Abstract
D-dimer is a widely used biomarker for indicating the activation of coagulation and fibrinolysis, and is reported to serve important roles in cancer progression. The aim of the current retrospective study was to investigate the association of D-dimer plasma level with the development of various cancers. Patients with breast (n=86), gastric (n=317), pancreatic (n=37), colon (n=153) and rectal (n=137) cancers and 92 healthy volunteers were assessed in the present study. Plasma levels of D-dimer in the patients and healthy controls were measured by immunoturbidimetric assays. The association of D-dimer levels with the clinicopathological features of patients were also determined. The plasma levels of D-dimer were significantly higher in patients with breast cancer (P=0.0022), gastric cancer (P<0.0001), pancreatic cancer (P=0.0003), colon cancer (P=0.0001) and rectal cancer (P=0.0028), compared with the healthy controls. It was also determined that the plasma D-dimer levels were positively associated with clinical cancer stage (P<0.05) and metastasis (P<0.05). These findings suggested that the plasma D-dimer level may be used as marker for predicting cancer metastasis and progression.
Keywords: D-dimer, biomarker, cancer, metastasis, progression
Introduction
D-dimer is the cleavage product of cross-linked fibrin that is formed by activation of the coagulation system, which signals hyperfibrinolysis in response to clot activation and fibrin formation (1). Elevated levels of D-dimer have been detected in patients exhibiting diffuse intravascular coagulation (2), vascular occlusion crisis in sickle cell disease (3), thromboembolic events (4,5) and myocardial infarction (6). D-dimer is a widely used biomarker for indicating the activation of coagulation and fibrinolysis (7,8). Coagulation disorders are among the most common complications in cancer patients (7,9). D-dimer levels are elevated in the plasma of patients with various solid cancers, including of the prostate (10–12), cervix (13–15) and esophageal squamous cells (16). However, the association of D-dimer levels and cancer progression remains to be a focus of study.
In the present retrospective study, the plasma levels of D-dimer in patients with breast, gastric, pancreatic, colon and rectal cancers and in healthy controls were firstly measured. Subsequently, associations between the D-dimer levels and clinical features were assessed. The results suggested that the plasma level of D-dimer was notably associated with the extent of tumor metastasis and tumor stage in cancer patients.
Materials and methods
Study population
Enrolled patients had been diagnosed with breast cancer (n=86; age range, 35–79 years; all female), gastric cancer (n=317; age range, 32–83 years; male: female, 208:109), pancreatic cancer (n=37; age range, 34–82 years; male: female, 22:15), colon cancer (n=153; age range, 26–90 years; male: female 73:80) or rectal cancer (n=137; age range, 44–79 years; male: female, 81:56) at the Affiliated Changzhou No. 2 People's Hospital of Nanjing Medical University, Changzhou, China, from January 2010 to December 2014. A total of 92 healthy volunteers (age range, 23–69 years; male: female, 43:49) undergoing physical examination at Changzhou No. 2 People's Hospital in January 2016 were also enrolled. The cancers were defined according to pathological findings. Other inclusion criteria were measurable disease by magnetic resonance imaging. Disease clinical staging (I–IV) depended on the systems recommended by the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (17–21). Exclusion criteria were intravascular disseminated coagulation, which was diagnosed on the parameters issued by the Chinese Hematology Society (22–24). The present retrospective study was approved by the Ethics Committee of Changzhou No. 2 People's Hospital, and written informed consent from all participants was obtained following full disclosure of the research aims and procedures.
D-dimer level assays
D-dimer expression levels were detected prior to and following treatment in cancer patients. In the healthy controls D-dimer was detected during a fasting (for 8 h prior) physical examination. A total of 3 ml whole blood was drawn from the antecubital vein of each subject and collected in 3.8% sodium citrate vacutainer collection tubes (Greiner Bio-One GmbH, Frickenhausen, Germany). Plasma D-dimer levels were analyzed with an INNOVANCE®D-Dimer Calibrator (Siemens AG, Munich, Germany). All samples were run in duplicate according to manufacturer's recommendations. The D-dimer cross-linkage region has a stereosymmetrical structure, meaning the epitope for the monoclonal antibody occurs twice. D-dimer levels >0.55 mg/l were considered to be elevated, since this was the upper limit of the 90% confidence interval for the average value determined in the healthy volunteers. The D-dimer levels in tumor patients can be affected following surgery (25,26), chemotherapy (27,28) and adjuvant therapy (29). Therefore, the data analyzed herein is the D-dimer expression level of all patients prior to treatment.
Statistical analysis
D-dimer levels were presented as the median and range. Statistically significant differences between healthy volunteers and cancer patients were determined using unpaired Student's t-tests. Cases were divided into two groups, high or low, according to the median D-dimer level as a cutoff. Associations of D-dimer level with clinicopathological characteristics were analyzed by using the χ2 test. P<0.05 was considered to indicate statistical significance. The statistical analysis and graphing of data were performed with GraphPad Prism version 7.00 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
D-dimer levels are significantly increased in patients with cancer
To investigate the role of D-dimer in the development of cancer, an INNOVANCE®D-Dimer Calibrator was used to analyze the D-dimer levels in patients with breast, gastric, pancreatic, colon and rectal cancers and in healthy controls. The results demonstrated that D-dimer levels were significantly higher in the patients with breast cancer (2.61±0.77 mg/l; n=86; P=0.0022), gastric cancer (2.85±0.21 mg/l; n=317; P<0.0001), pancreatic cancer (5.65±1.39 mg/l; n=37; P=0.0003), colon cancer (4.48±0.83 mg/l; n=153; P=0.0001) and rectal cancer (2.00±0.46 mg/l; n=137; P=0.0028), compared with in healthy volunteers (0.29±0.01 mg/l, n=92; Fig. 1). These results indicated that D-dimer is expressed to a high level and may serve important roles during cancer development.
Figure 1.
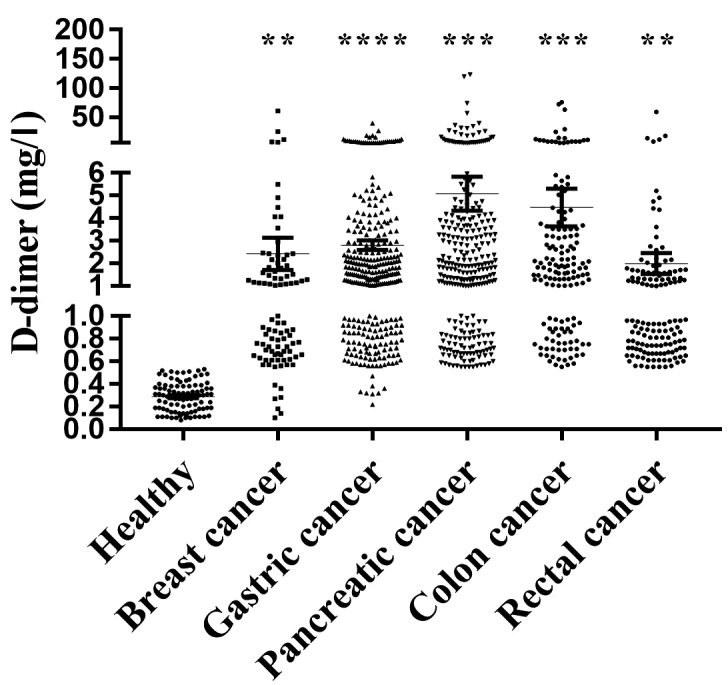
D-dimer levels in healthy volunteers and patients with different cancers. The graph shows the median, quartiles and range of the data. **P<0.01, ***P<0.001 and ****P<0.0001 vs. healthy volunteers.
D-dimer levels are associated with the clinicopathological characteristics of patients with cancer
Additionally, associations of D-dimer level with the clinicopathological characteristics of patients were assessed. Specimens were divided into two groups according to the expression level of D-dimer (≤ or >0.55 mg/l). The data indicated that the D-dimer plasma level differed significantly according to metastasis (P=0.0181) and TNM stage (P=0.0101) in patients with breast cancer (Table I); metastasis (P<0.0001) and TNM stage (P=0.0063) in patients with gastric cancer (Table II); TNM stage (P=0.0395) in patients with pancreatic cancer (Table III); metastasis (P=0.0120), TNM stage (P=0.0039) and sex (P=0.0145) in patients with colon cancer (Table IV); and metastasis (P=0.0104) and TNM stage (P=0.0002) in patients with rectal cancer (Table V). No differences were identified between D-dimer level and other clinical features (Tables I–V). These data suggested that the plasma level of D-dimer may be used as a marker in cancer progression.
Table I.
Clinical association between D-dimer level and clinicopathological variables of breast cancer patients.
| Cases with low and high D-dimer level, n (%) | ||||
|---|---|---|---|---|
| Characteristic | Cases (n=86) | Low | High | P-value |
| Age, years | 1 | |||
| ≥60 | 46 | 35 (76.1) | 11 (23.9) | |
| <60 | 40 | 31 (77.5) | 9 (22.5) | |
| Metastasis | 0.0181 | |||
| Positive | 70 | 20 (28.6) | 50 (71.4) | |
| Negative | 16 | 10 (62.5) | 6 (37.5) | |
| TNM stage | 0.0101 | |||
| I&II | 17 | 11 (64.7) | 6 (35.3) | |
| III&IV | 69 | 20 (28.9) | 49 (71.1) | |
For patients with breast cancer, since there were no male patients it was not possible to perform statistics for male vs. female patients. TNM, tumor-node-metastasis.
Table II.
Clinical association between D-dimer level and clinicopathological variables of gastric cancer patients.
| Cases with low and high D-dimer level, n (%) | ||||
|---|---|---|---|---|
| Characteristic | Cases (n=317) | Low | High | P-value |
| Age, years | 0.5337 | |||
| ≥60 | 171 | 119 (60.0) | 52 (40.0) | |
| <60 | 146 | 107 (69.4) | 39 (30.6) | |
| Gender | 0.4341 | |||
| Male | 208 | 145 (69.7) | 63 (30.7) | |
| Female | 109 | 81 (74.3) | 28 (25.7) | |
| Metastasis | <0.0001 | |||
| Positive | 215 | 117 (54.5) | 98 (45.6) | |
| Negative | 102 | 79 (77.5) | 23 (22.5) | |
| TNM stage | 0.0063 | |||
| I&II | 32 | 26 (84.2) | 6 (15.8) | |
| III&IV | 285 | 174 (61.1) | 111 (38.9) | |
TNM, tumor-node-metastasis.
Table III.
Clinical association between D-dimer level and clinicopathological variables of pancreatic cancer patients.
| Cases with low and high D-dimer level, n (%) | ||||
|---|---|---|---|---|
| Characteristic | Cases (n=37) | Low | High | P-value |
| Age, years | 1 | |||
| ≥60 | 23 | 10 (43.5) | 13 (56.5) | |
| <60 | 14 | 6 (42.9) | 8 (57.1) | |
| Gender | 1 | |||
| Male | 22 | 10 (45.5) | 12 (54.5) | |
| Female | 15 | 6 (40.0) | 9 (60.0) | |
| Metastasis | 0.5541 | |||
| Positive | 34 | 13 (38.2) | 21 (61.8) | |
| Negative | 3 | 2 (66.7) | 1 (33.3) | |
| TNM stage | 0.0395 | |||
| I&II | 14 | 8 (57.1) | 6 (42.9) | |
| III&IV | 23 | 5 (21.7) | 18 (78.3) | |
TNM, tumor-node-metastasis.
Table IV.
Clinical association between D-dimer level and clinicopathological variables of colon cancer patients.
| Cases with low and high D-dimer level, n (%) | ||||
|---|---|---|---|---|
| Characteristic | Cases (n=153) | Low | High | P-value |
| Age, years | 0.7127 | |||
| ≥60 | 113 | 64 (56.6) | 49 (43.4) | |
| <60 | 40 | 21 (52.5) | 19 (47.5) | |
| Gender | 0.0145 | |||
| Male | 73 | 48 (65.8) | 25 (34.2) | |
| Female | 80 | 36 (45.0) | 44 (55.0) | |
| Metastasis | 0.0120 | |||
| Positive | 119 | 51 (42.9) | 68 (57.1) | |
| Negative | 34 | 23 (67.7) | 11 (32.3) | |
| TNM stage | 0.0039 | |||
| I&II | 17 | 15 (88.2) | 2 (11.8) | |
| III&IV | 136 | 70 (51.5) | 66 (48.5) | |
TNM, tumor-node-metastasis.
Table V.
Clinical association between D-dimer level and clinicopathological variables of rectal cancer patients.
| Cases with low and high D-dimer level, n (%) | ||||
|---|---|---|---|---|
| Characteristic | Cases (n=137) | Low | High | P-value |
| Age, years | 0.8477 | |||
| ≥60 | 100 | 48 (48.0) | 52 (52.0) | |
| <60 | 37 | 19 (51.4) | 18 (48.6) | |
| Gender | 1 | |||
| Male | 81 | 40 (49.4) | 41 (50.6) | |
| Female | 56 | 27 (48.2) | 29 (51.8) | |
| Metastasis | 0.0104 | |||
| Positive | 109 | 47 (43.1) | 62 (56.9) | |
| Negative | 28 | 20 (71.4) | 8 (28.6) | |
| TNM stage | 0.0002 | |||
| I&II | 44 | 30 (68.2) | 14 (31.8) | |
| III&IV | 93 | 32 (34.4) | 61 (65.6) | |
TNM, tumor-node-metastasis.
Discussion
In the present study, it was demonstrated that elevated plasma levels of D-dimer were associated with clinical cancer stages and metastasis in patients with breast, gastric, colon and rectal cancers. It was also identified that D-dimer plasma level was associated with cancer stages in patients with pancreatic cancer and with gender in patients with colon cancer. These findings suggest that D-dimer level has a potential use in predicting the likelihood of metastasis and progression in various cancers.
Distant metastasis is the main cause of poor prognosis and leads to inefficacy in treatments in cancer patients (30). D-dimer is a widely used biomarker for indicating the activation of coagulation and fibrinolysis. It is established that activated coagulation, which is common in malignancy, plays an important role in cancer metastasis (31). The coagulation/fibrinolytic system is activated in cancer patients and may contribute to cancer progression (14). Thus, tumor-related degradation products of the coagulation and fibrinolytic system have been proposed to predict tumor load and prognosis (14,32). Plasma D-dimer is a pro-coagulation factor that may reflect the presence of micro-metastases or circulating tumor cells, which may be responsible for tumor recurrence (30,33,34). Recent studies have reported a positive association between circulating tumor cells and plasma D-dimer levels in patients with metastatic breast cancer (35,36). Diao et al (37) observed that plasma D-dimer levels were markedly increased in gastric cancer patients with distant metastases, particularly in patients with visceral metastases. In fact, they suggested D-dimer to be a promising predictor of clinical stage in the gastric cancer patients (37). Lee et al (38) reported that increased plasma D-dimer and fibrinogen degradation product levels were significantly associated with TNM stage in colorectal cancer patients. Furthermore, preoperative plasma D-dimer levels have been associated with larger tumor size and lymph node metastasis in colorectal cancer patients (39). Other recent studies have shown that plasma D-dimer values may predict overall survival and progression free survival in patients with pancreatic cancer (40,41). In accordance with the above studies, the present study confirmed a positive association between D-dimer level and tumorigenesis. It was further identified that D-dimer level was associated with cancer stages but not with metastasis in patients with pancreatic cancer. Among the patients with pancreatic cancer, the majority presented with metastasis (34/37), and fibrinogen and carbohydrate antigen 19-9 (CA19-9) were significantly elevated in the patients with metastatic pancreatic cancer, compared with patients with non-metastatic cancer (data not shown), suggesting that a combination of D-dimer, fibrinogen and CA19-9 may be used for predicting metastasis of pancreatic cancer.
In conclusion, the present retrospective study demonstrated that D-dimer plasma level was significantly higher in patients with cancer and associated with clinical stages and metastasis. The current study was limited in that it only identified the association of D-dimer with tumor stage and metastasis. We will further investigate the sensitivity and specificity of D-dimer as an indicator of tumorigenesis, and the correlation between D-dimer and the survival and prognosis of cancer patients, to clarify whether D-dimer level may be considered as an important factor in the prognosis of cancer patients.
Acknowledgements
Not applicable.
Funding
The present work was supported by grants from the Project of Jiangsu Provincial Medical Youth Talent (grant no. QNRC2016267 to TF) and the Projects of Nanjing Medical University Science and Technology Development Fund Key (grant no. 2016NJMUZD103 to PZ).
Availability of data and materials
All data generated or analyzed in this study are included in this published article.
Authors' contributions
PZ, HD and TF designed the study. HZ, YS, ZX and SW performed the experiments and collected and analyzed data. PZ, HD and TF wrote, reviewed and revised the manuscript. All authors read and approved the final version to be published.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Changzhou No. 2 People's Hospital of Nanjing Medical University, Changzhou, China, and written informed consent permitting the use of patient samples in the current study was obtained prior to treatment.
Consent for publication
All patients consented to the publication of any relevant data on the basis of anonymization of all personal data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jiang X, Mei X, Wu H, Chen X. D-dimer level is related to the prognosis of patients with small cell lung cancer. Ann Transl Med. 2017;5:394. doi: 10.21037/atm.2017.07.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobayashi T, Kajiki M, Nihashi K, Honda G. Surveillance of the safety and efficacy of recombinant human soluble thrombomodulin in patients with obstetrical disseminated intravascular coagulation. Thromb Res. 2017;159:109–115. doi: 10.1016/j.thromres.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed S, Siddiqui AK, Iqbal U, Sison CP, Shahid RK, Sheth M, Patel DV, Russo LA. Effect of low-dose warfarin on D-dimer levels during sickle cell vaso-occlusive crisis: A brief report. Eur J Haematol. 2004;72:213–216. doi: 10.1111/j.0902-4441.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- 4.Lowe GD, Haverkate F, Thompson SG, Turner RM, Bertina RM, Turpie AG, Mannucci PM. Prediction of deep vein thrombosis after elective hip replacement surgery by preoperative clinical and haemostatic variables: The ECAT DVT Study. European Concerted Action on Thrombosis. Thromb Haemost. 1999;81:879–886. doi: 10.1055/s-0037-1614592. [DOI] [PubMed] [Google Scholar]
- 5.Kwietniak M, Al-Amawi T, Błaszkowski T, Sulżyc-Bielicka V, Kładny J. The usefulness of D-dimer in diagnosis and prediction of venous thromboembolism in patients with abdominal malignancy. Pol Przegl Chir. 2017;89:27–30. doi: 10.5604/01.3001.0010.1018. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Qiu B, Zhang Y, Cao Y, Zhang X, Wu Z, Wang S, Mei L. The Value of Pre-Infarction Angina and Plasma D-Dimer in Predicting No-Reflow After Primary Percutaneous Coronary Intervention in ST-Segment Elevation Acute Myocardial Infarction Patients. Med Sci Monit. 2018;24:4528–4535. doi: 10.12659/MSM.909360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horowitz NA, Blevins EA, Miller WM, Perry AR, Talmage KE, Mullins ES, Flick MJ, Queiroz KC, Shi K, Spek CA, et al. Thrombomodulin is a determinant of metastasis through a mechanism linked to the thrombin binding domain but not the lectin-like domain. Blood. 2011;118:2889–2895. doi: 10.1182/blood-2011-03-341222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Im JH, Fu W, Wang H, Bhatia SK, Hammer DA, Kowalska MA, Muschel RJ. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res. 2004;64:8613–8619. doi: 10.1158/0008-5472.CAN-04-2078. [DOI] [PubMed] [Google Scholar]
- 9.Huang W, Kim HR. Dynamic regulation of platelet-derived growth factor D (PDGF-D) activity and extracellular spatial distribution by matriptase-mediated proteolysis. J Biol Chem. 2015;290:9162–9170. doi: 10.1074/jbc.M114.610865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamzah AB, Choo YM, Hassali MA, Saleem F, Verma AK. Disseminated Intravascular Coagulation and Excessive Fibrinolysis (DIC XFL) Syndrome in Prostate Cancer: A Rare Complicated Disorder. J Clin Diagn Res. 2017;11:XD01–XD02. doi: 10.7860/JCDR/2017/22582.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong SK, Ko DW, Park J, Kim IS, Doo SH, Yoon CY, Park H, Lee WK, Kim DS, Jeong SJ, et al. Alteration of Antithrombin III and D-dimer Levels in Clinically Localized Prostate Cancer. Korean J Urol. 2010;51:25–29. doi: 10.4111/kju.2010.51.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoury JD, Adcock DM, Chan F, Symanowski JT, Tiefenbacher S, Goodman O, Paz L, Ma Y, Ward DC, Vogelzang NJ, et al. Increases in quantitative D-dimer levels correlate with progressive disease better than circulating tumor cell counts in patients with refractory prostate cancer. Am J Clin Pathol. 2010;134:964–969. doi: 10.1309/AJCPH92SXYLIKKTS. [DOI] [PubMed] [Google Scholar]
- 13.Luo YL, Chi PD, Zheng X, Zhang L, Wang XP, Chen H. Preoperative D-dimers as an independent prognostic marker in cervical carcinoma. Tumour Biol. 2015;36:8903–8911. doi: 10.1007/s13277-015-3650-5. [DOI] [PubMed] [Google Scholar]
- 14.Satoh T, Matsumoto K, Tanaka YO, Akiyama A, Nakao S, Sakurai M, Ochi H, Onuki M, Minaguchi T, Sakurai H, et al. Incidence of venous thromboembolism before treatment in cervical cancer and the impact of management on venous thromboembolism after commencement of treatment. Thromb Res. 2013;131:e127–e132. doi: 10.1016/j.thromres.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 15.Sun YH, Cui L, Chen J, Wang M, Liu JJ, Liu XX, Huang XE. Analysis of Relationships Between Prethrombotic States and Cervical Cancer. Asian Pac J Cancer Prev. 2015;16:6163–6166. doi: 10.7314/APJCP.2015.16.14.6163. [DOI] [PubMed] [Google Scholar]
- 16.Feng JF, Yang X, Chen S, Zhao Q, Chen QX. Prognostic Value of Plasma D-dimer in Patients with Resectable Esophageal Squamous Cell Carcinoma in China. J Cancer. 2016;7:1663–1667. doi: 10.7150/jca.15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Forero A, Giordano SH, et al. National Comprehensive Cancer Network: Invasive breast cancer. J Natl Compr Canc Netw. 2011;9:136–222. doi: 10.6004/jnccn.2011.0016. [DOI] [PubMed] [Google Scholar]
- 18.Ettinger DS, Akerley W, Bepler G, Blum MG, Chang A, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Govindan R, et al. National Comprehensive Cancer Network: Thymic malignancies. J Natl Compr Canc Netw. 2010;8:1302–1315. doi: 10.6004/jnccn.2010.0096. [DOI] [PubMed] [Google Scholar]
- 19.Romanus D, Weiser MR, Skibber JM, Ter Veer A, Niland JC, Wilson JL, Rajput A, Wong YN, Benson AB, III, Shibata S, et al. Concordance with NCCN Colorectal Cancer Guidelines and ASCO/NCCN Quality Measures: An NCCN institutional analysis. J Natl Compr Canc Netw. 2009;7:895–904. doi: 10.6004/jnccn.2009.0059. [DOI] [PubMed] [Google Scholar]
- 20.Benson AB, III, Arnoletti JP, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Dilawari RA, Engstrom PF, Enzinger PC, et al. National Comprehensive Cancer Network: Colon cancer. J Natl Compr Canc Netw. 2011;9:1238–1290. doi: 10.6004/jnccn.2011.0104. [DOI] [PubMed] [Google Scholar]
- 21.Engstrom PF, Arnoletti JP, Benson AB, III, Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS, Enzinger PC, et al. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Rectal cancer. J Natl Compr Canc Netw. 2009;7:838–881. doi: 10.6004/jnccn.2009.0057. [DOI] [PubMed] [Google Scholar]
- 22.Ma J, Qiu LG. Guidelines for treatment of myeloma bone disease. Zhonghua Xue Ye Xue Za Zhi. 2011;32:721–723. (In Chinese) [PubMed] [Google Scholar]
- 23.Lymphoma Working Party of Chinese Society of Hematology, corp-author. Chinese guidelines for the diagnosis and management of chronic lymphocytic leukemia. Zhonghua Xue Ye Xue Za Zhi. 2011;32:498–501. (In Chinese) [PubMed] [Google Scholar]
- 24.Shen ZX, Chinese Society of Hematology. Chinese Medical Association Chinese guidelines for the diagnosis and treatment of acute promyelocytic leukemia. Zhonghua Xue Ye Xue Za Zhi. 2011;32:885–886. (In Chinese) [PubMed] [Google Scholar]
- 25.Shi A, Huang J, Wang X, Li M, Zhang J, Chen Y, Huang Y. Postoperative D dimer predicts venous thromboembolism in patients undergoing urologic tumor surgery. Urol Oncol. 2018;36:307 e315 307 e321. doi: 10.1016/j.urolonc.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Yao Y, Shen H, Zhou Y, Yang Z, Huang H. Efficacy of thoracoscopic surgery in the treatment of lung cancer in the perioperative period and its effects on serum D-dimer. Oncol Lett. 2018;15:4397–4403. doi: 10.3892/ol.2018.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang B, Liu G, Liu X, Zheng S, Dong K, Dong R. Circulating D-dimer level correlates with disease characteristics in hepatoblastoma patients. Medicine (Baltimore) 2017;96:e8798. doi: 10.1097/MD.0000000000008798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pace A, Mandoj C, Antenucci A, Villani V, Sperduti I, Casini B, Carosi M, Fabi A, Vidiri A, Koudriavtseva T, et al. A predictive value of von Willebrand factor for early response to Bevacizumab therapy in recurrent glioma. J Neurooncol. 2018;138:527–535. doi: 10.1007/s11060-018-2820-x. [DOI] [PubMed] [Google Scholar]
- 29.Khangarot SS, Gupta N, Goswami B, Hadke NS, Lal P, Gupta N, Khurana N. Correlation of D dimer and factor VIII levels with histopathology in patients with breast carcinoma. Cancer Biomark. 2010;7:305–314. doi: 10.3233/CBM-2010-0196. [DOI] [PubMed] [Google Scholar]
- 30.Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, Key NS, Barcel DA, Scheithauer W, Kornek G, et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363–1370. doi: 10.1111/j.1538-7836.2012.04754.x. [DOI] [PubMed] [Google Scholar]
- 31.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 32.Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, Degen JL, Bugge TH. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302–3309. [PubMed] [Google Scholar]
- 33.Batschauer AP, Figueiredo CP, Bueno EC, Ribeiro MA, Dusse LM, Fernandes AP, Gomes KB, Carvalho MG. D-dimer as a possible prognostic marker of operable hormone receptor-negative breast cancer. Ann Oncol. 2010;21:1267–1272. doi: 10.1093/annonc/mdp474. [DOI] [PubMed] [Google Scholar]
- 34.Blackwell K, Haroon Z, Broadwater G, Berry D, Harris L, Iglehart JD, Dewhirst M, Greenberg C. Plasma D-dimer levels in operable breast cancer patients correlate with clinical stage and axillary lymph node status. J Clin Oncol. 2000;18:600–608. doi: 10.1200/JCO.2000.18.3.600. [DOI] [PubMed] [Google Scholar]
- 35.Cui LN, Li N, Fu S, Zhang X, Wang X, Wang RT. Combination of preoperative D-dimer and mean platelet volume predicts postoperative deep venous thrombosis in breast cancer patients. Cancer Biomark. 2018;21:909–913. doi: 10.3233/CBM-170975. [DOI] [PubMed] [Google Scholar]
- 36.Mego M, Zuo Z, Gao H, Cohen EN, Giordano A, Tin S, Anfossi S, Jackson S, Woodward W, Ueno NT, et al. Circulating tumour cells are linked to plasma D-dimer levels in patients with metastatic breast cancer. Thromb Haemost. 2015;113:593–598. doi: 10.1160/TH14-07-0597. [DOI] [PubMed] [Google Scholar]
- 37.Diao D, Cheng Y, Song Y, Zhang H, Zhou Z, Dang C. D-dimer is an essential accompaniment of circulating tumor cells in gastric cancer. BMC Cancer. 2017;17:56. doi: 10.1186/s12885-016-3043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S, Huh SJ, Oh SY, Koh MS, Kim SH, Lee JH, Han JY, Choi HJ, Kim SJ, Kim HJ. Clinical significance of coagulation factors in operable colorectal cancer. Oncol Lett. 2017;13:4669–4674. doi: 10.3892/ol.2017.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stender MT, Larsen TB, Lundbye-Christensen S, Yilmaz MK, Thorlacius-Ussing O. Haemostatis activity in rectal cancer patients exposed to preoperative radiotherapy: A clinical prospective cohort study. Blood Coagul Fibrinolysis. 2009;20:276–282. doi: 10.1097/MBC.0b013e328329e4ae. [DOI] [PubMed] [Google Scholar]
- 40.Durczynski A, Skulimowski A, Hogendorf P, Szymanski D, Kumor A, Marski K, Juliebø SØ, Poznanska G, Strzelczyk J. The concentration of D-dimers in portal blood positively correlates with overall survival in patients with non-resectable pancreatic cancer. World J Surg Oncol. 2017;15:223. doi: 10.1186/s12957-017-1291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao J, Fu Z, Gao L, Wang X, Cheng S, Wang X, Ren H. Evaluation of serum D-dimer, fibrinogen, and CA19-9 for postoperative monitoring and survival prediction in resectable pancreatic carcinoma. World J Surg Oncol. 2017;15:48. doi: 10.1186/s12957-017-1104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in this study are included in this published article.


