j.str.2008.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boesgaard T. W., Zilinskaite J., Vänttinen M., Laakso M., Jansson P. A., Hammarstedt A., Smith U., Stefan N., Fritsche A., Häring H., Hribal M., Sesti G., Zobel D. P., Pedersen O., Hansen T., and EUGENE 2 Consortium (2008) The common SLC30A8 Arg325Trp variant is associated with reduced first-phase insulin release in 846 non-diabetic offspring of type 2 diabetes patients–the EUGENE2 study. Diabetologia 51, 816–820 10.1007/s00125-008-0955-6 [DOI] [PubMed] [Google Scholar]
- 37. Huang Q., Merriman C., Zhang H., and Fu D. (2017) Coupling of insulin secretion and display of a granule-resident zinc transporter ZnT8 on the surface of pancreatic beta cells. J. Biol. Chem. 292, 4034–4043 10.1074/jbc.M116.772152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lim H. H., Fang Y., and Williams C. (2011) High-efficiency screening of monoclonal antibodies for membrane protein crystallography. PLoS One 6, e24653 10.1371/journal.pone.0024653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rohou A., and Grigorieff N. (2015) CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 10.1016/j.jsb.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scheres S. H. (2012) RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 10.1016/j.jsb.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Punjani A., Rubinstein J. L., Fleet D. J., and Brubaker M. A. (2017) cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 10.1038/nmeth.4169 [DOI] [PubMed] [Google Scholar]
- 42. Vyas N. K., Vyas M. N., Chervenak M. C., Johnson M. A., Pinto B. M., Bundle D. R., and Quiocho F. A. (2002) Molecular recognition of oligosaccharide epitopes by a monoclonal Fab specific for Shigella flexneri Y lipopolysaccharide: X-ray structures and thermodynamics. Biochemistry 41, 13575–13586 10.1021/bi0261387 [DOI] [PubMed] [Google Scholar]
- 43. Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., and Ferrin T. E. (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]