We evaluated the in vivo efficacy of human-simulated WCK 5222 (cefepime-zidebactam) against cefepime-resistant Acinetobacter baumannii strains (n = 13) in the neutropenic murine lung infection model. Twelve isolates were meropenem resistant.
KEYWORDS: Acinetobacter, carbapenem resistant, lung infection
ABSTRACT
We evaluated the in vivo efficacy of human-simulated WCK 5222 (cefepime-zidebactam) against cefepime-resistant Acinetobacter baumannii strains (n = 13) in the neutropenic murine lung infection model. Twelve isolates were meropenem resistant. In control animals and those that received cefepime or zidebactam alone, the mean bacterial growth at 24 h was >2 log10 CFU/lung compared with 0-h controls (6.32 ± 0.33 log10 CFU/lung). WCK 5222 produced a decline in the bacterial burden for all isolates (mean reduction, −3.34 ± 0.85 log10 CFU/lung) and demonstrated remarkable potency.
TEXT
Pneumonia caused by carbapenem-resistant Acinetobacter baumannii is associated with increased mortality compared with carbapenem-susceptible cases (1, 2). Combination antimicrobial therapy is a preferred treatment modality, yet synergy observed in vitro has inconsistently translated to improved patient outcomes (1). Thus, novel agents are needed to combat these multidrug-resistant pulmonary pathogens. One such agent undergoing development, cefepime-zidebactam combination (WCK 5222; Wockhardt Bio AG, Switzerland), has displayed increased in vitro activity compared with cefepime alone against Gram-negative pathogens (3). Zidebactam inhibits Ambler class A and C β-lactamases and has intrinsic activity against Enterobacteriaceae and Pseudomonas aeruginosa via the inhibition of penicillin binding protein 2 (PBP-2) (3, 4). Although zidebactam has no direct antibacterial activity against A. baumannii, bacterial killing is observed when combined with cefepime. This activity is secondary to the β-lactam-enhancing effect of zidebactam, mediated through complementary PBP binding, as cefepime inhibits PBP-1a and PBP-3, while zidebactam inhibits PBP-2 (5).
The purpose of this study was to describe the in vivo efficacy of human-simulated WCK 5222 over 24 h against A. baumannii strains (n = 13, Table 1) in a neutropenic murine lung infection model (6). All strains were meropenem resistant, with the exception of ACBN 163. Female ICR mice weighing 20 to 22 g (Envigo RMS, Inc., Frederick, MD), cefepime hydrochloride with arginine (Qilu Antibiotics, Jinan, China), and zidebactam (Wockhardt Bio AG, Switzerland) were used throughout the study. For in vivo assessments, six mice comprised each pharmacokinetic time point and each treatment and control group in the efficacy analyses. Animals were administered all treatments by subcutaneous injection (0.1 to 0.2 ml), and the protocol was approved by the Hartford Hospital Institutional Animal Care and Use Committee. The MICs of cefepime, zidebactam, and WCK 5222 (cefepime and zidebactam, 1:1 concentration ratio) were determined in triplicate by broth microdilution using cation-adjusted Mueller-Hinton broth, in accordance with Clinical and Laboratory Standards Institute procedures (7).
TABLE 1.
Phenotypic profiles and resistance mechanisms of isolates selected for the in vivo efficacy studies
| Isolatea | β-Lactamases encoded | Resistance genes detected | MIC by treatment (mode [range]) (μg/ml)b |
|
|---|---|---|---|---|
| Cefepime | WCK 5222 | |||
| ACBN 160 | OXA-24, OXA-65, TEM-1B | aac(3)-IIa, strA, strB, sul2 | >512 (512, >512) | 32 (32, 32) |
| ACBN 163 | TEM-1D, ADC-25, OXA-66 | aac(3)-Ia, aph(3′)-Ic, strA, strB, sul1 | 32 (32, 64) | 16 (16, 16) |
| ACBN 171 | ADC-25, OXA-23, OXA-66 | armA, catB8, mph(E), msr(E), strA, strB, sul1 | 256 (256, 256) | 64 (64, 64) |
| ACBN 179 | ADC-25, OXA-23, OXA-223 | aadA2, aadB, sul1 | 256 (256, 256) | 32 (32, 32) |
| ACBN 182 | PER-7, OXA-23, OXA-203 | aph(3′)-VIa, armA, ARR-3, cmlA1, mph(E), msr(E), strA, strB, sul1, sul2, tet(B) | 256 (256, 512) | 16 (16, 32) |
| ACBN 189 | OXA-24, OXA-65, TEM-1B | aac(3)-IIa, strA, strB, sul2 | 128 (128, 256) | 32 (32, 32) |
| ACBN 194 | ADC-25, OXA-23, OXA-82 | aph(3′)-Ic, armA, catB8, mph(E), msr(E), strA, strB, sul1 | 512 (512, >512) | 16 (16, 32) |
| ACBN JJ1-1 | ADC-81-like, OXA-24, OXA-65, TEM-1 | aac(3)-IIa, aac(6′)-Ian, aph(3′)-VIa-like, aph(6)-Ia, aph(6)-Id, sul2 | >512 (>512, >512) | 64 (64, 64) |
| ACBN JJ3-20 | ADC-81-like, OXA-24, OXA-65, TEM-1 | aac(3)-IIa, aac(6′)-Ian, aph(6)-Ia, aph(6)-Id, sul2 | 512 (512, 512) | 32 (32, 32) |
| ACBN JJ4-25 | ADC-30, OXA-66, OXA-72 | aac(3)-I, aacA16, aadA1, aph(6)-Ia, aph(6)-Id, sul2, tet(B) | 256 (256, 256) | 64 (64, >64) |
| ACBN JJ5-13 | ADC-33, OXA-23, OXA-82 | aac(3)-I, ant(3″)-Ia, sul1 | 256 (256, 512) | 32 (32, 64) |
| ACBN JJ12-1 | ADC-81-like, OXA-23, OXA-69 | armA, mph(E), msr(E), sul1 | 256 (128, 256) | 32 (32, 32) |
| ACBN JJ13-11 | ADC-96-like, CARB-16, OXA-10, OXA-23-like, OXA-58, OXA-68, OXA-72 | aac(3)-IId, aadA1, ant(2″)-Ia, aph(6)-Ia, aph(6)-Id, arr-2, cmlA5, floR, mph(E), msr(E), sul1, sul2, tet(X) | >512 (>512, >512) | 32 (32, 32) |
Isolates with prefix “JJ” originated from a clinical respiratory culture (each from a different U.S. hospital system to minimize the inclusion of clonal isolates) and were selected from the Center for Anti-Infective Research and Development library; genotypic profiling was performed by JMI Laboratories (North Liberty, IA). All other isolates were acquired from and phenotypically and molecularly characterized by the FDA-CDC Antimicrobial Resistance Isolate Bank (Atlanta, GA).
Modal zidebactam MIC was >512 μg/ml for all isolates studied.
Plasma and lung epithelial lining fluid (ELF) pharmacokinetic analyses were performed following intranasal inoculation with a meropenem-susceptible A. baumannii strain, and the methods were consistent with those we have previously described (6, 8). Drug concentrations in plasma and bronchoalveolar lavage (BAL) fluid were determined using a validated liquid chromatography-tandem mass spectrometry method (9); drug concentrations in ELF were determined following normalization of drug concentrations in BAL fluid to the urea content in BAL fluid and plasma (8). Following administration of zidebactam single doses at 12.5 mg/kg and 37.5 mg/kg of body weight (based on mean body weight of the study population), the ELF-to-plasma penetration ratios were 0.81 and 0.91, respectively, calculated by dividing the ELF 24-h area under the concentration-time curve (AUC0–24) by the calculated murine plasma unbound (free) drug AUC0–24 (fAUC0–24). Murine zidebactam plasma pharmacokinetic parameters were best described by a one-compartment model (WinNonlin v5.0.1; Pharsight Corp., Mountain View, CA), yielding a volume of distribution (V) of 0.23 liter/kg, absorption rate constant (ka) of 6.95 h−1, and elimination rate constant (ke1) of 0.72 h−1.
Cefepime, zidebactam, and WCK 5222 human-simulated regimens used in the efficacy analyses were constructed to describe free drug concentrations observed in humans following 1-h intravenous infusions of 2 g cefepime and 1 g zidebactam administered every 8 h in phase 1 studies (Wockhardt, Ltd., unpublished data). Specifically, these murine regimens were based on the percentage of the dosing interval during which the unbound drug concentrations exceed the MIC (%fT>MIC) in human plasma (Table 2) following administration of the aforementioned target doses. Murine cefepime pharmacokinetic parameters that we derived previously were used to determine the cefepime regimen, accounting for differences in protein binding in humans and mice (10); for zidebactam, the parameters reported above were utilized, accounting for murine (12.6%) and human (4.7%) protein binding (Wockhardt, Ltd., unpublished data). Confirmatory plasma pharmacokinetic studies demonstrated that murine free plasma exposures predicted by the mathematical model were achieved following administration of the human-simulated regimens. The cefepime human-simulated regimen was confirmed first (Fig. 1), and the zidebactam regimen was confirmed in a separate study dosed in combination with the confirmed cefepime regimen (Fig. 2). There was no evidence of pharmacokinetic interaction between cefepime and zidebactam in plasma or ELF (Fig. 1).
TABLE 2.
Comparison of %fT>MIC values achieved in human plasma and murine plasma after treatmenta
| MIC (μg/ml) | Plasma %fT>MIC for: |
|||
|---|---|---|---|---|
| Cefepime |
Zidebactam |
|||
| Humans | Mice | Humans | Mice | |
| 4 | 100.00 | 100.00 | 92.50 | 92.50 |
| 8 | 92.92 | 92.50 | 67.50 | 70.00 |
| 16 | 66.25 | 66.25 | 42.08 | 41.25 |
| 32 | 41.25 | 41.25 | 19.17 | 20.42 |
| 64 | 18.33 | 19.58 | 0.00 | 3.75 |
| 128 | 0.00 | 0.00 | 0.00 | 0.00 |
Cefepime (2 g) and zidebactam (1 g) were administered to humans intravenously over 1 h every 8 h in a phase 1 study. Human-simulated regimens were administered subcutaneously to mice.
FIG 1.
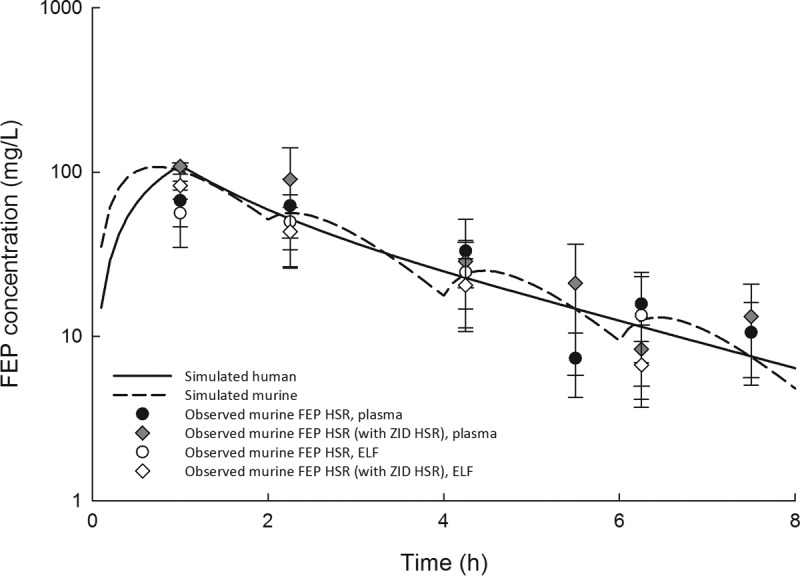
Observed cefepime (FEP) free plasma and epithelial lining fluid (ELF) concentrations in the neutropenic lung infection model following dosing of the cefepime human-simulated regimen (42 mg/kg at 0 h, 9 mg/kg at 2 h, 6 mg/kg at 4 h, and 3 mg/kg at 6 h, every 8 h) alone or in combination with that of zidebactam compared with the simulated human cefepime exposure following a 2-g 1-hour intravenous infusion.
FIG 2.
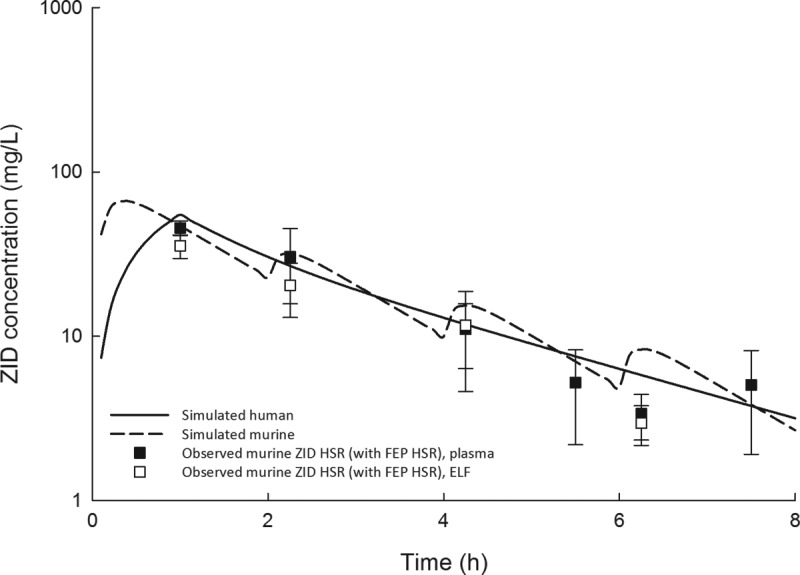
Observed zidebactam (ZID) free plasma and epithelial lining fluid (ELF) concentrations in the neutropenic lung infection model following dosing of the zidebactam human-simulated regimen (22.5 mg/kg at 0 h, 4.5 mg/kg at 2 h, 2.5 mg/kg at 4 h, and 1.5 mg/kg at 6 h, every 8 h) and the cefepime human-simulated regimen compared with the simulated human zidebactam exposure following a 1-g 1-hour intravenous infusion.
In the 24-h efficacy studies, groups of animals received either human-simulated cefepime alone, human-simulated zidebactam alone, saline injections at each treatment time point (i.e., 24-h controls), or human-simulated WCK 5222 (i.e., the combination of cefepime and zidebactam human-simulated regimens, administered as a cefepime dose followed immediately by a separate injection of the corresponding zidebactam dosage). Efficacy was defined as the change in log10 CFU/lung at 24 h from the 0-h control groups, composed of animals sacrificed 2 h postinoculation with each isolate (mean among all isolates, 6.32 ± 0.33 log10 CFU/lung). While the control and monotherapy groups experienced bacterial growth over 24 h for each isolate, WCK 5222 produced a decline in bacterial burden (mean bacterial reduction, −3.34 ± 0.75 log10 CFU/lung; Fig. 3). WCK 5222 produced substantial bacterial killing against 13 genotypically diverse A. baumannii isolates, inclusive of the isolates that expressed OXA-23/24, oxacillinases often responsible for carbapenem resistance in A. baumannii (11). Moreover, four isolates harbored armA, which encodes a methyltransferase that confers high-level resistance to all aminoglycosides (12).
FIG 3.
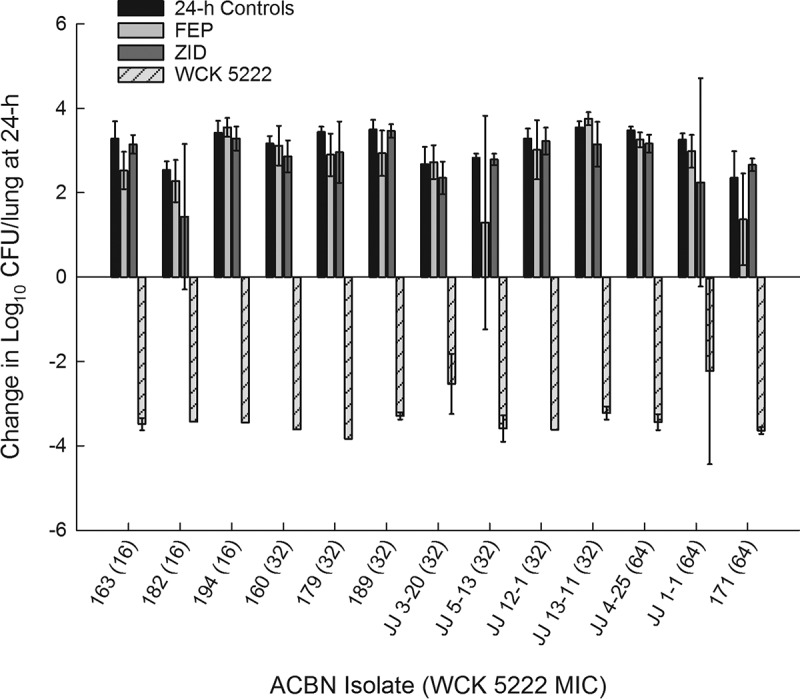
Average change in log10 CFU/lung (± standard deviation) at 24 h from 0 h burden in a neutropenic murine lung infection model.
The pharmacodynamic driver of cephalosporin efficacy is %fT>MIC, and 50 to 70% is the target exposure for bactericidal activity (13). We demonstrated that WCK 5222 freely penetrated the murine ELF compartment without preferential accumulation. Thus, it is remarkable that potent bacterial killing with human-simulated WCK 5222 was observed for isolates with MICs of 64 μg/ml when the %fT>MIC in plasma was only ∼20% and ∼4% for cefepime and zidebactam, respectively. This in vivo potentiation of cefepime activity is discordant with a previously reported observation of minimal in vitro potentiation in A. baumannii (3). This may be secondary to the limitations of an MIC-based assessment of synergy for β-lactam–β-lactam enhancer combinations or the use of artificial media that may underestimate the in vivo potency of antimicrobials (14–16). Moreover, Bhagwat and colleagues proposed that WCK 5222 activity correlated with novel markers of antibacterial efficacy (17). Cefepime concentrations that were substantially lower than the MIC induced bacterial cell elongation, and this minimum elongation concentration (MEC) predicted efficacy in combination with zidebactam. Likewise, zidebactam induced spheroplast formation, and the minimum spheroplastation concentration (MSC) was reportedly associated with efficacy against A. baumannii. Evidence of pharmacodynamic relevance of these novel markers was based on the observation that rather than the MIC, these sub-MICs (MSC and MEC) provided an optimal exposure-response correlation (17). The MEC and MSC values were not assessed in this study, as our experiments were not designed to further validate this theory.
To summarize, human-simulated WCK 5222 demonstrated potent antibacterial activity against carbapenem-susceptible and carbapenem-resistant A. baumannii in the neutropenic murine lung infection model. Importantly, the extent of activity observed with the addition of zidebactam to cefepime was greater than expected, as each drug alone displayed a lack of antibacterial activity against all isolates tested. These observations support the role of zidebactam as a β-lactam enhancer. Overall, the results of this in vivo pharmacodynamic assessment support the continued clinical development of WCK 5222 for the treatment of lung infections caused by A. baumannii.
ACKNOWLEDGMENTS
We thank Sara Giovagnoli, whose technical expertise played an integral role in completing the work. We also thank Safa Abuhussain, Tomefa Asempa, Janice Cunningham, Elizabeth Cyr, Kim Greenwood, Michelle Insignares, James Kidd, Lauren McLellan, Alissa Padgett, Debora Santini, Sean Stainton, Christina Sutherland, and Jennifer Tabor-Rennie from the Center for Anti-Infective Research and Development, Hartford, CT, for their dedication to exceptional technical quality and assistance with conducting this study.
This study was funded by Wockhardt Bio AG, Switzerland.
REFERENCES
- 1.Wenzler E, Goff DA, Humphries R, Goldstein EJC. 2017. Anticipating the unpredictable: a review of antimicrobial stewardship and Acinetobacter infections. Infect Dis Ther 6:149–172. doi: 10.1007/s40121-017-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng YL, Wan YF, Zhou LY, Ye ML, Liu S, Xu CQ, He YQ, Chen JH. 2013. Risk factors and mortality of patients with nosocomial carbapenem-resistant Acinetobacter baumannii pneumonia. Am J Infect Control 41:e59–e63. doi: 10.1016/j.ajic.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N. 2017. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother 72:1373–1385. doi: 10.1093/jac/dkw593. [DOI] [PubMed] [Google Scholar]
- 4.Sader HS, Rhomberg PR, Flamm RK, Jones RN, Castanheira M. 2017. WCK 5222 (cefepime/zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant β-lactamases. J Antimicrob Chemother 72:1696–1703. doi: 10.1093/jac/dkx050. [DOI] [PubMed] [Google Scholar]
- 5.Palwe SR, Biniwale SS, Khande HN, Joshi PR, Bhagwat SS, Patel MV. 2016. Cefepime (FEP) and zidebactam (ZID) mediated dual PBP engagement at sub-MIC concentrations drive cidality against diverse β-lactamase expressing Gram-negatives, abstr 448. ASM Microbe 2016, 16 to 20 June 2016, Boston, MA. [Google Scholar]
- 6.Housman ST, Crandon JL, Nichols WW, Nicolau DP. 2014. Efficacies of ceftazidime-avibactam and ceftazidime against Pseudomonas aeruginosa in a murine lung infection model. Antimicrob Agents Chemother 58:1365–1371. doi: 10.1128/AAC.02161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing, 28th ed CLSI document M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.Keel RA, Crandon JL, Nicolau DP. 2012. Pharmacokinetics and pulmonary disposition of tedizolid and linezolid in a murine pneumonia model under variable conditions. Antimicrob Agents Chemother 56:3420–3422 doi: 10.1128/AAC.06121-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patil K, Tambe H, Zope V, Chavan R, Yeole R, Patel M. 2018. Simultaneous determination of zidebactam and cefepime in dog plasma by LC-MS/MS and its application to pre-clinical pharmacokinetic study. Biomed Chromatogr 32:e4249. doi: 10.1002/bmc.4249. [DOI] [PubMed] [Google Scholar]
- 10.Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. 2017. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of Gram-negative bacteria in the murine thigh infection model. Antimicrob Agents Chemother 61:e01022-17. doi: 10.1128/AAC.01022-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee C-R, Lee J, Park M, Park K, Bae IK, Kim Y, Cha C-J, Jeong B, Lee S. 2017. Biology of Acinetobacter baumannii: pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front Cell Infect Microbiol 7:1–35. doi: 10.3389/fcimb.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaiskos I, Souli M, Giamarellou H. 2015. Plazomicin: an investigational therapy for the treatment of urinary tract infections. Expert Opin Invest Drugs 24:1501–1511. doi: 10.1517/13543784.2015.1095180. [DOI] [PubMed] [Google Scholar]
- 13.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 14.Lin L, Nonejuie P, Munguia J, et al. 2015. Azithromycin synergizes with cationic antimicrobial peptides to exert bactericidal and therapeutic activity against highly multidrug-resistant Gram-negative bacterial pathogens. EBioMedicine 2:690–698. doi: 10.1016/j.ebiom.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stainton SM, Monogue ML, Nicolau DP. 2017. In vitro-In vivo discordance with humanized piperacillin-tazobactam exposures against piperacillin-tazobactam-resistant/pan-β-lactam-susceptible Klebsiella pneumoniae strains. Antimicrob Agents Chemother 61:e00491-17. doi: 10.1128/AAC.00491-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monogue ML, Nicolau DP. 2016. In vitro-in vivo discordance with humanized piperacillin-tazobactam exposures against piperacillin-tazobactam-resistant/pan-β-lactam-susceptible Escherichia coli. Antimicrob Agents Chemother 60:7527–7529. doi: 10.1128/AAC.01208-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhagwat SS, Takalkar SS, Chavan RP, Friedland HD, Patel MV. 2017. WCK 5222: unravelling sub-MIC PD effects employing in vivo dose fractionation studies and translating into MIC based PK/PD target for carbapenem-resistant A. baumannii, abstr P282. ASM Microbe 2017, 1 to 5 June 2017, New Orleans, LA. [Google Scholar]


