In Clostridium difficile, the signaling molecule c-di-GMP regulates multiple processes affecting its ability to cause disease, including swimming and surface motility, biofilm formation, toxin production, and intestinal colonization. In this study, we used RNA-seq to define the transcriptional regulon of c-di-GMP in C. difficile. Many new targets of c-di-GMP regulation were identified, including multiple putative colonization factors. Transcriptional analyses revealed a prominent role for riboswitches in c-di-GMP signaling. Only a subset of the 16 previously predicted c-di-GMP riboswitches were functional in vivo and displayed potential variability in their response kinetics to c-di-GMP. This work underscores the importance of studying c-di-GMP riboswitches in a relevant biological context and highlights the role of the riboswitches in controlling gene expression in C. difficile.
KEYWORDS: Clostridium difficile, biofilms, c-di-GMP, cyclic diguanylate, flagellar motility, riboswitch
ABSTRACT
The intracellular signaling molecule cyclic diguanylate (c-di-GMP) regulates many processes in bacteria, with a central role in controlling the switch between motile and nonmotile lifestyles. Recent work has shown that in Clostridium difficile (also called Clostridioides difficile), c-di-GMP regulates swimming and surface motility, biofilm formation, toxin production, and intestinal colonization. In this study, we determined the transcriptional regulon of c-di-GMP in C. difficile, employing overexpression of a diguanylate cyclase gene to artificially manipulate intracellular c-di-GMP. Consistent with prior work, c-di-GMP regulated the expression of genes involved in swimming and surface motility. c-di-GMP also affected the expression of multiple genes encoding cell envelope proteins, several of which affected biofilm formation in vitro. A substantial proportion of the c-di-GMP regulon appears to be controlled either directly or indirectly via riboswitches. We confirmed the functionality of 11 c-di-GMP riboswitches, demonstrating their effects on downstream gene expression independent of the upstream promoters. The class I riboswitches uniformly functioned as “off” switches in response to c-di-GMP, while class II riboswitches acted as “on” switches. Transcriptional analyses of genes 3′ of c-di-GMP riboswitches over a broad range of c-di-GMP levels showed that relatively modest changes in c-di-GMP levels are capable of altering gene transcription, with concomitant effects on microbial behavior. This work expands the known c-di-GMP signaling network in C. difficile and emphasizes the role of the riboswitches in controlling known and putative virulence factors in C. difficile.
IMPORTANCE In Clostridium difficile, the signaling molecule c-di-GMP regulates multiple processes affecting its ability to cause disease, including swimming and surface motility, biofilm formation, toxin production, and intestinal colonization. In this study, we used RNA-seq to define the transcriptional regulon of c-di-GMP in C. difficile. Many new targets of c-di-GMP regulation were identified, including multiple putative colonization factors. Transcriptional analyses revealed a prominent role for riboswitches in c-di-GMP signaling. Only a subset of the 16 previously predicted c-di-GMP riboswitches were functional in vivo and displayed potential variability in their response kinetics to c-di-GMP. This work underscores the importance of studying c-di-GMP riboswitches in a relevant biological context and highlights the role of the riboswitches in controlling gene expression in C. difficile.
INTRODUCTION
Cyclic diguanylate (c-di-GMP) is nearly ubiquitous in bacteria and well known to regulate a variety of processes, particularly the switch between motile and sessile states (1–4). In some pathogens, c-di-GMP also controls virulence factor production (5–10). In response to largely undefined extracellular cues, the intracellular c-di-GMP concentration is modulated through the opposing activities of diguanylate cyclases (DGCs) and phosphodiesterases (PDEs). These enzymes may be controlled at the transcriptional and posttranslational level to adjust intracellular c-di-GMP. Many bacteria encode multiple DGCs and PDEs leading to complex c-di-GMP signaling networks. Changes in intracellular c-di-GMP are sensed by specific receptors that respond with changes to their activity or function. Multiple protein receptors of c-di-GMP receptors have been identified, including PilZ domains, diguanylate cyclases containing I-sites, GIL proteins, MshEN domains, and the Cle subfamily of CheY proteins (11–16). c-di-GMP can also bind to certain transcription factors and alter their activity (17–19). In addition to these protein receptors, c-di-GMP can bind to two distinct RNA structures, the class I (GEMM) and class II c-di-GMP riboswitches, to carry out its regulatory function (20, 21). Riboswitches are found in the 5′ untranslated region (UTR) of some transcripts. Binding of a ligand to the riboswitch alters the secondary structure of the RNA to promote or inhibit transcript termination, mRNA stability, or translation initiation (22, 23). Characterization of gene regulation by c-di-GMP riboswitches has largely relied on experiments using purified c-di-GMP and transcripts generated through in vitro transcription or using heterologous hosts (20, 25). However, little work has been done to directly test the role of c-di-GMP riboswitches in their native genetic contexts in vivo.
Clostridium difficile is an obligate anaerobe capable of causing intestinal disease ranging from mild diarrhea to potentially fatal pseudomembranous colitis and toxic megacolon. Recent work suggests that c-di-GMP plays a key role in controlling the production of surface structures involved in host colonization by C. difficile. For example, C. difficile produces peritrichous flagella that are involved in intestinal colonization, and the flagellum itself may serve as an adhesin (26–28). The expression of flagellar genes, flagellum biosynthesis, and motility are negatively regulated by c-di-GMP (29). C. difficile also produces type IV pili (TFP) that participate in autoaggregation, biofilm formation, adherence to epithelial cells, and persistence of C. difficile in the mammalian intestine (30–32). The production of TFP is positively regulated by c-di-GMP, also at the level of transcriptional regulation (30). In addition, two sortase-dependent surface proteins, encoded by CD630_28310 and CD630_32460, are positively regulated by c-di-GMP (21, 33), although the contribution of these proteins to adherence and host colonization has not been reported. Interestingly, the ZmpI zinc-dependent metalloprotease, which cleaves the CD630_28310 and CD630_32460 proteins, is negatively regulated by c-di-GMP (33).
C. difficile strain 630 encodes 37 proteins with putative or demonstrated DGC or PDE activity (25, 29, 34). Additionally, C. difficile 630 encodes 16 predicted class I and class II c-di-GMP sensing riboswitches, more than any sequenced bacterial genome outside a few deltaproteobacteria, suggesting an important role for c-di-GMP signaling through riboswitches in this organism (2, 20, 21). The presence of truncated RNAs corresponding to 7 of the c-di-GMP riboswitches has been confirmed by Northern blotting (35). Notably, each of the loci described above, CD630_28310, CD630_32460, zmpI, and pilA1 (TFP major pilin gene), and the flgB operon (flagellar genes) are preceded by a c-di-GMP riboswitch (20, 21). Regulation of pilA1 expression has been demonstrated to occur through the Cdi-2-4 riboswitch in the 5′ UTR of the transcript (30–32). In the presence of c-di-GMP, the Cdi-2-4 riboswitch assumes a secondary structure that allows transcription read-through and expression of the downstream TFP genes, consistent with the observed positive regulation of pilus gene expression and TFP production by c-di-GMP in vivo (30, 31). Conversely, c-di-GMP binding to the Cdi-1-3 riboswitch in the 5′ UTR of the flgB operon mRNA causes transcript termination, leading to a decrease in the transcription of flgB and other genes in the early-stage flagellar operon (20, 29, 35).
The processes regulated by c-di-GMP in C. difficile identified to date have been identified based on the presence of putative c-di-GMP riboswitches upstream of the relevant loci, and studies have specifically focused on those loci affecting measurable phenotypes. Yet evidence suggests that additional c-di-GMP-regulated factors remain to be identified. For example, the adherence behaviors that occur in C. difficile in response to elevated c-di-GMP can be attributed only in part to regulation of TFP and flagellar biosynthesis (29–32). Inactivation of TFP genes reduces, but does not eliminate, biofilm formation in response to c-di-GMP in C. difficile 630Δerm (31). Similarly, c-di-GMP-mediated increases in adherence to epithelial cells in vitro can be only partly explained by inhibition of flagellum biosynthesis (27, 32). The broader role of c-di-GMP signaling in C. difficile has not been determined.
In this study, we used RNA-seq to identify additional factors regulated by c-di-GMP in C. difficile. The analysis took advantage of a previously developed strategy for artificial manipulation of c-di-GMP in C. difficile, which allowed us to compare the transcriptomes of C. difficile strain 630Δerm with elevated intracellular c-di-GMP concentrations and basal c-di-GMP levels. Transcriptional analyses demonstrated a prominent role for riboswitches in the c-di-GMP signaling system of C. difficile and allowed us to examine the in vivo functionality of the 16 predicted c-di-GMP riboswitches in this organism. Eleven showed an ability to regulate gene expression in response to c-di-GMP, with class I riboswitches functioning as “off” switches and class II riboswitches functioning as “on” switches. Differences in responsiveness to changes in c-di-GMP were observed, suggesting that individual riboswitches of the same class display distinct thresholds of activation. This work underscores the importance of examining c-di-GMP riboswitch function in a relevant biological context and highlights the role of the riboswitches in controlling known and putative virulence factors in C. difficile.
RESULTS
c-di-GMP controls the transcription of a large number of genes in C. difficile.
To identify the genes regulated by c-di-GMP, we used RNA-seq to compare the transcriptomes of C. difficile 630Δerm with wild-type or elevated c-di-GMP levels. C. difficile with pMC-Pcpr (vector control) served as the wild type, which we previously determined to contain low levels of c-di-GMP (near the limit of detection by UPLC-MS) (29). To increase intracellular c-di-GMP, the diguanylate cyclase gene dccA was expressed from a plasmid, pDccA, under the control of the nisin-inducible cpr promoter (29). C. difficile 630Δerm bearing pDccA or vector control was grown to mid-exponential phase with 1 μg/ml nisin, and RNA was processed for sequencing. Genes were considered to be regulated using the following criteria: changes in reads mapped per kilobase per million reads (RPKM) of >2-fold between the two conditions and P < 0.05 by Bonferroni’s correction. A complete list of the differentially expressed genes is found in Table S3 in the supplemental material. These genes were grouped according to the predicted Riley functional class of their encoded proteins (Fig. 1) (36, 37).
FIG 1.
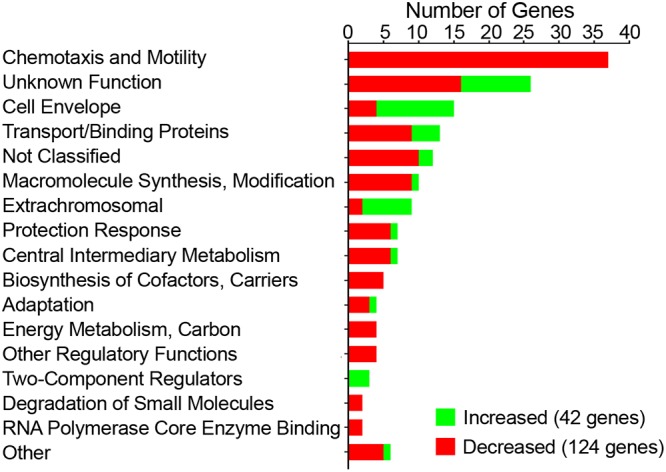
C. difficile genes regulated by c-di-GMP grouped by Riley classification of predicted gene products. Genes were included if the fold change in expression (differences in RPKM) was greater than 2-fold and P value was <0.05 after Bonferroni’s correction for multiple comparisons.
A total of 166 genes met the criteria, with 124 genes negatively regulated and 42 positively regulated by c-di-GMP (Fig. 1). The largest class of genes regulated by c-di-GMP were genes involved in chemotaxis and flagellar motility (37 total genes). These genes account for nearly 25% of the c-di-GMP-regulated genes identified. Consistent with previous work showing that c-di-GMP negatively regulates flagellar motility, all these genes showed decreased expression in response to increased c-di-GMP (pDccA strain) (Fig. 1). The next largest set of c-di-GMP-regulated genes are known or predicted to encode proteins that localize to the cell envelope. Among this set are genes involved in biosynthesis of TFP, which showed increased expression in response to c-di-GMP, consistent with previous studies (30, 32). Three genes encoding putative surface proteins, CD630_27970, CD630_28310, and CD630_32460, were also in this category, and all three genes are preceded by predicted c-di-GMP riboswitches. The third major class of c-di-GMP-regulated genes encode proteins predicted to be involved in transport/binding. Of these, seven are predicted PTS system proteins that are likely involved in importing sugars or sugar alcohols, and six are predicted components of ABC-type transporters. Across the remaining classes, several of the remaining genes are predicted to encode phage proteins, proteins involved in the transfer of mobile genetic elements, and proteins involved in iron regulation (e.g., FeoB1) or oxidative stress (e.g., Rbr1 and TrxA1).
Increased intracellular c-di-GMP moderately affects transcription of c-di-GMP metabolism genes.
Because ectopic expression of dccA greatly increases intracellular c-di-GMP, we considered that the C. difficile c-di-GMP signaling system might compensate by altering other c-di-GMP enzymes to buffer against altered c-di-GMP. To address this possibility, we examined the RNA-seq data for genes encoding the GGDEF and/or EAL domains that synthesize and degrade c-di-GMP, respectively. As expected, transcription of dccA was significantly elevated (8.6-fold) in C. difficile pDccA (Fig. S1). One other locus, CD630_07570 encoding a GGDEF-EAL domain phosphodiesterase (25, 34), showed a significant (5.1-fold) decrease in transcription, which suggests a further increase in intracellular c-di-GMP. Several other c-di-GMP metabolism genes showed modest changes in expression but did not meet the defined statistical cutoff. As a group, those containing GGDEF domains, most of which are confirmed DGCs (25, 34), showed no particular upward or downward trend in transcription in C. difficile pDccA. However, those with tandem GGDEF-EAL domains, most of which function as PDEs (25, 34), showed a trend toward increased transcription. When analyzed individually by t test, many of the genes showed statistically significant changes in expression. These results suggest that C. difficile may cumulatively buffer against increased c-di-GMP by moderately increasing the expression of multiple phosphodiesterase genes. It also remains possible that existing DGC and PDE enzymes are regulated posttranslationally in response to elevated c-di-GMP.
The effect of artificially increased c-di-GMP on GGDEF and EAL domain-encoding genes. Shown are the means and standard deviations of reads normalized per kilobase per million reads (RPKM) for genes encoding GGDEF and/or EAL domain proteins. #, at least 2-fold change and P < 0.05 after Bonferroni’s correction; *, P < 0.05 by Student’s t test comparing RPKM values in C. difficile with vector versus pDccA. Download FIG S1, PDF file, 0.3 MB (285KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genes predicted to be controlled by class I and class II riboswitches are well represented among c-di-GMP-regulated genes.
A total of 16 c-di-GMP riboswitches are predicted in C. difficile 630: twelve class I (GEMM) riboswitches and four class II riboswitches (20, 21). Of the 12 class I riboswitches, transcripts corresponding to the genes downstream of 7 were significantly altered (Table 1). Transcript abundances for six of these loci were significantly lower in C. difficile pDccA than the vector control, with fold changes ranging from −4.66 to −62.61. These results suggest that these class I riboswitches generally function as “off” switches in response to c-di-GMP. The negatively regulated loci encode flagella (Cdi-1-3), a calcium binding putative adhesin (Cdi-1-2), and the zinc-dependent metalloprotease ZmpI (Cdi-1-12) (38). The remaining three encode hypothetical proteins (Cdi-1-8, Cdi-1-9, and Cdi-1-11). In contrast, the expression of CD630_19900, the gene downstream of Cdi-1-1, was increased more than 30-fold, and this gene is the sole class I riboswitch-regulated gene positively regulated by c-di-GMP.
TABLE 1.
Changes in transcript abundance for c-di-GMP riboswitches and the downstream genes
| Riboswitcha | Chromosome region starta,b |
Fold change RS (pDccA/vector)c |
Downstream gene | Chromosome regionb | Fold change gene (pDccA/vector)d |
|---|---|---|---|---|---|
| Cdi-1-1 | (−) 2296134 | 13.44f | CD630_19900 | (−) 2295867...2296352 | 31.90 |
| Cdi-1-2 | (−) 3266578 | −15.63f | CD630_27970 | (−) 3260792...3266755 | −7.15 |
| Cdi-1-3 | (+) 308778 | −2.74 | CD630_02450 (flgB) | (+) 309272...309589 | −15.06 |
| Cdi-1-4 | (+) 3379981 | 1.02 | NDe | ||
| Cdi-1-5 | (−) 1142269 | 1.03 | ND | ||
| Cdi-1-6 | (+) 2285923 | −6.41 | ND | ||
| Cdi-1-7 | (+) 2907226 | 1.19 | ND | ||
| Cdi-1-8 | (+) 2297492 | −9.25 | CD630_19903 | (+) 2297643...2297819 | −7.78 |
| Cdi-1-9 | (+) 2671809 | −4.81 | CD630_23090 | (+) 2671951...2672127 | −4.66 |
| Cdi-1-10 | (−) 1653520 | −2.07 | ND | ||
| Cdi-1-11 | (+) 3936240 | −8.49 | CD630_33682 | (+) 3936389...3936565 | −8.90 |
| Cdi-1-12 | (−) 3303074 | −31.30 | CD630_28300 (zmpI) | (−) 3302613...3303275 | −62.61 |
| Cdi-2-1 | (−) 3801063 | 4.48 | CD630_32460 | (−) 3798299...3800482 | 4.29 |
| Cdi-2-2 | (−) 3826609 | 2.34 | CD630_32670 | (−) 3825352...3826029 | 18.78 |
| Cdi-2-3 | (−) 3306681 | 1.10 | CD630_28310 | (−) 3303646...3306564 | 42.51 |
| Cdi-2-4 | (−) 4105796 | 4.10 | CD630_35130 (pilA1) | (−) 4105120...4105635 | 11.74 |
Riboswitch naming and start sites based on predictions by Sudarsan et al. (20) and Lee et al. (21).
(+/−) indicates sense versus antisense strand.
Fold change for the riboswitch region only.
Fold change for the gene 3′ of the riboswitch.
ND, no downstream genes or transcripts detected.
Boldface and italic indicate significantly increased and decreased abundance relative to the vector control condition, respectively.
For the remaining five class I riboswitches (Cdi-1-4, Cdi-1-5, Cdi-1-6, Cdi-1-7, and Cdi-1-10), no coding sequence was detectable downstream of the riboswitch based on either the genome annotation or analysis of the reads in the regions (Table 1 and Fig. S2). Transcript reads corresponding to the riboswitch sequences themselves were detected (Fig. S2), albeit at very low levels. Transcripts for annotated genes overlapping riboswitches Cdi-1-6 and Cdi-1-10 significantly differed between C. difficile pDccA and the control. These results suggest these mRNAs are nonfunctional riboswitches or have functions other than gene regulation in cis.
Evidence of transcription corresponding to the class I riboswitches lacking a downstream open reading frame. Reads in red correspond to the sense strand; reads in green, to the antisense strand. Reads in yellow map to more than one location of the genome. Top panels of each image show reads from C. difficile 630 pDccA, and bottom panels show reads from the vector control. The genome positions are indicated at the top of each image. The annotated riboswitch is shown in blue block arrows, as are genes in the region. The number of reads can be estimated using the logarithmic scale on the left. Download FIG S2, PDF file, 0.6 MB (664.9KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The class I c-di-GMP riboswitches are generally highly conserved in C. difficile, with Cdi-1-1, Cdi-1-6, Cdi-1-8, Cdi-1-9, Cdi-1-10, Cdi-1-11, and Cdi-1-12 present in all 54 complete genomes available on NCBI (Fig. S3). Cdi-1-2 and Cdi-1-3 are absent in a subset of strains that also lack the downstream CD630_27970 and flgB genes, respectively. Notably, the nonfunctional Cdi-1-4 and Cdi-1-5 and their downstream regions appear to be duplications in C. difficile 630 and 630Δerm, and they are absent from most available C. difficile genomes (Fig. S3). Cdi-1-4 and Cdi-1-5 are most similar to Cdi-1-7, which may explain their common lack of function.
Conservation of riboswitch regions across C. difficile genomes. The conservation of c-di-GMP riboswitch sequences in the C. difficile complete genomes available through NCBI was determined using BLASTn with query sequences from C. difficile 630. Because of high sequence identity among the aptamers and some sequence duplications, ∼250 nucleotides downstream of the riboswitches were included to distinguish between them. Conservation of select c-di-GMP riboswitch-controlled genes, CD630_02450 (flgB), CD630_32460, and CD630_32670, was also examined; their respective riboswitches are noted above the locus numbers. Black squares indicate the presence of the sequence (greater than or equal to 90% identity and >97% coverage). Gray squares indicate overall conservation, but with reduced identity (80% to 90% identity and >97% coverage). White squares indicate the absence of the riboswitch. Download FIG S3, PDF file, 0.3 MB (325.6KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In contrast to the majority of class I riboswitch loci, the transcript abundances for the four genes downstream of class II riboswitches were significantly higher (4.29- to 42.51-fold) in C. difficile pDccA than in the vector control, indicating that class II riboswitches are uniformly “on” switches in C. difficile. Three of these riboswitches positively regulate known or putative adhesins in response to c-di-GMP. Cdi-2-1 regulates CD630_32460, which encodes a protein predicted to localize to the bacterial surface. Cdi-2-3 regulates CD630_28310, a putative adhesin with a predicted collagen binding domain (33). Notably, the products of these genes have been shown to be sortase-dependent proteins cleaved by the ZmpI metalloprotease that is negatively regulated by c-di-GMP through Cdi-1-12 (33, 38). Cdi-2-4 controls expression of genes encoding TFP, which are involved in adherence to epithelial cells and intestinal colonization (32). Cdi-2-3 and Cdi-2-4 and their downstream genes are highly conserved in C. difficile genomes (Fig. S3). In contrast, Cdi-2-1 and the downstream CD630_32460 are present in approximately 2/3 of the genomes. Interestingly, though CD630_32670 is highly conserved in all of the available genomes, about 1/3 lack the upstream Cdi-2-2 riboswitch, suggesting alternative modes of regulation in some strains.
To validate the RNA-seq results, we performed qRT-PCR using RNA isolated from C. difficile with vector, pDccA, or pDccAmut grown in the presence or absence of 1 µg/ml nisin. The pDccAmut vector encodes a catalytically inactive form of DccA and allows us to attribute regulation specifically to increased c-di-GMP and not another potential function of the DccA protein (29). Four out of the seven genes adjacent to class I riboswitches showed decreased transcript levels in C. difficile pDccA (Table 2). Expression of all four of the genes 3′ of the class II c-di-GMP riboswitches was significantly increased in C. difficile with pDccA compared to the vector and pDccAmut controls (Table 3). The average transcript levels for the other three genes was decreased in agreement with the RNA-seq data, but the differences did not meet statistical significance.
TABLE 2.
Fold changes in transcripts for genes controlled by class I (GEMM) riboswitches as determined by qRT-PCR
| Location and growthc |
19900,a Cdi-1-1 | 27970, Cdi-1-2 | 02450 (flgB), Cdi-1-3 |
19903, Cdi-1-8 | 23090, Cdi-1-9 | 33682, Cdi-1-11 | 28300 (zmpI), Cdi-1-12 |
|---|---|---|---|---|---|---|---|
| Vector − | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Vector + | 1.09 | 1.46 | 1.21 | 0.81 | 0.85 | 0.61 | 0.85 |
| pDccAmut − | 1.28 | 2.02 | 1.50 | 1.61 | 1.48 | 1.27 | 1.44 |
| pDccAmut + | 1.27 | 1.82 | 1.57 | 2.04 | 2.12 | 1.25 | 0.90 |
| pDccA − | 3.25b | 0.70 | 1.12 | 1.75 | 1.58 | 1.18 | 1.72 |
| pDccA + | 149.50 | 0.24 | 0.18 | 0.59 | 0.31 | 0.34 | 0.15 |
Locus tag from C. difficile 630 genome sequence (GenBank accession no. AM180355.1) and upstream riboswitch.
Bold numbers indicate values significantly different (P < 0.05) from C. difficile with vector grown without nisin, by 2-way ANOVA.
+/− indicates cultures grown with or without 1 µg/ml nisin, respectively.
TABLE 3.
Fold changes in transcripts for genes controlled by class II c-di-GMP riboswitches as determined by qRT-PCR
| Location and growthc | 32670,a Cdi-2-1 | 32460, Cdi-2-2 | 28310, Cdi-2-3 | 35130 (pilA1), Cdi-2-4 |
|---|---|---|---|---|
| Vector − | 1 | 1 | 1 | 1 |
| Vector + | 0.87 | 0.75 | 0.99 | 0.82 |
| pDccAmut − | 1.14 | 1.07 | 1.27 | 1.12 |
| pDccAmut + | 1.16 | 0.76 | 0.88 | 0.60 |
| pDccA − | 1.25 | 1.88 | 2.43 | 2.22 |
| pDccA + | 4.76b | 17.35 | 40.86 | 18.44 |
Locus tag from C. difficile 630 genome sequence (GenBank accession no. AM180355.1) and upstream riboswitch.
Bold numbers indicate values significantly different (P < 0.05) from C. difficile with vector grown without nisin, by 2-way ANOVA.
+/− indicates cultures grown with or without 1 µg/ml nisin.
Expression of genes downstream of predicted c-di-GMP riboswitches is altered in response to small changes in c-di-GMP.
The RNA-seq analysis was done following induction of dccA expression with 1 µg/ml nisin to ensure a robust increase in c-di-GMP and to maximize identification of regulated genes. However, previous studies found that this induction level increased intracellular c-di-GMP up to 2,000-fold, which is likely not a biologically meaningful change (29). We thus sought to determine the effect of more subtle changes in c-di-GMP on C. difficile gene expression. In addition, the presence of a large set of genes regulated (both positively and negatively) by c-di-GMP riboswitches provides the opportunity to examine the differences in responsiveness of this class of c-di-GMP receptor to changes in intracellular c-di-GMP. For example, differences between riboswitches of the same type may result in changes to ligand affinity, resulting in responses to different threshold concentrations of c-di-GMP (20, 39).
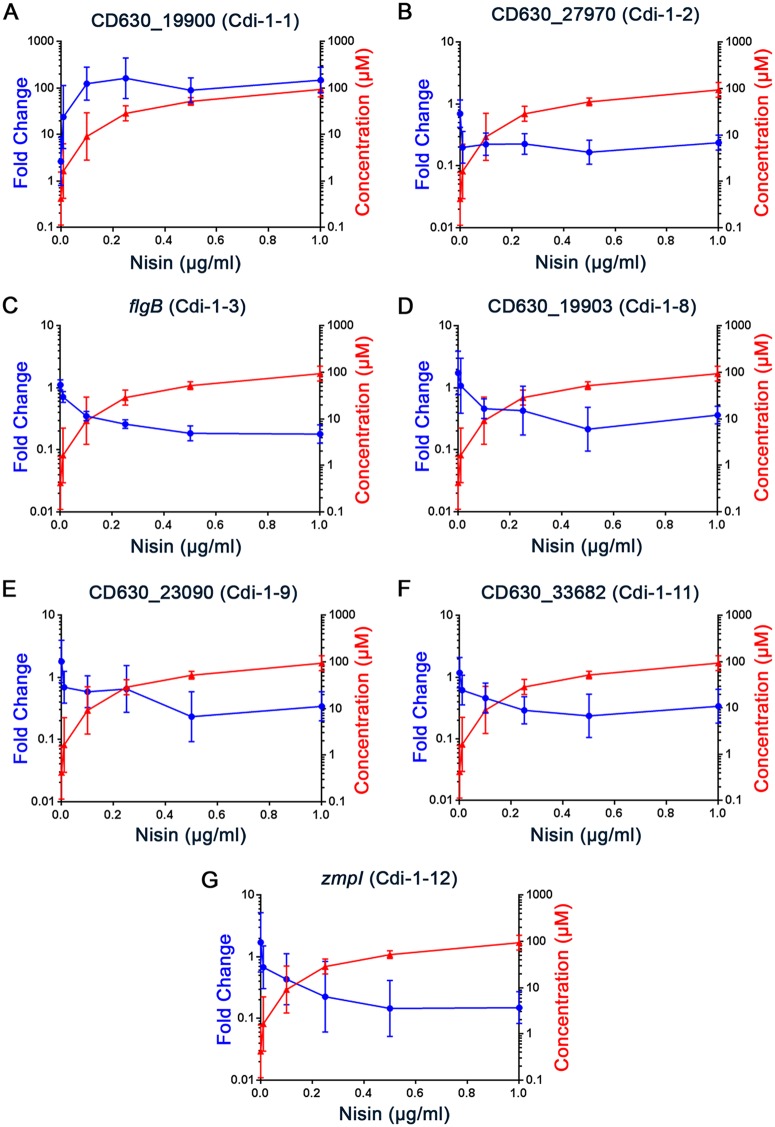
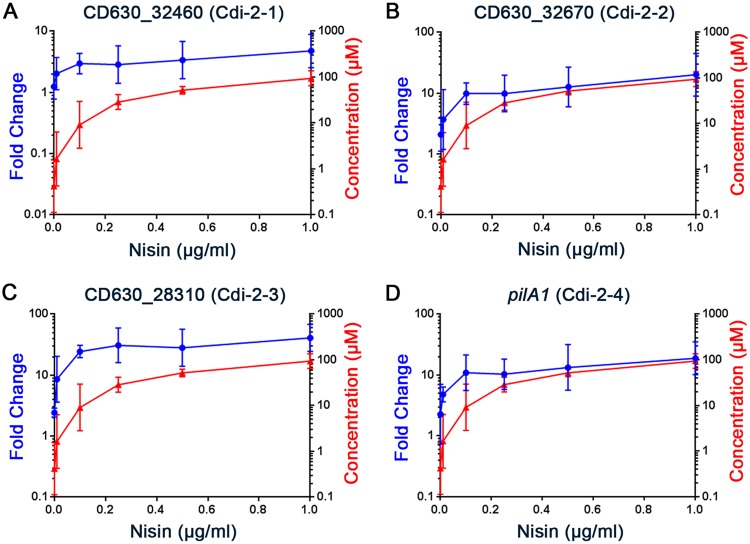
To evaluate the responsiveness of the riboswitches to c-di-GMP, we measured the transcript abundance of the gene immediately 3′ of each riboswitch in C. difficile with a range of intracellular c-di-GMP concentrations. To achieve a range of intracellular c-di-GMP concentrations, C. difficile pDccA was grown with nisin inducer in concentrations ranging from 0 to 1 μg/ml. This range of nisin concentrations resulted in a dose-dependent decrease in swimming motility of the C. difficile pDccA strain, with 0.1 µg/ml resulting in a downward trend in motility and 0.25 µg/ml significantly inhibiting motility (Fig. S4). As controls, C. difficile with vector or pDccAmut was grown with 0 or 1 µg/ml nisin. The cultures were divided, with one portion used for nucleotide extraction and measurement of intracellular c-di-GMP concentration by UPLC-MS, and the other portion used for RNA extraction and evaluation of transcript abundance by qRT-PCR. The control strains contained low intracellular c-di-GMP concentrations (131 nM ± 268 nM) regardless of nisin addition, consistent with previously reported data for C. difficile (29). Induction of dccA with increasing levels of nisin led to a dose-dependent increase in c-di-GMP, yielding a near-linear 156-fold range from 630 nM with no nisin to 99 µM at the highest nisin concentration (Fig. 2 and 3 and Fig. S5). The corresponding effects of c-di-GMP on transcript abundance for the downstream gene were expressed as the fold change compared to vector control grown without nisin. We note that the nonnormalized abundances are affected by the strength of the respective promoters and vary greatly between the loci (Fig. S6).
FIG 2.
Regulation of genes downstream of class I (GEMM) riboswitches by c-di-GMP. C. difficile with vector or pDccA was grown with a range of nisin concentrations (µg/ml). Cultures were divided for quantification of intracellular c-di-GMP concentration by LC-MS (red) or measurement of transcript abundance for the downstream open reading frame by qRT-PCR (blue). Lines and error bars represent the geometric mean and geometric standard deviation. Not shown are those for which no downstream open reading frame or transcript was identified.
FIG 3.
Genes 3′ of class II c-di-GMP riboswitches are positively regulated by c-di-GMP. C. difficile with vector or pDccA was grown with a range of nisin concentrations (µg/ml). Cultures were split for quantification of intracellular c-di-GMP concentration by LC-MS (red) or measurement of transcript abundance for the downstream open reading frame by qRT-PCR (blue). Lines and error bars represent the geometric mean and geometric standard deviation.
Dose-dependent decrease in C. difficile motility in response to c-di-GMP. C. difficile 630Δerm with pDccA or vector was assayed for motility in 0.5× BHIS-0.3% agar medium supplemented with 10 µg/ml Tm, and the indicated concentrations of nisin to induce dccA expression and c-di-GMP biosynthesis. Diameters of motility were measured after 48 hours of growth at 37°C. Shown are the means and standard deviations for 4 independent replicates. **, P < 0.01; ***, P < 0.001, by unpaired t test. Download FIG S4, PDF file, 0.2 MB (222.9KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Change in intracellular c-di-GMP in response to induction of dccA expression with nisin. C. difficile pDccA was grown with 0, 0.01, 0.1, 0.25, 0.5, or 1.0 µg/ml nisin, and then nucleotides were extracted for quantification of c-di-GMP by UPLC-MS. Data are expressed as concentration of c-di-GMP in cell volume extracted as described previously (5, 7). Shown are the means and standard errors for three independent samples. Download FIG S5, PDF file, 0.2 MB (208.5KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The effect of artificially increased c-di-GMP on riboswitch gene expression. Shown are the means and standard deviations of the reads normalized per kilobase per million reads (RPKM) for genes downstream of c-di-GMP riboswitches. All meet P < 0.05 after Bonferroni’s correction. Download FIG S6, PDF file, 0.2 MB (242.4KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
For most of the genes, transcript abundance was significantly different between C. difficile pDccA grown with 0.1 µg/ml nisin and C. difficile with vector grown without nisin (Fig. 2 and 3). These conditions correspond to a 19-fold difference in intracellular c-di-GMP, as well as a detectable decrease in swimming motility (see Fig. S4 and S5). Increasing nisin concentrations above 0.1 µg/ml generally resulted in modest additional effects on the expression of these genes, though there were differences in the range of responses to c-di-GMP. For example, the abundances of the CD630_27970, CD630_19903, CD630_23090, and CD630_33682 transcripts decreased between 0, 0.01, and 0.1 µg/ml nisin (632 nM, 2.54 µM, and 13.67 µM c-di-GMP, respectively) and then remained constant up to 1 µg/ml nisin (99 µM c-di-GMP) (Fig. 2). In contrast, the transcript abundances of flgB and zmpI continued to decrease with increasing c-di-GMP. One possible explanation for these differences is that the baseline gene expression varies, affecting the number of target RNA molecules available for saturation of binding by c-di-GMP. However, despite dissimilar expression kinetics in response to c-di-GMP, CD630_27970, flgB, and zmpI showed comparable baseline transcript levels (Fig. S6). These differences reflect potential variability in the responsiveness of class I riboswitches to c-di-GMP.
Regulation through class II c-di-GMP riboswitches similarly shows the greatest response between 632 nM (baseline), 2.54 µM, and 13.67 µM c-di-GMP (Fig. 3). CD640_32670, CD630_28310, and pilA1 increased 4.4-, 10.1-, and 3.9-fold in this range and reached 11.7-, 18.6-, and 10.8- fold increases in C. difficile with 99 µM c-di-GMP, respectively. CD630_32460 transcript abundance increased more gradually, reaching a maximum 4.9-fold increase in C. difficile with 99 µM c-di-GMP. Taken together, these results indicate that gene regulation via c-di-GMP riboswitches occurs in response to small and likely physiologically relevant changes in c-di-GMP in vivo.
Regulation of CD630_19900 expression by c-di-GMP occurs at multiple levels.
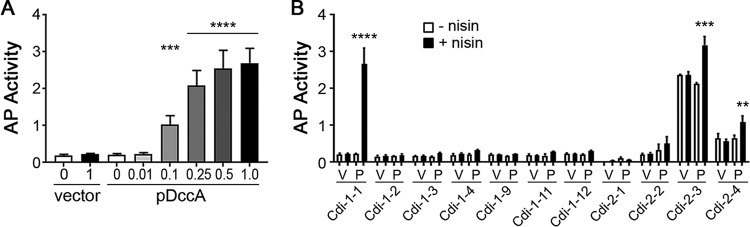
Based on RNA-seq and qRT-PCR analyses, class I c-di-GMP riboswitches in C. difficile appeared to function as “off” switches with the exception of Cdi-1-1, for which expression of the downstream CD630_19900 gene increased in response to c-di-GMP. However, recent work on the Vc2 c-di-GMP riboswitch in Vibrio cholerae demonstrated that c-di-GMP can impact expression of a gene at multiple levels (40). In the case of Vc2, positive regulation by c-di-GMP via the promoter could override inhibition of expression through Vc2, leading to an observed increase in gene expression in response to c-di-GMP. To address the possibility that c-di-GMP also regulates transcription initiation of CD630_19900, and possibly other genes, we generated transcriptional fusions of the alkaline phosphatase (AP) reporter gene phoZ to the regions encompassing each of the promoters but excluding the riboswitches. The fragments consisted of ∼200 bp encompassing the promoter (location estimated based on RNA-seq read mapping) and at most 10 bases of the 5′ end of the riboswitch sequences such that no functional riboswitch is present, allowing us to specifically determine the extent of c-di-GMP regulation via the promoters. These fusions were integrated into the C. difficile 630Δerm chromosome, and then pDccA or vector was introduced. The strains were grown with a range of nisin concentrations to induce dccA expression and c-di-GMP production, and then AP activity was assayed as previously described (41).
For the CD630_19900 promoter fusion, AP activity increased in response to nisin in a dose-dependent manner, up to a 13-fold increase in activity between the lowest and highest c-di-GMP levels tested (Fig. 4A). These results indicate that transcription initiation is positively regulated by c-di-GMP. c-di-GMP may still inhibit transcription read-through via the riboswitch, but the increased expression from the promoter is dominant under these conditions. Fusions to promoter regions for the remaining loci showed no change or up to a 2-fold change in expression at the highest concentration of c-di-GMP (Fig. 4B), suggesting that c-di-GMP does not substantially affect their transcription initiation.
FIG 4.
Alkaline phosphatase reporter assays of riboswitch-adjacent gene promoters. C. difficile 630Δerm bearing promoter-phoZ reporter fusions and either vector or pDccA was grown to mid-exponential phase with the indicated concentration of nisin (µg/ml). (A) Reporter activity for C. difficile with the Cdi-1-1 (CD630_19900) promoter fusion, grown with a range of nisin concentrations. (B) Reporter activity for C. difficile with fusions to the promoter regions upstream of the indicated riboswitches (but lacking the riboswitches themselves), grown with or without 1 µg/ml nisin (black bars and white bars, respectively). The means and standard deviations of 3 biological replicates are shown. **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001, using one-way ANOVA and Dunnett’s posttest, compared to the induced vector control for the respective reporter fusion.
c-di-GMP broadly regulates the composition of the C. difficile cell surface to influence surface interactions.
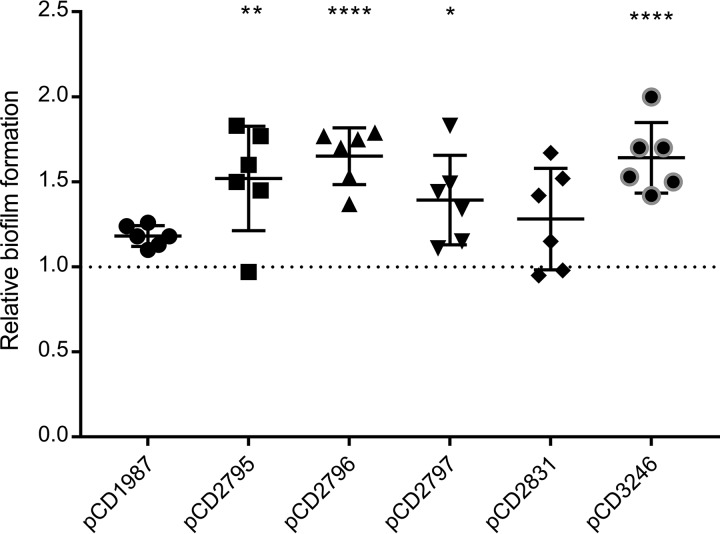
The RNA-seq analysis supported prior studies showing that c-di-GMP negatively regulates the production of flagella by inhibiting flagellar gene expression, and positively regulates type IV pilus biosynthesis by promoting TFP gene expression. In addition, c-di-GMP positively regulates CD630_28310 and CD630_32460, which encode sortase-dependent surface proteins, and inhibits expression of the ZmpI zinc-dependent metalloprotease that cleaves them (21, 33). Transcriptional analysis identified additional c-di-GMP-regulated candidate surface proteins involved in C. difficile adherence: CD630_19870, CD630_27950, CD630_27960, and CD630_27970. While the last three are adjacent on the chromosome, they are divergently transcribed and do not compose an operon. The fold changes in transcript levels for these six genes under elevated c-di-GMP conditions, based on the RNA-seq analysis, are listed in Table 4. We speculated that one or more of these proteins plays a role in biofilm formation, a process that is promoted by c-di-GMP in C. difficile (31, 35). To test this, genes encoding the individual cell envelope proteins were expressed ectopically in C. difficile from an ATc-inducible promoter (Ptet) during growth in static culture for 24 h to allow biofilm development (31). Four of the six genes (CD630_27950, CD630_27960, CD630_27970, and CD630_32460) significantly increased biofilm formation compared to the vector control (Fig. 5). The remaining two genes (CD630_19870 and CD630_28310) also increased biofilm formation somewhat, but the results did not achieve statistical significance.
TABLE 4.
Putative cell envelope proteins whose expression is regulated by c-di-GMP
| Locus | Fold changea (pDccA/vector) | Predicted function | Riboswitch upstream? |
|---|---|---|---|
| CD630_19870 | 4.32 | Cell wall protein 28 | No |
| CD630_27950 | 2.90 | Cell wall protein 11 | No |
| CD630_27960 | −4.06 | Cell wall protein 10 | No |
| CD630_27970 | −7.15 | Calcium-binding adhesion protein | Yes |
| CD630_28310 | 42.51 | Putative adhesin | Yes |
| CD630_32460 | 4.29 | Surface protein | Yes |
Boldface and italic indicate significantly increased and decreased abundance relative to the vector control, respectively, as determined using RNA-seq.
FIG 5.
Expression of genes encoding cell envelope proteins promotes biofilm formation. Genes encoding putative cell envelope proteins were expressed under the control of an ATc-inducible promoter in C. difficile 630Δerm. The strains were grown in 24-well plates in buffered BHIS containing 1% glucose and 20 ng/ml ATc to induce gene expression during biofilm development. Biofilm formation was assayed by crystal violet after 24-h incubation. Values are normalized to the induced vector control. Symbols indicate values from independent biological replicates, and bars indicate the means and standard deviations for six independent replicates. *, P < 0.05; **, P < 0.01; and ****, P < 0.0001, by 1-way ANOVA and Dunnett’s posttest, compared to the vector control.
DISCUSSION
In this work, we set out to define the transcriptional regulon of c-di-GMP in the human pathogen C. difficile. We found that c-di-GMP regulates a total of 166 genes under the growth conditions tested. While many of these genes were previously identified members of the c-di-GMP regulon in C. difficile (29, 30, 33), numerous additional c-di-GMP-regulated cellular processes were identified. Known and putative colonization factors were prominent in the regulon, consistent with a role for c-di-GMP in adherent behaviors of C. difficile (29–33).
Genes directly regulated via c-di-GMP riboswitches were highly represented, including 29 flagellar genes contained in the 23 kb flgB operon directly regulated by Cdi-1-3 and the TFP genes directly regulated by Cdi-2-4. For many of the remaining genes, the mechanism of regulation by c-di-GMP is unclear. The only predicted transcriptional regulators identified were SigD and two putative response regulators containing DNA binding domains (CD630_32650 and CD630_32670). These three regulators are encoded in operons directly controlled by c-di-GMP riboswitches and could relay changes in intracellular c-di-GMP to modulate expression of other loci. For example, SigD coordinates the indirect activation of flagellar genes carried outside the flgB operon, as well as a number of other genes involved in metabolism, membrane transport, and toxin biosynthesis (8, 29, 42). The partial overlap between the c-di-GMP regulon and the previously reported SigD regulon indicates the subset of genes controlled by the SigD sigma factor (42). The response regulators encoded by CD630_32650 and CD630_32670 likely mediate c-di-GMP regulation for additional genes. In addition, although no protein receptors of c-di-GMP have been identified in C. difficile to date, it is possible that c-di-GMP posttranslationally regulates the function of proteins that in turn modulate gene expression, as described in other species (18, 19, 43).
Of the 16 predicted c-di-GMP riboswitches in C. difficile, 11 are carried near genes on the same coding strand, positioning them to regulate the expression of these downstream genes. Consistent with this, expression of these 11 genes was altered by increasing the c-di-GMP concentration in vivo in a dose-dependent manner, with a 19-fold increase in c-di-GMP resulting in a significant change in gene expression. The normal dynamic range of c-di-GMP in C. difficile in response to extracellular stimuli is unknown, so it is unclear whether the 19-fold increase reflects a biologically meaningful change. Motility assays showed that a comparable level of dccA induction results in detectable changes in C. difficile swimming in vitro. These results indicate that even small increases in c-di-GMP are capable of altering gene expression, with consequent effects on C. difficile physiology and behavior. One limitation of our study is that it was not possible to determine quantitatively the effects of reducing c-di-GMP on gene expression, as basal c-di-GMP levels present in the control strain were already near the limit of detection. In addition, the measurements described averaged c-di-GMP concentrations and transcript levels across the population. Prior work in C. difficile and other species suggests heterogeneity in c-di-GMP levels, and concomitantly in gene expression, among individual bacteria (44–47). Thus, measurements of c-di-GMP and mRNA in bulk populations may under- or overestimate the responses of single cells.
Initial transcriptional analyses indicated that class II riboswitches are uniformly “on” switches, while all but one class I riboswitch are “off” switches in response to c-di-GMP in C. difficile. However, there are multiple examples of ligands also affecting the activity of the promoter upstream of their cognate riboswitch (39, 40, 48, 49). For example, in vivo studies of class I c-di-GMP riboswitches in Vibrio cholerae, Vc1 and Vc2, showed that c-di-GMP regulates the expression the downstream genes gbpA and tfoY at two levels: by controlling transcription initiation via the upstream promoters and via the riboswitches (39, 40). Moreover, the two control mechanisms acted in opposing directions (39, 49). Using reporter fusions to the regions upstream of the functional c-di-GMP riboswitches in C. difficile, we found that c-di-GMP controls expression of CD630_19900 via the promoter. These results may explain the observation that CD630_19900 is overall positively regulated by c-di-GMP; despite being preceded by a class I riboswitch, which presumably functions as an “off” switch, regulation via the promoter supersedes riboswitch control under the conditions tested. Thus, while in vitro transcription can be used to measure interactions between c-di-GMP and mRNA, these interactions need to be placed in the appropriate biological context before making conclusions about c-di-GMP regulation in vivo.
Of six genes encoding cell wall-localized proteins, four significantly increased in vitro biofilm formation when overexpressed. Despite negative regulation by c-di-GMP, CD630_27960 and CD960_27970 increased biofilm formation when overexpressed. We speculate that overexpression caused unexpected changes to the composition of the cell surface or overall bacterial physiology, resulting in more adherent bacteria independent of the individual gene expressed. Ongoing studies examining mutants in these genes, individually and in combination, will determine the roles of these loci in the surface behaviors of C. difficile. Several studies have described C. difficile biofilm development in vitro (50–54), and a few microbial factors that contribute to this process have been reported, including cell surface proteins, their regulators, and more (31, 35, 55–62). Some of the factors involved in biofilm formation include surface proteins regulated by c-di-GMP, including TFP, flagellin, CD630_28310, and CD630_32460 (31, 55, 56, 63). The contribution of biofilm formation to C. difficile disease remains largely speculative. In vitro biofilms are reservoirs for spores, accumulate toxins, and show increased antibiotic tolerance, suggesting that biofilms may aid in persistent colonization and disease recurrence (51, 56, 64). Recent work using animal models supports that C. difficile is present in microbial communities with biofilm-like characteristics (65, 66). Future studies examining factors required for biofilm development, including those regulated by c-di-GMP, could be used to determine the importance of biofilms to C. difficile disease.
In summary, this work expands the known c-di-GMP signaling network, demonstrates the functionality of c-di-GMP riboswitches in this organism, and highlights the role of the riboswitches in controlling known and putative virulence factors in C. difficile.
MATERIALS AND METHODS
Bacterial growth conditions.
C. difficile cultures were grown at 37°C in an atmosphere of 5% CO2, 5% H2, and 90% N2 using a Coy anaerobic chamber. Overnight cultures of C. difficile were grown in 2 to 5 ml of TY medium (30 g/liter Bacto tryptone, 20 g/liter yeast extract, 1 g/liter thioglycolate) with antibiotics as necessary for maintenance of plasmids. For experiments, overnights cultures were diluted in BHIS medium (37 g/liter brain heart infusion, 5 g/liter yeast extract). Antibiotics were used at the following concentrations: thiamphenicol (Tm), 10 μg/ml; chloramphenicol (Cm), 10 μg/ml; ampicillin (Amp), 100 μg/ml; and kanamycin (Kn), 100 μg/ml. Nisin was added at 0.01 to 2.0 µg/ml as indicated to induce expression from the cpr promoter. Strains and plasmids used in this study are described in Table S1 in the supplemental material.
Strains and plasmids used in this study. Download Table S1, PDF file, 0.7 MB (727.1KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
RNA sequencing.
Single colonies of 630Δerm bearing vector or pDccA were inoculated in TY-Tm medium and grown for ∼16 h, with 4 independent replicates per strain. Cultures were diluted 1:100 in 5 ml of BHIS-Tm and grown to an optical density at 600 nm (OD600) of 0.2. Nisin was then added to each culture at a final concentration of 1 μg/ml. Cultures were then grown to an OD600 of 1.0. Cells were collected by centrifugation at 3,000 × g for 10 min. Supernatants were removed, and RNA was extracted using TriSure (Bioline) and chloroform as described previously (29, 55). RNA was precipitated with 100% isopropanol and centrifugation at 13,000 × g for 10 min at 4°C. Supernatants were removed, and RNA pellets were washed with cold 70% ethanol and subjected to centrifugation at 13,000 × g for 10 min at 4°C. Air-dried RNA pellets were suspended in 50 μl of nuclease-free water. RNA was treated with Turbo DNA-free (Ambion) to remove DNA, and RNA integrity was checked using a Bioanalyzer assay. All RNA samples had RNA integrity numbers greater than 8. rRNA was depleted using a Ribo Zero (bacteria) rRNA removal kit (Illumina). Libraries were prepared using the TruSeq Ribo Zero Gold kit (Illumina). Samples were pooled for single-end sequencing on a Hi-Seq 2500 sequencer (Illumina). A combined 46.9 million (46.9M) 50-nt reads were obtained for vector control samples, and 63.9M 50-nt reads for pDccA samples. Base calling and demultiplexing of the data were done using Illumina bcl2fastq v.2.17.0.
RNA sequencing analysis was performed using CLC Genomic Workbench version 9.0 (Qiagen). Transcripts were mapped to the C. difficile 630 genome (AM180355.1), on which annotations for the c-di-GMP riboswitches were manually added based on the predictions by Sudarsan et al. and Lee et al. (20, 21). Reads were mapped to the reference genome with the software’s default scoring penalties for mismatch, deletion, and insertion differences from the reference genome. Transcript reads for each gene were normalized to the total number of reads and gene length (expressed as reads per kilobase of transcript, per million mapped reads [RPKM]) before calculating the fold change. Fold decreases are expressed as negative numbers. Genes were considered to be regulated by c-di-GMP if the fold change between the means of the pDccA- and vector-bearing strains was greater than 2 and P < 0.05 following Bonferroni correction for multiple comparisons.
qRT-PCR.
Cultures of C. difficile 630Δerm bearing vector, pDccA, or pDccAmut were grown as above but in a 25-ml volume of BHIS to allow splitting of the cultures for c-di-GMP quantification (see below) and quantitative reverse transcription-PCR (qRT-PCR). Cultures were grown with a range of nisin concentrations to achieve a range of dccA expression and intracellular c-di-GMP levels (29). For the vector and pDccAmut control strains, two nisin concentrations, 0 µg/ml and 1 µg/ml, were used. For the pDccA strain, 7 concentrations were used: 0, 0.01, 0.1, 0.25, 0.5, 1.0, and 2.0 µg/ml. The cultures were grown to exponential phase (OD600 of 1.0), and 3-ml samples were collected for qRT-PCR. Following RNA purification as above, cDNA was synthesized using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). A total of 10 ng of cDNA template was used in each qRT-PCR with SensiMix SYBR Green (Bioline). Primers, designated as gene-qF and qR for the forward and reverse primers, respectively, were used at a final concentration of 300 nM. All PCR primers were designed to yield comparably sized products from the first 500 bp (or less) of the gene, and all sets were determined to have >95% amplification efficiency. The data were analyzed using the 2−ΔΔCT method, with rpoC as a reference gene and normalization to the stated reference condition or strain (29, 55, 67).
Quantification of intracellular c-di-GMP.
The remaining 22 ml of the above cultures was collected as matched samples for c-di-GMP quantification. Serial dilutions were plated in BHIS agar for enumeration of CFU. Cells were then collected by centrifugation at 2,500 × g for 10 min. Supernatants were removed, and cell pellets were suspended in 1 ml PBS and transferred to a 1.5 -ml microcentrifuge tube. Samples were centrifuged at 10,000 × g, and supernatants were removed. Pellets were suspended in 200 μl of extraction buffer (40% acetonitrile, 40% methanol, 0.1 N formic acid) and placed at −20°C for 30 min. Samples were centrifuged at 12,000 × g for 5 min at 4°C, and 200-μl aliquots of the supernatant were transferred to clean tubes and immediately neutralized by adding 8 μl of 15% (wt/vol) NH4HCO3. The c-di-GMP concentration in these samples was determined by UPLC/MS as described previously (55). Intracellular c-di-GMP was calculated by normalizing measured c-di-GMP concentrations to the total cytoplasmic volume extracted, estimated using enumerated CFU in each sample, as described previously (29, 55).
Construction of plasmids for expression of genes encoding cell wall proteins.
Primers used for strain construction are listed in Table S2 in the supplemental material. The tet promoter from pRPF185 (68) was amplified by PCR using primers ATc_F + ATc_R. The PCR product was digested with the restriction enzymes EcoRI and SacI, ligated into similarly digested pMC123, and transformed into Escherichia coli DH5α. This plasmid (pRT1648) served as the vector control. The cell wall protein genes were amplified from 630Δerm genomic DNA using primers named according to LOCUSTAG_F and LOCUSTAG_R for the forward and reverse primers, respectively. The PCR products were ligated into pRT1648 using the SacI and BamHI restriction sites. These plasmids were conjugated into C. difficile 630Δerm as described previously (29).
Oligonucleotides used in this study. Download Table S2, PDF file, 0.5 MB (519.8KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genes regulated by c-di-GMP in C. difficile. Download Table S3, PDF file, 0.6 MB (619.6KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Biofilm assay.
Biofilm formation was assayed as described previously (31, 32, 55). Briefly, biofilms were grown in 1 ml modified BHIS (BHIS + 1% glucose + 50 mM sodium phosphate, pH 7.5) containing 10 µg/ml thiamphenicol and 20 ng/ml ATc. Bacteria were grown statically for 24 h at 37°C anaerobically. After 24 h, biofilm formation was measured using crystal violet staining.
Motility assay.
Swimming motility was assayed as described previously (29, 44). Briefly, 2 µl of overnight cultures were inoculated into 0.5× BHIS-0.3% agar medium supplemented with Tm10 to maintain plasmids and incubated at 37°C. Diameters of motility were measured after 24 h.
Construction of reporter fusions.
Promoter regions upstream of each riboswitch were amplified from 630Δerm genomic DNA using primers named cdiX-XpromF and cdiX-XpromR (Table S2). For example, for Cdi-1-1, the promoter was amplified using Cdi1-1promF and Cdi1-1promR. Amplified promoter regions were cloned into SalI- and SphI-digested pRT1346 (pSMB47::phoZ) (44) and then transformed into E. coli DH5α cells. Correct clones were identified by PCR and sequencing. Purified plasmids were transformed into Bacillus subtilis BS49, which contains Tn916 (69, 70). Single colonies of the BS49 strains containing the reporter fusions were used in conjugations with C. difficile 630Δerm as described previously (44, 71). This method results in semirandom integration of the reporter fusion on the C. difficile chromosome. Plasmids pMC-Pcpr (vector) and pDccA were introduced into these C. difficile strains via conjugation with E. coli HB101(pRK24) donors (29).
Alkaline phosphatase assay.
Single colonies of reporter fusion strains bearing vector or pDccA were inoculated into 2 ml TY-Tm and grown for ∼16 h. Cultures were diluted 1:100 in 3 ml of BHIS-Tm with various concentrations of nisin (0, 0.01, 0.1, 0.25, 0.5, 1.0, and 2.0 μg/ml). At mid-exponential phase (OD600 of ∼1.0), 1 ml of each culture was collected, and cells were collected by centrifugation for 1 min at 12,000 × g. Supernatants were discarded, and bacteria were stored at −20°C. Alkaline phosphatase activity was measured as described by Edwards et al. (41).
Accession number(s).
The raw and processed data files are accessible through NCBI Geo (accession number GSE120198).
ACKNOWLEDGMENTS
This work was supported by NIH award R01-AI107029 to R.T. The funding agency had no role in the design or execution of this study, the analysis or interpretation of the data, or the decision to publish the results.
The RNA libraries were made and sequencing was performed by the High Throughput Sequencing Facility at UNC.
REFERENCES
- 1.Galperin MY, Nikolskaya AN, Koonin EV. 2001. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol Lett 203:11–21. [DOI] [PubMed] [Google Scholar]
- 2.Romling U, Galperin MY, Gomelsky M. 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenal U, Reinders A, Lori C. 2017. Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol 15:271–284. doi: 10.1038/nrmicro.2016.190. [DOI] [PubMed] [Google Scholar]
- 4.Purcell EB, Tamayo R. 2016. Cyclic diguanylate signaling in Gram-positive bacteria. FEMS Microbiol Rev 40:753–773. doi: 10.1093/femsre/fuw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tischler AD, Camilli A. 2004. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol Microbiol 53:857–869. doi: 10.1111/j.1365-2958.2004.04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hisert KB, MacCoss M, Shiloh MU, Darwin KH, Singh S, Jones RA, Ehrt S, Zhang Z, Gaffney BL, Gandotra S, Holden DW, Murray D, Nathan C. 2005. A glutamate-alanine-leucine (EAL) domain protein of Salmonella controls bacterial survival in mice, antioxidant defence and killing of macrophages: role of cyclic diGMP. Mol Microbiol 56:1234–1245. doi: 10.1111/j.1365-2958.2005.04632.x. [DOI] [PubMed] [Google Scholar]
- 7.Kulasakara H, Lee V, Brencic A, Liberati N, Urbach J, Miyata S, Lee DG, Neely AN, Hyodo M, Hayakawa Y, Ausubel FM, Lory S. 2006. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3′-5′)-cyclic-GMP in virulence. Proc Natl Acad Sci U S A 103:2839–2844. doi: 10.1073/pnas.0511090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKee RW, Mangalea MR, Purcell EB, Borchardt EK, Tamayo R. 2013. The second messenger cyclic di-GMP regulates Clostridium difficile toxin production by controlling expression of sigD. J Bacteriol 195:5174–5185. doi: 10.1128/JB.00501-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L-H, Köseoğlu VK, Güvener ZT, Myers-Morales T, Reed JM, D’Orazio SEF, Miller KW, Gomelsky M. 2014. Cyclic di-GMP-dependent signaling pathways in the pathogenic Firmicute Listeria monocytogenes. PLoS Pathog 10:e1004301. doi: 10.1371/journal.ppat.1004301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen DA, Twentyman J, Hunstad DA. 2018. High levels of cyclic di-GMP in Klebsiella pneumoniae attenuate virulence in the lung. Infect Immun 86:e00647-17. doi: 10.1128/IAI.00647-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amikam D, Galperin MY. 2006. PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics 22:3–6. doi: 10.1093/bioinformatics/bti739. [DOI] [PubMed] [Google Scholar]
- 12.Christen B, Christen M, Paul R, Schmid F, Folcher M, Jenoe P, Meuwly M, Jenal U. 2006. Allosteric control of cyclic di-GMP signaling. J Biol Chem 281:32015–32024. doi: 10.1074/jbc.M603589200. [DOI] [PubMed] [Google Scholar]
- 13.Fang X, Ahmad I, Blanka A, Schottkowski M, Cimdins A, Galperin MY, Romling U, Gomelsky M. 2014. GIL, a new c-di-GMP-binding protein domain involved in regulation of cellulose synthesis in enterobacteria. Mol Microbiol 93:439–452. doi: 10.1111/mmi.12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones CJ, Utada A, Davis KR, Thongsomboon W, Sanchez DZ, Banakar V, Cegelski L, Wong GCL, Yildiz FH. 2015. C-di-GMP regulates motile to sessile transition by modulating MshA pili biogenesis and near-surface motility behavior in Vibrio cholerae. PLoS Pathog 11:e1005068. doi: 10.1371/journal.ppat.1005068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou SH, Galperin MY. 2016. Diversity of cyclic Di-GMP-binding proteins and mechanisms. J Bacteriol 198:32–46. doi: 10.1128/JB.00333-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nesper J, Hug I, Kato S, Hee CS, Habazettl JM, Manfredi P, Grzesiek S, Schirmer T, Emonet T, Jenal U. 2017. Cyclic di-GMP differentially tunes a bacterial flagellar motor through a novel class of CheY-like regulators. Elife 6:e28842. doi: 10.7554/eLife.28842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krasteva PV, Fong JC, Shikuma NJ, Beyhan S, Navarro MV, Yildiz FH, Sondermann H. 2010. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science 327:866–868. doi: 10.1126/science.1181185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava D, Harris RC, Waters CM. 2011. Integration of cyclic di-GMP and quorum sensing in the control of vpsT and aphA in Vibrio cholerae. J Bacteriol 193:6331–6341. doi: 10.1128/JB.05167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hickman JW, Harwood CS. 2008. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol Microbiol 69:376–389. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, Breaker RR. 2008. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science 321:411–413. doi: 10.1126/science.1159519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee ER, Baker JL, Weinberg Z, Sudarsan N, Breaker RR. 2010. An allosteric self-splicing ribozyme triggered by a bacterial second messenger. Science 329:845–848. doi: 10.1126/science.1190713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winkler WC, Breaker RR. 2005. Regulation of bacterial gene expression by riboswitches. Annu Rev Microbiol 59:487–517. doi: 10.1146/annurev.micro.59.030804.121336. [DOI] [PubMed] [Google Scholar]
- 23.Sherwood AV, Henkin TM. 2016. Riboswitch-mediated gene regulation: novel RNA architectures dictate gene expression responses. Annu Rev Microbiol 70:361–374. doi: 10.1146/annurev-micro-091014-104306. [DOI] [PubMed] [Google Scholar]
- 24.Reference deleted.
- 25.Gao X, Dong X, Subramanian S, Matthews PM, Cooper CA, Kearns DB, Dann CE. 2014. Engineering of Bacillus subtilis strains to allow rapid characterization of heterologous diguanylate cyclases and phosphodiesterases. Appl Environ Microbiol 80:6167–6174. doi: 10.1128/AEM.01638-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aubry A, Hussack G, Chen W, Kuolee R, Twine SM, Fulton KM, Foote S, Carrillo CD, Tanha J, Logan SM. 2012. Modulation of toxin production by the flagellar regulon in Clostridium difficile. Infect Immun 80:3521–3532. doi: 10.1128/IAI.00224-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dingle TC, Mulvey GL, Armstrong GD. 2011. Mutagenic analysis of the Clostridium difficile flagellar proteins, FliC and FliD, and their contribution to virulence in hamsters. Infect Immun 79:4061–4067. doi: 10.1128/IAI.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baban ST, Kuehne SA, Barketi-Klai A, Cartman ST, Kelly ML, Hardie KR, Kansau I, Collignon A, Minton NP. 2013. The role of flagella in Clostridium difficile pathogenesis: comparison between a non-epidemic and an epidemic strain. PLoS One 8:e73026. doi: 10.1371/journal.pone.0073026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purcell EB, McKee RW, McBride SM, Waters CM, Tamayo R. 2012. Cyclic diguanylate inversely regulates motility and aggregation in Clostridium difficile. J Bacteriol 194:3307–3316. doi: 10.1128/JB.00100-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bordeleau E, Purcell EB, Lafontaine DA, Fortier LC, Tamayo R, Burrus V. 2015. Cyclic di-GMP riboswitch-regulated type IV pili contribute to aggregation of Clostridium difficile. J Bacteriol 197:819–832. doi: 10.1128/JB.02340-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purcell EB, McKee RW, Bordeleau E, Burrus V, Tamayo R. 2016. Regulation of type IV pili contributes to surface behaviors of historical and epidemic strains of Clostridium difficile. J Bacteriol 198:565–577. doi: 10.1128/JB.00816-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKee RW, Aleksanyan N, Garrett EM, Tamayo R. 2018. Type IV pili promote Clostridium difficile adherence and persistence in a mouse model of infection. Infect Immun 86:e00943-17. doi: 10.1128/IAI.00943-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peltier J, Shaw HA, Couchman EC, Dawson LF, Yu L, Choudhary JS, Kaever V, Wren BW, Fairweather NF. 2015. Cyclic-di-GMP regulates production of sortase substrates of Clostridium difficile and their surface exposure through ZmpI protease-mediated cleavage. J Biol Chem 290:24453–24469. doi: 10.1074/jbc.M115.665091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bordeleau E, Fortier LC, Malouin F, Burrus V. 2011. c-di-GMP turn-over in Clostridium difficile is controlled by a plethora of diguanylate cyclases and phosphodiesterases. PLoS Genet 7:e1002039. doi: 10.1371/journal.pgen.1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soutourina OA, Monot M, Boudry P, Saujet L, Pichon C, Sismeiro O, Semenova E, Severinov K, Le Bouguenec C, Coppee JY, Dupuy B, Martin-Verstraete I. 2013. Genome-wide identification of regulatory RNAs in the human pathogen Clostridium difficile. PLoS Genet 9:e1003493. doi: 10.1371/journal.pgen.1003493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riley M. 1993. Functions of the gene products of Escherichia coli. Microbiol Rev 57:862–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pettit LJ, Browne HP, Yu L, Smits WK, Fagan RP, Barquist L, Martin MJ, Goulding D, Duncan SH, Flint HJ, Dougan G, Choudhary JS, Lawley TD. 2014. Functional genomics reveals that Clostridium difficile Spo0A coordinates sporulation, virulence and metabolism. BMC Genomics 15:160. doi: 10.1186/1471-2164-15-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hensbergen PJ, Klychnikov OI, Bakker D, van Winden VJ, Ras N, Kemp AC, Cordfunke RA, Dragan I, Deelder AM, Kuijper EJ, Corver J, Drijfhout JW, van Leeuwen HC. 2014. A novel secreted metalloprotease (CD2830) from Clostridium difficile cleaves specific proline sequences in LPXTG cell surface proteins. Mol Cell Proteomics 13:1231–1244. doi: 10.1074/mcp.M113.034728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kariisa AT, Weeks K, Tamayo R. 2016. The RNA domain Vc1 regulates downstream gene expression in response to cyclic diguanylate in Vibrio cholerae. PLoS One 11:e0148478. doi: 10.1371/journal.pone.0148478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pursley BR, Maiden MM, Hsieh ML, Fernandez NL, Severin GB, Waters CM. 2018. Cyclic di-GMP regulates TfoY in Vibrio cholerae to control motility by both transcriptional and posttranscriptional mechanisms. J Bacteriol 200:e00578-17. doi: 10.1128/JB.00578-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards AN, Pascual RA, Childress KO, Nawrocki KL, Woods EC, McBride SM. 2015. An alkaline phosphatase reporter for use in Clostridium difficile. Anaerobe 32:98–104. doi: 10.1016/j.anaerobe.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Meouche I, Peltier J, Monot M, Soutourina O, Pestel-Caron M, Dupuy B, Pons JL. 2013. Characterization of the SigD regulon of C. difficile and its positive control of toxin production through the regulation of tcdR. PLoS One 8:e83748. doi: 10.1371/journal.pone.0083748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shikuma NJ, Fong JC, Yildiz FH. 2012. Cellular levels and binding of c-di-GMP control subcellular localization and activity of the Vibrio cholerae transcriptional regulator VpsT. PLoS Pathog 8:e1002719. doi: 10.1371/journal.ppat.1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anjuwon-Foster BR, Tamayo R. 2017. A genetic switch controls the production of flagella and toxins in Clostridium difficile. PLoS Genet 13:e1006701. doi: 10.1371/journal.pgen.1006701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serra DO, Richter AM, Hengge R. 2013. Cellulose as an architectural element in spatially structured Escherichia coli biofilms. J Bacteriol 195:5540–5554. doi: 10.1128/JB.00946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulasekara BR, Kamischke C, Kulasekara HD, Christen M, Wiggins PA, Miller SI. 2013. c-di-GMP heterogeneity is generated by the chemotaxis machinery to regulate flagellar motility. Elife 2:e01402. doi: 10.7554/eLife.01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nair HA, Periasamy S, Yang L, Kjelleberg S, Rice SA. 2017. Real time, spatial, and temporal mapping of the distribution of c-di-GMP during biofilm development. J Biol Chem 292:477–487. doi: 10.1074/jbc.M116.746743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cromie MJ, Groisman EA. 2010. Promoter and riboswitch control of the Mg2+ transporter MgtA from Salmonella enterica. J Bacteriol 192:604–607. doi: 10.1128/JB.01239-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kariisa AT, Grube A, Tamayo R. 2015. Two nucleotide second messengers regulate the production of the Vibrio cholerae colonization factor GbpA. BMC Microbiol 15:166. doi: 10.1186/s12866-015-0506-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donelli G, Vuotto C, Cardines R, Mastrantonio P. 2012. Biofilm-growing intestinal anaerobic bacteria. FEMS Immunol Med Microbiol 65:318–325. doi: 10.1111/j.1574-695X.2012.00962.x. [DOI] [PubMed] [Google Scholar]
- 51.Dapa T, Unnikrishnan M. 2013. Biofilm formation by Clostridium difficile. Gut Microbes 4:397–402. doi: 10.4161/gmic.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crowther GS, Chilton CH, Todhunter SL, Nicholson S, Freeman J, Baines SD, Wilcox MH. 2014. Development and validation of a chemostat gut model to study both planktonic and biofilm modes of growth of Clostridium difficile and human microbiota. PLoS One 9:e88396. doi: 10.1371/journal.pone.0088396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pantaleon V, Monot M, Eckert C, Hoys S, Collignon A, Janoir C, Candela T. 2018. Clostridium difficile forms variable biofilms on abiotic surface. Anaerobe . doi: 10.1016/j.anaerobe.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Poquet I, Saujet L, Canette A, Monot M, Maihajlovic J, Ghigo JM, Soutourina O, Briandet R, Martin-Verstraete I, Dupuy B. 2018. Clostridium difficile biofilm: remodeling metabolism and cell surface to build a sparse and heterogeneously aggregated architecture. Front Microbiol 9 . doi: 10.3389/fmib.2018.02084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Purcell EB, McKee RW, Courson DS, Garrett EM, McBride SM, Cheney RE, Tamayo R. 2017. A nutrient-regulated cyclic diguanylate phosphodiesterase controls Clostridium difficile biofilm and toxin production during stationary phase. Infect Immun 85:e00347-17. doi: 10.1128/IAI.00347-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ðapa T, Leuzzi R, Ng YK, Baban ST, Adamo R, Kuehne SA, Scarselli M, Minton NP, Serruto D, Unnikrishnan M. 2013. Multiple factors modulate biofilm formation by the anaerobic pathogen Clostridium difficile. J Bacteriol 195:545–555. doi: 10.1128/JB.01980-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dawson LF, Valiente E, Faulds-Pain A, Donahue EH, Wren BW. 2012. Characterisation of Clostridium difficile biofilm formation, a role for Spo0A. PLoS One 7:e50527. doi: 10.1371/journal.pone.0050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boudry P, Gracia C, Monot M, Caillet J, Saujet L, Hajnsdorf E, Dupuy B, Martin-Verstraete I, Soutourina O. 2014. Pleiotropic role of the RNA chaperone protein Hfq in the human pathogen Clostridium difficile. J Bacteriol 196:3234–3248. doi: 10.1128/JB.01923-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pantaleon V, Soavelomandroso AP, Bouttier S, Briandet R, Roxas B, Chu M, Collignon A, Janoir C, Vedantam G, Candela T. 2015. The Clostridium difficile protease Cwp84 modulates both biofilm formation and cell-surface properties. PLoS One 10:e0124971. doi: 10.1371/journal.pone.0124971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walter BM, Cartman ST, Minton NP, Butala M, Rupnik M. 2015. The SOS response master regulator LexA is associated with sporulation, motility and biofilm formation in Clostridium difficile. PLoS One 10:e0144763. doi: 10.1371/journal.pone.0144763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maldarelli GA, Piepenbrink KH, Scott AJ, Freiberg JA, Song Y, Achermann Y, Ernst RK, Shirtliff ME, Sundberg EJ, Donnenberg MS, von Rosenvinge EC. 2016. Type IV pili promote early biofilm formation by Clostridium difficile. Pathog Dis 74:ftw061. doi: 10.1093/femspd/ftw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Girinathan BP, Ou J, Dupuy B, Govind R. 2018. Pleiotropic roles of Clostridium difficile sin locus. PLoS Pathog 14:e1006940. doi: 10.1371/journal.ppat.1006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valiente E, Bouche L, Hitchen P, Faulds-Pain A, Songane M, Dawson LF, Donahue E, Stabler RA, Panico M, Morris HR, Bajaj-Elliott M, Logan SM, Dell A, Wren BW. 2016. Role of glycosyltransferases modifying type B flagellin of emerging hypervirulent Clostridium difficile lineages and their impact on motility and biofilm formation. J Biol Chem 291:25450–25461. doi: 10.1074/jbc.M116.749523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Semenyuk EG, Laning ML, Foley J, Johnston PF, Knight KL, Gerding DN, Driks A. 2014. Spore formation and toxin production in Clostridium difficile biofilms. PLoS One 9:e87757. doi: 10.1371/journal.pone.0087757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Semenyuk EG, Poroyko VA, Johnston PF, Jones SE, Knight KL, Gerding DN, Driks A. 2015. Analysis of bacterial communities during Clostridium difficile infection in the mouse. Infect Immun 83:4383–4391. doi: 10.1128/IAI.00145-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soavelomandroso AP, Gaudin F, Hoys S, Nicolas V, Vedantam G, Janoir C, Bouttier S. 2017. Biofilm structures in a mono-associated mouse model of Clostridium difficile infection. Front Microbiol 8:2086. doi: 10.3389/fmicb.2017.02086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peltier J, Soutourina O. 2017. Identification of c-di-GMP-responsive riboswitches. Methods Mol Biol 1657:377–402. doi: 10.1007/978-1-4939-7240-1_29. [DOI] [PubMed] [Google Scholar]
- 68.Fagan RP, Fairweather NF. 2011. Clostridium difficile has two parallel and essential Sec secretion systems. J Biol Chem 286:27483–27493. doi: 10.1074/jbc.M111.263889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christie PJ, Korman RZ, Zahler SA, Adsit JC, Dunny GM. 1987. Two conjugation systems associated with Streptococcus faecalis plasmid pCF10: identification of a conjugative transposon that transfers between S. faecalis and Bacillus subtilis. J Bacteriol 169:2529–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Browne HP, Anvar SY, Frank J, Lawley TD, Roberts AP, Smits WK. 2015. Complete genome sequence of BS49 and draft genome sequence of BS34A, Bacillus subtilis strains carrying Tn916. FEMS Microbiol Lett 362:1–4. doi: 10.1093/femsle/fnu050. [DOI] [PubMed] [Google Scholar]
- 71.Bouillaut L, McBride SM, Sorg JA. 2011. Genetic manipulation of Clostridium difficile. Curr Protoc Microbiol Chapter 9:Unit 9A.2. doi: 10.1002/9780471729259.mc09a02s20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The effect of artificially increased c-di-GMP on GGDEF and EAL domain-encoding genes. Shown are the means and standard deviations of reads normalized per kilobase per million reads (RPKM) for genes encoding GGDEF and/or EAL domain proteins. #, at least 2-fold change and P < 0.05 after Bonferroni’s correction; *, P < 0.05 by Student’s t test comparing RPKM values in C. difficile with vector versus pDccA. Download FIG S1, PDF file, 0.3 MB (285KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Evidence of transcription corresponding to the class I riboswitches lacking a downstream open reading frame. Reads in red correspond to the sense strand; reads in green, to the antisense strand. Reads in yellow map to more than one location of the genome. Top panels of each image show reads from C. difficile 630 pDccA, and bottom panels show reads from the vector control. The genome positions are indicated at the top of each image. The annotated riboswitch is shown in blue block arrows, as are genes in the region. The number of reads can be estimated using the logarithmic scale on the left. Download FIG S2, PDF file, 0.6 MB (664.9KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Conservation of riboswitch regions across C. difficile genomes. The conservation of c-di-GMP riboswitch sequences in the C. difficile complete genomes available through NCBI was determined using BLASTn with query sequences from C. difficile 630. Because of high sequence identity among the aptamers and some sequence duplications, ∼250 nucleotides downstream of the riboswitches were included to distinguish between them. Conservation of select c-di-GMP riboswitch-controlled genes, CD630_02450 (flgB), CD630_32460, and CD630_32670, was also examined; their respective riboswitches are noted above the locus numbers. Black squares indicate the presence of the sequence (greater than or equal to 90% identity and >97% coverage). Gray squares indicate overall conservation, but with reduced identity (80% to 90% identity and >97% coverage). White squares indicate the absence of the riboswitch. Download FIG S3, PDF file, 0.3 MB (325.6KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Dose-dependent decrease in C. difficile motility in response to c-di-GMP. C. difficile 630Δerm with pDccA or vector was assayed for motility in 0.5× BHIS-0.3% agar medium supplemented with 10 µg/ml Tm, and the indicated concentrations of nisin to induce dccA expression and c-di-GMP biosynthesis. Diameters of motility were measured after 48 hours of growth at 37°C. Shown are the means and standard deviations for 4 independent replicates. **, P < 0.01; ***, P < 0.001, by unpaired t test. Download FIG S4, PDF file, 0.2 MB (222.9KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Change in intracellular c-di-GMP in response to induction of dccA expression with nisin. C. difficile pDccA was grown with 0, 0.01, 0.1, 0.25, 0.5, or 1.0 µg/ml nisin, and then nucleotides were extracted for quantification of c-di-GMP by UPLC-MS. Data are expressed as concentration of c-di-GMP in cell volume extracted as described previously (5, 7). Shown are the means and standard errors for three independent samples. Download FIG S5, PDF file, 0.2 MB (208.5KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The effect of artificially increased c-di-GMP on riboswitch gene expression. Shown are the means and standard deviations of the reads normalized per kilobase per million reads (RPKM) for genes downstream of c-di-GMP riboswitches. All meet P < 0.05 after Bonferroni’s correction. Download FIG S6, PDF file, 0.2 MB (242.4KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains and plasmids used in this study. Download Table S1, PDF file, 0.7 MB (727.1KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Oligonucleotides used in this study. Download Table S2, PDF file, 0.5 MB (519.8KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genes regulated by c-di-GMP in C. difficile. Download Table S3, PDF file, 0.6 MB (619.6KB, pdf) .
Copyright © 2018 McKee et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.