The drug resistance of Helicobacter pylori is gradually becoming a serious problem. Biofilm formation is an important factor that leads to multidrug resistance (MDR) in bacteria.
KEYWORDS: Helicobacter pylori, SpoT, efflux pump, biofilm, antibiotic resistance, GluP
ABSTRACT
The drug resistance of Helicobacter pylori is gradually becoming a serious problem. Biofilm formation is an important factor that leads to multidrug resistance (MDR) in bacteria. The ability of H. pylori to form biofilms on the gastric mucosa is known. However, there are few studies on the regulatory mechanisms of H. pylori biofilm formation and multidrug resistance. Guanosine 3′-diphosphate 5′-triphosphate and guanosine 3′,5′-bispyrophosphate [(p)ppGpp] are global regulatory factors and are synthesized in H. pylori by the bifunctional enzyme SpoT. It has been reported that (p)ppGpp is involved in the biofilm formation and multidrug resistance of various bacteria. In this study, we found that SpoT also plays an important role in H. pylori biofilm formation and multidrug resistance. Therefore, it was necessary to carry out some further studies regarding its regulatory mechanism. Considering that efflux pumps are of great importance in the biofilm formation and multidrug resistance of bacteria, we tried to determine whether efflux pumps controlled by SpoT participate in these activities. We found that Hp1174 (glucose/galactose transporter [gluP]), an efflux pump of the major facilitator superfamily (MFS), is highly expressed in biofilm-forming and multidrug-resistant (MDR) H. pylori strains and is upregulated by SpoT. Through further research, we determined that gluP is involved in H. pylori biofilm formation and multidrug resistance. Furthermore, the average expression level of gluP in the clinical MDR strains (C-MDR) was considerably higher than that in the clinical drug-sensitive strains (C-DSS). Taken together, our results revealed a novel molecular mechanism of H. pylori resistance to multidrug exposure.
INTRODUCTION
Helicobacter pylori, the rate of infection with which is over 50% throughout the world, is highly associated with a wide range of upper gastrointestinal diseases, especially gastric carcinoma (1). Contemporaneously, the most common method of treatment of infections caused by this bacterium is called triple therapy, which consists of a proton pump inhibitor and two antibiotics, namely, macrolides, nitroimidazoles, or β-lactams (2). However, in recent years, eradication of H. pylori has become increasingly difficult because the rate of antibiotic resistance acquisition by H. pylori has generally increased (3). In addition, with the extended use of antibiotics, the appearance of multidrug-resistant (MDR) H. pylori strains, which are resistant to multiple antibiotics, has been reported (4).
There are numerous molecular mechanisms that contribute to multidrug resistance in the bacteria, including decreased drug permeation, efflux pumps, alteration or bypass of the drug target, production of antibiotic-modifying enzymes, and other physiological states, such as the formation of biofilms (5). Biofilms are communities of microorganisms that are anchored to a surface and live in an extracellular matrix made up of extracellular polymeric substances (EPS); this matrix is produced by the microorganisms to form their immediate environment (6). The property that makes biofilms distinct from planktonic cells is their increased resistance to antimicrobial agents. It has recently become widely accepted that biofilms play an important role in the pathogenesis of some chronic human infections, as well as bacterial multidrug resistance (6, 7). Furthermore, H. pylori has the ability to form biofilms in vitro (8). In 2006, using scanning electron microscopy (SEM), Carron et al. first proposed that H. pylori could form biofilms in vivo (9). The formation of biofilms in vivo is an important cause of H. pylori resistance to multiple antibiotics (10), as is its stringent response to a stressful environment lacking nutrients (11).
The stringent response is a bacterial stress response that controls bacterial adaptation to stress signals, such as nutrient deprivation (12). In bacteria, the signal molecules guanosine 3′-diphosphate 5′-triphosphate and guanosine 3′,5′-bispyrophosphate [(p)ppGpp], which are induced by diverse stresses, activate the stringent response (12, 13). The phenomenon of (p)ppGpp affecting bacterial multidrug resistance has already been reported for some other bacteria (14). Maisonneuve et al. reported that under antibiotic stress, Escherichia coli can produce rare cells that transiently become multidrug tolerant (15). In these rare cells, the level of (p)ppGpp was high (15). In addition, it has also been proven that (p)ppGpp can affect the formation of the bacterial biofilm. For example, Sugisaki et al. determined that the accumulation of (p)ppGpp accelerated the formation of biofilms in Bordetella pertussis (16), and Li et al. reported that the low level of (p)ppGpp contributed to the formation of biofilms in Actinobacillus pleuropneumoniae S8 (17). Nevertheless, there are still no reports certifying the relationship between (p)ppGpp and the formation of biofilms in H. pylori.
In many bacteria, such as E. coli, (p)ppGpp is synthesized by two enzymes, RelA and SpoT (18), and SpoT is a bifunctional enzyme with both (p)ppGpp synthetase and hydrolase activity (18). However, there is only one member of the RelA/SpoT family, SpoT, in the H. pylori genome (19–21), and it is also a bifunctional enzyme (22); therefore, in H. pylori, we focused on whether SpoT is involved in H. pylori biofilm formation and multidrug resistance.
There are a series of transport proteins in bacteria that can acquire nutrients and extrude metabolic by-products, and some of these proteins are called efflux pumps (23). Efflux pumps have the ability to expel a broad range of antibiotics and have been recognized as significant components of multidrug resistance in many bacteria (24, 25), such as H. pylori (26–32). It has been demonstrated that efflux pumps work as one of the mechanisms that contribute to the antimicrobial resistance of biofilms (33), and evidence can be found in several microorganisms, such as Pseudomonas aeruginosa (34), E. coli (35), and Candida albicans (36). Moreover, Yonezawa et al. reported that in H. pylori clinical MDR strains (C-MDR), the expression of some efflux pump genes was elevated in biofilm cells compared to that in planktonic cells (37). While some efflux transporters have been detected in H. pylori 26695 (21), their functions, especially their functions in H. pylori biofilm formation, must be further studied.
Considering that SpoT is a global regulator, we suppose that SpoT can influence the formation of biofilms and multidrug resistance by regulating the expression of the efflux pump in H. pylori.
RESULTS
SpoT is involved in H. pylori biofilm formation and multidrug resistance.
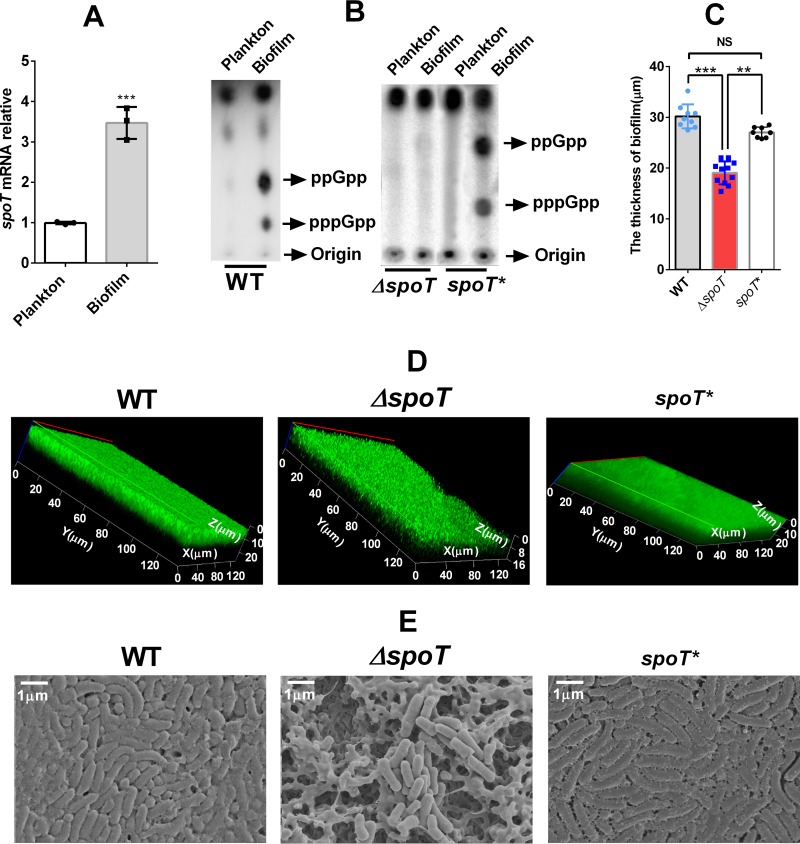
As a global regulatory factor, SpoT has been proven to participate in the formation of bacterial biofilms (16, 17, 38), while no related studies regarding H. pylori have been done. Therefore, we analyzed the difference in spoT expression between biofilm-forming and planktonic cells by real-time PCR (RT-PCR). The spoT gene is highly expressed in the latter (Fig. 1A). The (p)ppGpp production assay showed that the H. pylori cells in the biofilm produced more (p)ppGpp than did the planktonic cells (Fig. 1B). By constructing an spoT mutant strain (the ΔspoT strain) and an spoT-complemented strain (the spoT* strain), we compared the biofilms of the wild-type (WT), ΔspoT, and spoT* strains by confocal laser scanning microscopy (CLSM) (Fig. 1C and D) and SEM (Fig. 1E). LIVE/DEAD cell viability assays showed that the ΔspoT strain formed a lighter biofilm than did the WT strain and the spoT*strain (Fig. 1C and D). The WT strain and the spoT* strain could form complete and compact biofilms on the nitrocellulose (NC) membrane, whereas for the biofilm of the ΔspoT strain, the bacteria were not packed tightly enough, the biofilm matrix was not complete, and some cavities could be seen (Fig. 1E).
FIG 1.
SpoT is involved in H. pylori biofilm formation. (A) mRNA expression of SpoT in biofilm-forming and planktonic cells determined by qRT-PCR. The levels of the signals were normalized to the 16S rRNA levels. Data are the means ± standard errors of the means from three independent experiments. Significance was determined by the unpaired Student's t test. ***, P < 0.001. (B) (p)ppGpp was induced in the biofilm-forming cells (of the wild-type [WT] and spoT-complemented [spoT*] strains) but not in the planktonic cells or the spoT mutant strain (the ΔspoT strain). 32P-labeled nucleotides of formic acid extracts of H. pylori were detected by thin-layer chromatography. Planktonic H. pylori bacteria were grown to the exponential phase. (C) Comparison of the biofilm thickness of the WT, ΔspoT, and spoT*strains, using data from the assay whose results are presented in panel D. The data presented are the means ± standard errors of the means from three independent experiments. Significance was determined by the paired Student's t test. **, P < 0.01; ***, P < 0.001; NS, not significant. (D) Confocal laser scanning microscopy (CLSM) images of WT, ΔspoT, and spoT* strain biofilms. Cells stained with membrane-permeant SYTO 9 (green) and membrane-impermeant propidium iodide (PI) (red) were visualized by confocal microscopy. (E) Scanning electron microscopy (SEM) images of WT, ΔspoT, and spoT*strain biofilms. The biofilm used in this experiment is a mature biofilm grown on a nitrocellulose membrane for 3 days, and the planktonic bacteria were from early exponential phase (OD600, approximately 0.4 to 0.5).
According to the MIC of the WT strain and the ΔspoT strain, for planktonic cells, the ΔspoT strain was apparently more sensitive to various antibiotics, not including ciprofloxacin, than was the WT and spoT* strain (Table 1). With regard to the biofilm-forming cells, after knocking out spoT, cell resistance to various antibiotics, especially that to penicillin G, was reduced (Table 1). Then, we treated the WT, ΔspoT, and spoT* strains with antibiotics (clarithromycin [CLA], amoxicillin [AMO], tetracycline [TET], and metronidazole [MET]) at their MICs. The growth inhibitory curve demonstrated that the growth ability of the ΔspoT strain was obviously inhibited (Fig. 2).
TABLE 1.
MICs determined for WT, MDR, ΔspoT, ΔgluP, spoT*, and gluP* strains in biofilm-forming and planktonic cells
| Drug | MIC (μg/ml) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Planktonic cells |
Biofilm-forming cells |
|||||||||||
| WT | ΔspoT | spoT* | ΔgluP | gluP* | MDR-H | MDR-H (ΔgluP) | WT | ΔspoT | spoT* | ΔgluP | gluP* | |
| Ampicillin (AMP) | 0.0625 | 0.03125 | 0.0625 | 0.0156 | 0.0625 | 0.3125 | 0.156 | 3.75 | 0.9375 | 3.75 | 0.6 | 3.75 |
| Penicillin G (PEN) | 0.125 | 0.0156 | 0.0625 | 0.0078 | 0.0625 | 0.625 | 0.3125 | 2.5 | 0.234 | 2.5 | 0.078 | 1.25 |
| Amoxicillin (AMO) | 0.0625 | 0.0156 | 0.0625 | 0.0039 | 0.0625 | 0.8 | 0.3125 | 2.5 | 0.486 | 1.25 | 0.312 | 1.25 |
| Clarithromycin (CLA) | 0.125 | 0.0625 | 0.125 | 0.125 | 0.125 | 0.75 | 0.625 | 5 | 1.56 | 2.5 | 2.5 | 3.125 |
| Tetracycline (TET) | 0.25 | 0.0625 | 0.125 | 0.125 | 0.125 | 5 | 2.5 | 10 | 1.875 | 5 | 1.25 | 6.25 |
| Ciprofloxacin (CIP) | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 4 | 1 | 5 | 2 | 5 | 3.75 | 5 |
| Metronidazole (MET) | 0.5 | 0.125 | 0.5 | 0.125 | 0.25 | 4 | 1.5 | 5 | 3.125 | 5 | 2.5 | 5 |
| Furazolidone (FUR) | 1 | 0.5 | 1 | 0.25 | 2 | 6 | 2 | 8 | 2.5 | 10 | 2 | 10 |
FIG 2.
Comparison of growth inhibition curve characteristics of the WT, ΔspoT, and spoT*strains with various antibiotics (AMO [0.125 μg/ml], CLA [0.125 μg/ml], MET [0.5 μg/ml], TET [0.25 μg/ml]). Data are the means ± standard errors of the means from three independent experiments. The significance of the difference between the WT and ΔspoT strains was determined using the paired Student's t test. *, P < 0.05; **, P < 0.01.
Comparing the efflux capacity of the wild-type, ΔspoT, and spoT* strains.
As SpoT is involved in the formation of the biofilm and multidrug resistance of H. pylori, along with an efflux pump (25, 33), we inferred that SpoT could possibly carry out those functions by regulating the expression of the efflux pump. Therefore, we compared the efflux activity of the WT, ΔspoT, and spoT* strains. The results revealed that the inactivation of SpoT, whether it was in biofilm-forming or planktonic cells, caused a distinct increase in the accumulation of Hoechst 33342, notably for planktonic cells, and the fluorescence values of the ΔspoT strain were >3-fold greater than those of the WT and spoT* strains. These results demonstrate that the efflux activity of the ΔspoT strain in response to Hoechst 33342 was weaker than that of the WT strain (Fig. 3), which suggests that SpoT likely regulates the efflux pump gene expression of H. pylori.
FIG 3.
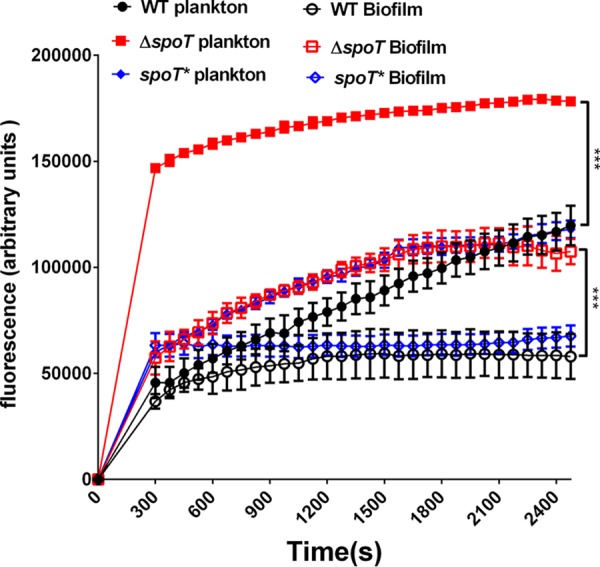
Comparison of the accumulation of Hoechst 33342 (2.5 M) in biofilm and planktonic cells of the WT, ΔspoT, and spoT*strains. The fluorescence intensity was recorded at excitation and emission wavelengths of 350 and 460 nm, respectively, over a 30-min incubation period. The data presented are the means ± standard errors of the means from three separate experiments. The paired Student's t test was performed to compare the accumulation of Hoechst 33342 between the WT and ΔspoT strains. ***, P < 0.001.
Screening efflux pumps involved in biofilm formation and multidrug resistance in H. pylori by qRT-PCR.
We analyzed the difference in expression of some efflux pumps belonging to the major facilitator superfamily (MFS) and ATP-binding cassette (ABC) superfamily between biofilm-forming and planktonic cells by using quantitative real-time PCR (qRT-PCR) (Table 2). As seen in Table 2, the expression levels of two particular genes increased greater than 3-fold in biofilm-forming cells compared to those in to planktonic cells: Hp1181 (multidrug transporter) and Hp1174 (gluP). Furthermore, we analyzed the difference in mRNA expression of these two genes in an artificially selected multidrug-resistant H. pylori strain (MDR-H), and only gluP was highly expressed compared to its expression in the sensitive strain (H. pylori 26695) (Fig. 4). Considering that SpoT is highly expressed in MDR-H (Fig. 4) and biofilm-forming cells, we proposed that SpoT may participate in the biofilm formation and multidrug resistance of H. pylori by upregulating gluP.
TABLE 2.
qRT-PCR analysis of efflux pump expression difference between biofilm-forming and planktonic H. pylori cells
| Efflux pump family | Locus tag | Predicted function | Fold change in expression between biofilm and planktonic cells | P value for significancea |
|---|---|---|---|---|
| Major facilitator superfamily (MFS) | Hp0313 | Nitrite | 0.46 ± 0.14 | * |
| Hp0936 | Proline/betaine | 1.08 ± 0.17 | ||
| Hp1091 | Alpha-ketoglutarate | 0.58 ± 0,03 | ** | |
| Hp1165 | Multidrug efflux | 2.66 ± 0.86 | ||
| Hp1174 | Glucose/galactose transporter | 5.75 ± 0.68b | *** | |
| Hp1181 | Multidrug efflux | 3.34 ± 0.31 | *** | |
| Hp1185 | Sugar efflux | 0.45 ± 0.27 | * | |
| ATP-binding cassette (ABC) superfamily | Hp1220 | Multidrug efflux | 2.99 ± 0.32 | ** |
| Hp1082 | Multidrug efflux | 2.02 ± 0.25 | ||
| Hp1486 | Multidrug efflux | 1.11 ± 0.29 | ||
| Hp1206 | Multidrug efflux | 1.93 ± 0.17 | ** |
Significance was determined using the paired Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The boldface data indicate that the expression levels of the two genes increased greater than 3-fold in biofilm-forming cells compared to those in planktonic cells.
FIG 4.
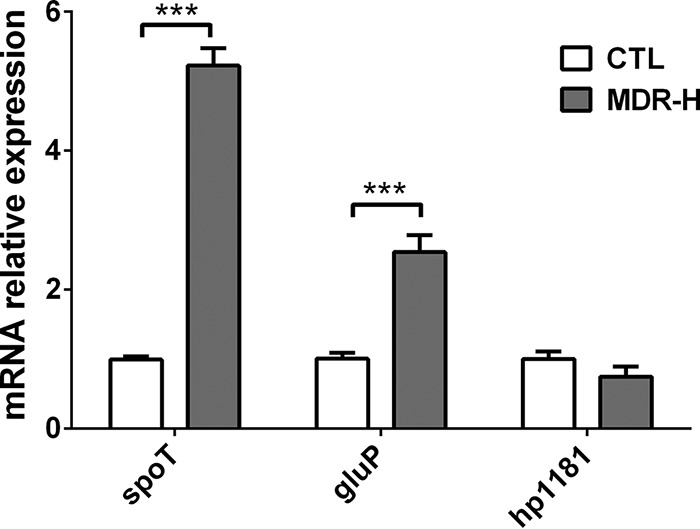
qRT-PCR analysis of the mRNA levels of spoT, gluP (Hp1174), and Hp1181 in the MDR-H strain (selected artificially) compared to those in the WT. The signals were normalized to the 16S rRNA levels. Data are the means ± standard errors of the means from three independent experiments. Significance was determined by the paired Student's t test. ***, P < 0.001. CTL, control.
SpoT regulates GluP expression.
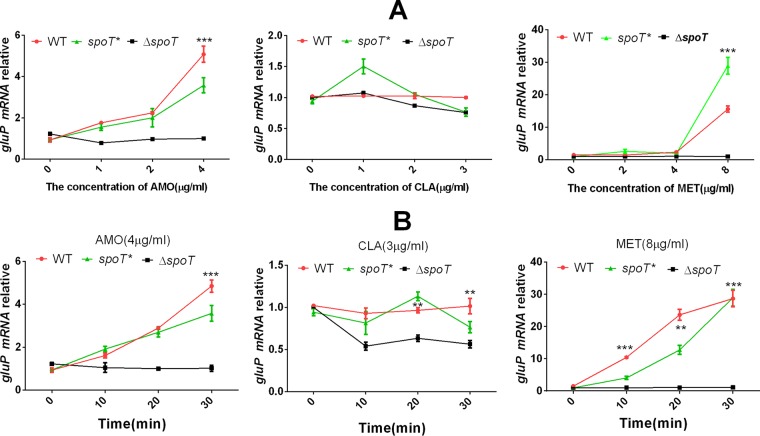
To determine whether GluP is regulated by SpoT, we chose three antibiotics (CLA, AMO, and MET) with which to treat the WT, ΔspoT, and spoT* strains. First, we applied the three antibiotics at different concentrations to the WT, ΔspoT, and spoT* strains for 10 min; then, we applied a specific concentration of the three antibiotics along a time gradient. The qRT-PCR results showed that both AMO and MET could induce the expression of gluP in the WT and spoT* strains but not in the control group (the WT strain without antibiotic treatment), doing so in a concentration- and time-dependent manner, while in the ΔspoT strain, GluP could barely be induced by these two antibiotics (Fig. 5). However, CLA could hardly induce the expression of gluP, and the sensitivity of the WT and ΔspoT strains to CLA was similar according to the MIC data. This suggests that gluP may not be involved in the resistance of H. pylori to CLA. In short, the results presented above indicate that SpoT upregulates GluP to cope with specific antibiotic stress.
FIG 5.
qRT-PCR analysis of the mRNA levels of gluP in the WT, ΔspoT, and spoT*strains. The WT, ΔspoT, and spoT*strains were exposed to different drug concentrations for 10 min (A) and exposed to specific drug concentrations for different time periods (B). The results were compared to those for the WT without drug treatment (control). The signal was normalized to the 16S rRNA levels. Data are the means ± standard errors of the means from three independent experiments. Significance was determined by the paired Student's t test between the WT and ΔspoT strains. **, P < 0.01; ***, P < 0.001.
GluP is involved in H. pylori efflux.
GluP, a glucose transporter, is responsible for glucose transport in H. pylori (21, 39). In addition, structural analysis demonstrates that GluP is an efflux pump belonging to the MFS, which suggests that GluP likely functions in drug efflux in H. pylori. Therefore, we successfully constructed a gluP mutant strain (the ΔgluP strain) and a gluP-complemented strain (the gluP* strain) and compared the efflux capacity of the WT, ΔgluP, and gluP* strains. These results revealed that the inactivation of GluP caused a distinct increase in the accumulation of Hoechst 33342, whether it was in biofilm-forming or planktonic cells but especially in planktonic cells, and the fluorescence values of the ΔgluP strain were >3-fold greater than those of the WT and gluP* strains. These results showed that the ΔgluP strain had a lower efflux capacity for Hoechst 33342 than the WT strain in both planktonic and biofilm-forming cells (Fig. 6).
FIG 6.
Comparison of the accumulation of Hoechst 33342 (2.5 M) in biofilm and planktonic cells of the WT, ΔgluP, and gluP* strains. The fluorescence intensity was recorded at excitation and emission wavelengths of 350 and 460 nm, respectively, over a 30-min incubation period. The data presented are the means ± standard errors of the means from three separate experiments. The paired Student's t test was performed to compare the accumulation of Hoechst 33342 between the WT and ΔgluP strains. ***, P < 0.001.
GluP is involved in H. pylori biofilm formation and multidrug resistance.
Studies have reported that efflux pumps participate in bacterial biofilm formation (33), so we compared the biofilms of the WT, ΔgluP, and gluP* strains by SEM. The images showed that compared with the bacteria in the biofilms of the WT and gluP* strains, the bacteria in the biofilm of the ΔgluP strain were not tightly packed, and the biofilm matrix was incomplete and showed more cavities (Fig. 7A). By CLSM, LIVE/DEAD cell viability assays showed that the ΔgluP strain formed a thinner biofilm (Fig. 7B and C).
FIG 7.
GluP is involved in H. pylori biofilm formation. (A) Scanning electron microscopy (SEM) images of WT, ΔgluP, and gluP* strain biofilms. (B) Confocal laser scanning microscopy (CLSM) images of WT, ΔgluP, and gluP* strain biofilms. Cells stained with membrane-permeant SYTO 9 (green) and membrane-impermeant propidium iodide (PI) (red) were visualized by confocal microscopy. (C) Comparison of the biofilm thickness between the WT, ΔgluP, and gluP* strains, using data from the assay whose results are presented in panel B. The data presented are the means ± standard errors of the means from three independent experiments. Significance was determined by the paired Student's t test. **, P < 0.01; ***, P < 0.001. The biofilm used in this experiment is a mature biofilm grown on a nitrocellulose membrane for 3 days, while the planktonic H. pylori bacteria were from the early exponential phase (OD600, approximately 0.4 to 0.5).
According to the MIC for planktonic cells of the WT strain and the ΔgluP strain (Table 1), the ΔgluP strain was apparently more sensitive to various antibiotics than the WT strain, except for its sensitivity to CLA and ciprofloxacin. After knocking out gluP in the MDR-H strain, its resistance to drugs, especially its resistance to AMO, also decreased significantly (Table 1). In addition, for those biofilm-forming cells, after knocking out gluP, their sensitivity to various antibiotics increased (Table 1).
We treated the WT, ΔgluP, and gluP* strains separately with three antibiotics (CLA, AMO, and MET) at the MIC (for the WT strain) and then observed their growth inhibition curves. The results showed that compared with the growth of the WT and gluP* strains, the growth of the ΔgluP strain was significantly inhibited by AMO and MET but not by CLA (Fig. 8). The results presented above suggest that GluP may not be involved in the tolerance of planktonic H. pylori bacteria to CLA, which is consistent with the results of MIC testing.
FIG 8.
Comparison of growth curves and growth inhibition curve characteristics of the WT, ΔgluP, and gluP* strains. (A) Growth curves of the WT and ΔgluP strains. (B, C, and D) Growth inhibition curves of the WT, ΔgluP, and gluP* strains exposed to AMO (0.125 μg/ml), CLA (0.125 μg/ml), and MET (0.5 μg/ml). Data are the means ± standard errors of the means from three independent experiments. Significance was determined by the paired Student's t test. *, P < 0.05; **, P < 0.01.
The relative mRNA expression levels of gluP were assessed by qRT-PCR in the clinical MDR strains (C-MDR) and clinical drug-sensitive strains (C-DSS). The average expression levels of gluP in the C-MDR were significantly higher than those in the C-DSS (Fig. 9).
FIG 9.
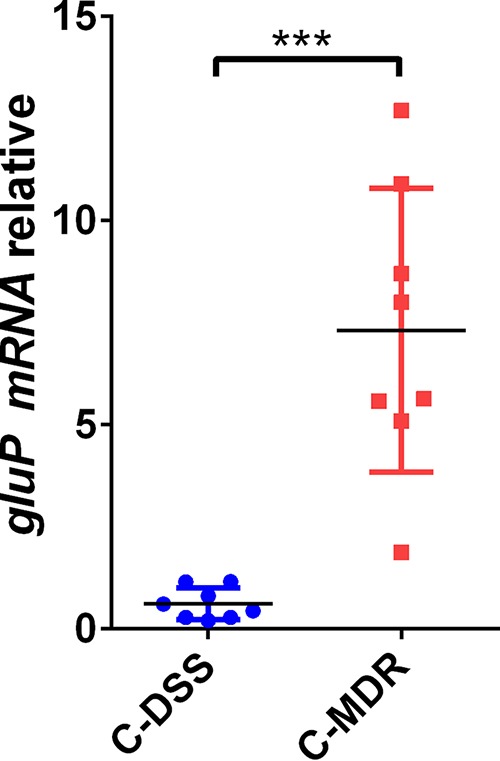
Analysis of gluP expression differences in clinical drug-sensitive strains (C-DSS) (n = 8) and clinical multidrug-resistant strains (C-MDR) (n = 8) by qRT-PCR. The results were compared to the value for gluP in the WT. The signal was normalized to the 16S rRNA levels. Data are the means ± standard errors of the means from three independent experiments. Significance was determined by the paired Student's t test. ***, P < 0.001.
DISCUSSION
Recently, the antibiotic resistance acquired by H. pylori has generally increased, and the formation of biofilms in vivo is an important cause leading to this multidrug resistance (10). In this research, we found that SpoT is involved in biofilm formation in H. pylori. While it has been reported that the efflux pump functions in the formation of biofilms and multidrug resistance (33), we found that gluP is involved in the biofilm formation and multidrug resistance of H. pylori. Further analysis showed that the expression of gluP is upregulated by SpoT. Considering that SpoT is a global regulator, our study partly explains the molecular mechanism by which SpoT is involved in the biofilm formation and multidrug resistance of H. pylori.
Antibiotics, as signaling molecules (40), can induce bacteria to produce (p)ppGpp and lead to a stringent response (14). Studies have shown that (p)ppGpp is involved in bacterial tolerance to various antibiotics, such as penicillin (41), vancomycin (42), and ampicillin (43); in H. pylori, (p)ppGpp is synthesized from SpoT (21). Our previous studies discovered that (p)ppGpp modulates the expression of H. pylori efflux transporters (44), accompanied by the importance of the efflux pump to multidrug resistance (24); thus, we wondered whether (p)ppGpp participates in the multidrug resistance of H. pylori. This research confirmed that inference.
Biofilm formation is another important factor that causes multidrug resistance in bacteria (7). Biofilms are communities of microorganisms anchored to a surface and live in an extracellular matrix that is made up of EPS produced by the organisms to form their immediate environment (7). Bacteria with biofilms have strong resistance to antibiotics and host immune defenses (6). It has been reported that (p)ppGpp is involved in the formation of biofilms in various bacteria, but the function of (p)ppGpp during this process varies from species to species. Several previous studies have shown that the lack of (p)ppGpp resulted in decreased biofilm formation in bacteria, such as Enterococcus faecalis (38), Vibrio cholerae (45), and Bordetella pertussis (16). However, other studies have proven that (p)ppGpp synthase deletion mutants of Actinobacillus pleuropneumoniae (17) and Francisella novicida (46) can produce significantly more biofilms than can the WT. Since spoT is the only gene that plays a part in the synthesis of (p)ppGpp in H. pylori, its ability to synthesize (p)ppGpp is totally lost after the knockout of spoT, consequently lowering its ability to adapt to stressful environments (19, 20, 22). In this study, we induced H. pylori to form a biofilm through nutrient starvation because nutrition deficiency is a significant factor that induces bacteria to form biofilms. After spoT knockout, the ability of H. pylori to form biofilms was greatly reduced.
Biofilm formation is an important cause of multidrug resistance in bacteria, and biofilms lead to continuous chronic infections, which add to the difficulty in providing a clinical cure (6). Drug resistance mechanisms include the following: poor diffusion of antibiotics through the biofilm polysaccharide matrix, physiological changes due to the low growth rate and starvation responses that form persistent cells, phenotypic changes of the cells forming the biofilm, and the expression of efflux pumps (7). Drug efflux is a key mechanism of drug resistance in Gram-negative bacteria (24). Microorganisms regulate the internal environment to adapt to their outer circumstances by excluding poisonous substances (antimicrobial agents, metabolites, and quorum-sensing chemical signals) via efflux pumps (25). There are six families of bacterial efflux pumps: (i) the ABC superfamily, (ii) the MFS, (iii) the multidrug and toxic compound extrusion (MATE) family, (iv) the small multidrug resistance (SMR) family, (v) the resistance-nodulation-division (RND) superfamily, and (vi) the drug metabolite transporter (DMT) superfamily (33). It is generally agreed that the ABC, MFS, and RND families play important roles in Gram-negative bacteria (33). As extensive studies regarding the RND family in H. pylori have been conducted (8, 28, 32, 37), we focused on the function of the MFS and the ABC superfamily.
Normally, the expression of efflux pumps in biofilm-forming bacteria is higher than that in planktonic cells (33). For example, in P. aeruginosa, the mechanism for tolerance to colistin is the upregulation of the MexAB-OprM efflux pump in biofilms (47). Moreover, in E. coli cells grown in biofilms and exposed to several antibiotics, the genes encoding the AcrAB-TolC efflux pump are upregulated (48). In addition, the expression levels of the acrA and acrB genes have been observed to increase in Salmonella biofilm cells (49). It has been reported that the RND family of efflux pumps is highly expressed in H. pylori biofilms (37), and recent studies have shown that Hp1165 and hefA are highly expressed in clinically isolated H. pylori biofilms (50). In our research, the expression of several efflux pumps was high in H. pylori biofilms. Some further studies of gluP found that efflux pump expression functioned in the formation of H. pylori biofilms. After gluP knockout, the H. pylori biofilm matrix is damaged and the bacteria are unable to form a well-structured biofilm. Some studies confirmed that the extremely low level of biofilm formation observed in the WT can be observed in E. coli mutants without emrD, emrE, emrK, acrD, acrE, and mdtE efflux pump-encoding genes (51). Efflux pumps are involved in biofilm formation, possibly due to the export or import of some substances related to biofilm formation. gluP is a glucose/galactose transporter belonging to the MFS (21), which is mainly responsible for the physiological uptake of sugars, such as d-glucose, into H. pylori (39). d-Glucose is involved in the synthesis of bacterial exopolysaccharides, while polysaccharides account for a major fraction of the biofilm matrix (52). Therefore, the deletion of gluP may affect the synthesis of the H. pylori biofilm matrix. We found that the matrix of the H. pylori biofilm was incomplete after gluP knockout, even if the concentration of glucose in the medium was increased (see Fig. S1 in the supplemental material). In addition, the biofilm matrix can limit the transport of some antimicrobial agents to cells within the biofilm (7).
The results of the growth inhibition curve (Fig. 8) and MIC (Table 1) analyses showed that knocking out gluP did not affect the sensitivity of H. pylori to CLA, suggesting that gluP may not be involved in the tolerance of H. pylori to CLA. In fact, although the efflux pump is an important weapon for multidrug resistance, it has a certain specificity for antibiotic substrates (53); for example, the AcrAB-TolC pump is not involved in telithromycin efflux in Enterobacter aerogenes or Escherichia coli (54). Our results suggest that CLA is not a good substrate for gluP.
Extensive studies concerning the regulatory mechanism of efflux pumps, such as two-component signal (TCS) transduction systems, local repressors, and global response regulators, have been conducted (55). In E. coli, (p)ppGpp can also regulate the expression of the efflux pump of YojI (56). There is one possible mechanism that explains how (p)ppGpp indirectly mediates global changes at the transcriptional level, such as by reducing the RpoD competitiveness for the core RNA polymerase. (p)ppGpp releases RpoD from the RNA polymerase and shifts the use of the alterative sigma factors (57). It is known that the H. pylori genome includes only two alterative sigma factors (σ54 and σ28) (21). According to the research of Niehus and coworkers, the promoter sequence of σ54-dependent genes is TTTGCTT (58). By analyzing the upstream sequence of the putative ATG start codon of the open reading frame of gluP, we discovered a possible conserved sequence (TTTGCAT) that was recognized by σ54 (Fig. S2), which suggests that gluP could be regulated by σ54. In further studies, antibiotic treatment could not induce high gluP expression in the σ54 mutant strain (Fig. S3). Therefore, SpoT may upregulate the expression of gluP by σ54-dependent transcription, but detailed mechanisms require more studies.
In conclusion, this research has discovered a new mechanism regarding biofilm formation and multidrug resistance in H. pylori. Further analysis is required to identify the specific mechanisms by which SpoT regulates H. pylori biofilm formation. On account of the present data, our research provides evidence and clues for the clinical treatment of patients infected with drug-resistant strains and epidemiological investigation.
MATERIALS AND METHODS
Bacterial strain, media, growth conditions, and clinical isolation of H. pylori.
H. pylori 26695, which was kindly provided by Zhang Jianzhong (Chinese Disease Control and Prevention Center), was used in this study. The strain was maintained at −80°C in brucella broth (BB; Difco, Detroit, MI) with 20% (vol/vol) glycerol and 10% fetal bovine serum (FBS). The strain was cultured under a microaerobic environment (5% O2, 10% CO2, 85% N2) at 37°C on brucella agar plates containing 7% lysed sheep blood. The liquid culture medium for H. pylori consisted of BB containing 10% FBS, and the cells were incubated in a shaker set at 120 rpm at 37°C. The mutant strains were cultured on agar plates with kanamycin (Sigma-Aldrich, St. Louis, MO) at 30 μg/ml. The E. coli strain was TOP10 (TransGen Biotech, Beijing, China) and was grown in Luria-Bertani medium at 37°C.
Eight MDR clinical isolates and eight sensitive clinical isolates were obtained from patients, including those with gastritis, gastric ulcers, duodenal ulcers, and gastric cancer, at Qiannan People's Hospital (Guizhou Province). All the patients provided informed consent before examination. The study was approved by the ethics committee of School of Medicine, Shandong University. MDR H. pylori strains from the patients could not be killed with repeated standard triple-therapy treatment. Isolated H. pylori strains were identified using universally accepted phenotypic tests: typical morphology on Gram-stained smears; and positive urease, oxidase, and catalase tests. The names of the C-DSS (n = 8) and C-MDR (n = 8) are listed in Table S1 in the supplemental material.
Assessment of susceptibility to antibiotics.
The MICs of various antibiotics for all the clinical and standard strains were determined by the Etest and the agar dilution method as reported by Osato et al. (59). The bacteria (optical density at 600 nm [OD600], 0.8) were inoculated on an agar plate containing 2-fold dilutions of antibiotics. All the plates were incubated at 37°C under microaerobic conditions, and MIC values were determined. The method used to determine the MICs for biofilm-forming bacteria was similar to that used for the planktonic bacteria. The biofilm, which was attached to a nitrocellulose (NC) membrane, was incubated in a liquid medium containing different concentrations of various antibiotics for 12 h. After being washed three times with phosphate-buffered saline (PBS), the biofilm bacteria were suspended in liquid culture medium, and this liquid was inoculated on an agar plate to determine the MIC values.
Construction of biofilm.
The biofilm could be cultivated under two kinds of conditions: the static condition or the continuous-flow condition. There are many ways to cultivate biofilms under the static condition; we used the colony biofilm (described in previous articles [60, 61]) with slight modification. To allow the adherence of H. pylori at the interface, 20 μl of bacteria was inoculated at 5 × 107 cells ml−1 onto an NC membrane (approximately 1 by 1 cm2), which was placed on an agar plate. The agar plate was cultured in a microaerobic environment (5% O2, 10% CO2, 85% N2) at 37°C for 3 days.
SEM.
To observe the H. pylori biofilm, scanning electron microscopy (SEM) was used. The samples for SEM were prepared using the following standard procedures. Planktonic bacteria were fixed with glutaraldehyde after centrifugation. For the biofilm-forming bacteria, the samples were gently washed with autoclaved PBS three times to remove the planktonic bacteria. The biofilms were fixed in 2.5% glutaraldehyde at 4°C for 2 h and then washed three times with cacodylate buffer and dehydrated through a series of graded ethanol solutions (25%, 50%, 75%, 95%, and 100%). Subsequently, the samples were freeze-dried, sputter coated with gold, and observed by SEM.
CLSM.
To determine bacterial shape and viability, planktonic H. pylori bacteria were stained with membrane-permeant and membrane-impermeant fluorescent dyes from LIVE/DEAD BacLight bacterial viability kits (Molecular Probes, Invitrogen, USA). Then, they were observed by confocal microscopy, which was performed as described in a previous study (44). For biofilm-forming cells, the NC membrane with the biofilm was removed from the agar plate and then gently washed three times with PBS. Subsequently, the NC membrane was stained in a 12-well plate with 1 ml of SYTO 9 dye for 20 min under dark conditions and gently washed three times with PBS. After that, the NC membrane was fixed on a slide, covered with a coverslip, and subsequently analyzed by confocal laser scanning microscopy (CLSM; Leica TCS SP5; Leica Microsystems GmbH, Wetzlar, Germany). SYTO 9 is a green fluorescent membrane-permeant dye that labels all bacteria by staining nucleic acid, whereas propidium iodide (PI) is a red fluorescent membrane-impermeant dye that labels only bacteria with damaged membranes.
Detection of (p)ppGpp accumulation patterns.
(p)ppGpp production was assayed according to the method described in a previous study with slight modification (44). The treatment of the planktonic bacteria was the same as previously described (44). The biofilm bacteria were washed with PBS, diluted to an OD600 of 0.2, and incubated for an additional 2 h. When all the strains reached an OD600 of approximately 0.3, samples from each culture plate were centrifuged at 10,000 rpm for 5 min and resuspended in 250 μl of liquid culture medium. 32P (Amersham) was added to 100 μCi ml−1, and the cultures were labeled for 2 h at 37°C. Then, 50 μl of each sample was added to an equal volume of 2 M formic acid. Afterward, at least four freeze-thaw cycles were conducted. The acid extracts were briefly centrifuged, and the supernatants were spotted onto polyethyleneimine-cellulose plates (Sigma-Aldrich), dried, and developed in 1.5 M KH2PO4 (pH 3.4) for approximately 2.5 h. The results were obtained under phosphor screen scanning (Bio-Rad).
Extraction of RNA and quantitative real-time PCR (qRT-PCR).
Total RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA). To reverse transcribe the total RNA, a PrimeScript RT reagent kit with gDNA Eraser (TaKaRa) was used. The primers used are shown in Table 3. The 20-μl quantitative PCR mixtures contained 5 μl of the resulting cDNA, which was already diluted; 10 μl of SYBR Premix Ex Taq (TaKaRa, Otsu, Shiga, Japan); 0.8 μl of the primer mixture; and 4.2 μl of double-distilled water. Then, RT-PCR was performed using an ABI Prism 7000 sequence detection system (Applied Biosystems, Carlsbad, CA) for 1 cycle at 95°C for 30 s and 40 cycles at 95°C for 5 s and 60°C for 31 s. Dissociation curve analysis was performed to verify the product homogeneity. The 16S rRNA amplicon was used as an internal control for data normalization. Changes in the transcript level were determined by applying the relative quantitative (ΔΔCT) method. The threshold cycle (CT) values for all three biological replicates for each strain were compiled.
TABLE 3.
Primers used in this studya
| Forward primers |
Reverse primers |
||
|---|---|---|---|
| Name | Sequence (5′–3′) | Name | Sequence (5′–3′) |
| hp1174SF | CGGGATCCTATTATTGGGGAGGCGCGAT | hp1174SR | CCATCGATAGCTCTGTCCTCATCAAATACTG |
| hp1174XF | AACTGCAGACTTCTAACACTCTGGCGCT | hp1174XR | CGGAATTCCACCGCCAAGCCCAAATAAG |
| hp1174CF | CGGAATTCATGCAAAAAACTTCTAACAC | hp1174CR | CGGGATCCTTAGGAGTTTTCTTCTTGCT |
| hp0313 | CGCATGCTTTTTACCCATTT | hp0313 | AGAAAACACCACCCACGAAG |
| hp0936 | ACGCGCCAAGTTTAGTCAAT | hp0936 | CAGGCGCTGCTATAGCTTTT |
| hp1091 | TGCGTTCTTAGCGCCTTATT | hp1091 | GCGCCATAAGGATAATGGAA |
| hp1165 | AGGGAGTTCTTTGGGATCGT | hp1165 | AAGACGGGCGTAATCAAATG |
| hp1174 | CCGCTGGTAATCCCTTTGTA | hp1174 | CTTGCATTATCGCCCATTTT |
| hp1181 | GGGGTGGCGTTTTTCTTTAT | hp1181 | CCCCAAATAAGCCCTAAAGC |
| hp1185 | ATCTCTGGGCATTTCACCAC | hp1185 | CCATCGCAAAAGCGATAAAT |
| hp1220 | CAAAAGGCATGAGGGAAAAA | hp1220 | TTGCGTTTTGGCTAAATTCC |
| hp1082 | TGCCGTTAGCTGCTATTCCT | hp1082 | ACGGCGATGTTTTTGATACC |
| hp1486 | AAATGAAGCCCACACCACTC | hp1486 | TAAATTCCGCATGCATTTGA |
| hp1206 | TTTTCCTGCTTGTGCTGATG | hp1206 | CCCCACCAAGCAAAAACTAA |
| hp0714 | GGGTTTTCCCCATTAAGCAT | hp0714 | AGAGGCGATGTTGAGCAGTT |
Underlining indicates nucleotides that were added at the 5′ end to create a restriction site.
Construction of Hp1174 (gluP) and spoT mutants and complemented strains.
In our previous studies, we successfully constructed an spoT mutant strain (the ΔspoT strain) (62) and an spoT-complemented strain (the spoT* strain) (44). The plasmids (pILL570 and pUC18K2) used to construct the mutant strain were kindly provided by Agnès Labigne (Unité de Pathogénie Bactérienne des Muqueuses, Institut Pasteur). The construction of the gluP mutant strain was identical to the construction of the ΔspoT strain, as described in a previous study (62). Briefly, the gluP gene from the genome of H. pylori 26695 was destroyed with the insertion of the nonpolar aphA-3 gene, encoding a kanamycin resistance cassette. The construction of the gluP-complemented strain was similar to the construction of the spoT* strain. Briefly, the gluP-complemented strain (the gluP* strain) was constructed using the chloramphenicol resistance cassette from pMcagA, which was kindly presented by Wei Hong (Department of Microbiology, Guizhou Medical University, China). Full-length gluP was cloned into pMcagA, and the resulting plasmid was inserted into the middle of the Hp0547 (cagA) gene, which provided homologous recombination sites in H. pylori. The vector-transformed gluP mutant strain was constructed by electroporation to obtain the gluP-complemented strain (the gluP* strain). The genotype of the complemented gluP* transformant was verified by PCR and sequencing of the genomic loci. The primers used in these studies are listed in Table 3.
Determination of growth curves and growth inhibition curves.
The growth curves were determined as described in a previous study (44). The growth profiles in BB with a preliminary OD600 of 0.08 were monitored, and then the bacteria were cultured for another 144 h at 37°C with shaking. Records were taken every 12 h by determining the OD600 of the test strain. The values stated are the mode values from at least three biological replicates performed on at least three independent occasions.
To analyze the growth inhibition curve, the H. pylori strains were inoculated into BB, which also contained different antibiotics (at the MIC for the WT strain), with a preliminary OD600 of 0.08, and then the bacteria were cultured for another 144 h at 37°C with shaking. Each experiment was repeated at least three times.
Hoechst 33342 accumulation assay.
For the planktonic bacteria, the accumulation assay was performed as described previously (44). The biofilm bacteria were first rinsed with PBS and subsequently suspended in PBS, with the final OD600 being 0.1. Then, 180 μl of this liquid and 20 μl of Hoechst 33342 (25 μM; Sigma-Aldrich) were added to each well of a 96-well plate. Recording began 5 min after the addition of Hoechst 33342. Excitation and emission were measured at 355 nm and 460 nm, respectively, using a FLUOstar Optima microplate reader (BMG LABTECH, Aylesbury, UK). Readings were taken every 75 s for 30 cycles, and the raw data were analyzed by use of the Excel program. Each experiment was repeated at least three times.
Generation of MDR-H.
In order to obtain artificially selected multidrug-resistant H. pylori (MDR-H), H. pylori 26695 was cultivated on an agar plate containing 0.5× MIC of chloramphenicol for 48 to 72 h under a microaerobic environment at 37°C, as described in a previous study (63). The resistant colonies were incubated with repeated doubling of the chloramphenicol concentration until no colony was seen. Colonies were maintained on agar plates containing 4× MICs of TET, ampicillin, penicillin G, and erythromycin. Then, the colonies were incubated for 48 to 72 h under a microaerobic environment.
Statistical analysis.
Data are presented as the means ± standard errors of the means. Statistical significance was determined using the unpaired Student's t test, and the P values were corrected by the Sidak-Bonferroni method for multiple comparisons. P values of <0.05 were considered statistically significant. The results were analyzed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA).
Supplementary Material
ACKNOWLEDGMENTS
The present research was supported by the National Natural Science Foundation of China (no. 81471991, 81671978, 81460314, 81374101, and 81571960).
We declare that we have no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00957-18.
REFERENCES
- 1.Kusters JG, van Vliet AHM, Kuipers EJ. 2006. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mégraud F. 2004. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 53:1374–1384. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo Y-T, Liou J-M, El-Omar EM, Wu J-Y, Leow AHR, Goh KL, Das R, Lu H, Lin J-T, Tu Y-K, Yamaoka Y, Wu M-S. 2017. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2:707–715. doi: 10.1016/S2468-1253(17)30219-4. [DOI] [PubMed] [Google Scholar]
- 4.Kwon DH, Dore MP, Kim JJ, Kato M, Lee M, Wu JY, Graham DY. 2003. High-level beta-lactam resistance associated with acquired multidrug resistance in Helicobacter pylori. Antimicrob Agents Chemother 47:2169–2178. doi: 10.1128/AAC.47.7.2169-2178.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blair JMA, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJV. 2015. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 6.Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. 2016. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 7.Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 8.Stark RM, Gerwig GJ, Pitman RS, Potts LF, Williams NA, Greenman J, Weinzweig IP, Hirst TR, Millar MR. 1999. Biofilm formation by Helicobacter pylori. Lett Appl Microbiol 28:121–126. doi: 10.1046/j.1365-2672.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 9.Carron MA, Tran VR, Sugawa C, Coticchia JM. 2006. Identification of Helicobacter pylori biofilms in human gastric mucosa. J Gastrointest Surg 10:712–717. doi: 10.1016/j.gassur.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Yonezawa H, Osaki T, Kamiya S. 2015. Biofilm formation by and its involvement for antibiotic resistance. Biomed Res Int 2015:914791. doi: 10.1155/2015/914791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fruci M, Poole K. 2016. Bacterial stress responses as determinants of antimicrobial resistance, p 115–136. In de Bruijn FJ. (ed), Stress and environmental regulation of gene expression and adaptation in bacteria. John Wiley & Sons, Inc, Hoboken, NJ. [Google Scholar]
- 12.Potrykus K, Cashel M. 2008. (p)ppGpp: still magical? Annu Rev Microbiol 62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 13.Srivatsan A, Wang JD. 2008. Control of bacterial transcription, translation and replication by (p)ppGpp. Curr Opin Microbiol 11:100–105. doi: 10.1016/j.mib.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Wu J, Long Q, Xie J. 2010. (p)ppGpp and drug resistance. J Cell Physiol 224:300–304. doi: 10.1002/jcp.22158. [DOI] [PubMed] [Google Scholar]
- 15.Maisonneuve E, Castro-Camargo M, Gerdes K. 2013. (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell 154:1140–1150. doi: 10.1016/j.cell.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 16.Sugisaki K, Hanawa T, Yonezawa H, Osaki T, Fukutomi T, Kawakami H, Yamamoto T, Kamiya S. 2013. Role of (p)ppGpp in biofilm formation and expression of filamentous structures in Bordetella pertussis. Microbiology 159:1379–1389. doi: 10.1099/mic.0.066597-0. [DOI] [PubMed] [Google Scholar]
- 17.Li G, Xie F, Zhang Y, Bossé JT, Langford PR, Wang C. 2015. Role of (p)ppGpp in viability and biofilm formation of Actinobacillus pleuropneumoniae S8. PLoS One 10:e0141501. doi: 10.1371/journal.pone.0141501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkinson GC, Tenson T, Hauryliuk V. 2011. The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS One 6:e23479. doi: 10.1371/journal.pone.0023479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mouery K, Rader BA, Gaynor EC, Guillemin K. 2006. The stringent response is required for Helicobacter pylori survival of stationary phase, exposure to acid, and aerobic shock. J Bacteriol 188:5494–5500. doi: 10.1128/JB.00366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells DH, Gaynor EC. 2006. Helicobacter pylori initiates the stringent response upon nutrient and pH downshift. J Bacteriol 188:1–5. doi: 10.1128/JB.188.1.1-18.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, Nelson K, Quackenbush J, Zhou L, Kirkness EF, Peterson S, Loftus B, Richardson D, Dodson R, Khalak HG, Glodek A, McKenney K, Fitzegerald LM, Lee N, Adams MD, Hickey EK, Berg DE, Gocayne JD, Utterback TR, Peterson JD, Kelley JM, Cotton MD, Weidman JM, Fujii C, Bowman C, Watthey L, Wallin E, Hayes WS, Borodovsky M, Karp PD, Smith HO, Fraser CM, Venter JC. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 22.Zhou YN, Coleman WG, Yang Z, Yang Y, Hodgson N, Chen F, Jin DJ. 2008. Regulation of cell growth during serum starvation and bacterial survival in macrophages by the bifunctional enzyme SpoT in Helicobacter pylori. J Bacteriol 190:8025–8032. doi: 10.1128/JB.01134-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maloney PC. 1994. Bacterial transporters. Curr Opin Cell Biol 6:571–582. [DOI] [PubMed] [Google Scholar]
- 24.Nikaido H. 1996. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol 178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webber MA, Piddock LJV. 2003. The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother 51:9–11. doi: 10.1093/jac/dkg050. [DOI] [PubMed] [Google Scholar]
- 26.Bina JE, Alm RA, Uria-Nickelsen M, Thomas SR, Trust TJ, Hancock REW. 2000. Helicobacter pylori uptake and efflux: basis for intrinsic susceptibility to antibiotics in vitro. Antimicrob Agents Chemother 44:248–254. doi: 10.1128/AAC.44.2.248-254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Amsterdam K, Bart A, Van Der Ende A. 2005. A Helicobacter pylori TolC efflux pump confers resistance to metronidazole. Antimicrob Agents Chemother 49:1477–1482. doi: 10.1128/AAC.49.4.1477-1482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu ZQ, Zheng PY, Yang PC. 2008. Efflux pump gene hefA of Helicobacter pylori plays an important role in multidrug resistance. World J Gastroenterol 14:5217–5222. doi: 10.3748/wjg.14.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirata K, Suzuki H, Nishizawa T, Tsugawa H, Muraoka H, Saito Y, Matsuzaki J, Hibi T. 2010. Contribution of efflux pumps to clarithromycin resistance in Helicobacter pylori. J Gastroenterol Hepatol 25(Suppl 1):S75–S79. doi: 10.1111/j.1440-1746.2009.06220.x. [DOI] [PubMed] [Google Scholar]
- 30.Trainor EA, Horton KE, Savage PB, Testerman TL, McGee DJ. 2011. Role of the HefC efflux pump in Helicobacter pylori cholesterol-dependent resistance to ceragenins and bile salts. Infect Immun 79:88–97. doi: 10.1128/IAI.00974-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsugawa H, Suzuki H, Muraoka H, Ikeda F, Hirata K, Matsuzaki J, Saito Y, Hibi T. 2011. Enhanced bacterial efflux system is the first step to the development of metronidazole resistance in Helicobacter pylori. Biochem Biophys Res Commun 404:656–660. doi: 10.1016/j.bbrc.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 32.Mehrabadi JF, Sirous M, Daryani NE, Eshraghi S. 2011. Assessing the role of the RND efflux pump in metronidazole resistance of Helicobacter pylori by RT-PCR assay. J Infect Dev Ctries 5:88–93. doi: 10.3855/jidc.1187. [DOI] [PubMed] [Google Scholar]
- 33.Soto SM. 2013. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 4:223–229. doi: 10.4161/viru.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Kievit TR, Parkins MD, Gillis RJ, Srikumar R, Ceri H, Poole K, Iglewski BH, Storey DG. 2001. Multidrug efflux pumps: expression patterns and contribution to antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 45:1761–1770. doi: 10.1128/AAC.45.6.1761-1770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamasaki S, Wang LY, Hirata T, Hayashi-Nishino M, Nishino K. 2015. Multidrug efflux pumps contribute to Escherichia coli biofilm maintenance. Int J Antimicrob Agents 45:439–441. doi: 10.1016/j.ijantimicag.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Ramage G. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother 49:973–980. doi: 10.1093/jac/dkf049. [DOI] [PubMed] [Google Scholar]
- 37.Yonezawa H, Osaki T, Hanawa T, Kurata S, Ochiai K, Kamiya S. 2013. Impact of Helicobacter pylori biofilm formation on clarithromycin susceptibility and generation of resistance mutations. PLoS One 8:e73301. doi: 10.1371/journal.pone.0073301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chávez de Paz LE, Lemos JA, Wickström C, Sedgley CM. 2012. Role of (p)ppGpp in biofilm formation by Enterococcus faecalis. Appl Environ Microbiol 78:1627–1630. doi: 10.1128/AEM.07036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Psakis G, Saidijam M, Shibayama K, Polaczek J, Bettaney KE, Baldwin JM, Baldwin SA, Hope R, Essen LO, Essenberg RC, Henderson PJF. 2009. The sodium-dependent d-glucose transport protein of Helicobacter pylori. Mol Microbiol 71:391–403. doi: 10.1111/j.1365-2958.2008.06535.x. [DOI] [PubMed] [Google Scholar]
- 40.Yim G, Wang HH, Davies J. 2007. Antibiotics as signalling molecules. Philos Trans R Soc Lond B Biol Sci 362:1195–1200. doi: 10.1098/rstb.2007.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodionov DG, Ishiguro EE. 1995. Direct correlation between overproduction of guanosine 3′,5′-bispyrophosphate (ppGpp) and penicillin tolerance in Escherichia coli. J Bacteriol 177:4224–4229. doi: 10.1128/jb.177.15.4224-4229.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abranches J, Martinez AR, Kajfasz JK, Chávez V, Garsin DA, Lemos JA. 2009. The molecular alarmone (p)ppGpp mediates stress responses, vancomycin tolerance, and virulence in Enterococcus faecalis. J Bacteriol 191:2248–2256. doi: 10.1128/JB.01726-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geiger T, Kästle B, Gratani FL, Goerke C, Wolz C. 2014. Two small (p)ppGpp synthases in Staphylococcus aureus mediate tolerance against cell envelope stress conditions. J Bacteriol 196:894–902. doi: 10.1128/JB.01201-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geng X, Li W, Chen Z, Gao S, Hong W, Ge X, Hou G, Hu Z, Zhou Y, Zeng B, Li W, Jia J, Sun Y. 2017. The bifunctional enzyme SpoT is involved in the clarithromycin tolerance of Helicobacter pylori by upregulating the transporters HP0939, HP1017, HP0497, and HP0471. Antimicrob Agents Chemother 61:e02011-16. doi: 10.1128/AAC.02011-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He H, Cooper JN, Mishra A, Raskin DM. 2012. Stringent response regulation of biofilm formation in Vibrio cholerae. J Bacteriol 194:2962–2972. doi: 10.1128/JB.00014-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dean SN, Chung MC, van Hoek ML. 2015. Burkholderia diffusible signal factor signals to Francisella novicida to disperse biofilm and increase siderophore production. Appl Environ Microbiol 81:7057–7066. doi: 10.1128/AEM.02165-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pamp SJ, Gjermansen M, Johansen HK, Tolker-Nielsen T. 2008. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol Microbiol 68:223–240. doi: 10.1111/j.1365-2958.2008.06152.x. [DOI] [PubMed] [Google Scholar]
- 48.Bailey AM, Webber MA, Piddock LJV. 2006. Medium plays a role in determining expression of acrB, marA, and soxS in Escherichia coli. Antimicrob Agents Chemother 50:1071–1074. doi: 10.1128/AAC.50.3.1071-1074.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eaves DJ, Ricci V, Piddock LJV. 2004. Expression of acrB, acrF, acrD, marA, and soxS in Salmonella enterica serovar Typhimurium: role in multiple antibiotic resistance. Antimicrob Agent Chemother 48:1145–1150. doi: 10.1128/AAC.48.4.1145-1150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Attaran B, Falsafi T, Ghorbanmehr N. 2017. Effect of biofilm formation by clinical isolates of Helicobacter pylori on the efflux-mediated resistance to commonly used antibiotics. World J Gastroenterol 23:1163–1170. doi: 10.3748/wjg.v23.i7.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumura K, Furukawa S, Ogihara H, Morinaga Y. 2011. Roles of multidrug efflux pumps on the biofilm formation of Escherichia coli K-12. Biocontrol Sci 16:69–72. doi: 10.4265/bio.16.69. [DOI] [PubMed] [Google Scholar]
- 52.Limoli DH, Jones CJ, Wozniak DJ, Cruz S. 2015. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectr 3(3):MB-0011-2014. doi: 10.1128/microbiolspec.MB-0011-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Bambeke F, Balzi E, Tulkens M. 2000. Antibiotic efflux pumps. Biochem Pharmacol 60:457–470. doi: 10.1016/S0006-2952(00)00291-4. [DOI] [PubMed] [Google Scholar]
- 54.Chollet R, Chevalier J, Bryskier A, Pagès JM. 2004. The AcrAB-TolC pump is involved in macrolide resistance but not in telithromycin efflux in Enterobacter aerogenes and Escherichia coli. Antimicrob Agents Chemother 48:3621–3624. doi: 10.1128/AAC.48.9.3621-3624.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun J, Deng Z, Yan A. 2014. Bacterial multidrug efflux pumps: mechanisms, physiology and pharmacological exploitations. Biochem Biophys Res Commun 453:254–267. doi: 10.1016/j.bbrc.2014.05.090. [DOI] [PubMed] [Google Scholar]
- 56.Pomares MF, Vincent PA, Farías RN, Salomón RA. 2008. Protective action of ppGpp in microcin J25-sensitive strains. J Bacteriol 190:4328–4334. doi: 10.1128/JB.00183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jishage M, Kvint K, Shingler V, Nyström T. 2002. Regulation of σ factor competition by the alarmone ppGpp. Genes Dev 16:1260–1270. doi: 10.1101/gad.227902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niehus E, Gressmann H, Ye F, Schlapbach R, Dehio M, Dehio C, Stack A, Meyer TF, Suerbaum S, Josenhans C. 2004. Genome-wide analysis of transcriptional hierarchy and feedback regulation in the flagellar system of Helicobacter pylori. Mol Microbiol 52:947–961. doi: 10.1111/j.1365-2958.2004.04006.x. [DOI] [PubMed] [Google Scholar]
- 59.Osato MS, Reddy R, Reddy SG, Penland RL, Graham DY. 2001. Comparison of the Etest and the NCCLS-approved agar dilution method to detect metronidazole and clarithromycin resistant Helicobacter pylori. Int J Antimicrob Agents 17:39–44. doi: 10.1016/S0924-8579(00)00320-4. [DOI] [PubMed] [Google Scholar]
- 60.Franklin MJ, Bothner B, Akiyama T, Chang C. 2015. New technologies for studying biofilms. Microbiol Spectr 3(4):MB-0016-2014. doi: 10.1128/microbiolspec.MB-0016-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merritt JH, Kadouri DE, O'Toole GA. 2005. Growing and analyzing static biofilms. Curr Protoc Microbiol Chapter 1:Unit 1B.1. doi: 10.1002/9780471729259.mc01b01s00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun Y, Li X, Li W, Zhao M, Wang L, Liu S, Zeng J, Liu Z, Jia J. 2012. Proteomic analysis of the function of spot in Helicobacter pylori anti-oxidative stress in vitro and colonization in vivo. J Cell Biochem 113:3393–3402. doi: 10.1002/jcb.24215. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Z. 2010. Influence of efflux pump inhibitors on the multidrug resistance of Helicobacter pylori. World J Gastroenterol 16:1279. doi: 10.3748/wjg.v16.i10.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.