Abstract
Objectives:
To investigate the clinical significance of VEGF, sVEGFR-1 in heart failure reduced ejection fraction (HFrEF) and heart failure mid-range ejection fraction (HFmrEF) patients.
Methods:
A total of 104 people consisting of HFrEF and HFmrEF patients (n=54) and healthy (n=50) subjects were included in this comparative cross-sectional study. The study took place in Gulhane Training and Research Hospital, Ankara, Turkey, between 2011 and 2013. Serum VEGF, sVEGFR-1, plasma pro-BNP analysis and transthoracic echocardiography were performed.
Results:
The average sVEGFR-1 level of the HFrEF and HFmrEF patients was significantly higher than the control group (0.185±0.122; 0.141±0.120; p=0.013). The average sVEGFR-1 level of the HFrEF and HFmrEF patients using beta-blocker was significantly higher than the HFrEF and HFmrEF patients not using it (p=0.015). There was a significant and positive correlation between sVEGFR-1 and N-terminal pro-brain natriuretic peptide (pro-BNP) levels in the group with HF (r=0.211, p=0.044).
Conclusion:
It increases awareness about the role of sVEGFR-1 in HFrEF anf HFmrEF patients and the need for further studies. Beta-blocker may have a negative effect on angiogenesis in HFrEF and HFmrEF via increasing sVEGFR-1 levels. Additionally, Pro-BNP may contribute to inhibiting angiogenesis by increasing sVEGFR-1 levels and sVEGFR-1 may be an important biomarker in HFrEF and HFmrEF.
Heart failure with reduced ejection fraction (HFrEF) is clinical syndrome with distinctive symptoms and signs caused by a cardiac abnormality. In this study, ejection fraction (EF) is lower than 40%. Patients with EF of 40% to 50% named as heart failure with mid-range ejection fraction (HFmrEF) which is considered as a subgroup of heart failure with preserved ejection fraction (HFpEF) rather than HFrEF.1,2 Neurohumoral mechanisms play the role of conformation after the deterioration of cardiac functions.3,4 Angiogenesis, the formation new vessels that are already present, is an essential part of remodelling. Angiogenic molecules and their receptors play critical roles in endothelial growth, microvascular permeability, and angiogenesis.5 Vascular endothelial growth factor (VEGF) is an angiogenic molecule that both supports vessel dilation and stimulates new blood vessel formation. The primary VEGF receptors are VEGFR-1 and VEGFR-2, which are transmembrane molecules. Neovascularization due to the action of VEGF may be restricted as a means of prevention of heart failure (HF).6 Vascular endothelial growth factor receptor-1-mediated signalling improves vascular permeability.7 Soluble VEGFR-1 (sVEGFR-1) is formed by alternative splicing of VEGFR-1 messenger RNA (mRNA) and plays a role as a decoy protein. It is probably an inhibitor of VEGF.8
Studies on the association between circulating VEGF or sVEGFR-1 levels and clinical information of HFrEF and HFmrEF patients are very limited and the role of various concentrations of VEGF and sVEGFR-1 in the process of angiogenesis is still unknown. This is a clinical study on the clinical significance of plasma VEGF and sVEGFR-1 levels in HFrEF, HFmrEF patients and in patients on medications and with comorbidities due to HF. We hypothesized that increased sVEGFR-1 levels may contribute to impaired angiogenesis in HFrEF and HFmrEF patients. The aim of the study was to assess the effect of HFrEF and HFmrEF on the serum levels of VEGF and sVEGFR-1 in patients with HFrEF and HFmrEF. The results of this study will identify a physiopathological unknown in HFrEF, HFmrEF patients and will illuminate future studies.
Methods
A total of 104 patients, 54 with HFrEF and HFmrEF and 50 healthy controls, in Gulhane Training and Research Hospital, Ankara, Turkey, were included in this cross-sectional comparative study between 2011 and 2013. Informed consent was obtained from each of the participants, and the local ethics committee of Gulhane Training and Research Hospital approved the research protocol. All of the procedures were followed in accordance with the ethical standards of the respective committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. We collected the blood samples as serums from all of the participants and the mean levels of serum VEGF, sVEGFR-1 of the HFrEF and HFmrEF patient group and the control group have been compared. The associations among VEGF, sVEGFR-1 and clinical findings such as demographic features, laboratory results, medications and comorbidities have been evaluated in HFrEF and HFmrEF group.
Each patient had undergone transthoracic echocardiography. A diagnosis of HFrEF was based on symptoms, physical examination findings, (European Society of Cardiology, 2016), echocardiographic findings, and N-terminal pro b-type natriuretic peptide levels (NT-proBNP). End stage renal disease (ESRD) patients have the estimated glomerular filtration rate (eGFR) lower than 15 mL/minute/1.73 m2. Also the patients with chronic kidney disease (CKD) had the eGFR lower than 60 mL/minute/1.73 m2 except ESRD patients. The HFrEF and HFmrEF patients were more than 18 years old and the ejection fraction in these patients was under 50%. Excluded from the study were patients with infections, acute or chronic inflammatory disease, high erythrocyte sedimentation rate or C-reactive protein (CRP), having or suspected malignancy, diabetes mellitus, or cerebrovascular accident.
Blood serum samples were collected from each of the participants. Serum VEGF and sVEGFR-1 levels were determined by using Quantikine ELISA kits (R&D Systems, Minneapolis, MN, USA) and a Synergy HT plate reader (Bio-Tek Instruments Inc, Winooski, VT, USA). N-terminal pro b-type natriuretic peptide levels were detected in the plasma quantitatively by the magnetic immunochromatographic technique. The mean levels of serum VEGF and sVEGFR-1 of patients in the HFrEF and HFmrEF group and those in the control group were compared.
Statistical analysis
Sample size has been calculated for the experimental design. Taking into account the summary statistics of our study variables, which shows the smallest difference between the control and patient groups; by considering the power of the test as 80% and the alpha as 5% the participant number must be included to the study has been calculated as 40. The Statistical Package for Social Science (SPSS) for Windows Version 22.00 (SPSS Inc., Chicago, IL., USA) was used to conduct the statistical analyses. For the descriptive statistics, the discontinuous variables were median and interquartile range (IQR, 25% and 75%); continuous variables were the mean ± standard deviation or median (minimum, maximum) as appropriate. Normality of the data was evaluated with the Kolmogorov-Smirnov test. A Chi-square test was used for categorical values and a student’s t-test was used for continuous variables that were distributed normally. For continuous variables that were not distributed normally, the statistical analysis was performed by the Mann-Whitney U-test. P-value less than 0.05 was considered significant.
Results
The differences in serum VEGF and sVEGFR-1 levels between patients with HFrEF and HFmrEF and the control group were analyzed. The demographics and clinical characteristics of the study population together with the laboratory findings are presented in Table 1. Urea, creatinine, white blood cells, NT-proBNP, sVEGFR-1 levels were significantly higher in patients with HFrEF and HFmrEF than the control group’s (p<0.005 for all). In addition, the glomerular filtration rate (GFR), ejection fraction (EF), and levels of hemoglobin, triglycerides, high-density lipoprotein (HDL-C), total cholesterol (TC) were significantly lower in HFrEF and HFmrEF patients than the control subjects (Table 1).
Table 1.
Comparison of demographic features and clinical laboratory results of the control group and HFrEF, HFmrEF group.

Correlation analysis showed that sVEGFR-1 levels were significantly positively correlated with NT-proBNP levels in HFrEF and HFmrEF patients (Figure 1). Serum VEGF and urea levels were significantly negatively correlated in HFrEF and HFmrEF patients.
Figure 1.
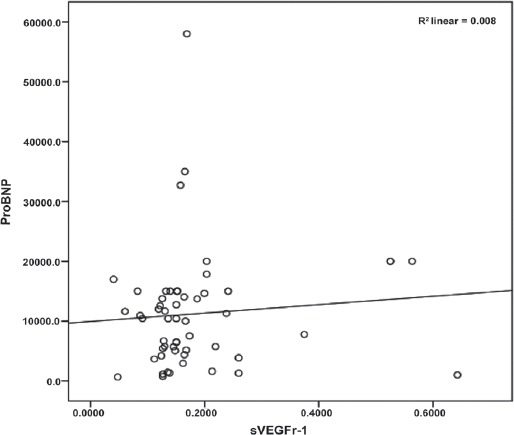
Scatter plot of the positive correlation between NT-proBNP and sVEGFR-1 levels.
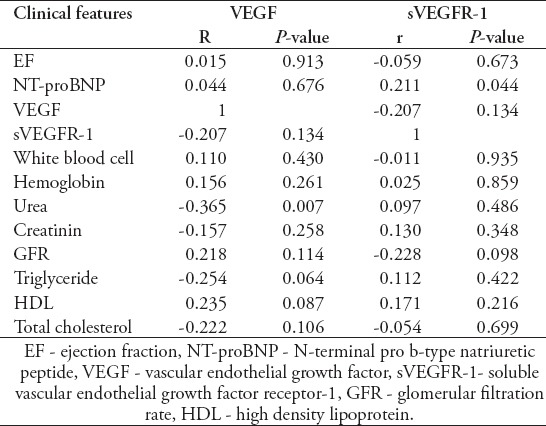
The average sVEGFR-1 level of HFrEF and HFmrEF patients with CKD was not detected to be significantly higher than patients without CKD. There were no significant results in the correlation analysis among sVEGFR-1, GFR, urea, creatinine levels in the HFrEF and HFmrEF patient group (Table 2).
Table 2.
The correlation of VEGF and sVEGFR-1 levels with clinical features which are found to be significantly different in HFrEF and HFmrEF group.
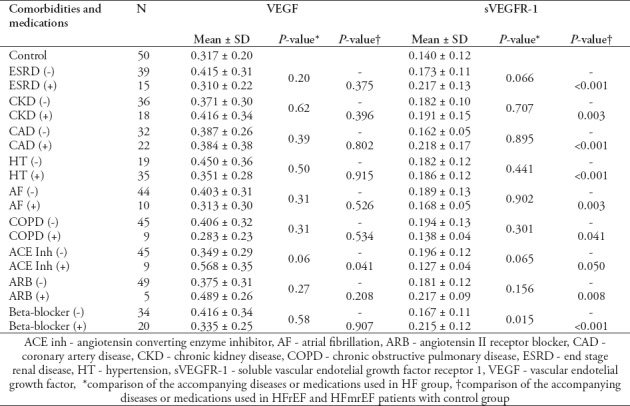
Also, significantly higher levels of sVEGFR-1 were observed in HFrEF and HFmrEF patients with beta-blocker therapy than in the HFrEF and HFmrEF patients without beta-blocker therapy. The mean VEGF level of HFrEF and HFmrEF patients using ACE inhibitor is significantly higher than the control group. Additionally, the mean sVEGFR-1 levels of HFrEF and HFmrEF patients with ESRD, CKD, CAD, HT, and AF are significantly higher than the control subjects. Also, the mean sVEGFR-1 levels of patients receiving beta-blocker and ARB are significantly higher than the control group (Table 3).
Table 3.
Comparison of VEGF and sVEGFR-1 levels between HFrEF and HFmrEF group and control group.
Discussion
This is the first study evaluating and demonstrating the importance of plasma VEGF and sVEGFR-1 levels in HFrEF and HFmrEF patients. Serum sVEGFR-1 levels were significantly higher in patients with HFrEF and HFmrEF than in the control group and no significant difference in the average levels of VEGF between the HFrEF group and the control group was detected.
The pathophysiological role of VEGF was introduced in HF patients in previous studies.9-11 The importance of VEGF was shown previously in animal studies as a mediator for inducing new blood vessels with regard to cardiac remodeling.12 Patel et al13 showed that VEGF levels did not differ among varying degrees of LV systolic functions or did not correlate with levels of EF. In this study, VEGF levels were not found to be significantly higher in HFrEF and HFmrEF patients and no significant correlation was detected with echocardiographic findings in patients and control groups, which was compatible with previous studies.
In 2010, Kaza et al6 demonstrated that up-regulation of the sVEGFR-1 in hypertrophied myocardium prevented capillary growth by inhibiting VEGF in rat models. Inhibition of sVEGFR-1 increased VEGF levels to stimulate angiogenesis and preserved contractility of cardiac muscles. This was an animal study and the data collected were used in literature for sVEGFR-1. It brings to mind that increasing sVEGFR-1 levels may be important in terms of angiogenesis and pathophysiology in HFrEF and HFmrEF patients. It was previously known that sVEGFR-1 is correlated with morbidity and mortality and is a potent marker of disease severity in septic or critically ill patients.14-16 We think that significantly higher levels of sVEGFR-1 in the patient group are not sufficient for us to speculate on the potency of the marker for disease severity.
The CRP and the erythrocyte sedimentation rates of both of the groups were not significantly different. Due to these results, it is thought that the difference in sVEGFR-1 between the 2 groups could not be explained by an inflammatory state.
Due to the results about the levels of sVEGFR-1 in CKD patients and the insignificant correlation analysis among sVEGFR-1, urea, creatinine and GFR, we believe that the level of sVEGFR-1 in the HFrEF and HFmrEF patient group was not affected by GFR.
Beta-blockers had lower mortality and morbidity in symptomatic HFrEF and HFmrEF, despite treatment with an ACEI and, in most cases, a diuretic.17,18 In our study, the level of sVEGFR-1 of HFrEF and HFmrEF patients using a beta-blocker is significantly higher than those who do not use a beta-blocker, which suggests that beta-blockers may significantly and negatively affect angiogenesis in patients with HFrEF and HFmrEF. The negative effects of carvedilol and propranolol on angiogenesis are known in the literature.19,20 In this study, our patients were especially on metoprolol treatment as a beta-blocker. In heart failure patients, this report regarding metoprolol, may demonstrate inhibiting angiogenesis via increasing sVEGFR-1 levels. This effect may be a negative effect of beta-blocker in HFrEF and HFmrEF patients. Further analyses are needed in this aspect.
N-terminal pro b-type natriuretic peptide, is an important molecule in diagnosing and excluding heart failure.21 It was found that there was a significant and positive correlation between NT-proBNP and sVEGFR-1 levels. Goetze et al22 found a significantly positive correlation between VEGF and BNP mRNA in an animal study. Kameda et al23 suggested that high sVEGFR-1 levels may be an effective biomarker to predict the progression of heart failure in patients with CAD. We speculate that an increasing level of sVEGFR-1 by an increasing level of NT-proBNP may be a physiological response to heart failure, designed to decrease angiogenesis in HFrEF and HFmrEF patients, unlike the previous literature. Nielsen et al24 demonstrated that pro-BNP predicts mortality and morbidity in HF patients. Also according to literature, sVEGFR-1 is correlated with morbidity and mortality and is a potent marker of disease severity in septic or critically ill patients.15,16 These findings may suggest that sVEGFR-1 could predict mortality and morbidity indirectly; therefore, further participants including clinical studies are needed in this respect. Also, a positive correlation between sVEGFR-1 and EF in the HFrEF and HFmrEF group was not found. Additionally, the clinical course of sVEGFR-1 cannot be exactly evaluated by only considering the positive correlation of sVEGFR-1 and NT-proBNP.
Pawlak et al25 found that urea and creatinine levels were independently associated with VEGF. There was a significant and negative correlation between VEGF and urea levels in HFrEF and HFmrEF patients contrary to the literature. We speculate that the increase of urea in HFrEF and HFmrEF patients may be one of the mechanisms of inhibition of angiogenesis in HFrEF and HFmrEF patients.
In summary, our study is limited to analysis of only one angiogenic molecule, VEGF and one anti-angiogenic molecule sVEGFR-1, which makes it difficult to evaluate the imbalance of angiogenic/anti-angiogenic factors in patients with HFrEF and HFmrEF due to the other angiogenic factors have not been evaluated at the same time. However, we think that our study is significant and worthy due to the fact that it has not been studied before in literature as a clinical study. This study establishes an association but not exactly a cause-and-effect relationship. Finally, this study is based on a limited number of patients; thus it is not possible to ascertain whether these findings apply to other patients with HFrEF and HFmrEF. Accordingly, larger clinical studies will be necessary to confirm these findings.
Beta-blocker may have a negative effect on angiogenesis in HFrEF and HFmrEF patients via increasing sVEGFR-1 levels while Pro-BNP may contribute to inhibiting angiogenesis by increasing sVEGFR-1 levels and sVEGFR-1 may predict HFrEF and HFmrEF as a biomarker. Our study increases awareness about the role of sVEGFR-1 in HFrEF and HFmrEF patients and the need for future studies.
Acknowledgment
We would like to thank our patients and their families who participated in the research helpfully and devotedly without expecting material compensation. We would like to thank Basaran Tercume and Scribendi for the professional English language and scientific editing.
Footnotes
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012 Jul;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 3.Xu B, Li H. Brain mechanisms of sympathetic activation in heart failure: Roles of the renin angiotensin system, nitric oxide and pro inflammatory cytokines (Review) Mol Med Rep. 2015;12:7823–7829. doi: 10.3892/mmr.2015.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanai E, Frantz S. Pathophysiology of Heart Failure. Compr Physiol. 2015;6:187–214. doi: 10.1002/cphy.c140055. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 6.Kaza E, Ablasser K, Poutias D, Griffiths ER, Saad FA, Hofstaetter JG, del Nido PJ, Friehs I. Up-regulation of soluble vascular endothelial growth factor receptor-1 prevents angiogenesis in hypertrophied myocardium. Cardiovasc Res. 2011;89:410–418. doi: 10.1093/cvr/cvq321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada T, Jesmin S, Gando S, Yanagida Y, Mizugaki A, Sultana SN, et al. Angiogenic factors and their soluble receptors predict organ dysfunction and mortality in post cardiac arrest syndrome. Crit Care. 2012;16:R171. doi: 10.1186/cc11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Failla CM, Carbo M, Morea V. Positive and negative regulation of angiogenesis by soluble vascular endothelial growth factor receptor-1. Int J Mol Sci. 2018;19:E1306. doi: 10.3390/ijms19051306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao M, Lu X, Chen W, Xiao GH, Zhang Y, Yu R, Li J. Randomized clinical trial of physiological ischemic training for patients with coronary heart disease complicated with heart failure: Safety of training, VEGF of peripheral blood and quality of life. Exp Ther Med. 2018;16:260–264. doi: 10.3892/etm.2018.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lottonen-Raikaslehto L, Rissanen R, Gurzeler E, Merentie M, Huusko J, Schneider JE, et al. Left ventricular remodeling leads to heart failure in mice with cardiac-specific overexpression of VEGF-B167: echocardiography and magnetic resonance imaging study. Physiol Rep. 2017;5:e13096. doi: 10.14814/phy2.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobusiak-Prokopowicz M, Jołda-Mydłowska B, Grzebieniak T, Początek K, Mysiak A. Expression of proinflammatory factors, proangiogenic factors and endostatin in patients with heart failure and different grades of collateral circulation development. Adv Clin Exp Med. 2015;24:987–994. doi: 10.17219/acem/33811. [DOI] [PubMed] [Google Scholar]
- 12.Lottonen-Raikaslehto L, Rissanen R, Gurzeler E, Merentie M, Huusko J, Schneider JE, et al. Left ventricular remodeling leads to heart failure in mice with cardiac-specific overexpression of VEGF-B167: echocardiography and magnetic resonance imaging study. Physiol Rep. 2017;5:e13096. doi: 10.14814/phy2.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel JV, Abraheem A, Chackathayil J, Gunning M, Creamer J, Hughes EA, Lip GY. J Intern Med. 2009;265:562–567. doi: 10.1111/j.1365-2796.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- 14.Wada T, Jesmin S, Gando S, Sultana SN, Zaedi S, Yokota H. Using angiogenic factors and their soluble receptors to predict organ dysfunction in patients with disseminated intravascular coagulation associated with severe trauma. Crit Care. 2012;16:R63. doi: 10.1186/cc11309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skibsted S, Jones AE, Puskarich MA, Arnold R, Sherwin R, Trzeciak S, Schuetz P, Aird WC, Shapiro NI. Biomarkers of endothelial cell activation in early sepsis. Shock. 2013;39:427–432. doi: 10.1097/SHK.0b013e3182903f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumnicka P, Sporek M, Mazur-Laskowska M, Ceranowicz P, Kuźniewski M, Drożdż R, et al. Serum soluble fms-like tyrosine kinase 1 (sflt-1) predicts the severity of acute pancreatitis. Int J Mol Sci. 2016;17:2038. doi: 10.3390/ijms17122038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam PH, Gupta N, Dooley DJ, Singh S, Deedwania P, Zile MR, et al. Role of high-dose beta-blockers in patients with heart failure with preserved ejection fraction and elevated heart rate: high-dose beta-blocker and outcomes in HFpEF. Am J Med. 2018;1 doi: 10.1016/j.amjmed.2018.07.008. pii: S0002-9343(18)30731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lund LH, Benson L, Dahlström U, Edner M, Friberg L. Association between use of ß-blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA. 2014;312:2008–20018. doi: 10.1001/jama.2014.15241. [DOI] [PubMed] [Google Scholar]
- 19.Ding Q, Tian XG, Li Y, Wang QZ, Zhang CQ. Carvedilol may attenuate liver cirrhosis by inhibiting angiogenesis through the VEGF-Src-ERK signaling pathway. World J Gastroenterol. 2015;21:9566–9576. doi: 10.3748/wjg.v21.i32.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan WK, Li P, Guo ZT, Huang Q, Gao Y. Propranolol induces regression of hemangioma cells via the down-regulation of the PI3K/Akt/eNOS/VEGF pathway. Pediatr Blood Cancer. 2015;62:1414–1420. doi: 10.1002/pbc.25453. [DOI] [PubMed] [Google Scholar]
- 21.Zaphiriou A, Robb S, Murray-Thomas T, Mendez G, Fox K, McDonagh T, et al. The diagnostic accuracy of plasma BNP and NTproBNP in patients referred from primary care with suspected heart failure: results of the UK natriuretic peptide study. Eur J Heart Fail. 2005;7:537–541. doi: 10.1016/j.ejheart.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Goetze JP, Gore A, Møller CH, Steinbrüchel DA, Rehfeld JF, Nielsen LB. Acute myocardial hypoxia increases BNP gene expression. FASEB J. 2004;18:1928–1930. doi: 10.1096/fj.03-1336fje. [DOI] [PubMed] [Google Scholar]
- 23.Kameda R, Yamaoka-Tojo M, Makino A, Wakaume K, Nemoto S, Kitasato L, et al. Soluble Fms-like tyrosine kinase 1 is a novel predictor of brain natriuretic peptide elevation. Int Heart J. 2013;54:133–139. doi: 10.1536/ihj.54.133. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen OW, Cowburn PJ, Sajadieh A, Morton JJ, Dargie H, McDonagh T. Value of BNP to estimate cardiac risk in patients on cardioactive treatment in primary care. Eur J Heart Fail. 2007;9:1178–1185. doi: 10.1016/j.ejheart.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Pawlak K, Ulazka B, Mysliwiec M, Pawlak D. Vascular endothelial growth factor and uPA/suPAR system in early and advanced chronic kidney disease patients: a new link between angiogenesis and hyperfibrinolysis? Transl Res. 2012;160:346–354. doi: 10.1016/j.trsl.2012.04.004. [DOI] [PubMed] [Google Scholar]


