Abstract
Nasopharyngeal carcinoma (NPC) is a prevalent tumor that affects the head and neck. Radiation therapy is typically used to treat NPC; however, poor prognoses and distant metastases are common due to radiation resistance. The antitumor activities of trichosanthin (TCS) have been reported in several types of tumors. The aim of the present study was to investigate whether TCS may serve as a potential radiosensitizer in the treatment of NPC tumors. In the present study, NPC cells were treated with radiation alone or together with TCS and radiosensitivity was compared. Clonogenic assay, flow cytometry and an animal study were performed to assess cell death in NPC. The clonogenic assay demonstrated that TCS had a significant radiosensitizing effect on NPC cells. Western blotting indicated that phosphorylated protein kinase B and signal-regulated kinase [phosphoinositide 3-kinase (PI3K) pathway] were downregulated, and that cleaved caspase-3 was upregulated by combined treatment with TCS and radiation. Furthermore, TCS potently radiosensitized NPC xenografts in vivo. In conclusion, TCS radiosensitized NPC in vitro and in vivo via downregulation of PI3K pathways and the upregulation of cleaved caspase-3.
Keywords: trichosanthin, nasopharyngeal carcinoma tumor, radiosensitivity, phosphoinositide 3-kinase pathway
Introduction
Trichosanthin (TCS) is purified from the root tuber of Trichosanthes kirilowii (1). In China, TCS has been used historically as an abortifacient agent in early and midterm pregnancy and to treat invasive moles, ectopic pregnancies and hydatidiform moles (1,2). TCS is a typical type I ribosome-inactivating protein, which attacks eukaryotic cells ribosomes via its rRNA N-glycosidase activities (2). In recent years, the biological and pharmacological activities of TCS have been investigated (1).
A previous study has reported that TCS may serve as a potential anticancer agent via suppressing the proliferation of various cancer cell types, including melanoma, colon carcinoma, lung cancer, stomach cancer, breast cancer, hepatoma and prostate cancer (3). TCS has been demonstrated to suppress the proliferation of HeLa cells by obstructing the protein kinase C/mitogen activated protein kinase signaling pathway and increasing cytosolic Ca2+ concentrations (2,4,5). TCS also successfully reduced human choriocarcinoma cell proliferation by upregulating reactive oxygen species production (4). TCS triggers apoptosis in human lung cancer cells via anti-telomerase effects and inhibiting cell migration and metastasis, as well as inducing G1 phase arrest (5).
Nasopharyngeal carcinoma (NPC) is a prevalent head and neck malignant tumor that arises in the nasopharyngeal epithelium, with annual incidence rate of 54.7 cases per 100,000 individuals in South East Asia, Southeast China, the Middle East, Northeast Africa and Alaska (6–8). The current NPC treatment strategy primarily relies on radiotherapy (9,10). In the 1960s and 1970s, the 5-year survival rate for NPC was 0% (10). In the 2000s, however, this was successfully increased to >80% (10). Although intensity-modulated radiation therapy and concurrent chemo-radiotherapy have proved effective for regional NPC control, the prognoses of patients with distant metastasis remains poor (8). Furthermore, typical radiation doses are ~70 Gy in adults and ~60 Gy in children and adolescents, with the additional application of chemotherapy (6). Such high doses may lead to late toxicities of xerostomia, neck fibrosis, dental caries, trismus, hypopituitarism, stunted growth, hearing loss and secondary malignancies in ~70% of patients (6).
The development of radiation-sensitizing agents may decrease the effective radiation dose in NPC, reducing the incidence of negative side effects. The aim of the present study therefore was to investigate the radiation-sensitizing effects of TCS in NPC and the underlying mechanisms responsible.
Materials and methods
Reagents
TCS (98% purity) was procured from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and dissolved in dimethylsulfoxide (DMSO) to generate a stock solution. The stock solution was then further diluted with RPMI-1640 complete medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) to make the final concentration of 0.05%. Antibodies against cleaved caspase-3 were provided by Cell Signaling Technology, Inc. (Danvers, MA, USA; cat. no 9661). Phosphorylated (p)-protein kinase B (AKT; cat. no. SC101629), p-extracellular signal-regulated kinase (ERK; cat. no. SC136521) and GAPDH (cat. no. SC293335) were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The secondary antibodies utilized were horseradish peroxidase (HRP) conjugated rabbit monoclonal antibodies (cat. no. 5571) and HRP conjugated anti-mouse immunoglobulin G (cat. no. 7076), which were both provided by Cell Signaling Technology, Inc.
Cell culture
The NPC cell line SUNE-1 was obtained from the Cancer Institute and Hospital, Chinese Academy of Medical Sciences (Beijing, China) and cultured in RPMI-1640 medium containing 5% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.), 100 U/l penicillin and 100 mg/ml streptomycin under standard conditions (37°C and 5% CO2). Radiation was administrated as a single treatment or combined with TCS. Combination Index (CI) analysis was used to investigate the synergy between two therapies based on the method of Chou et al (11).
Clonogenic survival assay
A Gulmay machine (FCS320; Gulmay Incorporated, Shanghai, China) operating with 250 kVp at 0.65 Gy/min X-ray radiation was used. SUNE-1 cells were seeded at a density of 2×102 cells/well and grown in 6-well plates for 5 h (37°C, 5% CO2). Cells were divided into five groups: DMSO control, 4 Gy irradiation alone, 20 µM TCS alone, 4 Gy irradiation + 10 µM TCS or 4 Gy irradiation + 20 µM TCS. Cells were first treated with DMSO or TCS and subsequently irradiated at room temperature. Following irradiation, cells were further incubated for 24 h for colony formation. Colonies with >50 cells were taken into counted to calculate the survival fraction. The linear-quadratic model of Kaleidagraph version 3.51 (Synergy Software, Reading, PA, USA) was used to analyze survival data. Experiments were repeated a minimum of three times and the mean survival fraction was calculated.
Cell apoptosis assay and flow cytometry
Cell apoptosis was measured using an Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit (Sigma-Aldrich; Merck KGaA). SUNE-1 cells were resuspended in PBS, seeded in 6-well plates (1×106 cells/well) and incubated overnight at 37°C to allow them to attach. Cells were treated with TCS or DMSO for 24 h at 37°C, harvested, washed twice with ice-cold PBS and resuspended in 250 µl binding buffer (as provided by the kit). Cells were stained with 5 µl Annexin V-FITC and propidium iodide (PI) in the dark for 30 min at room temperature, following which they were analyzed using a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
Total cell proteins were extracted for western blot analysis. NPC cells were harvested and lysed in cell lysis buffer (Bio-Rad Laboratories, Inc., Hercules, CA, USA), including complete protease inhibitor cocktail (Roche Diagnostics, Basel, Switzerland) and phosphatase inhibitor cocktail (Sigma-Aldrich; Merck KGaA) for 2 h, followed by centrifugation at a speed of 1,000 × g) for 30 min at 4°C. Protein concentrations were determined using a bicinchoninic acid Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal quantities of protein (50 mg) were separated by 10 and 15% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Polyvinylidene difluoride membranes were blocked in 5% skimmed milk at room temperature for 1 h and incubated sequentially with the primary (1:1,000) and secondary (1:5,000) antibodies (listed in the reagents section) overnight at 4°C. Membranes were washed three times with TBS supplemented with Tween-20 (pH 7.4; 10 mM Tris-HCl, 150 mM NaCl, 0.1% Tween-20) and bands were visualized using a chemiluminescence reagent (EMD Millipore, Billerica, MA, USA) and BioMax® XAR film (Kodak, Rochester, NY, USA). ImageJ software (version 1.45; National Institutes of Health, Bethesda, MD, USA) was used to analyze band intensities.
In vivo experiments
A total of 30 female BALB/c nude mice (7-week old, 20–25 g) were supplied by Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and housed under a 12 h light/dark cycle kept at 22°C with a humidity of 60%. Mice received free access to an autoclaved laboratory rodent diet and water. All animal experiments were approved by the Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou Animal Research Ethics Committee (Zhengzhou, UK). SUNE-1 human NPC cells were suspended in 100 ml RPMI-1640 medium mixed with 100 ml Matrigel™ Matrix (BD Biosciences). To construct the xenograft tumor model, cells were inoculated subcutaneously into the right flanks of BALB/c nude mice. Once the tumors reached ~200 mm3, the mice were randomly assigned into four groups (n=6 in each). Groups received the following treatment: 50 mg/kg/day TCS via oral gavage (12), 4 Gy irradiation, 4 Gy irradiation + daily 50 mg/kg/day TCS via oral gavage or normal saline via oral gavage for 5 weeks. Tumor volumes were monitored weekly and measured in two dimensions (length and width) with calipers. Tumor volumes were calculated using the following formula: Tumor size=(length × width2)/2. After 5 weeks, mice were sacrificed and tumors were harvested and weighed.
Statistical analysis
Data were analyzed using one-way analysis of variance or Student's t-test with PRISM 5.0 (GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference. Data are presented as the mean ± standard deviation.
Results
Combined treatment with TCS and radiation reduced the survival fraction of SUNE-1 cells
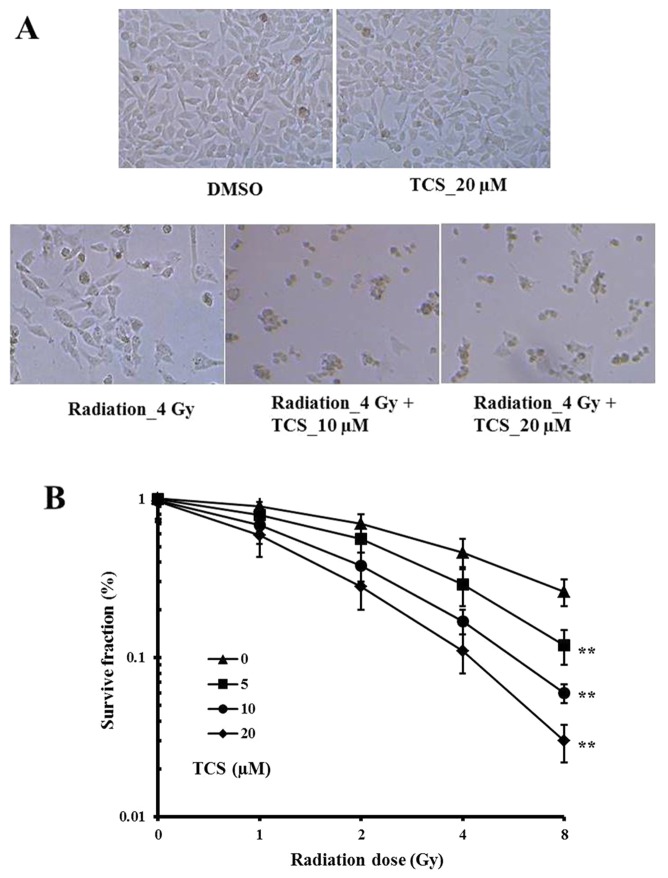
The survival fraction of SUNE-1 cells was calculated to assess the anti-proliferative effects of TCS and radiation. The results demonstrated that SUNE-1 cell growth was markedly inhibited by irradiation alone compared with the control cells (Fig. 1A). In addition, the combination of TCS with radiation inhibited cell growth markedly more than radiation alone (Fig. 1A). No marked survival inhibition was observed with TCS treatment alone (Fig. 1A). The survival rate was significantly decreased when the TCS dosage or radiation was increased (Fig. 1B). The CI value for the combination treatment was 0.678, which indicates a synergistic anti-proliferation effect.
Figure 1.
Combined treatment with TCS and radiation reduced the survival of SUNE-1 cells in a dose-dependent manner. Human SUNE-1 cells were cultured with DMSO, 4 Gy radiation alone, 20 µM TCS alone, 4 Gy radiation + 10 µM TCS or 4 Gy radiation + 20 µM TCS for 24 h. (A) Morphological differences in SUNE-1 cell colonies were observed under a microscope. Magnification, ×200. (B) Colonies of >50 cells were counted to calculate the survival fraction. **P<0.01 vs. radiation alone. TCS, trichosanthin; DMSO, dimethylsulfoxide.
Combined treatment with TCS and radiation increased the apoptosis of SUNE-1 cells
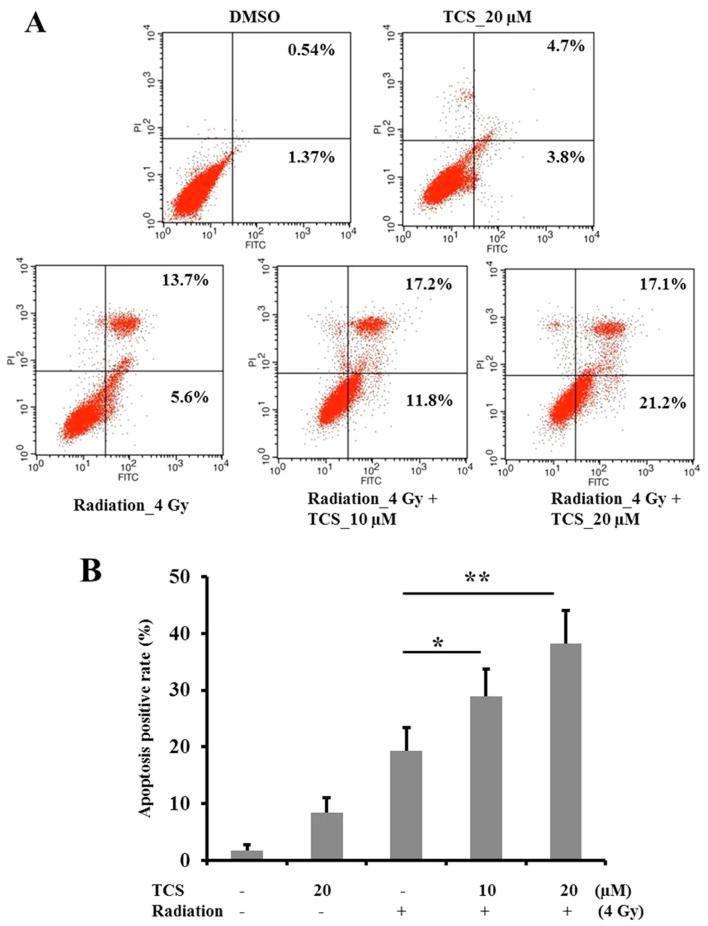
Annexin V/PI staining was performed to measure the apoptosis rate in SUNE-1 cells (Fig. 2A). The results revealed that treatment with TCS alone (20 µM) had no significant effect on apoptosis (Fig. 2B). Treatment with radiation alone significantly induced apoptosis compared with the control group (Fig. 2B), while combined treatment with TCS and radiation significantly increased apoptosis compared with radiation alone. These results indicate that TCS is able to radiosensitize NCP cells to enhance the apoptosis-inducing effects of radiation. Furthermore, the radiosensitization effect of TCS was demonstrated to be dose-dependent (Fig. 2B).
Figure 2.
Combined treatment with TCS and radiation increased the apoptosis rate in SUNE-1 cells. SUNE-1 cells (1×106) were incubated with TCS and/or exposed to radiation for 24 h. Apoptosis was assessed by (A) Annexin V/PI flow cytometry and (B) quantified. Cells in the right lower quadrant and right upper quadrant were in early and late apoptosis, respectively. *P<0.05 and **P<0.01 vs. radiation alone. TCS, trichosanthin; PI, propidium iodide; FITC, fluorescein isothiocyanate; DMSO, dimethylsulfoxide.
TCS radiosensitizes NPC cells via downregulating the phosphoinositide 3-kinase (PI3K) signaling pathway
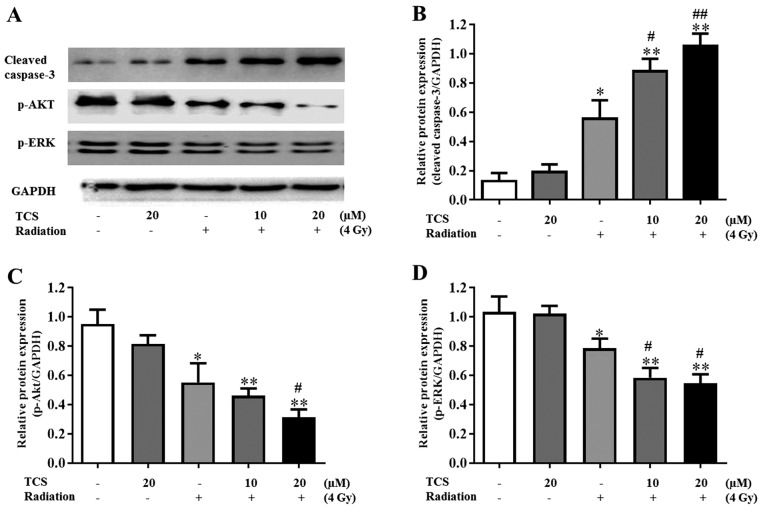
To further explore the mechanism underlying the radiosensitization effect of TCS, the expression of autophagy-associated proteins cleaved caspase-3, p-AKT and p-ERK were assessed. The role of ERK and AKT in NPC cell apoptosis has previously been reported (7). Western blot results revealed that TCS treatment had no significant effect of the expression of p-ERK and p-AKT in SUNE-1 cells (Fig. 3A). However, compared with the control group, p-ERK and p-AKT were downregulated following exposure to radiation and were further downregulated by combined treatment with TCS and radiation (Fig. 3A). The results of western blotting also indicated that combined treatment with TCS and radiation significantly increased the expression of cleaved caspase-3 compared with radiation alone and the control group (Fig. 3B). Cells treated with 20 µM TCS and radiation therapy had significantly lower p-ERK and p-AKT expression compared with the 10 µM TCS combined treatment group, which was consistent with previous apoptosis analysis and survive fraction analysis results (Fig. 3C and D). These results suggest that TCS was able to radiosensitize SUNE-1 cells via inhibiting the PI3K/AKT signaling pathway.
Figure 3.
TCS radiosensitized SUNE-1 cells via downregulating the phosphoinositide 3-kinase signaling pathway. Cells were treated with 0, 10 or 20 µM TCS and/or exposed to 4 Gy radiation with dimethylsulfoxide as the control. (A) The expression of p-AKT, AKT, p-ERK, ERK and cleaved caspase-3 was assessed using western blotting. GAPDH expression was used as a loading control. (B-D) The semi-quantification of proteins concentrations were tested using with ImageJ software. The results are presented as the mean ± SD of three independent experiments. *P<0.05 and **P<0.01 vs. control. #P<0.05 and ##P<0.01 vs. radiation alone. TCS, trichosanthin; p, phosphorylated; AKT, protein kinase B; ERK, extracellular signal-regulated kinase.
TCS in combination with radiation inhibits tumor growth in SUNE-1 ×enografts
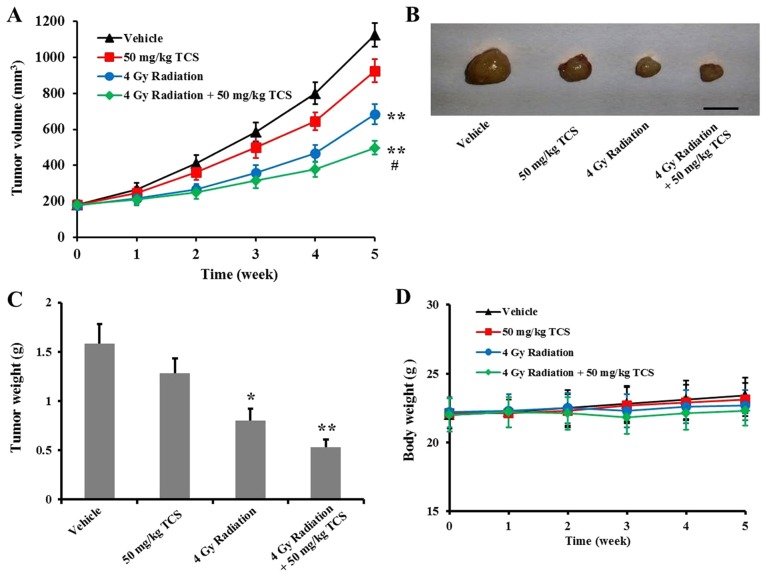
In order to further confirm the radiosensitization ability of TCS in vivo, SUNE-1 ×enografts were constructed. Consistent with the in vitro results, combined treatment with TCS and radiation inhibited SUNE-1 tumor growth in a xenograft tumor model (Fig. 4A). At the end of week 5, mice were sacrificed and tumors were resected for weighing. Tumor weight was significantly lower in the radiation only and TCS combination with radiation groups compared with the control group (Fig. 4B and C). Changes in body weight were recorded throughout the experimental period and no significant differences were observed between groups (Fig. 4D). In conclusion, radiation therapy alone significantly inhibits NPC tumor growth in mice, whereas combined treatment with TCS and radiation may be more effective.
Figure 4.
Combined treatment with TCS and radiation inhibited tumor growth in SUNE-1 ×enografts. (A) 7-week old BALB/c mice were subcutaneously injected with SUNE-1 cells and treated with vehicle, 50 mg/kg/day TCS, 4 Gy radiation or 50 mg/kg/day TCS + 4 Gy radiation for 5 weeks and tumor volumes were monitored. Scale bar=1 cm. At the end of week 5, mice were sacrificed and tumors were (B) excised and (C) weighed. (D) Mice were weighed weekly throughout the experimental period. *P<0.05 and **P<0.01 vs. control; #P<0.05 vs. radiation. TCS, trichosanthin.
Discussion
Radiotherapy is currently the primary treatment for patients with NPC, however radioresistance results in poor patient prognoses (13,14) and the relapse rate is as high as 82% (15,16). Once relapse or metastases occur, the 5-year survival rate is greatly reduced (16). Low radiosensitivity contributes to the poor overall survival of patients with recurrent NPC (17). Cancer cells exhibit different levels of radiosensitivity due to tumor cell heterogeneity. Even when NPC cells are of the same histological differentiation status, radiosensitivity varies (17). The development of novel therapeutic strategies to overcome radioresistance and enhance the radiosensitivity of NPC are urgently required.
The antitumor effect of TCS has been reported in a number of previous studies. Fang et al (5) reported that TCS inhibits breast cancer cell proliferation and induces apoptosis via the activation of both caspase-8 and caspase-9 regulated pathways. Miao et al (4) reported that TCS successfully induced apoptosis in glioma cells in a dose- and time-dependent manner by targeting leucine-rich repeat-containing G-protein coupled receptor 5 and downregulating the Wnt/β-catenin signaling pathway. It has also been reported that recombinant TCS inhibits the proliferation of PC3 cells in a dose-dependent manner in vitro, as well as significantly reducing prostate tumor weight and prostate tumor volume in vivo (2). In the present study, it was demonstrated that TCS potently radiosensitizes NPC xenografts in vitro and in vivo via downregulation of the p-AKT and p-ERK signaling pathways. Considering the clinical usage of TCS on other human diseases, these results suggest that TCS may be used as a novel treatment for NPC.
Recombinant TCS has been demonstrated to inhibit prostate cancer by activating the caspase-8-regulatory pathway to induce the apoptosis of PC3 cells (2). Further research is required to explore the antitumor effects of TCS in other tumor types. Furthermore, the mechanism underlying the antitumor effects of TCS has not been fully elucidated. A more systematic and comprehensive analysis of these mechanisms is essential to facilitate the application of TCS as a clinical tumor treatment strategy.
In addition to TCS, other reagents have been reported to radiosensitize NPC cells. Rad51 is a protein that serves a critical role in the repair of DNA double-strand breaks following irradiation. One study reported that the radiosensitivity of NPC cells could be enhanced by the inhibition of autophagy via reducing Rad51 expression (17). As a result, Rad51 may be a potential novel adjuvant treatment reagent for NPC (17). It has also been reported that curcumin is able to radiosensitize NPC cells both in vivo and in vitro via the upregulation of microRNA (miR)-593 (18). MiR-593 reduces multi-drug resistance gene 1 expression and therefore promotes radiosensitivity in NPC cells (18). According to Liu et al (19), the inhibitors GSK2126458 and PKI-587 represses the progression of four NPC cell lines (SUNE-1, CNE2, 5-8F and 6-10B) and inhibits tumor growth in vitro via the PI3K/mammalian target of rapamycin (mTOR) signaling pathway. NPC cell apoptosis was increased via the suppression of AKT, mTOR and 4EBP1 phosphorylation by GSK2126458 and PKI-587. Furthermore, NPC cells were sensitized to radiation by DNA damage induced by GSK2126458 and PKI-587, which enhanced G2-M cell-cycle delay and induced apoptosis (19).
In conclusion, a number of novel reagents including TCS are able to reduce the radioresistance of NPC cancer cells and may be used in combination with radiotherapy to improve the prognosis of patients with cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
All authors performed the experiments. WW, JW, RQ, XLi, DL and XLiu analyzed the data. WW and JW designed the study, and BT and JZ drafted the manuscript.
Ethics approval and consent to participate
All animal experiments were approved by the Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou Animal Research Ethics Committee (Zhengzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wei B, Huang Q, Huang S, Mai W, Zhong X. Trichosanthin-induced autophagy in gastric cancer cell MKN-45 is dependent on reactive oxygen species (ROS) and NF-κB/p53 pathway. J Pharmacol Sci. 2016;131:77–83. doi: 10.1016/j.jphs.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Li H, Zhang Z, Wang N, Zhang Y. The anticancerous activity of recombinant trichosanthin on prostate cancer cell PC3. Biol Res. 2016;49:21. doi: 10.1186/s40659-016-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sha O, Niu J, Ng TB, Cho EY, Fu X, Jiang W. Antitumor action of trichosanthin, a type 1 ribosome-inactivating protein, employed in traditional Chinese medicine: A mini review. Cancer Chemother Pharmacol. 2013;71:1387–1393. doi: 10.1007/s00280-013-2096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miao J, Jiang Y, Wang D, Zhou J, Fan C, Jiao F, Liu B, Zhang J, Wang Y, Zhang Q. Trichosanthin suppresses the proliferation of glioma cells by inhibiting LGR5 expression and the Wnt/β-catenin signaling pathway. Oncol Rep. 2015;34:2845–2852. doi: 10.3892/or.2015.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang EF, Zhang CZ, Zhang L, Wong JH, Chan YS, Pan WL, Dan XL, Yin CM, Cho CH, Ng TB. Trichosanthin inhibits breast cancer cell proliferation in both cell lines and nude mice by promotion of apoptosis. PLoS One. 2012;7:e41592. doi: 10.1371/journal.pone.0041592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makowska A, Eble M, Prescher K, Hoss M, Kontny U. Chloroquine sensitizes nasopharyngeal carcinoma cells but not nasoepithelial cells to irradiation by blocking autophagy. PLoS One. 2016;11:e0166766. doi: 10.1371/journal.pone.0166766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu QP, Chen WD, Peng JR, Xu YD, Cai Q, Feng GK, Ding K, Zhu XF, Guan Z. Antitumor activity of 7RH, a discoidin domain receptor 1 inhibitor, alone or in combination with dasatinib exhibits antitumor effects in nasopharyngeal carcinoma cells. Oncol Lett. 2016;12:35988–36008. doi: 10.3892/ol.2016.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen X, Tang X, Li Y, Ren X, He Q, Yang X, Zhang J, Wang Y, Ma J, Liu N. Microarray expression profiling of long non-coding RNAs involved in nasopharyngeal carcinoma metastasis. Int J Mol Sci. 2016;17:E1956. doi: 10.3390/ijms17111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi G, Chen J, Shi C, Wang Y, Mi S, Shao W, Yu X, Ma Y, Ling J, Huang J. Cinnamic acid (CINN) induces apoptosis and proliferation in human nasopharyngeal carcinoma cells. Cell Physiol Biochem. 2016;40:589–596. doi: 10.1159/000452572. [DOI] [PubMed] [Google Scholar]
- 10.Yi W, Liu ZG, Li X, Tang J, Jiang CB, Hu JY, Tu ZW, Wang H, Niu DL, Xia YF. CT-diagnosed severe skull base bone destruction predicts distant bone metastasis in early N-stagenasopharyngeal carcinoma. Onco Targets Ther. 2016;9:7011–7017. doi: 10.2147/OTT.S99717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YH, Wang Y, Yusufali AH, Ashby F, Zhang D, Yin ZF, Aslanidi GV, Srivastava A, Ling CQ, Ling C. Cytotoxic genes from traditional Chinese medicine inhibit tumor growth both in vitro and in vivo. J Integr Med. 2014;12:483–494. doi: 10.1016/S2095-4964(14)60057-1. [DOI] [PubMed] [Google Scholar]
- 13.Lu ZX, Ma XQ, Yang LF, Wang ZL, Zeng L, Li ZJ, Li XN, Tang M, Yi W, Gong JP, et al. DNAzymes targeted to EBV-encoded latent membrane protein-1 induce apoptosis and enhance radiosensitivity in nasopharyngeal carcinoma. Cancer Lett. 2008;265:226–238. doi: 10.1016/j.canlet.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Chan AT, Felip E, ESMO Guidelines Working Group Nasopharyngeal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):S123–S125. doi: 10.1093/annonc/mdp150. [DOI] [PubMed] [Google Scholar]
- 15.Suarez C, Rodrigo JP, Rinaldo A, Langendijk JA, Shaha AR, Ferlito A. Current treatment options for recurrent nasopharyngeal cancer. Eur Arch Otorhinolaryngol. 2010;267:1811–1824. doi: 10.1007/s00405-010-1385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Zhang R, Claret FX, Yang H. Involvement of microRNA-24 and DNA methylation in resistance of nasopharyngeal carcinoma to ionizing radiation. Mol Cancer Ther. 2014;13:3163–3174. doi: 10.1158/1535-7163.MCT-14-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo N, Lu YK, Xie WM, Liu Y, Zhou WX, Wang HX, Nong L, Jia YX, Tan AH, Chen Y, et al. Inhibition of autophagy enhances the radiosensitivity of nasopharyngeal carcinoma by reducing Rad51 expression. Oncol Rep. 2014;32:1905–1912. doi: 10.3892/or.2014.3427. [DOI] [PubMed] [Google Scholar]
- 18.Fan HN, Shao M, Huang S, Liu Y, Liu J, Wang Z, Diao J, Liu Y, Tong LI, Fan Q. MiR-593 mediates curcumin-induced radiosensitization of nasopharyngeal carcinoma cells via MDR1. Oncol Lett. 2016;11:3729–3734. doi: 10.3892/ol.2016.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, Sun Q, Li Q, Yang H, Zhang Y, Wang R, Lin X, Xiao D, Yuan Y, Chen L, Wang W. Dual PI3K/mTOR inhibitors, GSK2126458 and PKI-587, suppress tumor progression and increase radiosensitivity in nasopharyngeal carcinoma. Mol Cancer Ther. 2015;14:429–439. doi: 10.1158/1535-7163.MCT-14-0548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.