Abstract
The DevR response regulator of Mycobacterium tuberculosis is an established regulator of the dormancy response in mycobacteria and can also be activated during aerobic growth conditions in avirulent strains, suggesting a complex regulatory system. Previously, we reported culture medium–specific aerobic induction of the DevR regulon genes in avirulent M. tuberculosis H37Ra that was absent in the virulent H37Rv strain. To understand the underlying basis of this differential response, we have investigated aerobic expression of the Rv3134c-devR-devS operon using M. tuberculosis H37Ra and H37Rv devR overexpression strains, designated as LIX48 and LIX50, respectively. Overexpression of DevR led to the up-regulation of a large number of DevR regulon genes in aerobic cultures of LIX48, but not in LIX50. To ascertain the involvement of PhoP response regulator, also known to co-regulate a subset of DevR regulon genes, we complemented the naturally occurring mutant phoPRa gene of LIX48 with the WT phoPRv gene. PhoPRv dampened the induced expression of the DevR regulon by >70–80%, implicating PhoP in the negative regulation of devR expression. Electrophoretic mobility shift assays confirmed phosphorylation-independent binding of PhoPRv to the Rv3134c promoter and further revealed that DevR and PhoPRv proteins exhibit differential DNA binding properties to the target DNA. Through co-incubations with DNA, ELISA, and protein complementation assays, we demonstrate that DevR forms a heterodimer with PhoPRv but not with the mutant PhoPRa protein. The study puts forward a new possible mechanism for coordinated expression of the dormancy regulon, having implications in growth adaptations critical for development of latency.
Keywords: Mycobacterium tuberculosis, bacterial signal transduction, gene expression, protein-protein interaction, protein-DNA interaction, DevR/DosR, dormancy regulon, negative regulation, PhoP, two-component system
Introduction
Despite global initiatives to control tuberculosis, it continues to be an enigma. Mycobacterium tuberculosis, the causative agent of tuberculosis has evolved into a highly efficient human pathogen due to its innate ability to adapt to the diverse host environments during infection. Although great strides have been made to elucidate the transcriptomic and proteomic profiles of M. tuberculosis in various cellular environments in vitro and in vivo, our understanding of the mechanisms that are responsible for growth adaptation are far from clear.
Long-term survival of mycobacteria in the host requires the bacteria to transition to a slow-growth phenotype resulting in dormant bacilli that may undergo reactivation when exposed to appropriate stimuli. The DevR (also called DosR) response regulator (RR)6 of the DevR-DevS (DevRS) TCS has been extensively studied for its role in mycobacterial dormancy and virulence (1–10). A wide variety of cellular stimuli are known to activate the DevRS TCS (11–16), highlighting its central role in metabolic and growth-adaptive strategies of mycobacteria. The DevR RR is activated via phosphorylation by two histidine sensor kinases, DevS and DosT (17), and also by Ser/Thr protein kinases, PknH (18) and PknB (19), that facilitate fine tuning of the dormancy response. This, in addition to the growing list of M. tuberculosis RRs that interact with DevR and/or co-regulate the DevR regulon, such as MprA, NarL, and PhoP, indicate overlapping regulatory pathways as a means for expansion of signaling networks (20–23). Indeed, such a strategy will enable coordinated gene expression across the perpetually changing metabolic status of the cell.
The constitutive aerobic induction of the DevR regulon in the hypervirulent M. tuberculosis W/Beijing (24) and avirulent H37Ra strains (25) has sparked significant interest in the underlying regulatory mechanisms governing DevR regulon expression during aerobic growth conditions. M. tuberculosis strains belonging to the W/Beijing lineage were shown to harbor point mutations in the devR promoter region and a naturally occurring frameshift mutation in the gene encoding the DosT sensor kinase (26) that were responsible for the constitutive expression of the regulon (27). These observations have enormous implications physiologically, particularly if the pre-induced dormancy regulon in W-lineage Beijing M. tuberculosis strains adds a survival advantage against intracellular stress signals, possibly contributing toward their hypervirulent and drug-tolerant phenotypes (24). Our previous studies have shown aerobic induction of the Rv3134c-devR-devS operon and DevR-regulated genes in M. tuberculosis H37Ra but not in H37Rv cultivated in Dubos Tween-albumin medium (25), highlighting differential regulation of this locus in these isogenic strains. Considering our limited understanding of the early transitioning events that lead to switching of growth rates and the onset of dormancy, it becomes relevant to understand the overall mechanisms directing expression of the DevR-dependent dormancy regulon.
The devRS genes are known to be differentially expressed in virulent M. tuberculosis H37Rv and its attenuated counterpart H37Ra strain (28, 29), with culture medium constituents like asparagine contributing to the difference in expression (25). Although the exact mechanism is presently unknown, annotation of the M. tuberculosis H37Ra genome sequence has provided valuable insights. Specifically, a point mutation at codon 219 (serine to leucine) in the DNA-binding domain of PhoP RR of H37Ra (PhoPRa) has been shown to be crucial for mycobacterial virulence (30–33). The complementation of avirulent M. tuberculosis H37Ra with a WT copy of the phoP gene from H37Rv (phoPRv) led to restoration of its virulence properties (32, 34). Among other notable differences is the up-regulation of the sigC gene encoding σ factor C in H37Ra, likely due to a mutation in its promoter region (33). Interestingly, both PhoP and SigC have been implicated in the regulation of DevR regulon genes (23, 35, 36). Whereas it has been suggested that PhoP participates in the transcriptional regulation of devRS TCS (23), direct evidence and the physiological role of such regulation has yet to be demonstrated.
The present study aims to investigate the aerobic expression of the devRS TCS in M. tuberculosis H37Ra and H37Rv to obtain insights into the mechanisms that regulate expression of the DevR regulon under aerobic (non-inducing) growth conditions. Transcription profiling of aerobically grown M. tuberculosis H37Ra and H37Rv devR overexpression strains, LIX48 and LIX50, respectively, revealed a distinct dysregulation of devRS genes in H37Ra that was conspicuously absent in H37Rv. Significantly, DevR overexpression resulted in up-regulation of 38 genes belonging to the DevR regulon in H37Ra in a phosphorylation-independent manner. Complementation of the LIX48 strain with an exogenous copy of phoPRv decreased aerobic expression of devR and its target gene, hspX, by >70–80%. Through DNA binding, and protein–protein interaction assays, we demonstrate PhoPRv binding to the Rv3134c promoter and, more importantly, formation of PhoPRv–DevR heterodimers as a plausible mechanism for co-regulation of the DevR regulon in H37Rv. A model highlighting the roles of PhoP and DevR RRs in regulating the dormancy regulon of M. tuberculosis H37Ra and H37Rv under aerobic and hypoxic growth conditions is discussed.
Results
Overexpression of devR in aerobic cultures of M. tuberculosis H37Ra and H37Rv results in significantly different transcription profiles
The transcription of devRS TCS in virulent M. tuberculosis H37Rv is marked by basal level expression under aerobic growth conditions and up-regulation upon exposure to hypoxia, NO, CO, vitamin C, and nitrite (1, 2, 11, 12, 14, 16, 37). In addition, we have shown that the Rv3134c-devR-devS operon in avirulent H37Ra is induced during aerobic growth in asparagine-containing medium (25). However, the question remained whether aerobic DevR regulon expression observed in H37Ra was directly due to DevR or other transcriptional regulators.
To avoid misinterpretation due to medium components, we simulated constitutively expressed devR by constructing recombinant M. tuberculosis H37Ra strain overexpressing the devR gene. Mycobacterial integrating plasmid pMG85 (38) overexpressing devR from the acetamide-inducible amidase promoter (Pamidase) was electroporated into M. tuberculosis H37Ra to construct strain LIX48 (Fig. 1). Because Pamidase in M. tuberculosis is constitutive (38), no acetamide was used in these studies. An analogous devR overexpression strain in M. tuberculosis H37Rv (LIX50; Fig. 1) was constructed in parallel. All experiments were done in Middlebrook 7H9 medium, a standard laboratory culture medium for mycobacteria cultivation that does not contain asparagine, to rule out any medium-specific induction of DevR regulon genes.
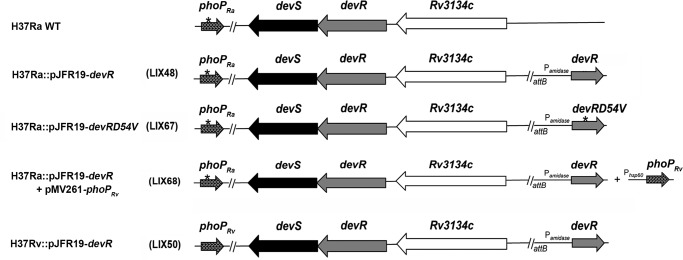
Figure 1.
Schematic representation of genetic backgrounds of the M. tuberculosis H37Ra and H37Rv strains used in this study. The genetic backgrounds of M. tuberculosis H37Ra and various recombinant derivatives thereof along with the M. tuberculosis H37Rv devR overexpression strain used in this study are presented as a schematic. The site-specific integration of mycobacterial integrative vector pJFR19 is shown at the attB site. The mutation in the phoP coding region in H37Ra is depicted with an asterisk.
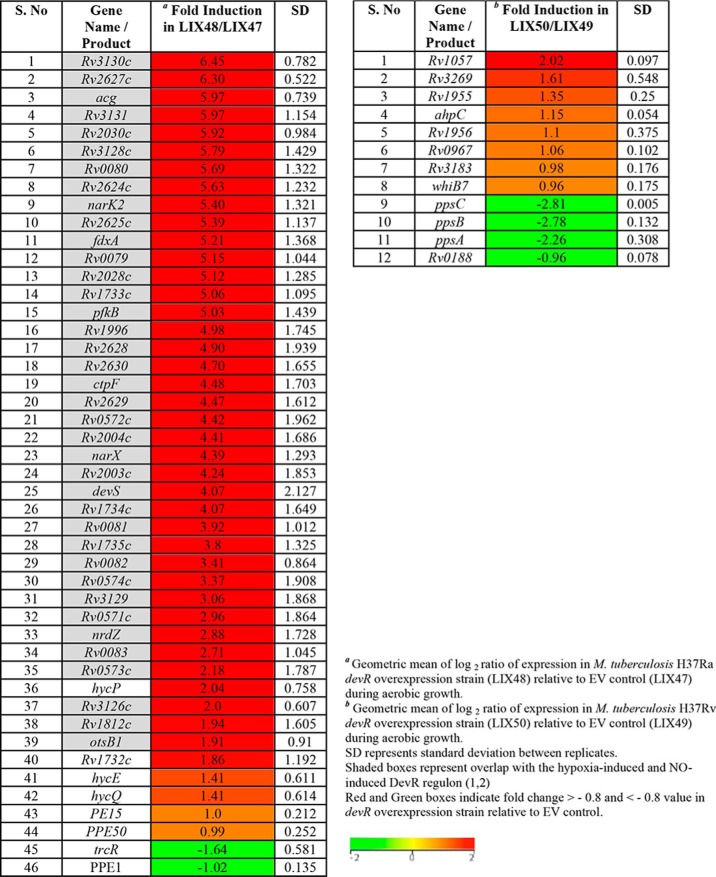
RNA isolated from logarithmic phase, aerobic cultures of LIX48 and LIX50, and their respective control strains LIX47 and LIX49 transformed with empty vector (EV) were subjected to microarray analysis as described under “Experimental procedures.” Forty-six genes exhibited differential expression (up-regulated, n = 44; down-regulated, n = 2) in M. tuberculosis H37Ra devR overexpression strain (LIX48) (Table 1, Tables S2 and S3), compared with the EV control strain (LIX47). By contrast, only eight genes were up-regulated in M. tuberculosis H37Rv devR overexpression strain (LIX50), relative to LIX49 control strain (Table 1, Tables S2 and S3). The DevR RR regulates expression of ∼50 genes that are collectively termed the DevR regulon (1). Of the 44 genes up-regulated in LIX48, 38 belonged to the DevR regulon (Table 1, shaded regions). Notably, no overlap among up-regulated genes was observed with the LIX50 strain. The drastic up-regulation of a majority of DevR regulon genes in LIX48 points toward a dysregulation of the devRS locus in H37Ra as a result of devR overexpression, which is absent in H37Rv.
Table 1.
Genes up-/down-regulated in M. tuberculosis H37Ra and H37Rv devR overexpression strains during aerobic growth
DevR overexpression–mediated aerobic induction of the DevR regulon in M. tuberculosis H37Ra is phosphorylation-independent
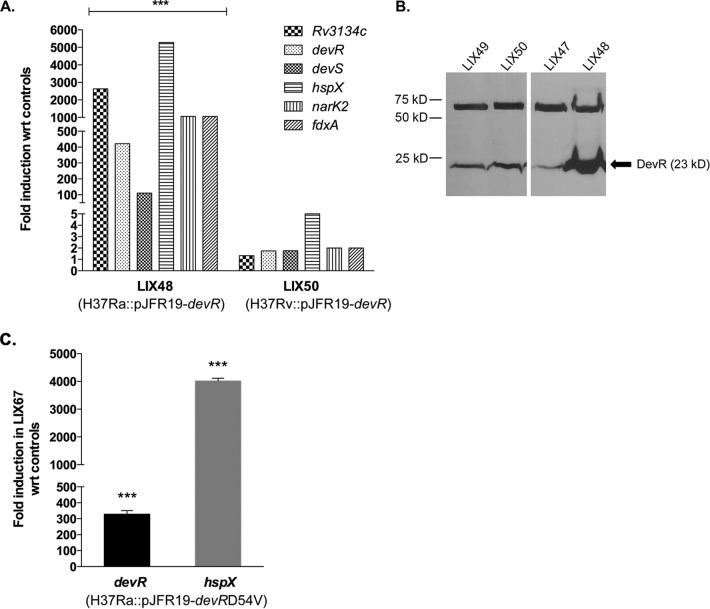
We validated the microarray results by analyzing expression of the Rv3134c-devR-devS operon and three known DevR-regulated genes, namely hspX, narK2, and fdxA, in LIX48 and LIX50 strains relative to the EV control strains. The up-regulation in the overexpression strains is reported as -fold induction with respect to baseline expression in the EV control strains (set at 1.0). As shown in Fig. 2A, qRT-PCR analysis revealed significant -fold induction of Rv3134c (∼2638-fold), devR (∼422-fold), devS (∼111-fold), hspX (∼5275-fold), fdxA (∼1055-fold), and narK2 (∼1000-fold) genes in the LIX48 strain. In contrast, only a marginal increase in transcript levels of Rv3134c (∼1.3-fold), devR (∼1.75-fold), devS (∼1.75-fold), hspX (∼5-fold), fdxA (∼2-fold), and narK2 (∼2-fold) was observed in the LIX50 strain (Fig. 2A). Western blot analysis of whole-cell lysates prepared from LIX48 and LIX50 strains corroborated the qRT-PCR results (Fig. 2B). Densitometric analysis revealed that the DevR protein increased ∼1.5-fold and at least 6-fold in H37Rv and H37Ra overexpression strains, respectively. These data suggest two things: first, that devR overexpression from the amidase promoter during aerobic growth of M. tuberculosis H37Ra leads to induction of the Rv3134c-devR-devS operon and DevR-regulated genes, and, second and more importantly, that there are significant differences in overexpression-mediated induction of the DevR regulon in M. tuberculosis H37Ra versus H37Rv strains.
Figure 2.
Expression analysis of DevR regulon genes in aerobically grown M. tuberculosis H37Rv and H37Ra devR overexpression strains. A, -fold induction of the Rv3134c-devR-devS operon and three DevR-regulated genes (narK2, hspX, and fdxA) in aerobic cultures of H37Ra devR (LIX48) and H37Rv devR (LIX50) overexpression strains relative to LIX47 and LIX49, EV control strains, respectively (baseline expression set to 1.0). ***, p < 0.001 for the differences in expression between LIX48 and LIX50 strains. B, Western blot analysis of whole-cell lysates of LIX49 (lane 1), LIX50 (lane 2), LIX47 (lane 3), and LIX48 (lane 4) with polyclonal rabbit anti-DevR antibodies (1:3000). Arrow, DevR protein. The top band in the blot was used as an internal control to normalize protein amounts in all lanes. C, -fold induction of devR and hspX in RNA isolated from aerobic cultures of M. tuberculosis H37Ra overexpressing phosphorylation-defective devRD54V gene (LIX67) relative to the EV control (baseline expression set to 1.0). ***, p < 0.001 for the differences in expression between LIX67 and control strains. Results are presented as mean ± S.D. (error bars) of three independent experiments.
To rule out possible involvement of DevR phosphorylation in the aerobic dysregulation of the devRS TCS in LIX48, we constructed an M. tuberculosis H37Ra strain overexpressing the devR gene carrying a mutation in aspartic acid residue at position 54 (LIX67; Fig. 1). The D54V substitution mutation in devR was shown previously to abolish phosphorylation of the DevR protein (3), thereby resulting in negligible expression of DevR regulon genes (1). The devRD54V gene was PCR-amplified from plasmid pKKPoperon devRD54V-Cmyc (57) and cloned into pJFR19 to yield plasmid pYA1626. The sequence-verified plasmid pYA1626 was electroporated into M. tuberculosis H37Ra to generate LIX67 strain (Fig. 1). Overexpression of devRD54V in LIX67 did not perturb gene induction. In fact, devR (∼329-fold) and hspX (∼4020-fold) (Fig. 2C) transcript levels in LIX67 were found to be comparable with those observed in LIX48 (Fig. 2A). Collectively, these observations dismiss any role for phosphoactivation of DevR through signal transduction as the underlying basis for the aerobic induction of DevR regulon genes in H37Ra devR overexpression strain.
Complementation of M. tuberculosis H37Ra devR overexpression strain with WT PhoPRv mitigates dysregulation of devR-devS TCS
In subsequent experiments, we investigated the possible role of PhoPRa in regulation of the DevR regulon in M. tuberculosis H37Ra. Toward this end, the phoPRv gene was amplified from M. tuberculosis H37Rv DNA and cloned downstream from an hsp60 promoter in pMV261 to yield the recombinant plasmid pYA1630. The M. tuberculosis H37Ra strain overexpressing devR (LIX48) was transformed with plasmid pYA1630 to generate LIX68 strain, wherein defective endogenous PhoPRa is complemented with WT PhoPRv. LIX47 strain transformed with the empty vector pMV261 served as control in this experiment. Expression of phoPRv in LIX68 led to a >70–80% reduction in the transcription of devR (∼120-fold versus 422-fold) and hspX (∼800-fold versus 5275-fold), compared with the levels obtained in LIX48 (Fig. 3). These observations establish that presence of WT PhoPRv down-regulates devR transcription. Based on these results, we propose that, in the M. tuberculosis H37Rv devR overexpression strain, WT PhoP negatively regulates aerobic expression of the devRS TCS and that this phenomenon is absent/inefficient in H37Ra owing to the presence of a mutant PhoP protein.
Figure 3.
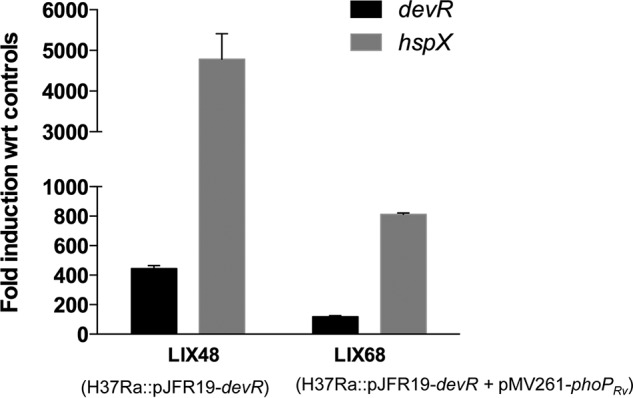
qRT-PCR analysis of M. tuberculosis H37Ra devR overexpression strain complemented with phoPRv gene (LIX68). -Fold induction of devR and hspX genes in LIX68 and its parent strain (LIX48) with respect to EV control strains (baseline expression set to 1.0) is shown. Results are presented as mean ± S.D. (error bars) of three independent experiments.
M. tuberculosis PhoPRv and DevR response regulators exhibit differential binding properties to Rv3134c promoter
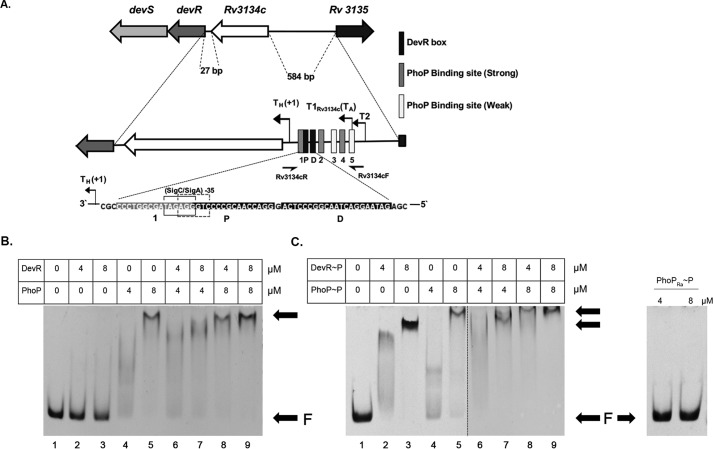
To determine whether PhoP mediates regulation of Rv3134c-devR-devS genes through direct binding or through indirect effects, we investigated the ability of PhoPRv and PhoPRa proteins to bind the Rv3134c promoter region. The consensus DNA-binding sequence for M. tuberculosis PhoPRv from PhoP ChIP-Seq data (39) was used to predict putative PhoP binding sites in a ∼350-bp upstream region of the Rv3134c-devR-devS operon. This region harbors multiple operon promoters (35, 40, 41) and DevR binding sites (35), and in addition, 5 putative PhoP-binding sites were detected (Fig. 4A). A 218-bp DNA fragment in the Rv3134c promoter region containing all five putative PhoP binding sites along with previously established DevR-binding sites was PCR-amplified from H37Rv DNA and examined for PhoPRv, PhoPRa, and DevR binding. We used small molecule phosphodonor acetyl phosphate to phosphorylate DevR and PhoP proteins because they can be phosphorylated by acetyl phosphate (42–44). All three proteins were confirmed to be phosphorylation-proficient (data not shown). Different concentrations (4 and 8 μm) of DevR, PhoPRv, and PhoPRa proteins were incubated separately with DNA. PhoPRv was found to bind the target DNA in a concentration-dependent but phosphorylation-independent manner (Fig. 4, B and C). In contrast, PhoPRa did not bind DNA (Fig. 4C, right). This result is consistent with the reported DNA-binding defect of PhoPRa protein (45). In agreement with previous data (43), unphosphorylated DevR bound to DNA poorly, whereas DevR∼P formed a stable complex with DNA (compare lanes 2 and 3 in Fig. 4, B and C). At equimolar concentrations, unphosphorylated PhoPRv exhibited a higher DNA binding property compared with unphosphorylated DevR (Fig. 4B, compare lanes 2 and 4 and lanes 3 and 5), suggesting differential binding affinities of the two proteins.
Figure 4.
DNA-binding studies of PhoP RR with Rv3134c-devR-devS promoter region. A, in silico analysis of the upstream region of Rv3134c-devR-devS operon revealed five putative sites for PhoP binding depicted as 1–5. Regulatory features including DevR binding boxes (P and D) and transcription start sites (aerobic and hypoxic) are indicated. Primers used for amplifying the 218-bp DNA probe for use in EMSAs are shown as half-arrows. B, binding of unphosphorylated DevR and PhoP proteins. C, binding of phosphorylated DevR, PhoP (left), and PhoPRa (right) proteins to target DNA. Arrows, free DNA (F) and bound DNA.
Next we examined whether DevR and PhoP affect each other's binding to target DNA. Significantly, co-incubation of phosphorylated DevR and PhoP resulted in a “supershift” of the protein–DNA complex (Fig. 4C, compare lanes 2 and 4 with lane 6, and compare lanes 3 and 5 with lanes 7–9), which was not distinctly evident when unphosphorylated proteins were co-incubated (Fig. 4B, lanes 6–9). Taken together, the electrophoretic mobility shift assay (EMSA) results validate the DNA-binding defect of PhoPRa and indicate that PhoPRv binds to the Rv3134c upstream region independent of phosphorylation. Moreover, the observed “supershift” in DNA binding suggests a possible interaction between DevR and PhoPRv RRs.
DevR and PhoPRv form heterodimers in vitro and in vivo
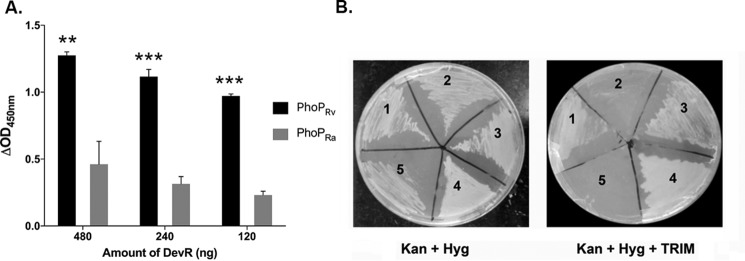
The interaction between M. tuberculosis DevR and PhoP RRs was assessed in vitro in an ELISA format using purified PhoPRa/PhoPRv-His6 protein (10 pmol) that was immobilized in triplicate in a 96-well plate overnight in coating buffer. DevR-His6 protein (480, 240, and 120 ng) was incubated with PhoPRa/PhoPRv, and the interaction was probed using anti-DevR antibody as primary antibody (1:5000) and horseradish peroxidase–conjugated anti-rabbit antibody as secondary antibody (1:10,000). The background values from control wells were subtracted from test well readings, and ΔA450 values were plotted as shown in Fig. 5A. A strong interaction was observed between DevR–PhoPRv as compared with DevR–PhoPRa proteins (p < 0.001; Fig. 5A).
Figure 5.
In vitro and in vivo protein–protein interaction of DevR and PhoP RRs. A, the interaction between M. tuberculosis DevR–PhoPRv (black bars) and DevR–PhoPRa (gray bars) proteins was assessed in vitro in an ELISA format using 10 pmol of purified PhoPRv/PhoPRa proteins as described under “Experimental procedures.” The background values from the control wells were subtracted from the test well readings, and ΔA450 values are plotted. ** and ***, p < 0.01 and 0.001, respectively, for the difference in interaction between DevR–PhoPRv and DevR–PhoPRa proteins. B, in vivo mycobacterial protein fragment complementation assay. Shown is M. smegmatis transformed with the following pairs of plasmid constructs encoding recombinant fusion proteins: recombinant PhoPRv-[F3]/DevR-[F1,2] (1) and PhoPRa-[F3]/DevR-[F1,2] (2). Positive controls (3 and 4) and negative control (5) were streaked on Kan + Hyg (left) and Kan + Hyg + TRIM plates (right). One representative picture from three independent experiments is shown. Error bars, S.D.
Because in vivo interaction in a related host, Mycobacterium smegmatis, is anticipated to provide functionally and physiologically relevant environment to study protein–protein interaction, we investigated DevR–PhoP interactions using the mycobacterial protein fragment complementation (M-PFC) assay that is well-established to study interactions between mycobacterial proteins in vivo (46). Briefly, when two mycobacterial interacting proteins are independently fused with domains of murine dihydrofolate reductase (mDHFR), functional reconstitution of the two mDHFR domains can occur in mycobacteria, allowing for the selection of mycobacterial resistance against trimethoprim (TRIM). The plasmid pairs (pUAB300::devR and pUAB400 carrying phoPRv and phoPRa, respectively) were generated as C-terminal fusions with the complementary fragments of mDHFR. The plasmid pairs were electroporated into M. smegmatis mc2155 to generate the following protein expression pairs, DevR/PhoPRv and DevR/PhoPRa. The cotransformants were screened on MB7H11 plates + kanamycin (Kan) (25 μg/ml) + hygromycin (Hyg) (50 μg/ml) with or without TRIM (30–50 μg/ml) (Fig. 5B). The protein pairs GCN4/GCN4 encoded by pUAB100/pUAB200 and GCN4/DevR encoded by pUAB100/pAVDevR200 were used as positive and negative control, respectively. Robust growth was observed in the positive controls (Fig. 5B, sections 3 and 4) as well as in transformants co-expressing DevR/PhoPRv (Fig. 5B, section 1), suggesting that these pairs of proteins interact in vivo. We observed interaction between DevR and PhoPRv even in the presence of 50 μg/ml TRIM, confirming strong interactions in vivo. Importantly, no growth was observed in the transformant co-expressing DevR/PhoPRa proteins (Fig. 5B, section 2). Furthermore, the negative control, co-expressing DevR and GCN4, did not show growth, ruling out a spontaneous association of mDHFRF[1,2] and F[3] (Fig. 5B, section 5). Based on these observations, we infer that PhoPRa is defective in interaction with DevR owing to the point mutation. Collectively, these findings indicate that formation of PhoPRv–DevR heterodimer and its interaction with DNA facilitate co-regulation of DevR regulon genes.
Discussion
The attenuated M. tuberculosis H37Ra strain is an isogenic variant of virulent H37Rv and thus provides a unique opportunity to understand and dissect regulatory mechanisms that might otherwise be difficult to unravel. Of significant interest is the culture medium-specific aerobic induction of the Rv3134c-devR-devS genes in M. tuberculosis H37Ra but not in H37Rv, suggesting differential regulation of these genes in H37Ra and H37Rv (25). Here, we have investigated aerobic expression of the Rv3134c-devR-devS genes by comparing transcription profiles of M. tuberculosis H37Ra and H37Rv devR overexpression strains. We report striking up-regulation of the Rv3134c-devR-devS operon and several members of the DevR regulon in M. tuberculosis H37Ra as a result of devR overexpression during aerobic growth that is absent in the analogous H37Rv strain. Our results implicate the inability of the mutant PhoPRa protein to bind DNA (Fig. 4C) and interact with DevR RR (Fig. 5) as the foremost factors responsible for aerobic dysregulation of the devRS TCS observed in H37Ra. Further, we identified a role for the WT PhoP RR in blocking unwarranted induction of the DevR regulon in M. tuberculosis H37Rv during aerobic growth.
Typically, TCS systems are responsive to specific stimuli, and activation usually leads to an increase in concentration of the phosphorylated RR species above a threshold. Thus, a balance in ratio between the unphosphorylated and phosphorylated RR molecules dictates the direction of response. Due to a dynamic equilibrium between the inactive and active forms, an increase in unphosphorylated RR above the threshold value can bypass activation requirements, resulting in transcriptional activation in the absence of any natural stimulus (47). Recent findings suggest that aerobic overexpression of devR in M. tuberculosis can override the need for signal activation (47, 48). Therefore, we utilized devR overexpression strains of M. tuberculosis H37Ra and H37Rv to investigate regulation of aerobic transcription of devRS genes. With pleiotropic effects associated with gene deletions and the fact that culture medium components modulate devRS expression, use of these strains provided an unequivocal advantage.
The net effect of devR overexpression driven from a strong promoter is an increased level of unphosphorylated DevR leading to signal-independent activation of the DevR regulon. In contrast to previous studies that have reported significant induction of the DevR regulon in aerobic cultures of M. tuberculosis H37Rv strains overexpressing devR (39, 47–49), we observed a fairly moderate response. We reason that promoters of varying strengths in overexpression constructs, differences in genetic backgrounds, and media used are sources of discrepancy between different studies (47–49). A critical concentration of DevR essential for activation of devRS TCS has been previously established (10). We rationalize that the marginal overexpression of DevR observed in H37Rv (Fig. 2) is probably insufficient to override the threshold for transcriptional activation. Thus, it follows that expression of the DevR regulon under non-inducing conditions, such as aerobic growth, is a function of cellular levels of the DevR protein. The substantially high levels of DevR in M. tuberculosis H37Ra devR overexpression strain used in the present study are indicative of a signal-independent, hyperactivated DevR regulon. It is noteworthy that such levels of DevR regulon have not been observed in any of the devR overexpression strains in H37Rv background (39, 47–49) or in W-Beijing lineage of M. tuberculosis (24) reported so far. Thus, it is not surprising that hyperactivation of the DevRS TCS in M. tuberculosis H37Ra devR overexpression strain resulted in significant clumping and poor aerobic growth (Fig. S1). Our results point toward differential autoregulatory effects of DevR in H37Ra and H37Rv overexpression strains. Because these strains were constructed identically, the differences in the level of DevR protein and downstream regulon expression are intriguing and may be due to disparities in their genetic backgrounds.
The M. tuberculosis Rv3134c-devR-devS operon characteristically contains multiple transcription start sites, aerobic (basal) and hypoxia-inducible promoters, binding sites of more than one transcriptional regulator, and/or alternative σ factors (such as SigA and SigC) that are suggestive of complex regulation of this operon (35, 36, 40, 41). However, little is known about how M. tuberculosis H37Rv precludes up-regulation of the DevR regulon during aerobic growth. Unlike the M. tuberculosis Beijing strain, wherein aerobic induction of the DevR regulon was linked to specific mutations (24, 26, 27), DNA sequences of the Rv3134c-devR-devS operon and its promoter region in H37Rv and H37Ra are identical. Thus, we hypothesized that aerobic dysregulation of this locus in M. tuberculosis H37Ra may be attributed to a defect in some regulatory protein(s). The marginal aerobic expression of DevR regulon genes in M. tuberculosis H37Ra ΔdevR (Fig. S2) and H37Rv ΔdevR mutant strains (35, 40) supports the role of another transcriptional regulator driving aerobic expression of these genes.
We focused on the possible involvement of M. tuberculosis PhoP in regulation of aerobic expression of devRS genes based on three key observations. First, a subset of the DevR regulon is down-regulated in aerobically grown M. tuberculosis H37Rv ΔphoP mutant bacteria (32); second, M. tuberculosis PhoP is implicated in co-regulation of the hypoxic response in mycobacteria, which is further supported by identification of multiple PhoP binding sites in the Rv3134c promoter region (this study), and third, PhoP in M. tuberculosis H37Ra has impaired DNA binding affinity as a result of a point mutation in its DNA recognition helix (45, 50). Partial restoration by complementation of the M. tuberculosis H37Ra devR overexpression strain with WT PhoPRv highlighted the role of mutant PhoPRa protein in aerobic dysregulation of the devRS TCS in H37Ra. A key finding of this study is that in addition to its positive regulatory effect on devR expression (23), PhoP is seen to exert a negative regulatory effect (Fig. 3). We propose that PhoPRv maintains the aerobic expression of devR gene to a level well below the threshold for DevR regulon activation. At present, it is unclear whether sigC promoter mutation in M. tuberculosis H37Ra also contributes toward regulation of devRS expression; thus, SigC involvement cannot be completely discounted. To the best of our knowledge, this is the first report demonstrating directin vitro binding of PhoPRv to the devRS upstream region. Although the significance of a strong PhoP binding site within the −35 element of a SigA/SigC promoter is not clear at present, interaction of PhoP with the transcriptional machinery is a distinct possibility. In line with this hypothesis, PhoP has been shown to interact with SigE in a recent report (51).
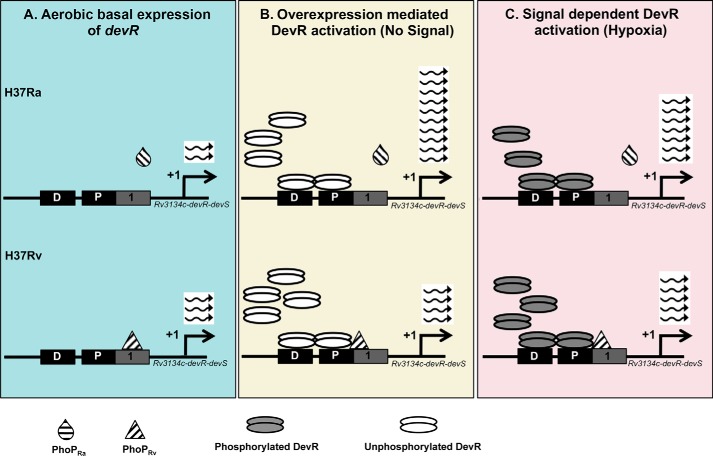
Based on our observations and previously reported data, we propose a model for possible regulatory mechanisms exerted by PhoP and DevR on expression of the Rv3134c-devR-devS operon in M. tuberculosis H37Ra and H37Rv (Fig. 6). During aerobic growth (non-inducing conditions) (Fig. 6A), DevR is unphosphorylated and does not bind to DNA (Fig. 4B). The devRS genes are expressed at lower basal level in H37Ra compared with H37Rv (29). Moreover, unlike PhoPRv that binds to Rv3134c upstream DNA (Fig. 4, B and C), PhoPRa mutant protein is defective in DNA binding (Fig. 4C, right). Under DevR overexpression conditions (Fig. 6B), unphosphorylated DevR mediates positive autoregulation (47), thereby increasing the level of total DevR protein, resulting in hyperactivation of the DevR regulon in H37Ra. By contrast, in the M. tuberculosis H37Rv overexpression strain, PhoPRv is bound to the Rv3134c promoter and interacts with DevR to block autoregulation. In the presence of inducing signals, such as hypoxia (Fig. 6C), DevR is phosphorylated in both M. tuberculosis H37Rv (1, 40) and H37Ra strains (32) and induces downstream expression of the DevR regulon, albeit to higher levels in H37Ra (32), highlighting the negative effect of PhoP. These findings suggest that PhoP plays a secondary but repressive role in hypoxia-induced DevR regulon expression.
Figure 6.
Model depicting the role of PhoP and DevR in regulating the expression of DevR regulon genes in M. tuberculosis H37Rv and H37Ra. The DevR binding sites upstream of Rv3134c-devR-devS genes responsible for activation of DevR regulon are depicted as described (39, 40). The most probable PhoP-binding site identified in this study (1, solid gray box) overlaps the proximal DevR-binding box (P, solid black box). A, aerobic basal devR expression. There is a basal level expression of the devR gene that is marginally lower in M. tuberculosis H37Ra as compared with H37Rv. B, overexpression-mediated activation of DevR. In M. tuberculosis H37Ra devR overexpression strain, the absence of PhoPRa binding enables unphosphorylated DevR to autoregulate and cause downstream expression of the DevR regulon. This sequence of events is prevented in M. tuberculosis H37Rv due to binding of PhoPRv to the Rv3134c upstream region and its interaction with DevR. C, signal dependent activation of DevR. Hypoxia leads to phosphorylation of DevR and its cooperative binding to the Dev boxes, resulting in induction of the DevR regulon genes in M. tuberculosis H37Rv and H37Ra strains. The inhibitory effect of PhoPRv on devR expression and differential interactions between these RRs explain the lesser number of transcripts in H37Rv compared with H37Ra during hypoxic growth conditions.
Heterodimers between RR proteins have now been discovered in multiple instances (52–55), suggesting that heterodimer formation may be a fundamental mechanism contributing to the concerted regulation of overlapping regulons in M. tuberculosis. We recently elucidated heterodimeric interactions between M. tuberculosis DevR and NarL RRs for co-regulation of gene expression during aerobic nitrate metabolism (22). Overlap of DevR- and PhoP-binding sites on the DNA raises the possibility of protein–protein interactions between them. Such a situation may arise during hypoxia, as both DevR and PhoP are known to regulate overlapping sets of genes during hypoxia (23). Our results establish that DevR and PhoPRv RRs interact in vitro and in vivo. Notably, DevR did not exhibit robust interaction with the mutant PhoPRa protein, suggesting that the S219L mutation disrupts heterodimer formation. Previous reports have suggested that the dimeric interface of PhoP monomers from B. subtilis retains a second surface that is available for further interactions (56), raising the likelihood that homodimer and heterodimer interfaces in PhoP may be different.
In conclusion, comparative analysis of the interplay between DevR and PhoP RRs in virulent H37Rv and avirulent H37Ra strains of M. tuberculosis provides novel insights into PhoPRv-mediated negative regulation of the devRS TCS, its interaction with DevR, and their differential DNA binding affinities as key regulatory mechanisms that fine-tune expression of the dormancy regulon in mycobacteria.
Experimental procedures
Bacterial strains and plasmids
Escherichia coli JM109 and DH5α strains were used as host strains for genetic manipulations and plasmid constructions. E. coli C43 (DE3) strain was used for overexpression and purification of His-tagged DevR and PhoPRv proteins. Purified His-tagged PhoPRa protein was obtained as a gift from Dr. Dibyendu Sarkar (IMTECH, Chandigarh, India). Virulent M. tuberculosis H37Rv, avirulent M. tuberculosis H37Ra, and M. smegmatis mc2155 strains were used in the present study. Table S1 summarizes bacterial strains and plasmids used in this study. Detailed plasmid constructions are available upon request to V. M.
Media, chemicals, and culture conditions
E. coli cultures were grown in 2× YT or Luria–Bertani (LB) broth or on LB-agar plates at 37 °C. M. smegmatis cultures were grown at 37 °C with aeration in Middlebrook 7H9 (Difco) or LB medium supplemented with 0.05% Tween 80 or on LB-agar plates. For the M-PFC assay, M. smegmatis strains were plated on Middlebrook 7H11 agar (MB7H11, Difco) supplemented with 0.5% glycerol, 0.5% glucose, and 0.2% Tween 80 and grown at 37 °C. M. tuberculosis strains were grown in Middlebrook 7H9 medium supplemented with 0.05% Tween 80 and 10% ADS (0.5% albumin, 0.2% dextrose, 0.085% Saline) or on Middlebrook 7H10 agar plates at 37 °C to an A600 ∼0.2–0.3 Antibiotics and chemicals were added as required at the following concentrations: ampicillin, 100 μg/ml; Kan, 50 μg/ml; Hyg, 50 and 100 μg/ml for M. smegmatis and M. tuberculosis, respectively, and 150 μg/ml for E. coli; isopropyl β-d-thiogalactopyranoside, 1 mm; and trimethoprim (TRIM), 20–50 μg/ml. All chemicals were obtained from Sigma-Aldrich, unless stated otherwise.
RNA isolation
RNA isolation of exponentially grown cultures of M. tuberculosis was performed as described previously (25). Total RNA integrity was assessed using RNA 6000 Nano Lab Chip on the 2100 Bioanalyzer (Agilent) according to the manufacturer's protocol. Total RNA purity was assessed by the NanoDrop® ND-1000 UV-visible spectrophotometer (NanoDrop Technologies).
Microarray slide design for M. tuberculosis H37Ra and H37Rv
The Agilent custom M. tuberculosis slide was designed by Genotypic Technology Private Ltd. to study the expression of all of the genes of M. tuberculosis H37Rv (source: NCBI Accession ID AL123456.2) and M. tuberculosis H37Ra strain (NCBI Accession ID NC_009525.1). Probes common to both M. tuberculosis H37Rv and M. tuberculosis H37Ra (n = 15,106) and probes specific to M. tuberculosis H37Ra (n = 18) and M. tuberculosis H37Rv (n = 84) were designed. All of the oligonucleotides were designed and synthesized in situ as per the standard algorithms and methodologies used by Agilent Technologies. The array format was 8 × 15K (AMADID: 023057) comprising a total number of 15,744 features encompassing 15,208 probes and 536 Agilent control probes.
Microarray hybridization and quantitative RT-PCR
RNA samples from two independent experiments were analyzed by microarrays. Poly(A) tails were added to the 3′-ends of RNA using the A-plus poly(A) polymerase tailing kit (Epicenter Biotechnologies). The samples were labeled using the Quick-Amp labeling Kit (Agilent). Five hundred nanograms of each sample was incubated with reverse transcription mix at 42 °C and converted to double-stranded cDNA primed by oligo(dT) with a T7 polymerase promoter. The cDNA was used as template for cRNA generation by in vitro transcription and incorporation of the dye Cy3-CTP and Cy5-CTP (Agilent). The cDNA synthesis and in vitro transcription steps were carried out at 40 °C. The quality of labeled cRNA was assessed for yields and specific activity followed by hybridization. Cy3 and Cy5-labeled samples (300 ng each) were hybridized using the Gene Expression Hybridization kit (Agilent) in Surehyb Chambers (Agilent) at 65 °C for 16 h. The hybridized slides were washed using Gene Expression wash buffers (Agilent) and scanned using the Microarray Scanner G2505C at 5 μm resolution. Data extraction from images was done using Feature Extraction software version 10.5.1.1 (Agilent), followed by analysis using the GeneSpring GX version 10 software (Agilent). Normalization of the data were done in GeneSpring GX using Lowess (locally weighted scatterplot smoothing) normalization. Samples were grouped based on the replicates, and genes showing up- or down-regulation >0.8-fold among the samples were identified. Results are reported as the geometric means of log2 expression ratios ± S.D. between the replicates.
Quantitative RT-PCR was performed to validate the microarray results as described previously (25). Briefly, 200 ng of RNA was reverse-transcribed into cDNA using the iScript cDNA synthesis kit (Bio-Rad). The cDNA was diluted 1:10 and served as the template in real-time PCR using gene-specific primers and SYBR Green dye (Bio-Rad). RNA from three independent experiments was used for expression analysis. Normalization of expression was done using 16S rRNA as an internal control, and the -fold change in expression between test and control samples was calculated using the iQ5 software.
Protein isolation and immunoblot analysis
Total protein was isolated from logarithmic phase cultures of M. tuberculosis H37Rv and H37Ra devR overexpression strains along with their respective EV control strains. The cell pellets were lysed using a bead beater and subjected to immunoblot analysis using anti-DevR antibody as described previously (3).
Overexpression and purification of His-tagged DevR and PhoPRv proteins
The phoPRv coding region (744 bp) was PCR-amplified from M. tuberculosis H37Rv genomic DNA and cloned into the NcoI and XhoI sites of the pET28a expression vector to create plasmid pGSPhoPRv-His6. Recombinant plasmid pAVDevR-His6 was used for purification of His-tagged DevR protein (43). E. coli C43 (DE3) cultures carrying recombinant pGSPhoPRv-His6 and pAVDevR-His6 plasmids were grown at 37 °C, 180 rpm in 600 ml of 2× YT medium containing Kan (50 μg/ml) to an A590 of 0.4–0.5. The production of recombinant proteins, DevR and PhoPRv, was induced by the addition of 1 mm isopropyl β-d-thiogalactopyranoside followed by overnight incubation at 25 °C followed by standing incubation at 4 °C for 4–5 h. Cells were harvested by centrifugation at 6000 rpm for 10 min (Sorvall GSA rotor, Sorvall RC6 Plus centrifuge (Thermo Scientific)). Briefly, the culture pellet was resuspended in 4 ml of lysis buffer containing 20 mm imidazole, 20 mm Na2HPO4, and 0.5 m NaCl, Complete EDTA-free protease inhibitor mixture (Roche Diagnostics). The resuspended pellet was sonicated, and the cell lysate was aliquoted into 1.5-ml sterile tubes and centrifuged at 12,000 rpm into Sorvall (F-20 microrotor, Thermo Scientific) for 40 min followed by filter sterilization of the supernatant through 0.45-μm and 0.22-μm filters. The supernatant was loaded onto the Ni2+-nitrilotriacetic acid–agarose column of 5 ml bed volume (Qiagen, GmBH, Germany) connected to an AKTA protein purifier system (GE Healthcare) via an injection valve with the help of a 10-ml Luerlok syringe. The pre-equilibration was done with 10 bed volumes of equilibration buffer (50 mm Na3PO4, pH 7.4, 300 mm NaCl). The flow rate was maintained at 0.5 ml/min. The column was washed with 40 bed volumes of equilibration buffer (as above) and then washed with 30 bed volumes of wash buffer (50 mm Na3PO4, pH 7.4, 300 mm NaCl, 50 mm imidazole, 10% glycerol). The Ni2+-nitrilotriacetic acid resin with bound protein was treated with the elution buffer (50 mm Na3PO4, pH 7.4, 300 mm NaCl, 300 mm imidazole), and the eluant was collected in fractions of 0.5 ml. The fractions were pooled, dialyzed against dialysis buffer (50 mm Tris-HCl, pH 8.0, 50 mm NaCl, 50% glycerol, 0.1 mm DTT), and stored at −20 °C. The purified proteins were subjected to SDS-PAGE followed by analysis through Coomassie Brilliant Blue R250 staining and immunoblotting with anti-His antibody (Sigma-Aldrich) at 1:2000 dilution and detected using diaminobenzidine-hydrogen peroxidase as substrate.
Electrophoretic mobility shift assays
In a standard EMSA reaction, phosphorylated protein and the target DNA probe were incubated with binding buffer containing 25 mm Tris-HCl (pH 8.0), 0.5 mm EDTA, 20 mm KCl, 6 mm MgCl2, 5% glycerol for 30 min on ice in a final reaction volume of 20 μl. The reaction was electrophoresed on a 5% nondenaturing acrylamide gel containing 1.5% glycerol at 120 V (constant) in 0.5× TBE buffer at 4 °C after pre-running the gel for 30 min under similar conditions. The gel was then stained with ethidium bromide, and DNA–protein complexes were visualized using the gel documentation system. For phosphorylation, DevR, PhoPRv, and PhoPRa were incubated with 50 mm acetyl phosphate (Sigma) for 30 min at 25–30 °C in 40 mm Tris-HCl (pH 8.0) and 5 mm MgCl2. After phosphorylation, a DNA-binding assay was performed in a 20-μl reaction. Negative control without any protein was included in all EMSA gels.
Enzyme-linked immunosorbent assay
PhoPRa/PhoPRv-His6 protein (10 pmol or 283 ng) was coated in triplicate in 100 μl of coating buffer in a 96-well polystyrene microtiter plate incubated overnight at 4 °C in a humidified chamber. After washing once with 1× PBS, the wells were blocked with blocking buffer (5% BSA, 0.25% Tween 20 in 1× PBS) for 2 h at 37 °C. Different amounts of DevR-His6 protein (in 100 μl of 1× PBS) were then added in triplicate in test (PhoP+) and control wells (PhoP−) and incubated for 1 h at room temperature. The plate was washed once with 1× PBS, followed by incubation with polyclonal rabbit anti-DevR antibody (1:5000) for 1 h under shaking conditions. The plate was washed three times with washing buffer (1× PBS containing 0.1% Tween 20). Detection was done using anti-goat anti-rabbit IgG-horseradish peroxidase–conjugated antibody (1:10,000) in 2% BSA in 1× PBS and 0.05% Tween 20 after incubation for 1 h at room temperature followed by washing and developed using TMB as substrate. Absorbance was recorded at 450 nm using a spectrofluorimeter (Spectra Max, Molecular Devices LLC). Suitable controls (namely antigen control (PhoP−, DevR−, coating buffer only), primary antibody control, secondary antibody control, and interacting partner control (PhoP−, DevR+)) were applied in the assay.
In vivo M-PFC assay
The M-PFC assay was performed to investigate DevR–PhoP interaction in vivo as described previously (46). The assay enables visualization of protein–protein interactions within mycobacteria through functional reconstitution of murine DHFR protein and selective targeting of mycobacterial DHFR by TRIM. Positive protein–protein interactions are scored by growth in the presence of TRIM concentrations that do not inhibit the recombinant mDHFR. Plasmids pSSDevR300, pGSPhoPRa400, and pGSPhoPRv400 encoding recombinant DevR-[F1,2], PhoPRa, and PhoPRv proteins fused with DHFR[F3] domains, respectively, were electroporated into M. smegmatis. The co-transformants obtained after electroporation into M. smegmatis were screened on MB7H11 plates containing Kan (50 μg/ml) + Hyg (100 μg/ml). Positive clones along with suitable controls were then subcultured or streaked on MB7H11 Kan (50 μg/ml) + Hyg (100 μg/ml) + TRIM (20–50 μg/ml) plates and incubated at 37 °C for 4–5 days to assess protein–protein interactions. Growth of colonies on TRIM-containing medium was indicative of protein–protein interaction. Homodimerization of the GCN4/GCN4 domains encoded by the plasmid pair pUAB100/pUAB200 served as the positive control. The plasmid pair pUAB100/pAVDevR200 was used as a negative control for the assay.
Statistical analyses
Statistical analyses were performed using Student's t test or one-way analysis of variance using GraphPad Prism version 6.0 software. A p value <0.05 was considered statistically significant.
Author contributions
A. V. and V. M. data curation; A. V., V. M., J. S. T., and J. E. C.-C. formal analysis; A. V., V. M., J. S. T., and J. E. C.-C. funding acquisition; A. V. and V. M. validation; A. V., V. M., and G. S. investigation; A. V. and V. M. methodology; A. V., V. M., J. S. T., and J. E. C.-C. writing-review and editing; V. M. conceptualization; V. M., J. S. T., and J. E. C.-C. supervision; V. M. visualization; V. M. writing-original draft; J. S. T. and J. E. C.-C. resources; J. S. T. and J. E. C.-C. project administration.
Supplementary Material
Acknowledgments
We acknowledge Genotypic Technology Pvt. Ltd. (Bangalore, India) for the microarray processing and related data analysis. We are grateful to Drs. Adrie Steyn for M-PFC vectors, Malini Rajagopalan for pMG85 plasmid, Kohinoor Kaur for the pKKPoperon devRD54V-Myc plasmid, and Saurabh Sharma for the pUAB300-devR plasmid. We are very thankful to Dr. Dibyendu Sarkar for the generous gift of purified PhoPRa protein. We thank our present and former colleagues of the Clark-Curtiss group (Arizona State University) and of the J. S. T. group (AIIMS) for critical analysis and valuable suggestions.
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The microarray data presented in this study have been deposited in the NCBI Gene Expression Omnibus (GEO) under the GEO series accession number GSE103551.
This article contains Figs. S1 and S2 and Tables S1–S3.
- RR
- response regulator
- EMSA
- electrophoretic mobility shift assay
- M-PFC
- mycobacterial protein fragment complementation
- mDHFR
- murine dihydrofolate reductase
- TCS
- two-component system
- TRIM
- trimethoprim
- qRT-PCR
- quantitative RT-PCR
- LB
- Luria broth
- Kan
- kanamycin
- Hyg
- hygromycin
- EV
- empty vector.
References
- 1. Park H. D., Guinn K. M., Harrell M. I., Liao R., Voskuil M. I., Tompa M., Schoolnik G. K., and Sherman D. R. (2003) Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 48, 833–843 10.1046/j.1365-2958.2003.03474.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Voskuil M. I., Schnappinger D., Visconti K. C., Harrell M. I., Dolganov G. M., Sherman D. R., and Schoolnik G. K. (2003) Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198, 705–713 10.1084/jem.20030205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saini D. K., Malhotra V., Dey D., Pant N., Das T. K., and Tyagi J. S. (2004) DevR-DevS is a bona fide two-component system of Mycobacterium tuberculosis that is hypoxia-responsive in the absence of the DNA-binding domain of DevR. Microbiology 150, 865–875 10.1099/mic.0.26218-0 [DOI] [PubMed] [Google Scholar]
- 4. Gautam U. S., McGillivray A., Mehra S., Didier P. J., Midkiff C. C., Kissee R. S., Golden N. A., Alvarez X., Niu T., Rengarajan J., Sherman D. R., and Kaushal D. (2015) DosS is required for the complete virulence of Mycobacterium tuberculosis in mice with classical granulomatous lesions. Am. J. Respir. Cell Mol. Biol. 52, 708–716 10.1165/rcmb.2014-0230OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehra S., Foreman T. W., Didier P. J., Ahsan M. H., Hudock T. A., Kissee R., Golden N. A., Gautam U. S., Johnson A. M., Alvarez X., Russell-Lodrigue K. E., Doyle L. A., Roy C. J., Niu T., Blanchard J. L., et al. (2015) The DosR regulon modulates adaptive immunity and is essential for M. tuberculosis persistence. Am. J. Respir. Crit. Care Med. 191, 1185–1196 10.1164/rccm.201408-1502OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boon C., and Dick T. (2002) Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J. Bacteriol. 184, 6760–6767 10.1128/JB.184.24.6760-6767.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boon C., and Dick T. (2012) How Mycobacterium tuberculosis goes to sleep: the dormancy survival regulator DosR a decade later. Fut. Microbiol. 7, 513–518 10.2217/fmb.12.14 [DOI] [PubMed] [Google Scholar]
- 8. Malhotra V., Sharma D., Ramanathan V. D., Shakila H., Saini D. K., Chakravorty S., Das T. K., Li Q., Silver R. F., Narayanan P. R., and Tyagi J. S. (2004) Disruption of response regulator gene, devR, leads to attenuation in virulence of Mycobacterium tuberculosis. FEMS Microbiol. Lett. 231, 237–245 10.1016/S0378-1097(04)00002-3 [DOI] [PubMed] [Google Scholar]
- 9. Majumdar S. D., Sharma D., Vashist A., Kaur K., Taneja N. K., Chauhan S., Challu V. K., Ramanathan V. D., Balasangameshwara V., Kumar P., and Tyagi J. S. (2010) Co-expression of DevR and DevR(N)-Aph proteins is associated with hypoxic adaptation defect and virulence attenuation of Mycobacterium tuberculosis. PLoS One 5, e9448 10.1371/journal.pone.0009448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Majumdar S. D., Vashist A., Dhingra S., Gupta R., Singh A., Challu V. K., Ramanathan V. D., Kumar P., and Tyagi J. S. (2012) Appropriate DevR (DosR)-mediated signaling determines transcriptional response, hypoxic viability and virulence of Mycobacterium tuberculosis. PLoS One 7, e35847 10.1371/journal.pone.0035847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taneja N. K., Dhingra S., Mittal A., Naresh M., and Tyagi J. S. (2010) Mycobacterium tuberculosis transcriptional adaptation, growth arrest and dormancy phenotype development is triggered by vitamin C. PLoS One 5, e10860 10.1371/journal.pone.0010860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar A., Toledo J. C., Patel R. P., Lancaster J. R. Jr, and Steyn A. J. (2007) Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor. Proc. Natl. Acad. Sci. U.S.A. 104, 11568–11573 10.1073/pnas.0705054104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sikri K., Batra S. D., Nandi M., Kumari P., Taneja N. K., and Tyagi J. S. (2015) The pleiotropic transcriptional response of Mycobacterium tuberculosis to vitamin C is robust and overlaps with the bacterial response to multiple intracellular stresses. Microbiology 161, 739–753 10.1099/mic.0.000049 [DOI] [PubMed] [Google Scholar]
- 14. Kumar A., Deshane J. S., Crossman D. K., Bolisetty S., Yan B. S., Kramnik I., Agarwal A., and Steyn A. J. (2008) Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J. Biol. Chem. 283, 18032–18039 10.1074/jbc.M802274200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shiloh M. U., Manzanillo P., and Cox J. S. (2008) Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe 3, 323–330 10.1016/j.chom.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Voskuil M. I., Honaker R. W., and Steyn A. J. (2009) Oxygen, nitric oxide, and carbon monoxide signaling. In Mycobacterium: Genomics and Molecular Biology. pp. 119–147, Caister Academic Press, Norfolk, UK [Google Scholar]
- 17. Saini D. K., Malhotra V., and Tyagi J. S. (2004) Cross talk between DevS sensor kinase homologue, Rv2027c, and DevR response regulator of Mycobacterium tuberculosis. FEBS Lett. 565, 75–80 10.1016/j.febslet.2004.02.092 [DOI] [PubMed] [Google Scholar]
- 18. Chao J. D., Papavinasasundaram K. G., Zheng X., Chávez-Steenbock A., Wang X., Lee G. Q., and Av-Gay Y. (2010) Convergence of Ser/Thr and two-component signaling to coordinate expression of the dormancy regulon in Mycobacterium tuberculosis. J. Biol. Chem. 285, 29239–29246 10.1074/jbc.M110.132894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bae H. J., Lee H. N., Baek M. N., Park E. J., Eom C. Y., Ko I. J., Kang H. Y., and Oh J. I. (2017) Inhibition of the DevSR two-component system by overexpression of Mycobacterium tuberculosis PknB in Mycobacterium smegmatis. Mol. Cells 40, 632–642 10.14348/molcells.2017.0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He H., Bretl D. J., Penoske R. M., Anderson D. M., and Zahrt T. C. (2011) Components of the Rv0081-Rv0088 locus, which encodes a predicted formate hydrogenlyase complex, are coregulated by Rv0081, MprA, and DosR in Mycobacterium tuberculosis. J. Bacteriol. 193, 5105–5118 10.1128/JB.05562-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bretl D. J., He H., Demetriadou C., White M. J., Penoske R. M., Salzman N. H., and Zahrt T. C. (2012) MprA and DosR coregulate a Mycobacterium tuberculosis virulence operon encoding Rv1813c and Rv1812c. Infect. Immun. 80, 3018–3033 10.1128/IAI.00520-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malhotra V., Agrawal R., Duncan T. R., Saini D. K., and Clark-Curtiss J. E. (2015) Mycobacterium tuberculosis response regulators, DevR and NarL, interact in vivo and co-regulate gene expression during aerobic nitrate metabolism. J. Biol. Chem. 290, 8294–8309 10.1074/jbc.M114.591800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gonzalo-Asensio J., Mostowy S., Harders-Westerveen J., Huygen K., Hernández-Pando R., Thole J., Behr M., Gicquel B., and Martín C. (2008) PhoP: a missing piece in the intricate puzzle of Mycobacterium tuberculosis virulence. PLoS One 3, e3496 10.1371/journal.pone.0003496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reed M. B., Gagneux S., Deriemer K., Small P. M., and Barry C. E. 3rd. (2007) The W-Beijing lineage of Mycobacterium tuberculosis overproduces triglycerides and has the DosR dormancy regulon constitutively upregulated. J. Bacteriol. 189, 2583–2589 10.1128/JB.01670-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malhotra V., Tyagi J. S., and Clark-Curtiss J. E. (2009) DevR-mediated adaptive response in Mycobacterium tuberculosis H37Ra: links to asparagine metabolism. Tuberculosis 89, 169–174 10.1016/j.tube.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fallow A., Domenech P., and Reed M. B. (2010) Strains of the East Asian (W/Beijing) lineage of Mycobacterium tuberculosis are DosS/DosT-DosR two-component regulatory system natural mutants. J. Bacteriol. 192, 2228–2238 10.1128/JB.01597-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Domenech P., Zou J., Averback A., Syed N., Curtis D., Donato S., and Reed M. B. (2017) Unique regulation of the DosR regulon in the Beijing lineage of Mycobacterium tuberculosis. J. Bacteriol. 199, e00696–16 10.1128/JB.00696-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dasgupta N., Kapur V., Singh K. K., Das T. K., Sachdeva S., Jyothisri K., and Tyagi J. S. (2000) Characterization of a two-component system, devR-devS, of Mycobacterium tuberculosis. Tuber. Lung Dis. 80, 141–159 10.1054/tuld.2000.0240 [DOI] [PubMed] [Google Scholar]
- 29. Kinger A. K., and Tyagi J. S. (1993) Identification and cloning of genes differentially expressed in the virulent strain of Mycobacterium tuberculosis. Gene 131, 113–117 10.1016/0378-1119(93)90678-V [DOI] [PubMed] [Google Scholar]
- 30. Das A. K., Pathak A., Sinha A., Datt M., Singh B., Karthikeyan S., and Sarkar D. (2010) A single-amino-acid substitution in the C terminus of PhoP determines DNA-binding specificity of the virulence-associated response regulator from Mycobacterium tuberculosis. J. Mol. Biol. 398, 647–656 10.1016/j.jmb.2010.03.056 [DOI] [PubMed] [Google Scholar]
- 31. Gonzalo-Asensio J., Malaga W., Pawlik A., Astarie-Dequeker C., Passemar C., Moreau F., Laval F., Daffé M., Martin C., Brosch R., and Guilhot C. (2014) Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proc. Natl. Acad. Sci. U.S.A. 111, 11491–11496 10.1073/pnas.1406693111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee J. S., Krause R., Schreiber J., Mollenkopf H. J., Kowall J., Stein R., Jeon B. Y., Kwak J. Y., Song M. K., Patron J. P., Jorg S., Roh K., Cho S. N., and Kaufmann S. H. (2008) Mutation in the transcriptional regulator PhoP contributes to avirulence of Mycobacterium tuberculosis H37Ra strain. Cell Host Microbe 3, 97–103 10.1016/j.chom.2008.01.002 [DOI] [PubMed] [Google Scholar]
- 33. Zheng H., Lu L., Wang B., Pu S., Zhang X., Zhu G., Shi W., Zhang L., Wang H., Wang S., Zhao G., and Zhang Y. (2008) Genetic basis of virulence attenuation revealed by comparative genomic analysis of Mycobacterium tuberculosis strain H37Ra versus H37Rv. PLoS One 3, e2375 10.1371/journal.pone.0002375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pérez E., Samper S., Bordas Y., Guilhot C., Gicquel B., and Martín C. (2001) An essential role for phoP in Mycobacterium tuberculosis virulence. Mol. Microbiol. 41, 179–187 10.1046/j.1365-2958.2001.02500.x [DOI] [PubMed] [Google Scholar]
- 35. Chauhan S., Sharma D., Singh A., Surolia A., and Tyagi J. S. (2011) Comprehensive insights into Mycobacterium tuberculosis DevR (DosR) regulon activation switch. Nucleic Acids Res. 39, 7400–7414 10.1093/nar/gkr375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun R., Converse P. J., Ko C., Tyagi S., Morrison N. E., and Bishai W. R. (2004) Mycobacterium tuberculosis ECF σ factor sigC is required for lethality in mice and for the conditional expression of a defined gene set. Mol. Microbiol. 52, 25–38 10.1111/j.1365-2958.2003.03958.x [DOI] [PubMed] [Google Scholar]
- 37. Roberts D. M., Liao R. P., Wisedchaisri G., Hol W. G., and Sherman D. R. (2004) Two sensor kinases contribute to the hypoxic response of Mycobacterium tuberculosis. J. Biol. Chem. 279, 23082–23087 10.1074/jbc.M401230200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fol M., Chauhan A., Nair N. K., Maloney E., Moomey M., Jagannath C., Madiraju M. V., and Rajagopalan M. (2006) Modulation of Mycobacterium tuberculosis proliferation by MtrA, an essential two-component response regulator. Mol. Microbiol. 60, 643–657 10.1111/j.1365-2958.2006.05137.x [DOI] [PubMed] [Google Scholar]
- 39. Galagan J. E., Minch K., Peterson M., Lyubetskaya A., Azizi E., Sweet L., Gomes A., Rustad T., Dolganov G., Glotova I., Abeel T., Mahwinney C., Kennedy A. D., Allard R., Brabant W., et al. (2013) The Mycobacterium tuberculosis regulatory network and hypoxia. Nature 499, 178–183 10.1038/nature12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chauhan S., and Tyagi J. S. (2008) Cooperative binding of phosphorylated DevR to upstream sites is necessary and sufficient for activation of the Rv3134c-devRS operon in Mycobacterium tuberculosis: implication in the induction of DevR target genes. J. Bacteriol. 190, 4301–4312 10.1128/JB.01308-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bagchi G., Chauhan S., Sharma D., and Tyagi J. S. (2005) Transcription and autoregulation of the Rv3134c-devR-devS operon of Mycobacterium tuberculosis. Microbiology 151, 4045–4053 10.1099/mic.0.28333-0 [DOI] [PubMed] [Google Scholar]
- 42. Gautam U. S., Sikri K., Vashist A., Singh V., and Tyagi J. S. (2014) Essentiality of DevR/DosR interaction with SigA for the dormancy survival program in Mycobacterium tuberculosis. J. Bacteriol. 196, 790–799 10.1128/JB.01270-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vashist A., Prithvi Raj D., Gupta U. D., Bhat R., and Tyagi J. S. (2016) The α10 helix of DevR, the Mycobacterium tuberculosis dormancy response regulator, regulates its DNA binding and activity. FEBS J. 283, 1286–1299 10.1111/febs.13664 [DOI] [PubMed] [Google Scholar]
- 44. Sinha A., Gupta S., Bhutani S., Pathak A., and Sarkar D. (2008) PhoP-PhoP interaction at adjacent PhoP binding sites is influenced by protein phosphorylation. J. Bacteriol. 190, 1317–1328 10.1128/JB.01074-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chesne-Seck M. L., Barilone N., Boudou F., Gonzalo Asensio J., Kolattukudy P. E., Martín C., Cole S. T., Gicquel B., Gopaul D. N., and Jackson M. (2008) A point mutation in the two-component regulator PhoP-PhoR accounts for the absence of polyketide-derived acyltrehaloses but not that of phthiocerol dimycocerosates in Mycobacterium tuberculosis H37Ra. J. Bacteriol. 190, 1329–1334 10.1128/JB.01465-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singh A., Mai D., Kumar A., and Steyn A. J. (2006) Dissecting virulence pathways of Mycobacterium tuberculosis through protein-protein association. Proc. Natl. Acad. Sci. U.S.A. 103, 11346–11351 10.1073/pnas.0602817103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sharma S., and Tyagi J. S. (2016) Mycobacterium tuberculosis DevR/DosR dormancy regulator activation mechanism: dispensability of phosphorylation, cooperativity and essentiality of α10 helix. PLoS One 11, e0160723 10.1371/journal.pone.0160723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Minch K., Rustad T., and Sherman D. R. (2012) Mycobacterium tuberculosis growth following aerobic expression of the DosR regulon. PLoS One 7, e35935 10.1371/journal.pone.0035935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Flores-Valdez M., Freches D., Bruffaerts N., Romano M., Schoolnik G., Dolganov G., and Huygen K. (2015) Overexpression of DosR in Mycobacterium tuberculosis does not affect aerobic replication in vitro or in murine macrophages. Ann. Microbiol. 65, 713–720 10.1007/s13213-014-0910-3 [DOI] [Google Scholar]
- 50. Wang S., Engohang-Ndong J., and Smith I. (2007) Structure of the DNA-binding domain of the response regulator PhoP from Mycobacterium tuberculosis. Biochemistry 46, 14751–14761 10.1021/bi700970a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bansal R., Anil Kumar V., Sevalkar R. R., Singh P. R., and Sarkar D. (2017) Mycobacterium tuberculosis virulence-regulator PhoP interacts with alternative σ factor SigE during acid-stress response. Mol. Microbiol. 104, 400–411 10.1111/mmi.13635 [DOI] [PubMed] [Google Scholar]
- 52. Al-Bassam M. M., Bibb M. J., Bush M. J., Chandra G., and Buttner M. J. (2014) Response regulator heterodimer formation controls a key stage in Streptomyces development. PLoS Genet. 10, e1004554 10.1371/journal.pgen.1004554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gao R., Tao Y., and Stock A. M. (2008) System-level mapping of Escherichia coli response regulator dimerization with FRET hybrids. Mol. Microbiol. 69, 1358–1372 10.1111/j.1365-2958.2008.06355.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Salscheider S. L., Jahn A., and Schnetz K. (2014) Transcriptional regulation by BglJ-RcsB, a pleiotropic heteromeric activator in Escherichia coli. Nucleic Acids Res. 42, 2999–3008 10.1093/nar/gkt1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pannen D., Fabisch M., Gausling L., and Schnetz K. (2016) Interaction of the RcsB response regulator with auxiliary transcription regulators in Escherichia coli. J. Biol. Chem. 291, 2357–2370 10.1074/jbc.M115.696815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen Y., Birck C., Samama J. P., and Hulett F. M. (2003) Residue R113 is essential for PhoP dimerization and function: a residue buried in the asymmetric PhoP dimer interface determined in the PhoPN three-dimensional crystal structure. J. Bacteriol. 185, 262–273 10.1128/JB.185.1.262-273.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kaur K. (2011) Gene regulation mediated by DevR-DevS Two-component system of Mycobacterium tuberculosis. Ph.D. thesis, All India Institute of Medical Sciences, New Delhi, India. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.