Abstract
Previous studies have suggested that ethanol is a fermentation product of microflora. However, it is unknown whether this ethanol production is elevated by intake of prebiotics. Prebiotics are considered to enhance the production of short-chain fatty acids (SCFAs) as a fermentation product of beneficial bacteria. In the present study, the effect of fructooligosaccharides (FOS) consumption on intestinal ethanol levels was investigated. Rats were fed a diet with or without 10% FOS for two weeks. Consequently, FOS intake significantly increased ethanol levels per gram of ileum and cecum digesta of the rats (3.5-fold and 1.9-fold, respectively, P<0.01). The numbers of cecum Bifidobacterium (producer of ethanol and lactate) were significantly increased by FOS intake (P<0.05) and correlated with the cecum ethanol levels per gram of cecum (r=0.626, P<0.05). FOS intake also led to a significant increase in the cecum levels of SCFAs, namely lactate, propionate and n-butyrate (P<0.05). Furthermore, ethanol levels were significantly correlated with lactate levels (r=0.691, P<0.01), but not with propionate or n-butyrate levels (r=0.449 and 0.493, respectively, P>0.05). The current study, to the best of our knowledge, is the first to indicate that FOS intake significantly increases the level of intestinal ethanol. Therefore, dietary FOS may affect the intestinal health status of animals by elevating their ethanol levels, without direct ethanol consumption.
Keywords: fructooligosaccharides, prebiotics, ethanol, Bifidobacterium, intestine, short-chain fatty acids, rat
Introduction
Previous studies have suggested that ethanol is a fermentation product of intestinal microflora (1–3). Additionally, it has been reported that ethanol is produced from glucose or fructooligosaccharides (FOS) by several intestinal bacteria, including Bifidobacterium, Lactobacillus fermentum and Weissella confusa (4). However, the extent to which these bacteria are responsible for ethanol production is unknown. Furthermore, there is a lack of studies on the effects of dietary factors, such as prebiotics, on intestinal ethanol production in animals without ethanol consumption. Consumption of prebiotics including FOS and inulin has been reported to enhance the intestinal fermentation process by elevating levels of probiotics including Bifidobacterium and Lactobacillus (5). It has been reported that addition of FOS to culture medium stimulates in vitro growth of Bifidobacterium (6,7). Dietary FOS is resistant to digestion and is metabolised by the microflora in the large intestine into short-chain fatty acids (SCFAs). Increasing data suggest that prebiotics may prevent several chronic diseases, including colon cancer, inflammatory bowel disease, alcoholic steatohepatitis and diabetes, by elevating probiotic and SCFA levels (8,9).
A high intake of ethanol is harmful due to increased risk of several diseases including coronary heart disease, brain diseases and colon cancer (10). Conversely, it has been suggested that low ethanol intake is associated with lower risk of such diseases (10,11). Therefore, it is of interest to elucidate the modulation of intestinal ethanol production by dietary factors. Considering the previous findings, the present study hypothesised that FOS intake may elevate ethanol production by the intestinal microflora. Indeed, the beneficial effects of dietary prebiotics on obese phenotype, impaired gut permeability, cardiac function and hyperlipidemia are particularly prominent in animals on a high-fat (HF) diet (12,13). Therefore, in the current study, the effect of FOS intake on intestinal ethanol levels in rats fed on a HF diet was investigated.
Materials and methods
Animals and diets
A total of 16 male, specific pathogen-free Sprague-Dawley rats (3 weeks old) were purchased from Hiroshima Laboratory Animal Centre (Hiroshima, Japan) and maintained according to the Guide for the Care and Use of Laboratory Animals of Hiroshima University (Hiroshima, Japan). The study was approved by the Research Ethics Committee of Hiroshima University (approval no.: C15-12). The rats were individually housed in an air-conditioned room at 23–24°C under a 12-h light/dark cycle (lights on from 08:00 a.m. to 8:00 p.m. Following acclimatization with a non-purified commercial rodent diet (MF diet for rat and mouse; Oriental Yeast Co., Ltd., Tokyo, Japan) for 3 days, the rats (mean body weight, 68.3±0.5 g) were randomly assigned to one of two groups (n=8 rats per group) based on experimental diet. The compositions of the experimental HF diets with and without FOS supplementation are summarised in Table I. In these diets, the 30% beef tallow and 10% FOS were in accordance with previous studies (14,15). The rats were randomly assigned to one of the two diets supplemented with or without 10% FOS (w/w; Wako Pure Chemical Industries, Ltd., Osaka, Japan). Equal quantities of the experimental diets were incorporated daily into food cups at 17:00 (9, 10, 12, 14 and 15 g on days 1, 2–4, 5–7, 8–12 and 13–14, respectively) to prevent differences in food intake. Food intake was determined from the food consumed each day until the next day's food was served. The weight of spilled food was recorded daily and appropriately incorporated into calculations of food intake. At the end of the feeding period, the rats were sacrificed by decapitation following brief exposure to 3–4% isoflurane gas (Wako Pure Chemical Industries, Ltd., Osaka, Japan) mixed with air in an anesthesia chamber. A total of 8 ml blood was collected from the neck, and serum was separated by centrifugation at 2,000 × g for 20 min at 4°C and stored at −80°C. The intestinal digesta was immediately isolated, weighed and stored at −80°C until the subsequent analyses of ethanol, organic acid and beneficial bacteria.
Table I.
Composition of the experimental diets.
| Proportion of diet (%) | ||
|---|---|---|
| Material | Control | 10% FOS |
| Beef tallowa | 30.0 | 30.0 |
| Caseinb | 20.0 | 20.0 |
| L-Cystinea | 0.3 | 0.3 |
| Vitamin mixturec | 1.0 | 1.0 |
| Mineral mixturec | 3.5 | 3.5 |
| Cellulosec | 5.0 | 5.0 |
| Sucrosea | 20.0 | 20.0 |
| Corn starchc | 20.2 | 10.2 |
| FOSd | − | 10.0 |
10% FOS Dietary components were from
Nacalai Tesque Inc., Kyoto, Japan
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany
Hiroshima Laboratory Animal Centre, Hiroshima, Japan
Wako Pure Chemical Industries, Ltd., Osaka, Japan. The vitamin and mineral mixtures were used according to the American Institute for Nutrition formulation (AIN-93) (16). FOS, fructooligosaccharides.
Cecum and serum ethanol
To determine ethanol levels by gas chromatography, 100 mg of the intestinal digesta and 100 µl serum were transferred into individual ice-cold 0.6 N perchloric acid solutions (PCA). The cecum PCA solution was homogenized on ice (Polytron RT-MR2100; Kinematica AG, Littau-Luzern, Switzerland), and the serum PCA solution was mixed for 10 sec on a vortex-mixer (17). The extracted samples were centrifuged at 10,000 × g at 4°C for 5 min, and the supernatant was stored as 1.0 ml samples collected in 20 ml glass vials at −80°C until measurement. 1-Propanol at a final concentration of 100 ppm was included as internal standard prior to analysis. A gas-chromatograph (GC-2014; Shimadzu Corporation, Kyoto, Japan) equipped with a flame ionization detector was used for analysis. A headspace autosampler (HT2000H; ALPHA M.O.S. Japan K.K., Tokyo, Japan) was used to load the analyses. DB-WAX capillary columns (1.0 µm thickness, 30 m length, 0.53 mm internal diameter) from Agilent Technologies Japan, Ltd. (Tokyo, Japan) were applied. Samples were incubated for 30 min at 65°C in the head-space autosampler. Samples (0.5 ml) were injected into the column at a rate of 1 ml/min with the injector maintained at 200°C and detector at 250°C. An initial temperature of 40°C was maintained for 1 min and then the column oven temperature was increased from 40 to 80°C at a rate of 8°C/min and then maintained at 80°C for 1 min. The column oven temperature was increased from 20 to 180°C at a rate of 20°C/min and then maintained at 180°C for 4 min. The flow rate of the carrier gas (N2) was 1.0 ml/min (split ratio 10:1). Standard curves were linear across four different concentrations (0.00, 3.25, 6.50 and 13.00 mmol/l) of ethanol (R2>0.99), and used for the assay of ethanol levels in the samples.
Cecum microflora
For the analysis of intestinal microflora, bacterial genomic DNA was isolated from the cecum digesta using an UltraClean™ Fecal DNA extraction kit (MO BIO Laboratories; Qiagen, Inc., Valencia, CA, USA) according to the manufacturer's instructions. The purified DNA was eluted in 100 µl elution buffer (5 mM Tris-HCl, pH 8.5), and the quality and quantity of DNA were determined by measuring the absorbance at 260 and 280 nm using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific, Inc., Wilmington, DE, USA). Samples were stored at −20°C. Real-time quantitative polymerase chain reaction (qPCR) was performed to investigate the variation in the total number of bacteria, including of Bifidobacterium spp. and Lactobacillus spp. The 16S rRNA primers for Bifidobacterium spp. and Lactobacillus spp. used for qPCR have been described previously (14). The total volume of reagent mixture for each PCR was 20 µl [4.4 µl distilled water, 10 µl Master mix (Takara Bio, Inc., Otsu, Japan), 2 µl Plus solution (Takara Bio, Inc., Otsu, Japan), 0.8 µl of each forward and reverse primer and 2 µl DNA]. The reaction conditions were as follows: 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec, 55°C for 15 sec and 72°C for 30 sec. The fluorescent products were detected at the last step of each cycle. Melting curve analysis was performed following amplification to distinguish the targeted PCR product from the non-targeted PCR products. Data were analysed using the second derivative maximum method of the StepOneTM Real-time PCR software version 2.3 (Applied Biosystems; Thermo Fisher Scientific, Inc.). The copy numbers of Bifidobacterium spp. and Lactobacillus spp./µl were determined for the standard plasmid solution of these bacteria [(cut standard plasmid mixture ng/µl) × (molecules bp/1.0 × 109 ng) × (1/660 DNA length bp/plasmid)=plasmid copies/µl] (14). Real-time qPCR products were run as five 10-fold serial dilutions of each standard mixture to compare the threshold cycle number with the copy number of the target sequence and to generate standard curves for the quantification of unknown samples. Typically, standard curves were linear across five orders of magnitude (R2>0.98).
Cecum SCFAs
Cecum SCFAs were measured according to an internal standard method using high-performance liquid chromatography (HPLC; L-2130; Hitachi, Ltd., Tokyo, Japan) equipped with an Aminex HPX-87H ion exclusion column (7.8 mm internal diameter × 30 cm; Bio-Rad, Laboratories, Hercules, CA, USA) (18). Briefly, 500 mg cecum digesta was homogenised in 5 ml 50 mmol/l H2SO4 containing 10 mmol/l 2,2-dimethyl butyric acid as an internal standard and subsequently centrifuged at 17,000 × g at 2°C for 20 min. The supernatant was ultrafiltered, and the filtrate was applied to the HPLC column.
Statistical analysis
Data are expressed as the mean ± standard error of the mean. Data were analyzed by Student's t-test after statistical outliers were removed using the Smirnov-Grubbs rejection test. In addition, association of cecum ethanol levels with cecum Bifidobacterium and SCFA levels was assessed using Spearman rank correlation analysis. The data analysis was performed using Excel Statistics 2010 for Windows (Microsoft Corporation, Redmond, WA, USA). For all tests, P<0.05 was considered to indicate statistical significance.
Results
Food intake and body weight
Dietary manipulation had no significant effect on total food intake over 14 days or on final body weight (P>0.05; Table II).
Table II.
Effects of dietary FOS on the copy numbers of beneficial bacteria and levels of SCFAs in the cecum digesta of rats.
| Dietary group | ||
|---|---|---|
| Variable | Control | 10% FOS |
| Final body weight, g | 198.1±1.9 | 193.9±3.2 |
| Total food intake over 14 days, g | 225.0±0.0 | 225.0±0.0 |
| Bacteria (numbers/g digesta) | ||
| Bifidobacterium spp. (×108) | 1.4±0.5 | 5.9±1.8a* |
| Lactobacillus spp. (×109) | 11.5±3.1 | 7.5±4.7 |
| Bacteria (numbers/total digesta) | ||
| Bifidobacterium spp. (×108) | 1.3±0.3 | 31.6±9.6a* |
| Lactobacillus spp. (×1010) | 1.5±0.3 | 3.5±2.3 |
| SCFAs (µmol/g digesta) | ||
| Succinate | 36.2±7.3* | 14.8±5.5a** |
| Lactate | 15.2±4.7* | 35.8±4.6b |
| Acetate | 66.3±6.9 | 24.4±6.2b |
| Propionate | 20.1±1.7 | 33.7±4.9a |
| n-Butyrate | 15.5±1.8 | 22.3±2.1a |
| Total SCFAs | 127.2±16.9** | 123.0±13.5** |
| SCFAs (µmol/total digesta) | ||
| Succinate | 49.0±11.2* | 73.1±23.9** |
| Lactate | 19.3±4.7* | 195.2±29.4b |
| Acetate | 86.0±7.5 | 119.7±23.7 |
| Propionate | 26.3±1.9 | 189.7±34.6b |
| n-Butyrate | 20.3±2.1 | 119.7±11.5b |
| Total SCFAs | 164.7±19.4** | 656.2±66.5b** |
Values represent the means ± standard error of the mean (n=6-8). * and ** indicate studies using 6 and 7 animals, respectively, and others are the data of 8 animals; outlier data were omitted from certain studies following confirmation with the Smirnov-Grubbs rejection test.
P<0.05
P<0.01 vs. control determined by Student's t-test. FOS, fructooligosaccharides; SCFAs, short-chain fatty acids.
Intestinal and serum ethanol levels
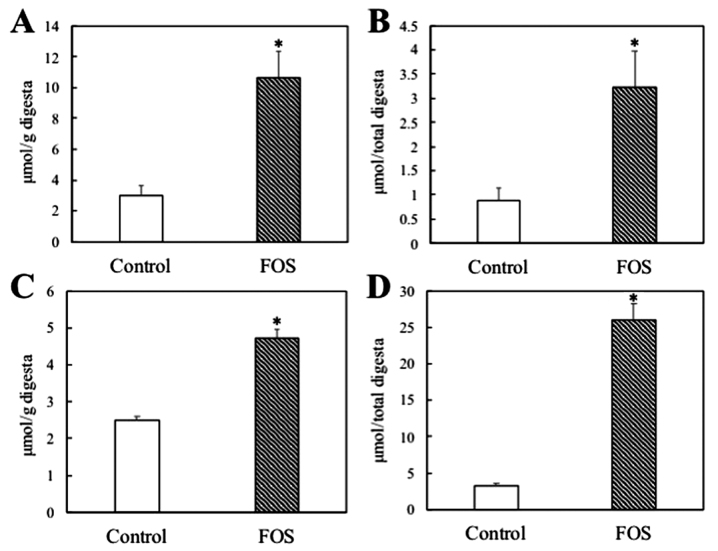
The weights of the ileum digesta were unaffected by FOS intake (control vs. FOS: 0.30±0.02 vs. 0.26±0.05 g; P>0.05). Ethanol levels per gram of ileum digesta were significantly increased 3.5-fold by FOS intake (P<0.01; Fig. 1A). In the total ileum digesta, FOS intake significantly increased ethanol levels by 3.6-fold (P<0.01; Fig. 1B). The weights of the cecum digesta were markedly increased 4.2-fold by FOS intake (control vs. FOS: 1.33±0.06 vs. 5.54±0.48 g; P<0.01). The ethanol levels per gram of cecum digesta were significantly increased 1.9-fold by FOS intake (P<0.01; Fig. 1C). In the total cecum digesta, ethanol levels were markedly increased by FOS intake by 7.9-fold (P<0.01; Fig. 1D). Serum ethanol levels were marginally but significantly increased by FOS intake (control vs. FOS: 2.63±0.05 vs. 2.84±0.07 mM; P<0.05).
Figure 1.
Effect of dietary FOS on ethanol levels in the ileum and cecum digesta of rats. (A) Εthanol levels per gram of ileum digesta; (B) ethanol levels in total ileum digesta; (C) ethanol levels per gram of cecum digesta; (D) ethanol levels in total cecum digesta. Values represent the means ± standard error of the mean (n=8). *P<0.01 vs. control group by Student's t-test. FOS, fructooligosaccharides.
Cecum bacteria and SCFAs
Compared with the control group, the copy numbers of Bifidobacterium per gram of cecum digesta were markedly increased 4.2-fold in the FOS group (P<0.05; Table II). However, the numbers of Lactobacillus per gram of cecum digesta were unaffected (P>0.05). The numbers of Bifidobacterium were positively correlated with ethanol level per gram of cecum digesta (r=0.626, P=0.017; Fig. 2).
Figure 2.
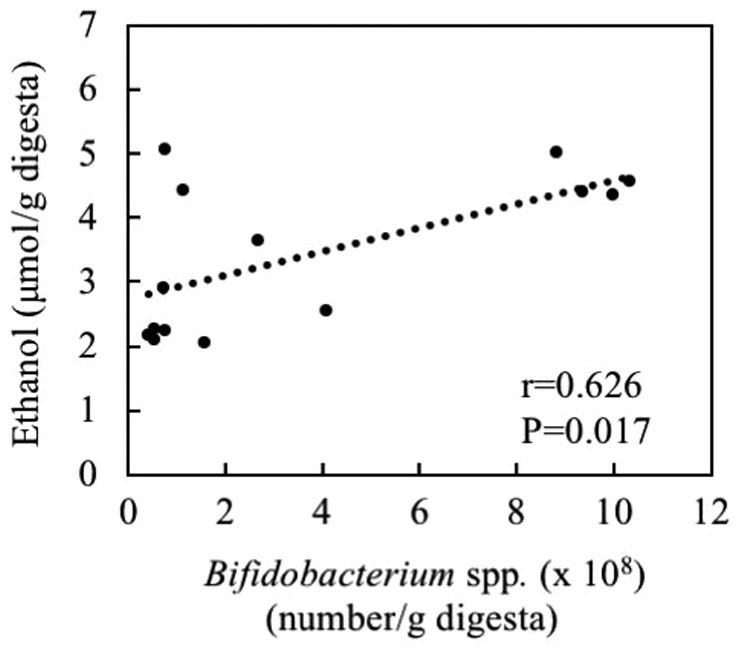
Association of ethanol levels with number of Bifidobacterium spp. per gram of cecum digesta.
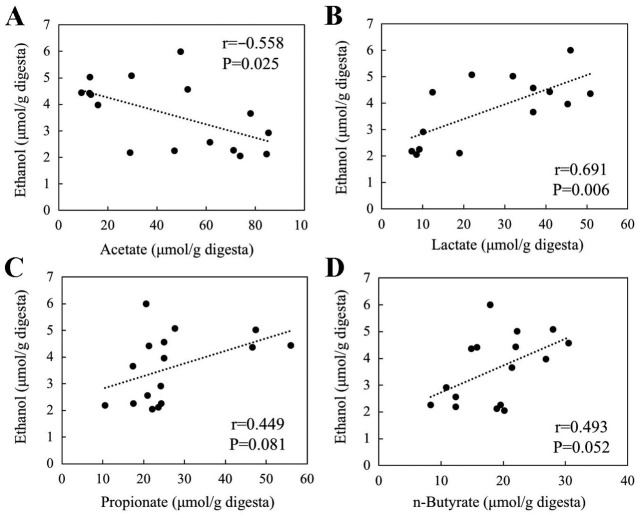
Succinate and acetate levels per gram of cecum digesta were decreased by FOS intake (2.5- and 2.7-fold decrease, respectively; P<0.05; Table II). By contrast, FOS intake significantly increased lactate, propionate and n-butyrate levels per gram of cecum digesta (2.4-, 1.7- and 1.4-fold, respectively; P<0.05; Table II). In the total cecum digesta, the levels of lactate, propionate, n-butyrate and total SCFAs were markedly increased by the FOS diet (10.3-, 7.3-, 6.0- and 4.0-fold, respectively, P<0.01). The ethanol levels per gram of cecum digesta were negatively correlated with acetate levels (r=−0.558, P=0.025; Fig. 3A) and positively correlated with lactate levels (r=0.691, P=0.006; Fig. 3B). However, ethanol levels were not significantly correlated with the level of propionate (r=0.449, P=0.081; Fig. 3C) or butyrate (r=0.493, P=0.052; Fig. 3D).
Figure 3.
Association of ethanol levels with the levels of (A) acetate, (B) lactate, (C) propionate and (D) n-butyrate per gram of cecum digesta.
Discussion
The present study demonstrated, to the best of our knowledge for the first time, that FOS intake significantly increased ethanol levels in the intestinal digesta of rats without direct consumption of ethanol. The results further indicated that the cecum levels of ethanol were associated with Bifidobacterium numbers. Previous in vitro studies have indicated that ethanol is produced by Bifidobacterium from glucose or FOS (2,3). Other in vitro studies have suggested that the addition of FOS stimulates the growth of Bifidobacterium (6,7). Therefore, the increase in intestinal ethanol levels may, at least in part, be mediated by an increased abundance of Bifidobacterium bacteria in the intestines of rats fed FOS. However, a previous study suggested ethanol is degraded to acetate by intestinal bacteria such as Bifidobacterium and Lactobacillus (19). Taken together, it may be hypothesized that Bididobacterium serves a role in the degradation of ethanol in rats following consumption of ethanol, while Bifidobacterium may stimulate the production of ethanol in rats lacking consumption of ethanol. Since the abundance of cecum Lactobacillus was unaffected by FOS in the current study, the possibility of the involvement of Lactobacillus in the mechanisms underlying the increase in intestinal ethanol was negated. At present, the possibilities of higher production and/or lower degradation of ethanol by other intestinal bacteria in rats fed FOS remains to be examined. A recent metagenome study demonstrated that FOS intake increased or decreased the abundance of a variety of bacteria in addition to Bifidobacterium in rats (20). Accordingly, further in vitro study is necessary to investigate if each of the intestinal bacteria present can produce or degrade ethanol.
Bifidobacterium is established to be a producer of lactate and ethanol (2,3). The current study also identified a significant positive correlation between the cecum levels of ethanol and lactate. It has been suggested that lactate is produced from pyruvate and that ethanol is produced from acetyl-CoA, a metabolite of pyruvate, in Bifidobacterium (2,3). Therefore, higher production of lactate may be associated with a higher production of ethanol. Further studies should be conducted to elucidate the underlying mechanisms of higher production of ethanol following elevated FOS intake.
High ethanol intake is well established to cause toxic effects and increase the risk of several diseases, including liver disease, coronary heart diseases and cancer (21). However, previous studies have suggested that low or moderate ethanol intake is associated with a lower risk of developing diseases including coronary heart disease, brain diseases and cancers (10,11). Previous experimental studies by our group have also suggested beneficial effects of a low ethanol dose on liver function and aging in rodents (22,23). Therefore, further studies are necessary to investigate whether increased ethanol levels in rats fed FOS are equivalent to the toxic or beneficial levels reported in animals, and to determine the overall ethanol production rate by intestinal microflora in rats fed FOS. In this regard, a study by Zhong et al (24) reported that rats administered a Lieber-DeCarli liquid diet mixed with 5% (w/v) ethanol for 8 weeks exhibited an elevation in cecum ethanol levels from 2–3 to 20–30 µmol/g digesta. The increased intestinal ethanol levels were associated with fatty liver disease pathology and reduced intestinal barrier function (24). Their results imply that increased levels of cecum ethanol (>20 µmol per gram of digesta) in rats fed ethanol may be harmful for the host. A more recent study using Caco-2 cells monolayers as an in vitro tight junction model indicated that the addition of 6% (v/v, 1 mmol/ml) ethanol to the culture medium decreased intestinal cell barrier function, although cell viability and lactate dehydrogenase release (cytotoxicity) were unaffected (25). The current study identified the production of 4–5 µmol ethanol per gram of cecum digesta in rats fed FOS. Thus, the levels of ethanol in FOS-fed rats appear to be lower than the harmful levels of ethanol reported (24,25). Further study is necessary to investigate whether the increased ethanol levels in the cecum of rats fed FOS are close to the beneficial levels in those administered low-dose ethanol in our previous studies (22,23).
In conclusion, the current study demonstrated that dietary FOS increased ethanol levels in the intestinal digesta of rats fed a HF diet. The results imply that FOS intake has an impact on intestinal health by increasing ethanol levels. The findings also indicate the importance of dietary prebiotics as an environmental factor in alcohol-use disorders and may provide novel insight into the link between prebiotics and diseases related to alcohol consumption.
Acknowledgements
This study was presented as part of an abstract at the 17th Global Dieticians and Nutritionists Annual Meeting October 3 2017 in Kuala Lumpur, Malaysia and published under Masahiro Yamaguchi et al in J Nutr Food Sci 7: 6, 2017.
Glossary
Abbreviations
- FOS
fructooligosaccharides
- SCFAs
short-chain fatty acids
- HF
high-fat
- qPCR
quantitative polymerase chain reaction
- HPLC
high-performance liquid chromatography
Funding
The current study was financially supported by the HIRAKU consortium, Hiroshima University.
Availability of data and materials
The analyzed data sets generated during the study are available from the correspondence author on reasonable request.
Authors' contributions
MY, YY and NK contributed to the study design, MY, YY, MA and YO acquired the data. MY and TK analyzed and interpreted the data. MY and NK wrote the manuscript and approved the contents of the manuscript. The final version of the manuscript has been read and approved by all authors.
Ethics approval and consent to participate
The study protocol was approved by the institutional ethics committee of Hiroshima University (approval no: C15-12).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: Implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119:1340–1347. doi: 10.1053/gast.2000.19267. [DOI] [PubMed] [Google Scholar]
- 2.van der Meulen R, Adriany T, Verbrugghe K, De Vuyst L. Kinetic analysis of bifidobacterial metabolism reveals a minor role for succinic acid in the regeneration of NAD+ through its growth-associated production. Appl Environ Microbiol. 2006;72:5204–5210. doi: 10.1128/AEM.00146-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979–990. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elshaghabee FM, Bockelmann W, Meske D, de Vrese M, Walte HG, Schrezenmeir J, Heller KJ. Ethanol production by selected intestinal microorganisms and lactic acid bacteria growing under different nutritional conditions. Front Microbiol. 2016;7:47–59. doi: 10.3389/fmicb.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barona M, Roy D, Vuillemard JC. Biochemical characteristics of fermented milk produced by mixed cultures of lactic starters and bifidobacteria. Lait. 2000;80:465–478. doi: 10.1051/lait:2000138. [DOI] [Google Scholar]
- 6.Sharp R, Fishbain S, Macfarlane GT. Effect of short-chain carbohydrates on human intestinal bifidobacteria and Escherichia coli in vitro. J Med Microbiol. 2001;50:152–160. doi: 10.1099/0022-1317-50-2-152. [DOI] [PubMed] [Google Scholar]
- 7.Mao B, Li D, Zhao J, Liu X, Gu Z, Chen YQ, Zhang H, Chen W. In vitro fermentation of fructooligosaccharides with human gut bacteria. Food Funct. 2015;6:947–954. doi: 10.1039/C4FO01082E. [DOI] [PubMed] [Google Scholar]
- 8.Cummings JH, Macfarlane GT, Englyst HN. Prebiotic digestion and fermentation. Am J Clin Nutr. 2001;73:415S–420S. doi: 10.1093/ajcn/73.2.415s. [DOI] [PubMed] [Google Scholar]
- 9.Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol. 2015;52:7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fekjaer HO. Alcohol - a universal preventive agent? A critical analysis. Addiction. 2013;108:2051–2057. doi: 10.1111/add.12104. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz G, Urrutia JC, Burgos CF, Silva V, Aguilar F, Sama M, Yeh HH, Opazo C, Aguayo LG. Low concentrations of ethanol protect against synaptotoxicity induced by Aβ in hippocampal neurons. Neurobiol Aging. 2015;36:845–856. doi: 10.1016/j.neurobiolaging.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton MK, Ronveaux CC, Rust BM, Newman JW, Hawley M, Barile D, Mills DA, Raybould HE. Prebiotic milk oligosaccharides prevent development of obese phenotype, impairment of gut permeability, and microbial dysbiosis in high fat-fed mice. Am J Physiol Gastrointest Liver Physiol. 2017;312:G474–G487. doi: 10.1152/ajpgi.00427.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tunapong W, Apaijai N, Yasom S, Tanajak P, Wanchai K, Chunchai T, Kerdphoo S, Eaimworawuthikul S, Thiennimitr P, Pongchaidecha A, et al. Chronic treatment with prebiotics, probiotics and synbiotics attenuated cardiac dysfunction by improving cardiac mitochondrial dysfunction in male obese insulin resistant rats. Eur J Nutr. 2018;57:2091–2104. doi: 10.1007/s00394-017-1482-3. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Iwamoto A, Kumrungsee T, Okazaki Y, Kuroda M, Yamaguchi S, Kato N. Consumption of an acid protease derived from Aspergillus oryzae causes bifidogenic effect in rats. Nutr Res. 2017;44:60–66. doi: 10.1016/j.nutres.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Coudray C, Tressol JC, Gueux E, Rayssiguier Y. Effects of inulin-type fructans of different chain length and type of branching on intestinal absorption and balance of calcium and magnesium in rats. Eur J Nutr. 2003;42:91–98. doi: 10.1007/s00394-003-0390-x. [DOI] [PubMed] [Google Scholar]
- 16.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 17.Heit C, Eriksson P, Thompson DC, Charkoftaki G, Fritz KS, Vasiliou V. Quantification of Neural Ethanol and Acetaldehyde Using Headspace GC-MS. Alcohol Clin Exp Res. 2016;40:1825–1831. doi: 10.1111/acer.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okazaki Y, Tomotake H, Tsujimoto K, Sasaki M, Kato N. Consumption of a resistant protein, sericin, elevates fecal immunoglobulin A, mucins, and cecal organic acids in rats fed a high-fat diet. J Nutr. 2011;141:1975–1981. doi: 10.3945/jn.111.144246. [DOI] [PubMed] [Google Scholar]
- 19.Nosova T, Jousimies-Somer H, Jokelainen K, Heine R, Salaspuro M. Acetaldehyde production and metabolism by human indigenous and probiotic Lactobacillus and Bifidobacterium strains. Alcohol Alcohol. 2000;35:561–568. doi: 10.1093/alcalc/35.6.561. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Kandasamy S, Zhang J, Kirby CW, Karakach T, Hafting J, Critchley AT, Evans F, Prithiviraj B, Prithiviraj B. Prebiotic effects of diet supplemented with the cultivated red seaweed Chondrus crispus or with fructo-oligo-saccharide on host immunity, colonic microbiota and gut microbial metabolites. BMC Complement Altern Med. 2015;15:279. doi: 10.1186/s12906-015-0802-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rehm J, Gmel GE, Sr, Gmel G, Hasan OSM, Imtiaz S, Popova S, Probst C, Roerecke M, Room R, Samokhvalov AV, et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction. 2017;112:968–1001. doi: 10.1111/add.13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osaki A, Okazaki Y, Kimoto A, Izu H, Kato N. Beneficial effect of a low dose of ethanol on liver function and serum urate in rats fed a high-fat diet. J Nutr Sci Vitaminol (Tokyo) 2014;60:408–412. doi: 10.3177/jnsv.60.408. [DOI] [PubMed] [Google Scholar]
- 23.Kimoto A, Izu H, Fu C, Suidasari S, Kato N. Effects of low dose of ethanol on the senescence score, brain function and gene expression in senescence-accelerated mice 8 (SAMP8) Exp Ther Med. 2017;14:1433–1440. doi: 10.3892/etm.2017.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong W, Li Q, Zhang W, Sun Q, Sun X, Zhou Z. Modulation of Intestinal Barrier and Bacterial Endotoxin Production Contributes to the Beneficial Effect of Nicotinic Acid on Alcohol-Induced Endotoxemia and Hepatic Inflammation in Rats. Biomolecules. 2015;5:2643–2658. doi: 10.3390/biom5042643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chopyk DM, Kumar P, Raeman R, Liu Y, Smith T, Anania FA. Dysregulation of junctional adhesion molecule-A contributes to ethanol-induced barrier disruption in intestinal epithelial cell monolayers. Physiol Rep. 2017;5:e13541. doi: 10.14814/phy2.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the correspondence author on reasonable request.