VT-1129 is a novel fungal enzyme-specific Cyp51 inhibitor with potent cryptococcal activity. Because of its long half-life (>6 days in mice) and our desire to quickly reach potent efficacy, we evaluated a VT-1129 loading dose-maintenance dose strategy against cryptococcal meningitis.
KEYWORDS: VT-1129, cryptococcal meningitis, Cryptococcus neoformans, in vivo efficacy, loading dose, maintenance dose, murine model
ABSTRACT
VT-1129 is a novel fungal enzyme-specific Cyp51 inhibitor with potent cryptococcal activity. Because of its long half-life (>6 days in mice) and our desire to quickly reach potent efficacy, we evaluated a VT-1129 loading dose-maintenance dose strategy against cryptococcal meningitis. VT-1129 plasma and brain pharmacokinetics were first studied in healthy mice, and these data were used to model loading dose-maintenance dose regimens to generate different steady-state concentrations. Mice were inoculated intracranially with Cryptococcus neoformans, and oral treatment began 1 day later. Treatment consisted of placebo or one of three VT-1129 loading dose-maintenance dose regimens, i.e., loading dose of 1, 3, or 30 mg/kg on day 1, followed by once-daily maintenance doses of 0.15, 0.5, or 5 mg/kg, respectively. In the fungal burden arm, therapy continued for 14 days and brains were collected on day 15 for fungal burden assessments. In the survival arm, treatment continued for 10 days, after which mice were monitored without therapy until day 30. VT-1129 plasma and brain concentrations were also measured. All VT-1129 doses significantly improved survival and reduced fungal burdens, compared to placebo. VT-1129 plasma and brain levels correlated with fungal burden reductions (R2 = 0.72 and R2 = 0.67, respectively), with a plasma concentration of 1 μg/ml yielding a reduction of ∼5 log10 CFU/g. With the highest loading dose-maintenance dose regimen, fungal burdens were undetectable in one-half of the mice in the fungal burden arm and in one-fourth of the mice in the survival arm, 20 days after the final dose. These data support a loading dose-maintenance dose strategy for quickly reaching highly efficacious VT-1129 concentrations for treating cryptococcal meningitis.
INTRODUCTION
Cryptococcal meningitis is a common and life-threatening invasive fungal infection in at-risk groups, including transplant recipients, lymphoma and leukemia patients, and individuals with HIV/AIDS (1). Current treatment recommendations include induction therapy with intravenously administered amphotericin B combined with 5-flucytosine, whose addition has been shown to lead to more rapid sterilization of the cerebrospinal fluid (2–5). Induction therapy is followed by consolidation and maintenance therapy phases, usually with orally administered fluconazole; maintenance therapy can be prolonged in the setting of continued immunosuppression. Even with appropriate therapy, mortality rates may range between 20% and 30% (6–8). In resource-limited settings, where amphotericin and 5-flucytosine may be unavailable, oral fluconazole therapy may be the only treatment option (9). However, this approach is suboptimal and results in high clinical failure rates, even when high doses are used (10, 11). While other triazoles, such as itraconazole, posaconazole, voriconazole, and isavuconazole, have activity against Cryptococcus species, experience with these agents in the treatment of cryptococcal meningitis is limited, and the triazoles may be associated with drug-drug interactions and adverse effects due to interactions with mammalian enzymes, including cytochrome P450 enzymes responsible for drug metabolism. Thus, there is a need for more efficacious, orally available agents for the treatment of cryptococcal meningitis.
VT-1129 is a novel tetrazole antifungal that can be administered orally. This agent prevents the synthesis of ergosterol, an important component of the fungal cell membrane, by selectively inhibiting the fungal cytochrome P450 enzyme Cyp51 (i.e., lanosterol 14α-demethylase) (12–14). Compared to clinically available triazoles that are used in the treatment of invasive fungal infections, VT-1129 is highly selective for fungal Cyp51 (14, 15). Thus, the potential for the drug-drug interactions and adverse effects that are often observed with the triazoles may be avoided. Our group demonstrated previously that the potent in vitro activity of VT-1129 observed against Cryptococcus species translated into in vivo efficacy over a wide range of fixed doses (12, 16). Given the prolonged half-life of VT-1129 (>6 days in mice), which dictates very slow achievement of steady-state levels, and the high concentrations of this tetrazole that are achieved within the central nervous system (CNS), a large loading dose on the first day of dosing should allow rapid achievement of an efficacious drug concentration, with lower daily maintenance doses minimizing the potential toxicities that could manifest late in therapy. The objective of this study was to evaluate the in vivo efficacy of a loading dose-maintenance dose administration strategy for VT-1129 in an established murine model of cryptococcal meningitis.
RESULTS
Pharmacokinetics and design of loading dose-maintenance dose treatment regimens.
Following single oral administrations of 10 and 100 mg/kg VT-1129 in mice, in vivo exposures (maximum plasma concentration [Cmax] and area under the concentration-time curve [AUC]) of VT-1129 appeared to be dose proportional (Tables 1 to 3 and Fig. 1). The Cmax increased from 3.33 μg/ml at 10 mg/kg to 30.8 μg/ml at 100 mg/kg, an approximately 10-fold increase. Similarly, the corresponding plasma AUC from 0 to 96 h (AUC0–96) increased from 252 μg · h/ml to 2,602 μg · h/ml. These data suggest that the in vivo exposure of VT-1129 increases linearly with the dose over the range tested. The brain concentration-time profiles showed patterns similar to those for the plasma profiles; in general, the brain concentrations were higher than the plasma concentrations at the same dose (Fig. 1). The average brain/plasma concentration ratio was approximately 1.5 at both doses. As shown in Fig. 1, a slow decline of VT-1129 concentrations was observed in mice. Thus, significant accumulation would be expected with repeat once-daily doses of either 10 mg/kg or 100 mg/kg.
TABLE 1.
Plasma and brain concentrations of VT-1129 in uninfected male CD-1 mice after single oral administration of VT-1129 at 10 and 100 mg/kg (n = 3 per time point)
| Time (h) | 10 mg/kg VT-1129 |
100 mg/kg VT-1129 |
||||
|---|---|---|---|---|---|---|
| Plasma level (mean ± SD) (μg/ml) | Brain level (mean ± SD) (μg/g) | Brain/plasma ratio | Plasma level (mean ± SD) (μg/ml) | Brain level (mean ± SD) (μg/g) | Brain/plasma ratio | |
| 0.25 | 0.486 ± 0.155 | 0.351 ± 0.056 | 0.7 | 5.827 ± 1.098 | 3.759 ± 0.374 | 0.6 |
| 0.5 | 1.024 ± 0.131 | 1.405 ± 0.302 | 1.4 | 7.944 ± 2.132 | 10.974 ± 3.391 | 1.4 |
| 1 | 1.783 ± 0.192 | 2.927 ± 0.205 | 1.6 | 13.122 ± 0.794 | 23.552 ± 3.353 | 1.8 |
| 2 | 2.088 ± 0.223 | 5.241 ± 1.770 | 2.5 | 14.933 ± 2.124 | 34.531 ± 3.905 | 2.3 |
| 4 | 2.832 ± 0.774 | 4.616 ± 0.353 | 1.6 | 18.315 ± 2.325 | 35.911 ± 1.214 | 2.0 |
| 6 | 2.996 ± 0.277 | 5.109 ± 0.084 | 1.7 | 15.964 ± 1.570 | 42.686 ± 6.603 | 2.7 |
| 24 | 3.329 ± 0.908 | 4.365 ± 0.109 | 1.3 | 30.608 ± 2.361 | 40.346 ± 1.484 | 1.3 |
| 48 | 2.562 ± 0.393 | 3.888 ± 0.291 | 1.5 | 30.787 ± 4.551 | 31.936 ± 8.658 | 1.0 |
| 96 | 2.057 ± 0.208 | 2.401 ± 0.244 | 1.2 | 25.742 ± 2.934 | 27.624 ± 3.263 | 1.1 |
| Average | 1.43 | 1.57 | ||||
TABLE 2.
Pharmacokinetic parameters of VT-1129 in uninfected male CD-1 mice after single oral administration of doses of 10 and 100 mg/kg
| Pharmacokinetic parametera | 10 mg/kg VT-1129 |
100 mg/kg VT-1129 |
||
|---|---|---|---|---|
| Plasma | Brain | Plasma | Brain | |
| Cmax | 3.33 μg/ml | 5.24 μg/g | 30.8 μg/ml | 42.7 μg/g |
| AUC0–96 | 252 μg · h/ml | 360 μg · h/g | 2,602 μg · h/ml | 3,231 μg · h/g |
Cmax and AUC0–96 values were calculated with the mean concentrations from 3 mice at each time point.
TABLE 3.
Pharmacokinetic parameters of VT-1129 in uninfected male CD-1 mice after oral administration of a 30-mg/kg loading dose on day 1 and 5-mg/kg maintenance doses on days 2 to 8
| Pharmacokinetic parametera | Day 1, plasma | Day 8 |
|
|---|---|---|---|
| Plasma | Brain | ||
| Cmax | 6.83 μg/ml | 11.6 μg/ml | 46.9 μg/g |
| AUC0–24 | 134 μg · h/ml | 251 μg · h/ml | 1,040 μg · h/g |
Cmax and AUC0–24 values were calculated with the mean concentrations from 3 mice at each time point.
FIG 1.
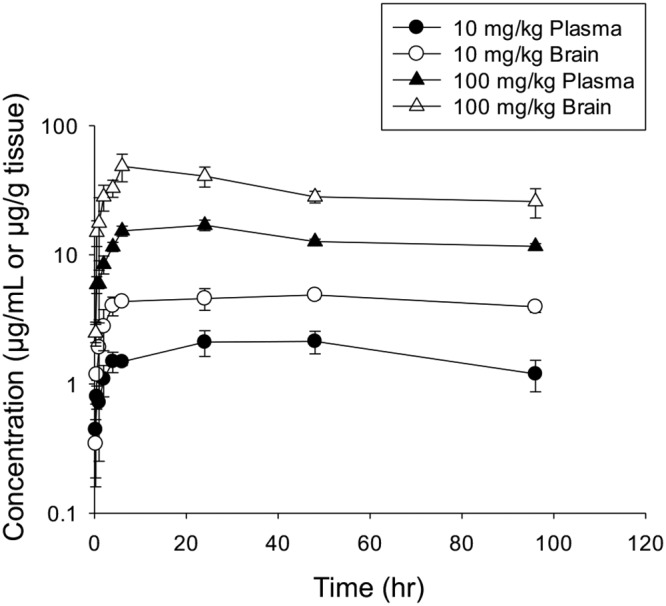
Mean plasma and brain concentrations of VT-1129 after single oral doses of 10 or 100 mg/kg (n = 3 per time point).
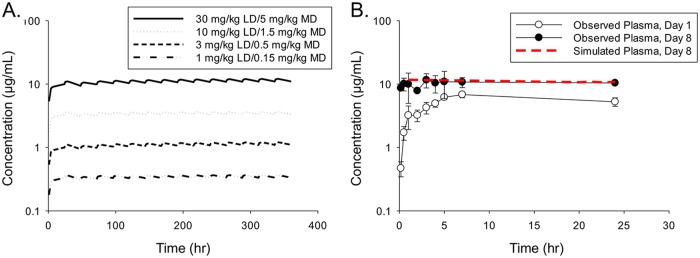
To apply a loading dose-maintenance dose strategy for the efficacy study, a simulation was conducted with different combinations of loading doses and maintenance doses in order to provide target plasma steady-state concentration (Css) values of 0.3, 1.0, 3.0, and 10.0 μg/ml (Fig. 2A). This goal could be achieved with the following dosing regimens: 1-mg/kg loading dose followed by 0.15-mg/kg daily maintenance doses to achieve a Css of 0.3 μg/ml; 3-mg/kg loading dose followed by 0.5-mg/kg daily maintenance doses to achieve a Css of 1.0 μg/ml; 10-mg/kg loading dose followed by 1.5-mg/kg daily maintenance doses to achieve a Css of 3.0 μg/ml; and 30-mg/kg loading dose followed by 5-mg/kg daily maintenance doses to achieve a Css of 10.0 μg/ml (Fig. 2B).
FIG 2.
(A) Simulated VT-1129 plasma concentrations with different loading dose (LD)-maintenance dose (MD) regimens in uninfected mice. (B) Comparison of simulated and observed plasma concentrations of VT-1129 with a loading dose of 30 mg/kg on day 1 and daily maintenance doses of 5 mg/kg on days 2 to 8. Mean concentrations from three mice at each time point were used.
To confirm the simulation results, an in-life animal study was conducted with a loading dose of 30 mg/kg on day 1, followed by daily maintenance doses of 5 mg/kg on days 2 to 8. Pharmacokinetic parameters are summarized in Table 3. The observed mean Cmax values were 6.83 μg/ml on day 1 and 11.6 μg/ml on day 8. The corresponding plasma AUC from 0 to 24 h (AUC0–24) values were 134 and 251 μg · h/ml on day 1 and day 8, respectively. The time-averaged daily concentration (Cmean) values, which were calculated from the AUC0–24/24 h ratios, were 5.58 μg/ml on day 1 and 10.5 μg/ml on day 8. These data suggested that the daily exposure (Cmax, AUC0–24, and Cmean) of VT-1129 could be maintained after reducing the dose from the initial loading dose of 30 mg/kg on day 1 to the daily maintenance dose of 5 mg/kg (a 6-fold dose reduction). The observed plasma Cmean value of 10.5 μg/ml on day 8 was in line with the predicted value of 10.0 μg/ml based on the simulation.
Survival.
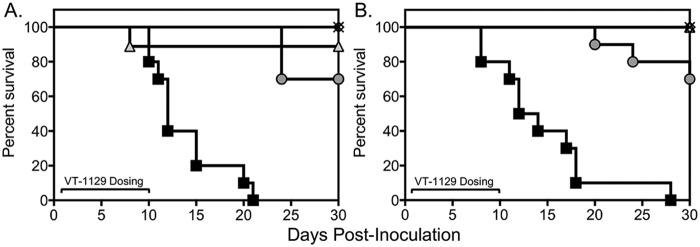
Treatment with VT-1129 resulted in a survival advantage in this experimental model of cryptococcal meningitis. Each VT-1129 loading dose-maintenance dose group and the positive-control group (10 mg/kg VT-1129 once daily, as a fixed dose) exhibited significant improvements in survival, compared to the vehicle control group. The median survival time was >30 days for each VT-1129 group, and the 30-day survival rates ranged from 70 to 100%. The median survival time and survival rate for the placebo group were 12 days and 0%, respectively. These results were highly reproducible between the two survival arm experiments (Fig. 3).
FIG 3.
Survival curves for mice inoculated intracranially with C. neoformans and treated with VT-1129. Treatment with oral therapy started 1 day postinoculation and continued for 10 days, after which mice were monitored without therapy until day 30. (A and B) Two independent experiments from the survival arm, with 10 mice per group per experiment. Black squares, vehicle control; gray circles, 1-mg/kg loading dose of VT-1129 on day 1 and 0.15-mg/kg daily maintenance doses thereafter; times signs, 3-mg/kg loading dose of VT-1129 on day 1 and 0.5-mg/kg daily maintenance doses thereafter; white diamonds, 30-mg/kg loading dose of VT-1129 on day 1 and 5-mg/kg daily maintenance doses thereafter; gray triangles, daily VT-1129 dose of 10 mg/kg.
Fungal burdens.
Significant reductions in fungal burdens were also observed with VT-1129. In the fungal burden arm, following 14 days of treatment, each VT-1129 loading dose-maintenance dose combination (mean log10 CFU/g range of 0.85 to 4.57) and the VT-1129 positive comparator dose of 10 mg/kg/day (mean log10 CFU/g of 0.46) significantly reduced fungal burdens, compared to the vehicle control (mean log10 CFU/g of 6.41; P < 0.0004) (Fig. 4A). For the VT-1129 loading dose-maintenance dose combinations of 3 mg/kg-0.5 mg/kg and 30 mg/kg-5 mg/kg and the VT-1129 daily dose of 10 mg/kg (no loading dose), CFU were undetectable in some animals (20%, 50%, and 70% of animals, respectively), even after the additional wash and filtration steps. Similar to the survival arm, the fungal burden results on day 15 were consistent between the two separate experiments.
FIG 4.
Brain tissue fungal burdens in mice with cryptococcal meningitis secondary to C. neoformans. (A) Brain tissue fungal burdens in mice in the fungal burden arm experiments. Treatment with oral therapy started 1 day postinoculation and continued for 14 days. CFU in brain tissue were measured on day 15 postinoculation. (B) Brain tissue fungal burdens in mice in the survival arm experiments. Treatment with oral therapy started 1 day postinoculation and continued for 10 days. Mice were monitored without therapy until day 30. CFU in brain tissue were measured on day 30 postinoculation or as the mice succumbed to infection. Combined results of two independent experiments, with 20 mice per group, are shown. Horizontal lines represent mean values. LD, loading dose; MD, maintenance dose. Light gray circles, CFU detected after vacuum filtration and washing to remove residual VT-1129; times signs, no fungal burden detected by any method. *, P < 0.001; **, P < 0.0001.
Fungal burdens were also assessed in the survival arm on day 30, 20 days after the cessation of therapy, or on the days the animals succumbed to infection or were moribund (Fig. 4B). The VT-1129 combinations of a 3-mg/kg loading dose followed by 0.5-mg/kg daily maintenance doses and a 30-mg/kg loading dose followed by 5-mg/kg daily maintenance doses significantly reduced fungal burdens (mean log10 CFU/g of 4.29 and 1.79, respectively), compared to the vehicle control (mean log10 CFU/g of 6.88; P < 0.0001). Fungal burdens remained undetectable in 5 of the 20 mice in the highest VT-1129 loading dose-maintenance dose group even after the homogenates were washed and filtered to remove antifungal carryover. Fungal burdens also remained significantly lower in the positive comparator group, i.e., VT-1129 at 10 mg/kg/day (mean log10 CFU/g of 3.14; P < 0.0001), compared to the vehicle control. The survival fungal burden results were reproducible between the two survival experiments.
VT-1129 concentrations.
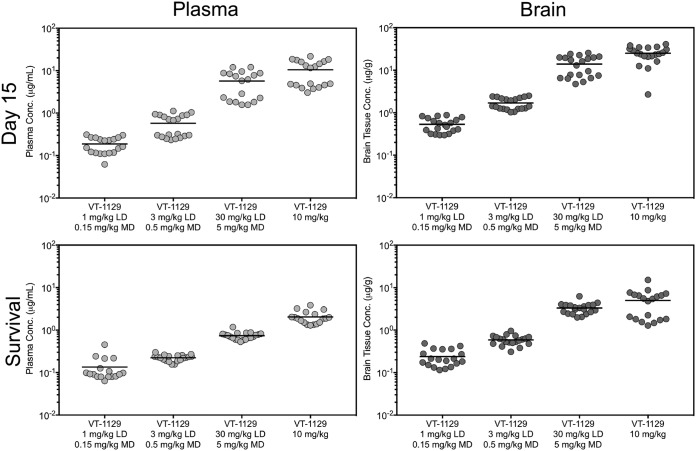
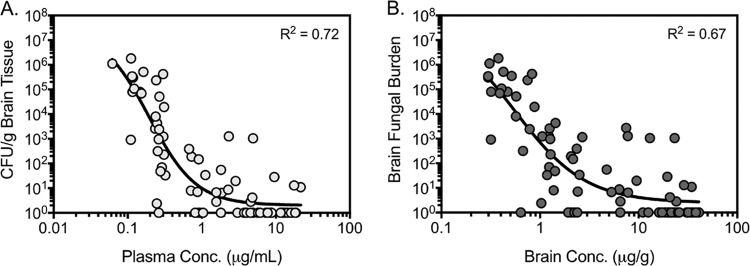
In the fungal burden arm, VT-1129 concentrations in both plasma and brain tissue on day 15 remained elevated and above the MIC for the Cryptococcus neoformans isolate used to establish infection (Fig. 5). Brain tissue concentrations of VT-1129 were higher than those measured in the plasma, with brain/plasma concentration ratios ranging from 2.90 to 3.51. Nonlinear regression analysis demonstrated a good correlation between the plasma and brain concentrations achieved on day 15 and reductions in fungal burdens, with coefficients of determination (R2) of 0.72 and 0.67, respectively (Fig. 6). The mean plasma concentrations observed from the end-bleed samples were comparable to the predicted values from the simulation based on the pharmacokinetic study in uninfected mice. This analysis showed that a plasma concentration of 1 μg/ml VT-1129 correlated with a reduction in fungal burden of ∼5 log10 CFU/g, with the VT-1129 group having a fungal burden at day 15 of ∼10 CFU/g, compared to a fungal burden of ∼1 × 106 CFU/g for the vehicle control group.
FIG 5.
VT-1129 plasma and brain tissue concentrations in mice with cryptococcal meningitis due to C. neoformans, on day 15 after 14 days of dosing in the fungal burden arm and on day 30 after 10 days of dosing (20 days after cessation of dosing) in the survival arm. Combined results of two independent experiments, with 20 mice per group, are shown. Horizontal lines represent mean values. LD, loading dose; MD, maintenance dose.
FIG 6.
Plasma (A) and brain (B) VT-1129 concentration-response curves on day 15 after 14 days of therapy in the fungal burden arm. Combined results of two independent experiments are shown.
In the survival arm, both plasma and brain tissue concentrations of VT-1129 remained elevated and above the MIC for the C. neoformans isolate used to establish infection in the mice in this study (≤0.015 μg/ml), although therapy had been stopped for 20 days (Fig. 5). Similar to the fungal burden arm results, concentrations of VT-1129 achieved within the brain tissue in the survival arm were higher than those measured in the plasma, with mean brain/plasma VT-1129 concentration ratios ranging from 2.01 to 4.60 in different dosing groups.
DISCUSSION
VT-1129 is an investigational tetrazole that is highly selective for the fungal Cyp51 enzyme responsible for the conversion of lanosterol to ergosterol in the fungal cell membrane and has little to no interactions with mammalian cytochrome P450 enzymes (14, 15). Our group demonstrated previously that VT-1129 had potent in vitro activity against Cryptococcus species, which translated into in vivo efficacy in experimental cryptococcal meningitis using fixed-dose treatment regimens (12, 16). In the current study, we demonstrated that a VT-1129 loading dose-maintenance dose administration strategy was also highly efficacious in this murine model of cryptococcal meningitis and the results were highly reproducible. The lowest loading-maintenance dose regimen evaluated was effective in improving survival and reducing fungal burdens within the brains of mice infected with C. neoformans. The in vivo efficacy observed in this study was enhanced with higher loading and maintenance doses and was maximized with the 30-mg/kg loading dose followed by 5-mg/kg daily maintenance doses. CFU were undetectable in brain homogenates of one-half of the mice in this group in the fungal burden arm. In these experiments, there was also a clear relationship between the concentrations of VT-1129 achieved in the plasma and brains and reductions in fungal burdens. In addition, CFU remained undetectable in one-fourth of the mice in the highest loading dose-maintenance dose group on day 30 of the survival arm, 20 days after the cessation of therapy. This continued efficacy nearly 3 weeks after the cessation of treatment can be attributed to the VT-1129 concentrations remaining elevated and well above the MIC for the infecting C. neoformans isolate, due to the long half-life of VT-1129 in mice (>6 days). This finding may have clinical implications, as VT-1129 may have the potential to clear Cryptococcus from the CNS or may be administered less frequently during the maintenance phase of treatment.
As with all studies that utilize animal models of invasive infections, this one is not without limitations. The C. neoformans isolate used to cause cryptococcal meningitis is a relatively susceptible strain, and the mice used in the model were immunocompetent; it is not known whether VT-1129 would have been as effective against infection caused by a less susceptible isolate or in the setting of immunosuppression. Previous work, in which members of our group participated, did demonstrate that the in vitro potency of VT-1129 was maintained against isolates with elevated fluconazole MICs (geometric mean VT-1129 MIC of 0.0506 μg/ml against isolates with fluconazole MICs of ≥8 μg/ml) (12). Thus, given its potent in vitro activity and the high level of exposure that is achieved due to its long half-life, it is possible that VT-1129 would maintain in vivo efficacy against isolates with reduced susceptibility to triazoles such as fluconazole.
In conclusion, this study is supportive of the continued evaluation of VT-1129 as a treatment against cryptococcal meningitis, and it indicates that a loading dose-maintenance dose regimen may be an effective administration strategy, due to the long half-life of VT-1129. Further work, including studies with immunocompromised hosts and clinical trials, are warranted to determine the place of VT-1129 therapy for cryptococcal meningitis.
MATERIALS AND METHODS
Isolate.
C. neoformans USC1597 was used in these experiments, as this isolate has been used previously by our group to establish CNS infections and to study responses to antifungal therapy (17–19). The MICs of VT-1129 and fluconazole against this isolate are ≤0.015 μg/ml and 1 μg/ml (50% inhibition of growth after 72 h), respectively, as measured using CLSI broth microdilution methodology (20). Following initial subculture from the stock onto Sabouraud dextrose agar (SDA), colonies of the strain were placed in brain heart infusion broth overnight at 37°C, in a shaking incubator, prior to inoculation. The cells were then collected by centrifugation and washed three times in sterile physiological saline. A hemocytometer was used to determine the concentrations of cells, and viability was confirmed by plating of serial dilutions and CFU enumeration.
Antifungal drugs and dosing formulations.
The benzenesulfonic acid (BSA) salt of VT-1129 (purity of >98%), manufactured by the National Center for Advancing Translational Sciences (National Institutes of Health, Bethesda, MD), was used in each experiment. The formulation was prepared by dissolving VT-1129 in 100% ethanol, with heating at 40°C, and stirring it as necessary to facilitate it going into solution. An equal volume of Cremophor EL was then added and mixed well, followed by the addition of sterile distilled water with frequent vortex-mixing. The stock solution was composed of 1, 1, and 8 parts of ethanol, Cremophor EL, and sterile distilled water, respectively. The maximum concentrations of ethanol and Cremophor EL in the doses of VT-1129 BSA and placebo administered to mice were 4% (vol/vol) each. The desired concentrations of VT-1129 BSA to obtain the equivalent doses of VT-1129 were achieved with further dilutions in sterile distilled water. The dosing solutions were prepared 1 day prior to the first dose, were kept refrigerated (2°C to 5°C), and were used for the duration of each study. The clinically available intravenous formulation of fluconazole was used. All dose administrations were by oral gavage.
Pharmacokinetic studies and simulations.
To determine the pharmacokinetic properties of VT-1129 BSA after oral administration, concentrations of VT-1129 were measured in uninfected male CD-1 mice after single oral doses of 10 and 100 mg/kg. This study was performed by Apredica/Cyprotex (Watertown, MA). The test article and the formulation used in the pharmacokinetic studies were the same as those used for the efficacy studies. Plasma and brain samples were collected from mice (n = 3 per time point) prior to dosing (0 h) and at 0.25, 0.5, 1, 2, 4, 6, 24, 48, and 96 h after oral administration. The concentrations of VT-1129 in plasma and brain samples were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (lower limit of quantification [LLOQ] of 0.005 μg/ml for plasma and 0.005 μg/g for brain tissues). Pharmacokinetic parameters for VT-1129 were calculated with the mean concentration from 3 mice at each time point, using noncompartmental analysis (NCA) (model 200) in the pharmacokinetic software package Phoenix WinNonlin (Certara, St. Louis, MO). The AUC was calculated using the linear trapezoidal method.
A simulation was performed for the loading dose-maintenance dose study design with the pharmacokinetic data obtained with the single oral dose of 10 mg/kg in mice, assuming linear kinetics for VT-1129. A nonparametric superposition method was applied in the simulation. A combination of different loading doses on day 1 and maintenance doses from day 2 to day 14 were used in the simulation, aiming at target VT-1129 Css values of 0.3, 1.0, 3.0, and 10.0 μg/ml.
To verify the simulation results, another pharmacokinetic study was conducted with male CD-1 mice after oral administration of a loading dose of 30 mg/kg on day 1, followed by daily maintenance doses of 5 mg/kg on days 2 to 8. Plasma samples were obtained on day 1, after oral administration of the loading dose, and day 8, after the last maintenance dose. Brain samples were also collected on day 8. The plasma and brain samples were analyzed by qualified LC-MS/MS methods for VT-1129. The pharmacokinetic parameters were calculated with the same method as described above.
Animal model.
An established murine model of cryptococcal meningitis was used to evaluate the in vivo efficacy of the loading dose-maintenance dose strategy for VT-1129 (17, 21, 22). Briefly, immunocompetent outbred male ICR mice (∼25 g; Envigo, Indianapolis, IN) were used and had access to food and water ad libitum. Mice were anesthetized with isoflurane and inoculated intracranially with 2,600 to 3,500 CFU/animal. The inoculum (volume of 0.06 ml) was delivered approximately 6 mm posterior to the orbit, through a midline puncture into the cranial vault, through a 27-gauge needle fastened to a tuberculin syringe with a cuff, to prevent penetration of more than 1 mm (17, 18). Following inoculation, mice were allowed to recover from anesthesia and then were randomized into experimental groups. This animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio, and all animals were maintained in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care guidelines.
Treatment groups.
Antifungal therapy began 1 day postinoculation. All treatments were administered by oral gavage, and treatment groups consisted of vehicle control and VT-1129 BSA at VT-1129 equivalent loading doses of 1, 3, and 30 mg/kg on day 1 and maintenance doses of 0.15, 0.5, and 5 mg/kg once daily. In addition, one group served as a positive control and received doses of 10 mg/kg VT-1129 once daily (i.e., without an initial loading dose on day 1).
Survival and fungal burdens.
In the survival arm, treatment continued for 10 days and survival was assessed on day 30. Any animal that appeared moribund prior to the designated endpoint was humanely euthanized, and death was recorded as occurring the next day. In the fungal burden arm, therapy continued for 14 days. On day 15, the day after cessation of therapy, mice were humanely euthanized and the brains were collected for fungal burden analysis by quantification of CFU. The brains were divided in half, with one half being weighed and homogenized in sterile saline and the other half being frozen (at −70°C within ∼1 h after collection) and used for determination of drug concentrations. Serial dilutions of the homogenates were prepared in sterile saline and plated on SDA. Following at least 72 h of incubation at 37°C, colonies were counted and the number of CFU per gram of brain tissue was calculated for each animal. For brain tissue samples in which no CFU were observed even in undiluted samples, vacuum filtration was performed to determine whether viable cryptococcal cells were present or whether antifungal carryover was occurring, which would suppress growth. Undiluted homogenate (0.5 ml) was pipetted onto a glass filter and the filter was washed with 5 ml of sterile water, followed by vacuum filtration. The filter was then removed, placed on SDA, and incubated at 37°C for at least 72 h, and the number of CFU was determined. Fungal burden analysis was performed in the same manner in the survival arm, on day 30 or on the day the mice succumbed to infection or were humanely euthanized due to morbidity. The experiments in the fungal burden and survival arms were conducted in duplicate, to evaluate the results for reproducibility.
Plasma and brain tissues were collected on day 15, the day after cessation of therapy, in the fungal burden arm and at the survival study endpoint on day 30 or on the day the mice succumbed to infection or were humanely euthanized due to morbidity in the survival arm. The plasma and brains were kept frozen and sent frozen, and VT-1129 was measured with an established LC-MS/MS assay (Apredica/Cyprotex).
Data analysis.
Survival data were plotted by Kaplan-Meier analysis, and differences in the median survival times and survival rates were analyzed with the log-rank test and Fisher's exact test, respectively. Differences in fungal burdens and VT-1129 concentrations between groups were assessed for significance by analysis of variance (ANOVA), with Tukey's posttest for multiple comparisons. Nonlinear regression was used to assess the relationship between VT-1129 plasma concentrations and fungal burdens. These data were fit to a four-parameter inhibitory sigmoid model (modified Hill equation) using curve-fitting software (Prism 6.0b; GraphPad Software, Inc., La Jolla, CA), and the goodness of fit was assessed by the coefficient of determination (R2).
ACKNOWLEDGMENTS
We thank Cyprotex for analysis of plasma and brain tissue concentrations of VT-1129.
This project was funded in whole or in part with federal funds from the National Cancer Institute, the National Institutes of Health (contract HHSN261200800001E), and the National Center for Advancing Translational Sciences.
N.P.W. has received research support to UT Health San Antonio from Astellas, bioMérieux, Cidara, F2G, Merck, Pfizer, and Viamet, has served on advisory boards for Merck, Astellas, Toyama, and Viamet, and has served as a speaker for Gilead. T.F.P. has received research grants to UT Health San Antonio from Astellas, Merck, and Revolution Medicines and has served as a consultant for Astellas, Gilead, Merck, Pfizer, Revolution Medicines, Toyama, Viamet, and Scynexis. L.K.N. has received travel support from Viamet Pharmaceuticals, Inc. E.P.G., W.J.H., and R.J.S. are employees of Viamet Pharmaceuticals, Inc. S.R.B. was an employee of Viamet Pharmaceuticals, Inc., at the time of the study and is now an employee of Mycovia Pharmaceuticals, Inc.
REFERENCES
- 1.Chayakulkeeree M, Perfect JR. 2006. Cryptococcosis. Infect Dis Clin North Am 20:507–544, v–vi. doi: 10.1016/j.idc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Horst CM, Saag MS, Cloud GA, Hamill RJ, Graybill JR, Sobel JD, Johnson PC, Tuazon CU, Kerkering T, Moskovitz BL, Powderly WG, Dismukes WE. 1997. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N Engl J Med 337:15–21. [DOI] [PubMed] [Google Scholar]
- 4.Dromer F, Bernede-Bauduin C, Guillemot D, Lortholary O. 2008. Major role for amphotericin B-flucytosine combination in severe cryptococcosis. PLoS One 3:e2870. doi: 10.1371/journal.pone.0002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brouwer AE, Rajanuwong A, Chierakul W, Griffin GE, Larsen RA, White NJ, Harrison TS. 2004. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet 363:1764–1767. doi: 10.1016/S0140-6736(04)16301-0. [DOI] [PubMed] [Google Scholar]
- 6.Dromer F, Mathoulin-Pelissier S, Launay O, Lortholary O. 2007. Determinants of disease presentation and outcome during cryptococcosis: the CryptoA/D study. PLoS Med 4:e21. doi: 10.1371/journal.pmed.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lortholary O, Poizat G, Zeller V, Neuville S, Boibieux A, Alvarez M, Dellamonica P, Botterel F, Dromer F, Chene G. 2006. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS 20:2183–2191. doi: 10.1097/01.aids.0000252060.80704.68. [DOI] [PubMed] [Google Scholar]
- 8.Day JN, Chau TTH, Wolbers M, Mai PP, Dung NT, Mai NH, Phu NH, Nghia HD, Phong ND, Thai CQ, Thai LH, Chuong LV, Sinh DX, Duong VA, Hoang TN, Diep PT, Campbell JI, Sieu TPM, Baker SG, Chau NVV, Hien TT, Lalloo DG, Farrar JJ. 2013. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med 368:1291–1302. doi: 10.1056/NEJMoa1110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. 2018. Guidelines for the diagnosis, prevention, and management of cryptococcal disease in HIV-infected adults, adolescents and children. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 10.Larsen RA, Leal MA, Chan LS. 1990. Fluconazole compared with amphotericin B plus flucytosine for cryptococcal meningitis in AIDS: a randomized trial. Ann Intern Med 113:183–187. doi: 10.7326/0003-4819-113-3-183. [DOI] [PubMed] [Google Scholar]
- 11.Saag MS, Powderly WG, Cloud GA, Robinson P, Grieco MH, Sharkey PK, Thompson SE, Sugar AM, Tuazon CU, Fisher JF, Hyslop N, Jacobson JM, Hafner R, Dismukes WE. 1992. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. N Engl J Med 326:83–89. doi: 10.1056/NEJM199201093260202. [DOI] [PubMed] [Google Scholar]
- 12.Lockhart SR, Fothergill AW, Iqbal N, Bolden CB, Grossman NT, Garvey EP, Brand SR, Hoekstra WJ, Schotzinger RJ, Ottinger E, Patterson TF, Wiederhold NP. 2016. The investigational fungal Cyp51 inhibitor VT-1129 demonstrates potent in vitro activity against Cryptococcus neoformans and Cryptococcus gattii. Antimicrob Agents Chemother 60:2528–2531. doi: 10.1128/AAC.02770-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen K, Vedula P, Smith KD, Meya DB, Garvey EP, Hoekstra WJ, Schotzinger RJ, Boulware DR. 2017. Activity of VT-1129 against Cryptococcus neoformans clinical isolates with high fluconazole MICs. Med Mycol 55:453–456. [DOI] [PubMed] [Google Scholar]
- 14.Warrilow AG, Parker JE, Price CL, Nes WD, Garvey EP, Hoekstra WJ, Schotzinger RJ, Kelly DE, Kelly SL. 2016. The investigational drug VT-1129 is a highly potent inhibitor of Cryptococcus species CYP51 but only weakly inhibits the human enzyme. Antimicrob Agents Chemother 60:4530–4538. doi: 10.1128/AAC.00349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoekstra WJ, Garvey EP, Moore WR, Rafferty SW, Yates CM, Schotzinger RJ. 2014. Design and optimization of highly-selective fungal CYP51 inhibitors. Bioorg Med Chem Lett 24:3455–3458. doi: 10.1016/j.bmcl.2014.05.068. [DOI] [PubMed] [Google Scholar]
- 16.Wiederhold NP, Najvar LK, Garvey EP, Brand SR, Xu X, Ottinger EA, Alimardanov A, Cradock J, Behnke M, Hoekstra WJ, Schotzinger RJ, Jaramillo R, Olivo M, Kirkpatrick WR, Patterson TF. 2018. The fungal Cyp-51 inhibitor VT-1129 is efficacious in an experimental model of cryptococcal meningitis. Antimicrob Agents Chemother doi: 10.1128/AAC.01071-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson GR III, Wiederhold NP, Najvar LK, Bocanegra R, Kirkpatrick WR, Graybill JR, Patterson TF. 2012. A murine model of Cryptococcus gattii meningoencephalitis. J Antimicrob Chemother 67:1432–1438. doi: 10.1093/jac/dks060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiederhold NP, Najvar LK, Bocanegra R, Kirkpatrick WR, Sorrell TC, Patterson TF. 2013. Limited activity of miltefosine in murine models of cryptococcal meningoencephalitis and disseminated cryptococcosis. Antimicrob Agents Chemother 57:745–750. doi: 10.1128/AAC.01624-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiederhold NP, Kovanda L, Najvar LK, Bocanegra R, Olivo M, Kirkpatrick WR, Patterson TF. 2016. Isavuconazole is effective for the treatment of experimental cryptococcal meningitis. Antimicrob Agents Chemother 60:5600–5603. doi: 10.1128/AAC.00229-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard— 3rd ed Document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.Nguyen MH, Najvar LK, Yu CY, Graybill JR. 1997. Combination therapy with fluconazole and flucytosine in the murine model of cryptococcal meningitis. Antimicrob Agents Chemother 41:1120–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding JC, Bauer M, Diamond DM, Leal MA, Johnson D, Williams BK, Thomas AM, Najvar L, Graybill JR, Larsen RA. 1997. Effect of severity of meningitis on fungicidal activity of flucytosine combined with fluconazole in a murine model of cryptococcal meningitis. Antimicrob Agents Chemother 41:1589–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]