Abstract
Inflammation reaction plays an important role in the pathogenesis of ankle fracture. The aim of the present study was to investigate the effect of RvD1 on the inflammatory response and underlying molecular mechanisms in MG-63 cells. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and ELISA were used to determine the mRNA and protein expression of cytokines extracted from peripheral blood in children with or without ankle fracture, respectively. MG-63 cells were pre-treated with/without RvD1 and stimulated with 1 µg/ml LPS. The cell viability was detected by MTT assay. The production of cytokines from MG-63 cells was assessed by RT-qPCR and western blot, respectively. The expression of p-p38, NF-κB (p50) and cyclooxygenase-2 (COX-2) mRNA and protein were detected by western blot and/or RT-qPCR. The levels of NLRP3, associated recruitment domain (ASC), cleaved caspase1, caspase-1 were measured by RT-qPCR and/or western blot. The levels of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF-α) mRNA and protein were up-regulated in children with ankle fracture compared with healthy children. RvD1 treatment did not induce cytotoxicity in MG-63 cells, but it can inhibit LPS induced MG-63 cell proliferation inhibition. RvD1 was able to dose-dependently reverse LPS induced up-regulation of TNF-α, IL-1β, IL-6 mRNA and protein expression. Furthermore, the LPS induced up-regulation of p-p38, NF-κB (p50), and NLRP3, ASC, cleaved caspase-1/caspase-1, and COX-2 was dose-dependently reversed by RvD1. In conclusion, The present study demonstrated that RvD1 inhibited inflammation though inhibiting MAPKp38/NF-κB pathway and NLRP3 inflammasome expression in MG-63 cells, indicating that it may be an effective drug for the treatment of ankle fracture.
Keywords: Resolvin D1, ankle fracture, NF-κB, p-p38, NLRP3 inflammasome
Introduction
Fracture is a very common bone injury. The incidence of fractures in the hands, forearm and feet of children is high, especially in the ankles, and the global accident rate is 187 out of every 100,000 people. However, there are differences in the incidence of fractures in different countries with different genders and ages (1–3). Childhood is the most vigorous and active stage of growth and development in a person's life. The growth and development of the skeletal system is significantly different from adults in terms of tissue anatomy, mechanical properties, injury and healing characteristics. At the same time, due to children's imperfect development of the nervous system, low tolerance, a strong reaction after fracture, manifested as anxiety, crying, kicking, etc., can lead to an extension of the range of fracture damage. Therefore, high-quality care for fractures is especially important. Nursing staff should be familiar with the above characteristics, combined with nursing professional skills and clinical experience, in order to take corresponding measures in the nursing work to prevent various complications and promote fracture healing. Owing to the special nature of children's bones, delay and/or improper treatment of ankle fractures in children may result in skeletal deformities and disability (4). The balance between the activity of osteoblasts and osteoclasts promotes bone formation, and the healing of fracture requires large amounts of osteoblasts (5). There is increasing evidence indicated that inflammation reaction plays an important role in the pathogenesis of ankle fracture. It has been reported that there is a link between osteoclasts and inflammatory cytokines, including a large number of TNF-α and IL-1β produced after fracture (6).
Resolvins (RVs) are a class of anti-inflammatory bioactive small molecules derived from ω-PUFA docosahexaenoic acid (DHA) under natural condition (7). Resolvin D1 (RvD1) is one of the most widely and intensively studied RVs with potent anti-inflammatory and erythropoietic activity (7,8). It has significant efficacy in the treatment of inflammation-related diseases. Pattern Recognition Receptors (PRRs) belong to the innate immune system and are responsible for identifying the pathogen-associated molecular pattern (PAMP) of pathogenic microorganisms, and then activating innate and adaptive immune responses (9). NLRP3 inflammasome is involved in the development of chronic inflammatory responses, which consists of NLRP3, ASC, and caspase-1 (10). Nuclear factor-κB (NF-κB), a significant therapeutic target for the control of inflammatory processes. RvD1 has been reported to inhibit NF-κB signaling pathway and cytokines to protect against ischemia-reperfusion kidney injury (11). Furthermore, RvD1 has been reported to inhibit NLRP3 inflammasome and NF-κB signaling pathway in STZ-induced diabetic retinopathy rats (12). However, the anti-inflammatory role of RvD1 in ankle fracture has not been clarified.
Therefore, the present study investigated the effects of RvD1on inflammatory response using MG-63 cells stimulated with LPS as an inflammatory process model. The findings of current study demonstrated that RvD1 reduced the expression of inflammatory cytokines (IL-1β, IL-6 and TNF-α) in lipopolysaccharide (LPS)-induced MG-63 cells. Moreover, RvD1 down-regulated the expression of p-p38, NF-κB (p50) and NLRP3 inflammasome in MG-63 cells treated with LPS. Therefore, the function of RvD1 may be a new direction for the treatment of ankle fractures.
Materials and methods
Reagents
LPS (Escherichia coli, 0111:B4) was obtained from Sigma-Aldrich; Merck KGaA, (Darmstadt, Germany). RvD1 was purchased from Cayman Chemical Company, (Ann Arbor, MI, USA). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco; Thermo Fisher Scientific, Inc., (Waltham, MA, USA). Primary antibodies: TNF-α (1:1,000; cat. no. 3707), IL-1β (1:1,000; cat. no. 12703), IL-6 (1:1,000; cat. no. 12153), p-p38 (1:1,000; cat. no. 1170), NF-κB (p50) (1:1,000; cat. no. 13586), NLRP3 (1:1,000; cat. no. 15101), ASC (1:1,000; cat. no. 67824), cleaved caspase-1 (1:1,000; cat. no. 4199), caspase-1 (1:1,000; cat. no. 3866), and COX-2 (1:1,000; cat. no. 12282), β-actin (1:5,000; cat. no. 4970), and the anti-rabbit and anti-mouse horseradish peroxidase-linked secondary antibody were purchased from Cell Signaling Technology, Inc., (Danvers, MA, USA). IL-1β (cat. no. E-EL-H0149c), IL-6 (cat. no. E-EL-H0102c) and TNF-α (cat. no. E-EL-H0109c) ELISA kits were purchased from Elabscience Biotechnology Co., Ltd, (Hubei, China).
Patients
A total of 35 peripheral blood samples (2 ml per individual) from 35 children with ankle fracture, as well as 35 peripheral blood samples from 35 healthy children were collected at our hospital from 2016 to 2017. The average age of children with ankle fracture and the healthy controls were 5.5±1.3 and 5.6±1.2 years old, respectively. Equal proportion of boys and girls in both groups. Informed consent was obtained from the parents or guardians of all children enrolled and the present study, and the present study was approved by the Ethics Committee of Children's Hospital Affiliated to Nanjing Medical University.
Cell culture
The human osteoblastic osteosarcoma cell line MG-63 was purchased from the American Type Culture Collection (cat. no. CRL-1427; Manassas, VA, USA). MG-63 cells were cultured in DMEM containing 10% FBS, 0.1 mM nonessential amino acids, 1% penicillin/streptomycin, and 1.0 mM sodium pyruvate (Gibco; Thermo Fisher Scientific, Inc.), and incubated in a humidified environment at 37°C with 5% CO2. For the experiments, MG-63 cells (1×105 cells per well) were seeded into 6-well plates and randomly divided into following six groups: 1) control group (medium only), 2) LPS group (LPS 1 µg/ml), 3) vehicle control+ LPS (0.1% ethanol +LPS 1 µg/ml) 4) RvD1 50+ LPS group (RvD1 50 nM + LPS 1 µg/ml), and 5) RvD1 100+ LPS group (RvD1 100 nM + LPS 1 µg/ml), RvD1 200+ LPS group (RvD1 200 nM + LPS 1 µg/ml). In RvD1 group, cells were pretreated with RvD1 (50, 100, 200 nM) (13) 2 h prior to LPS administration.
Cell viability assay
Cell viability was tested using MTT according to the manufacturer's protocol. Briefly, MG-63 cells (5×104 cells/well) were seeded in 24-well plates, pretreated with different concentrations of RvD1 (50, 100, 200 nM) for 2 h, then these cells were treated with or without 1 µg/ml LPS for 48 h, then removed the medium and incubated with 0.5 mg/ml MTT (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C for 4 h. Then, removed the supernatant and added the DMSO (Sigma-Aldrich; Merck KGaA) for a further 30 min at 37°C in the dark. The absorbance at 540 nm was measured using Bio-Rad absorbance reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
ELISA assay
The supernatants were collected from the peripheral blood of healthy controls and children with ankle fracture by centrifugation at 3,000 × g for 15 min at 4°C. The levels of IL-1β (cat. no. E-EL-H0149c), IL-6 (cat. no. E-EL-H0102c) and TNF-α (cat. no. E-EL-H0109c) in the peripheral blood of healthy controls and children with ankle fracture was detected by ELISA kits according to the manufacturer's protocol.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from MG-63 cells was extracted using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) after 24 h of incubation, according to the manufacturer's protocol. 1 µg of total RNA was reverse-transcribed into cDNA using TaqMan microRNA Reverse Transcription kit (Invitrogen; Thermo Fisher Scientific, Inc), following the manufacturer's protocol. TaqMan® Universal PCR Master Mix kit (Thermo Fisher Scientific, Inc.) was used to analyze the mRNA levels. The amplification conditions were as following: 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 60 sec. The primer sequences used were as following: IL-1β, forward, 5′-TGTGAAATGCCACCTTTTGA-3′ and reverse, 5′-TGAGTGATACTGCCTGCCTG-3′; IL-6, forward, 5′-CCGGAGAGGAGACTTCACAG-3′ and reverse, 5′-CAGAATTGCCATTGCACA-3′; TNF-α, forward, 5′-GAACTGGCAGAAGAGGCACT-3′ and reverse, 5′-GGTCTGGGCCATAGAACTGA-3′; cyclooxygenase (COX)-2, forward, 5′-TCCATTGACCAGAGCAGAGA-3′ and reverse, 5′-TCTGGACGAGGTTTTTCCAC-3′; GAPDH was used as internal controls, forward, 5′-GGCATTGCTCTCAATGACAA-3′ and reverse, 5′-TGTGAGGGAGATGCTCAGTG-3′. The 2−ΔΔCq method was used to quantify relative gene expression (14).
Western blot analysis
Total protein from MG-63 cells and the corresponding cell culture medium was extracted using lysis buffer (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). BCA assay (Thermo Fisher Scientific, Inc.) was used to measure the protein concentrations. Equal amount of proteins (25 µg/lane) were subjected to 10% SDS-PAGE and than electro-transferred onto PVDF membranes (EMD Millipore, Billerica, MA, USA). Then, the membranes were blocked with 5% skim milk for 1 h at room temperature, and subsequently incubated with primary antibodies: TNF-α, IL-1β, IL-6, p-p38, NF-κB (p50), NLRP3, ASC, cleaved caspase-1, caspase-1, COX-2, and β-actin overnight at 4°C. Then the membranes were incubated with an appropriate horseradish peroxidase-linked secondary antibody at room temperature for 1 h. Protein bands were visualized using an enhanced chemiluminescence system (Pierce) according to the manufacturer's instructions and quantified using Quantity One Version 4.6 Image software (Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analyses were performed using SPSS v.19.0 (SPSS, Inc., Chicago, IL, USA). Student's t-tests and one-way ANOVA followed by NSK tests were performed to analyze the differences between groups. Data were expressed as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
Levels of TNF-α, IL-1β and IL-6 in children with ankle fracture are higher than those in healthy children
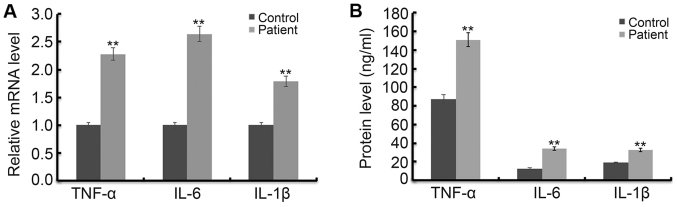
In order to determine the increase in inflammatory response in fractured children, the level of TNF-α, IL-1β and IL-6 was detected using RT-qPCR and western blot assay respectively. As shown in Fig. 1A, the mRNA levels of TNF-α, IL-1β and IL-6 were significantly increased in children with ankle fractures compared with the healthy children. In addition, the results of ELISA assay indicated that protein levels of TNF-α, IL-1β and IL-6 were significantly increased in children with ankle fractures compared with the healthy children (Fig. 1B).
Figure 1.
Levels of TNF-α, IL-1β and IL-6 in children with or without fractured ankle. In order to determine the increase in inflammatory response in fractured children, the level of TNF-α, IL-1β and IL-6 was detected using RT-qPCR and western blot assay. The mRNA (A) and protein (B) levels of TNF-α, IL-1β and IL-6 in children with or without fractured ankle were detected by RT-qPCR and ELISA, respectively. **P<0.01 vs. Control.Control, healthy children; Patient, children with fractured ankle.
RvD1 represses LPS induced proliferation inhibition in MG-63 cells
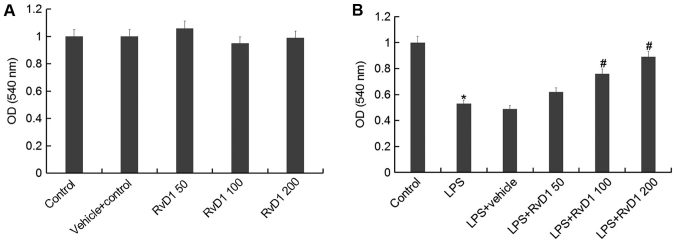
To study whether RvD1 has toxic effects on the human osteoblastic osteosarcoma cell line MG-63, MG-63 cells were pretreated with different concentrations of RvD1 (50, 100, 200 nM) for 2 h, then these cells were treated with or without 1 µg/ml LPS for 48 h. Then, cell viability was detected using MTT. The results showed that there was no significant changes in absorbance between RvD1 treatment groups and the control group, indicating that RvD1 did not induce cytotoxicity in MG-63 cells (Fig. 2A). However, compared with the control group, cell viability was significantly reduced by LPS treatment, and these reductions were markedly reversed by RvD1 treatment, indicating RvD1 inhibited LPS mediated cell proliferation inhibition (Fig. 2B).
Figure 2.
RvD1 shows no cytotoxicity on MG-63 cells. To study whether RvD1 has toxic effects on the human osteoblastic osteosarcoma cell line MG-63, MG-63 cells were pretreated with different concentrations of RvD1 (50, 100, 200 nM) for 2 h, then these cells were treated without (A) or with (B) 1 µg/ml LPS for 48 h. Then, cell viability of MG-63 cells was measured by MTT assay. Control, Cells Without any treatment; Vehicle control, Cells treated with 0.1% ethanol; RvD1 50, RvD1 100, RvD1 200: 50, 100 and 200 nM RvD1 treatment group. LPS, 1 µg/ml LPS treatment group; LPS + vehicle, 1 µg/ml LPS + 0.1% ethanol treatment group; LPS + RvD1 50, 1 µg/ml LPS + 50 nM RvD1 treatment group; LPS + RvD1 100, 1 µg/ml LPS + 100 nM RvD1 treatment group; LPS + RvD1 200, 1 µg/ml LPS + 200 nM RvD1 treatment group. *P<0.05 vs. Control; #P<0.05 vs. LPS. RvD1, resolvin D1.
RvD1 significantly inhibits the LPS induced increase of TNF-α, IL-1β and IL-6
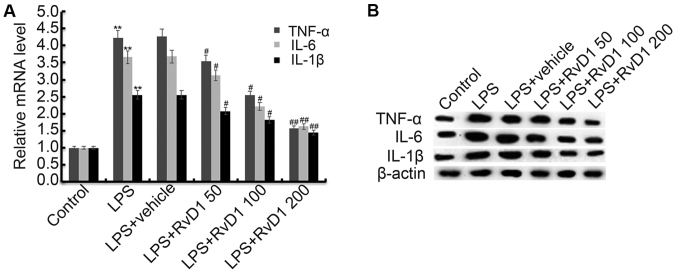
We then investigated the effect of RvD1 on inflammatory response of MG-63 cells. MG-63 cells were pretreated with different concentrations of RvD1 (50, 100, 200 nM) for 2 h, then these cells were treated with or without 1 µg/ml LPS for 48 h. The mRNA and protein levels of TNF-α, IL-1β and IL-6 were determined using RT-qPCR and western blot respectively. As shown in Fig. 3, the mRNA and protein levels of TNF-α, IL-1β and IL-6 were significantly increased in the LPS treatment alone group compare with the control group, and these increases were dose-dependently reversed following treatment with RvD1.
Figure 3.
Effect of RvD1 on TNF-α, IL-1β and IL-6 expression. MG-63 cells were pretreated with different concentrations of RvD1 (50, 100, 200 nM) for 2 h, then these cells were treated with or without 1 µg/ml LPS for 48 h. The mRNA (A) and protein (B) levels of TNF-α, IL-1β and IL-6 were determined using RT-qPCR and western blot respectively. Control, Cells without any treatment; LPS, 1 µg/ml LPS treatment group; LPS + vehicle, 1 µg/ml LPS + 0.1% ethanol treatment group; LPS + RvD1 50, 1 µg/ml LPS + 50 nM RvD1 treatment group; LPS + RvD1 100, 1 µg/ml LPS + 100 nM RvD1 treatment group; LPS + RvD1 200, 1 µg/ml LPS + 200 nM RvD1 treatment group. **P<0.01 vs. Control; #P<0.05 vs. LPS; ##P<0.01 vs. LPS. RvD1, resolvin D1.
RvD1 significantly inhibits the LPS induced activation of p38, NF-κB (p50) and increase of COX-2
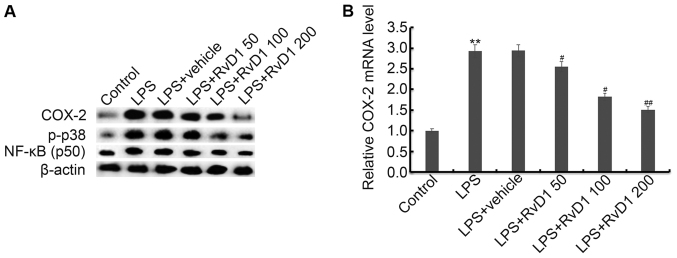
To investigate the molecular mechanism of anti-inflammatory effect of RD1 on LPS treated MG-63 cells, p38, NF-κB (p50) and COX-2 were analyzed in our present study. As shown in Fig. 4, the protein levels of p-p38, and NF-κB (p50) were significantly up-regulated compare with the control group. And the mRNA and protein levels of COX-2 markedly up-regulated compare with the control group. RvD1 treatment markedly inhibited the LPS induced up-regulation of p-p38, NF-κB (p50) and COX-2 expression.
Figure 4.
Effect of RvD1 on activation of p38MAPK-NF-κB pathway and COX-2 expression. MG-63 cells were pretreated with different concentrations of RvD1 (50, 100, 200 nM) for 2 h, then these cells were treated with or without 1 µg/ml LPS for 48 h. Then, protein (A) levels of COX-2, p-p38, NF-κB (p50) and p-p65 were detected by western blot analysis. The mRNA level of COX-2 was detected using RT-qPCR (B). Control, Cells without any treatment; LPS, 1 µg/ml LPS treatment group; LPS + vehicle, 1 µg/ml LPS + 0.1% ethanol treatment group; LPS + RvD1 50, 1 µg/ml LPS + 50 nM RvD1 treatment group; LPS + RvD1 100, 1 µg/ml LPS + 100 nM RvD1 treatment group; LPS + RvD1 200, 1 µg/ml LPS + 200 nM RvD1 treatment group. **P<0.01 vs. Control; #P<0.05 vs. LPS; ##P<0.01 vs. LPS. RvD1, resolvin D1
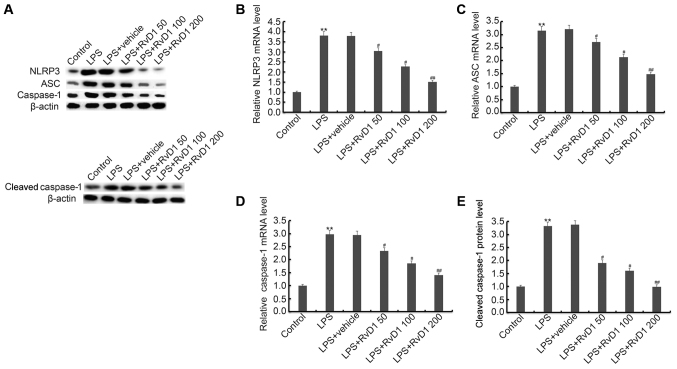
RvD1 markedly inhibits the LPS induced activation of NLRP3 inflammasome
Previous studies indicated that NLRP3 inflammasome activation can be inhibited by RvD1. Therefore, in our study we finally investigated the effect of RvD1 on NLRP3 inflammasome activation in MG-63 cells. Our results indicated that compared with the control group, the expression of NLRP3, ASC, cleaved caspase-1 and caspase-1 were all markedly up-regulated in the LPS treatment alone group. RvD1 treatment markedly inhibited LPS induced up-regulation of NLRP3, ASC, cleaved caspase-1 and caspase-1 (Fig. 5).
Figure 5.
(A-E) Effect of RvD1 on NLRP3 inflammasome. MG-63 cells were pretreated with different concentrations of RvD1 (50, 100, 200 nM) for 2 h, then these cells were treated with or without 1 µg/ml LPS for 48 h. Then, protein (A, E) level of NLRP3, ASC and cleaved caspase-1/caspase-1, and mRNA (B-D) levels of NLRP3, ASC and caspase-1 were detected by western blot analysis and RT-qPCR. Control, cells without any treatment; LPS, 1 µg/ml LPS treatment group; LPS + vehicle, 1 µg/ml LPS + 0.1% ethanol treatment group; LPS + RvD1 50, 1 µg/ml LPS + 50 nM RvD1 treatment group; LPS + RvD1 100, 1 µg/ml LPS + 100 nM RvD1 treatment group; LPS + RvD1 200, 1 µg/ml LPS + 200 nM RvD1 treatment group. **P<0.01 vs. Control; #P<0.05 vs. LPS; ##P<0.01 vs. LPS. RvD1, resolvin D1.
Discussion
In the present study, we demonstrated that the levels of IL-1β, IL-6 and TNF-α were up-regulated in children with ankle fracture compared with the healthy children. Pretreatment of RvD1 dose-dependently reversed the LPS induced up-regulation of IL-1β, IL-6, TNF-α, and COX-2 mRNA and protein levels, and also decreased expression of the p-p38, NF-κB (50), and NLRP3 inflammasome, including NLRP3, ASC, cleaved caspase-1/caspase-1 induced by LPS treatment in MG-63 cells. These findings suggested that RvD1 may relieve ankle fracture by inhibiting inflammatory response via repressing NLRP3 inflammasome and NF-κB signaling pathway. The anti-inflammatory function of RvD1 may reduce osteoclast and increase osteoblast thus promoting bone formation. Therefore, the present study identified a potential new method for the treatment of ankle fractures.
Inflammation plays a critical role in ankle fracture. Persistent elevation of inflammatory cytokines such as IL-1β, IL-6 and TNF-α is associated with poor prognosis in children with ankle fracture (15). LPS administration significantly increased the level of IL-1β, IL-6 and TNF-α, which was used to establish the cellar inflammation model of ankle fractures. RvD1 has been shown to down-regulated the level of TNF-α and IL-6 in BALF of mice with LPS induced ALI (16). In the present study, we found that RvD1 dose-dependently down-regulated level of IL-1β, IL-6 and TNF-α in LPS induced MG-63 cells.
In order to better understand the immediate cellular response to an ankle fracture and further study the anti-inflammatory function by the application of RvD1, we hypothesized that it may be possible to counteract the inflammatory signaling pathway involved in the injury-mediated response by RvD1. NLRP plays a critical role in the development of chronic inflammatory response through the up-regulation of the pro-inflammatory cytokines IL-18 and IL-1β (17). Activation of the NLRP3 inflammasome has been demonstrated in a wide variety of diseases, including liver injury (18). Previous study has reported that RvD1 abrogates hHcys-induced podocyte injury by inhibition of the NLRP3 inflammasome activation (19). RvD1 plays a protective role in STZ induced diabetic retinopathy by inhibiting the level of activation of the NLRP3 inflammasome (12). Our results demonstrated that RvD1 inhibited the expression of NLRP3, ASC, cleaved caspase-1 and caspase-1 induced by LPS in MG-63 cells. There results indicated that RvD1 alleviated ankle fracture may be by inhibiting the NLRP3 inflammasome.
NF-κB, a ubiquitously expressed transcription factor that regulates many inflammatory cytokines. It has been reported that activation of NF-κB participates in NLRP3 inflammasome activation (20). Previous study has reported that RvD1 markedly reduced acute lung injury associated mortality through inhibiting the activation of NF-κB pathway (21). In addtion, RvD1 has been shown to regulate NF-κB activation to reduce mucosal inflammation (22). Furthermore, RvD1 markedly inhibited IMQ induced activation of ERK1/2, p38, JNK (c-Jun N-terminal protein kinase, a subfamily of MAPKs), and NF-κB (23). Our present study found that RvD1 could repress LPS induced NF-κB and p38 activation.
Ankle injury commonly result in persistent pain, muscle wasting, functional impairments and stiffness (24). Till now, there is insufficient evidence to favor any particular rehabilitation approach after ankle fracture. The key to reducing pain and fracture healing is timely diagnosis, treatment and high-quality care. The Ottawa Ankle Rule (OAR) (25,26) has been reported to help to determine whether radiography suitable for the diagnosis of ankle fracture in children with ankle pain. However, when pediatric emergency department (ED) nurses accurately apply and interpret OAR, children in the hospital ED receive treatment from the nurse Cooperative Practice Program (CPP) to minimize ankle radiography, waiting times, and costs without an increased rate of missed fractures (27).
In summary, the present study demonstrated that RvD1 decreased the level of IL-1β, IL-6 and TNF-α in LPS treated MG-63 cells, which may be mediated by inhibiting the activation of p38/NF-κB/NLRP3 inflammation signaling pathway, may therefore relieve ankle fracture. RvD1 may be a potential therapeutic agent for ankle fracture treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DC, JP and YS designed the study, and performed data collection and analysis. YT and PZ were responsible for interpreting results. All authors collaborated to develop the manuscript.
Ethics approval and consent to participate
Written informed consent was obtained from the parents or guardians of all children enrolled and the present study, and the present study was approved by the Ethics Committee of Children's Hospital Affiliated to Nanjing Medical University.
Patient consent for publication
All patients provided consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.MacIntyre NJ, Dewan N. Epidemiology of distal radius fractures and factors predicting risk and prognosis. J Hand Ther. 2016;29:136–145. doi: 10.1016/j.jht.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Moon RJ, Harvey NC, Curtis EM, de Vries F, van Staa T, Cooper C. Ethnic and geographic variations in the epidemiology of childhood fractures in the United Kingdom. Bone. 2016;85:9–14. doi: 10.1016/j.bone.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hedstrom EM, Svensson O, Bergström U, Michno P. Epidemiology of fractures in children and adolescents. Acta Orthop. 2010;81:148–153. doi: 10.3109/17453671003628780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chevalley T, Bonjour JP, van Rietbergen B, Rizzoli R, Ferrari S. Fractures in healthy females followed from childhood to early adulthood are associated with later menarcheal age and with impaired bone microstructure at peak bone mass. J Clin Endocrinol Metab. 2012;97:4174–4181. doi: 10.1210/jc.2012-2561. [DOI] [PubMed] [Google Scholar]
- 5.He Z, Selvamurugan N, Warshaw J, Partridge NC. Pulsed electromagnetic fields inhibit human osteoclast formation and gene expression via osteoblasts. Bone. 2018;106:194–203. doi: 10.1016/j.bone.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Huang H, Zhao N, Xu X, Xu Y, Li S, Zhang J, Yang P. Dose-specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 2011;44:420–427. doi: 10.1111/j.1365-2184.2011.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 9.Werners AH, Bryant CE. Pattern recognition receptors in equine endotoxaemia and sepsis. Equine Vet J. 2009;44:490–498. doi: 10.1111/j.2042-3306.2012.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori MA, Bezy O, Kahn CR. Metabolic syndrome: Is Nlrp3 inflammasome a trigger or a target of insulin resistance? Circ Res. 2011;108:1160–1162. doi: 10.1161/RES.0b013e318220b57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 12.Yin Y, Chen F, Wang W, Wang H, Zhang X. Resolvin D1 inhibits inflammatory response in STZ-induced diabetic retinopathy rats: Possible involvement of NLRP3 inflammasome and NF-κB signaling pathway. Mol Vis. 2017;23:242–250. [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Gao X, Yang C, Chen L, Chen Z. Resolvin D1 attenuates Mpp+-induced parkinson disease via inhibiting inflammation in PC12 Cells. Med Sci Monit. 2017;23:2684–2691. doi: 10.12659/MSM.901995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Adams SB, Leimer EM, Setton LA, Bell RD, Easley ME, Huebner JL, Stabler TV, Kraus VB, Olson SA, Nettles DL. Inflammatory microenvironment persists after bone healing in intra-articular ankle fractures. Foot Ankle Int. 2017;38:479–484. doi: 10.1177/1071100717690427. [DOI] [PubMed] [Google Scholar]
- 16.Liao Z, Dong J, Wu W, Yang T, Wang T, Guo L, Chen L, Xu D, Wen F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARγ/NF-κB pathway. Respir Res. 2012;13:110. doi: 10.1186/1465-9921-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 18.Menzel CL, Sun Q, Loughran PA, Pape HC, Billiar TR, Scott MJ. Caspase-1 is hepatoprotective during trauma and hemorrhagic shock by reducing liver injury and inflammation. Mol Med. 2011;17:1031–1038. doi: 10.2119/molmed.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G, Chen Z, Bhat OM, Zhang Q, Abais-Battad JM, Conley SM, Ritter JK, Li PL. NLRP3 inflammasome as a novel target for docosahexaenoic acid metabolites to abrogate glomerular injury. J Lipid Res. 2017;58:1080–1090. doi: 10.1194/jlr.M072587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao A, Wu H, Hong Y, Tu S, Sun X, Wu Q, Zhao Q, Zhang J, Sheng J. Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: possible involvement of NF-κB pathway and NLRP3 inflammasome. Mol Neurobiol. 2016;53:3462–3476. doi: 10.1007/s12035-015-9242-y. [DOI] [PubMed] [Google Scholar]
- 21.Wang B, Gong X, Wan JY, Zhang L, Zhang Z, Li HZ, Min S. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm Pharmacol Ther. 2011;24:434–441. doi: 10.1016/j.pupt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Colby JK, Abdulnour RE, Sham HP, Dalli J, Colas RA, Winkler JW, Hellmann J, Wong B, Cui Y, El-Chemaly S, et al. Resolvin D3 and aspirin-triggered resolvin D3 are protective for injured epithelia. Am J Pathol. 2016;186:1801–1813. doi: 10.1016/j.ajpath.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Duan X, Hu F, Poorun D, Liu X, Wang X, Zhang S, Gan L, He M, Zhu K, et al. Resolvin D1 attenuates imiquimod-induced mice psoriasiform dermatitis through MAPKs and NF-κB pathways. J Dermatol Sci. 2018;89:127–135. doi: 10.1016/j.jdermsci.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Zwipp H, Hoffmann R, Thermann H, Wippermann BW. Rupture of the ankle ligaments. Int Orthop. 1991;15:245–249. doi: 10.1007/BF00192302. [DOI] [PubMed] [Google Scholar]
- 25.Stiell IG, Greenberg GH, McKnight RD, Nair RC, McDowell I, Reardon M, Stewart JP, Maloney J. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269:1127–1132. doi: 10.1001/jama.1993.03500090063034. [DOI] [PubMed] [Google Scholar]
- 26.Stiell IG, McKnight RD, Greenberg GH, McDowell I, Nair RC, Wells GA, Johns C, Worthington JR. Implementation of the Ottawa ankle rules. JAMA. 1994;271:827–832. doi: 10.1001/jama.271.11.827. [DOI] [PubMed] [Google Scholar]
- 27.Karpas A, Hennes H, Walsh-Kelly CM. Utilization of the Ottawa ankle rules by nurses in a pediatric emergency department. Acad Emerg Med. 2002;9:130–133. doi: 10.1197/aemj.9.2.130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.