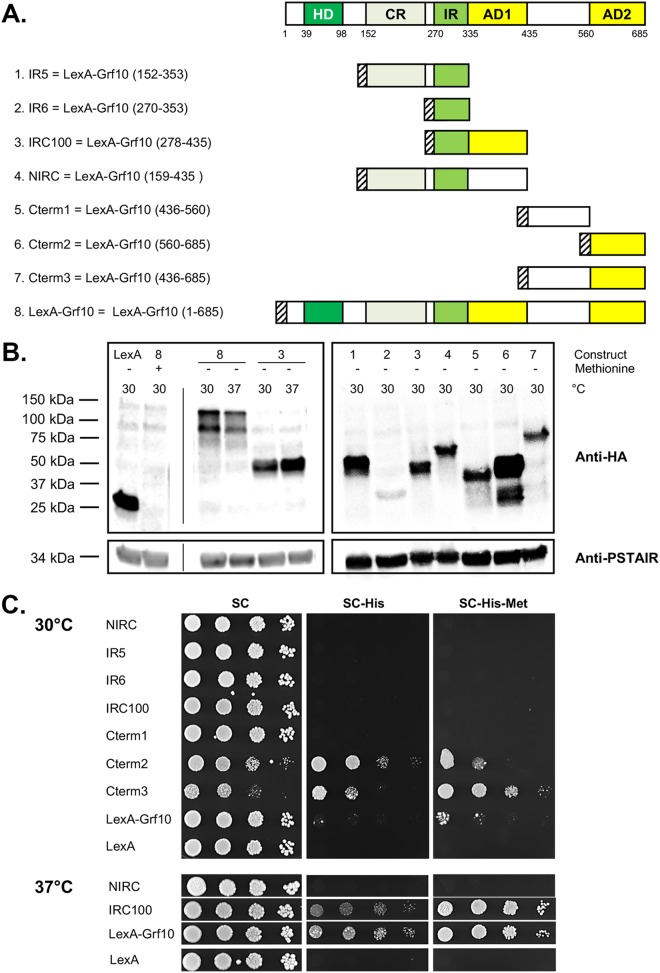
FIG 2.
Grf10 contains two activation domains. (A) Schematic (top) depicts the conserved regions (in green, see Fig. 1) and newly mapped functional domains (in yellow) of Grf10; numbers below the box indicate the amino acid number. Below, schematic representations of the Grf10 fragments cloned into pC2HB. LexA-HA is depicted as a striped box. AD1 and AD2, activation domain 1 and 2, respectively. (B) Immunoblot assay to determine protein stability. Left, LexA is the empty vector; 8 and 3 indicate the full-length LexA-Grf10 and IRC100 constructs from panel A, respectively. The presence or absence of methionine in the SC growth medium is represented by + or −, respectively, and the temperature for growth of the strains is indicated by 30 for 30°C or 37 for 37°C. The thin vertical line indicates position of lanes deleted by Photoshop to allow juxtaposition of control and experimental lanes. Experiments were performed twice, and band intensities were quantified using the ImageJ software, normalized to PSTAIRE, and averaged; a representative immunoblot is shown. Right, lanes marked 1 to 7 correspond to the same numbers in panel A to indicate the LexA fusions with portions of Grf10. Anti-HA (top) detects the LexA-Grf10 fusion proteins, and the anti-PSTAIR (bottom) was used to detect the cyclin-dependent kinase Cdc28 as a protein loading control. (C) Growth on medium lacking histidine to detect expression of the HIS1 reporter gene. Strains were grown overnight in YPD medium, diluted in sterile water (see Materials and Methods), serially diluted 1:10, and spotted on SC medium (containing histidine) or on SC-His medium with or without methionine, as indicated; the absence of methionine induces expression of LexA proteins. The plates were incubated at 30°C (top) or at 37°C (bottom) and photographed at 48 h. Representative plates are shown; at least three transformants of each construct were assayed.