Diabetes mellitus (DM) and tuberculosis (TB) are two common diseases with increasing geographic overlap and clinical interactions. The effect of DM and hemoglobin A1c (HbA1c) values on the pharmacokinetics (PK) and pharmacodynamics (PD) of anti-TB drugs remains poorly characterized.
KEYWORDS: diabetes mellitus, pharmacodynamics, pharmacokinetics, tuberculosis
ABSTRACT
Diabetes mellitus (DM) and tuberculosis (TB) are two common diseases with increasing geographic overlap and clinical interactions. The effect of DM and hemoglobin A1c (HbA1c) values on the pharmacokinetics (PK) and pharmacodynamics (PD) of anti-TB drugs remains poorly characterized. Newly diagnosed TB patients with and without DM starting fixed-dose, thrice-weekly treatment underwent sampling for PK assessments (predose and 0.5, 2, and 6 h postdose) during the intensive and continuation phases of treatment. The effect of DM and HbA1c values on the maximum concentration (Cmax) of rifampin, isoniazid, and pyrazinamide and the association between drug concentrations and microbiologic and clinical outcomes were assessed. Of 243 patients, 101 had DM. Univariate analysis showed significant reductions in the Cmax of pyrazinamide and isoniazid (but not rifampin) with DM or increasing HbA1c values. After adjusting for age, sex, and weight, DM was associated only with reduced pyrazinamide concentrations (adjusted geometric mean ratio = 0.74, P = 0.03). In adjusted Cox models, female gender (adjusted hazards ratio [aHR] = 1.75, P = 0.001), a lower smear grade with the Xpert assay (aHR = 1.40, P < 0.001), and the pyrazinamide Cmax (aHR = 0.99, P = 0.006) were independent predictors of sputum culture conversion to negative. Higher isoniazid or rifampin concentrations were associated with a faster time to culture conversion in patients with DM only. A pyrazinamide Cmax above the therapeutic target was associated with higher unfavorable outcomes (treatment failure, relapse, death) (odds ratio = 1.92, P = 0.04). DM and higher HbA1c values increased the risk of not achieving therapeutic targets for pyrazinamide (but not rifampin or isoniazid). Higher pyrazinamide concentrations, though, were associated with worse microbiologic and clinical outcomes. DM status also appeared to influence PK-PD relationships for isoniazid and rifampin.
INTRODUCTION
Tuberculosis (TB) remains a global health threat, with over 10.4 million incident cases in 2016, and it is now the leading infectious disease killer in the world (1). At the same time, the diabetes mellitus (DM) prevalence is exploding, with its rate increasing worldwide from 4.7% in 1980 to 8.5% in 2014, with 422 million persons currently affected (2). A recent meta-analysis showed that the risk of TB disease is increased over 3-fold in people with diabetes compared to people without DM (3). As the geographic overlap of areas with high burdens of each disease increases, the population risk of TB attributable to DM is also rising and now surpasses the attributable risk from HIV infection, particularly in countries like India, where the DM prevalence substantially outnumbers the HIV infection prevalence. It is estimated that up to 14% of patients with DM are infected with Mycobacterium tuberculosis, the causative agent of TB, and in some settings, up to 45% of patients with TB also have DM; the overall prevalence is about 4% (4). Moreover, patients with high hemoglobin A1c (HbA1c) values or who require insulin therapy, markers for poorly controlled or severe DM, seem to be at a particularly high risk for TB disease (5, 6).
DM has been shown to negatively impact TB treatment outcomes. One possible explanation for these findings is that patients with DM tend to have a higher mycobacterial burden at treatment initiation than patients without DM, perhaps because of immune dysregulation (7). The time that it takes for sputum cultures to convert from positive to negative with treatment may be longer in people with DM (8, 9). More importantly, the risk of treatment failure or relapse appears to be higher in people with DM than in those without DM in some studies (10–12) but not in others (13, 14). In one meta-analysis, the risk of death or treatment failure was 1.7 times higher in TB patients with DM than in those without DM (15).
If TB treatment outcomes are, indeed, worse in patients with DM, it is important to uncover potential causal pathways so that appropriate interventions can be employed. TB is treated with multidrug therapy—rifampin, isoniazid, pyrazinamide, and ethambutol—for the first 2 months of treatment (the intensive phase), followed by rifampin and isoniazid for the 4-month continuation phase. If drug concentrations are lower in patients with DM (or in the subgroup with high HbA1c values), then this may, in part, explain the poor treatment responses. In that case, higher drug doses might be beneficial. If, on the other hand, poor outcomes are related simply to increased age, increased weight, or poor glycemic control, then lengthening the treatment duration or treating DM more aggressively may be a better strategy for improving outcomes in patients with DM-associated TB. To complicate things further, metformin (used globally as a first-line treatment for DM) has immunomodulatory properties and may decrease TB-related mortality (16).
A few studies have evaluated the effect of DM on the pharmacokinetics (PK) of antituberculosis drugs, and the results have been mixed, with some reporting lower anti-TB drug concentrations in people with DM (17–20) and others showing no effect of DM on first-line drug exposures (21–23). In the context of a large prospective cohort study involving patients with and without DM in India, we evaluated the effects of DM (and the HbA1c value) on first-line anti-TB drug pharmacokinetics. We then assessed the pharmacokinetic-pharmacodynamic (PD) relationships, specifically looking at the association between drug exposures and microbiologic outcomes (culture conversion over time on treatment), taking into account key factors that may influence outcomes, such as DM. Lastly, we performed exploratory analyses to investigate the relationships between drug concentrations and clinical outcomes in patients with and without DM.
RESULTS
Study subjects.
Of the patients enrolled into the longitudinal cohort study, 243 individuals received thrice-weekly dosing and completed at least one PK visit. Among them, 223 (92%) had PK sampling during the intensive phase and 200 (82%) participated in PK sampling during the continuation phase. Of the 243 participants, 101 (41.6%) had DM. Of these, 45 (44.6%) were taking metformin. Participants with DM were older (median age, 48 versus 26 years), heavier (50.8 versus 45.0 kg), and more likely to be male (77% versus 61%) than participants without DM (Table 1). The baseline bacterial burden and the proportion of patients with cavitary disease were similar between the two groups. The median value of HbA1c during the intensive phase was 7.9% (interquartile range [IQR]: 6.5, 10.1) in the DM group and 5.3% (IQR: 5.0, 5.6) in the group without DM (P < 0.001). The median value of HbA1c during the continuation phase was 10.8% (IQR: 5.8, 12.6) in the DM group and 5.4% (IQR: 5.0, 5.7) in the patients without DM. The average doses of rifampin, isoniazid, and pyrazinamide were 9.9 mg/kg of body weight (95% confidence interval [CI], 9.7, 10.1 mg/kg), 13.20 mg/kg (95% CI = 12.9, 13.5 mg/kg), and 33.0 mg/kg (95% CI, 32.3, 33.7 mg/kg), respectively.
TABLE 1.
Baseline demographic information for Indian patients with pulmonary TB, comparing those with diabetes to those without diabetes
| Characteristic | Values for patients with: |
P value | |
|---|---|---|---|
| No DM (n = 142) | DM (n = 101) | ||
| Median (IQR) age (yr) | 26.0 (23.0, 35.0) | 48.0 (40.0, 55.0) | <0.001 |
| No. (%) of patients by sex | |||
| Male | 86 (60.6) | 78 (77.2) | 0.006 |
| Female | 56 (39.4) | 23 (22.8) | |
| Median (IQR) wt (kg) | 45.0 (38.9, 49.0) | 50.8 (44.0, 60.0) | <0.001 |
| Median (IQR) ht (cm) | 160.0 (152.0, 166.0) | 160.0 (152.0, 165.0) | 0.61 |
| Median (IQR) BMIa | 17.0 (15.6, 19.1) | 20.5 (18.0, 23.5) | <0.001 |
| Median (IQR) HbA1c value (%) | 5.6 (5.3, 5.9) | 8.9 (6.8, 11.6) | <0.001 |
| No. (%) of patients with the following bacterial burden: | |||
| High | 18 (12.9) | 9 (9.4) | |
| Medium | 54 (38.6) | 47 (49.0) | 0.33 |
| Low | 47 (33.6) | 25 (26.0) | |
| Very low | 19 (13.6) | 15 (15.6) | |
| No. (%) of patients with cavitary disease: | |||
| No | 70 (55.1) | 38 (48.7) | 0.37 |
| Yes | 57 (44.9) | 40 (51.3) | |
| No. (%) of patients with: | |||
| Infiltrate | |||
| Unilateral | 19 (15.1) | 11 (14.7) | 0.54 |
| Bilateral | 105 (83.3) | 64 (85.3) | |
| Unknown | 2 (1.6) | 0 (0.0) | |
| No. (%) of patients with pleural disease | |||
| No | 105 (82.7) | 71 (91.0) | 0.096 |
| Yes | 22 (17.3) | 7 (9.0) | |
BMI, body mass index.
Effect of DM and HbA1c values on the PK of anti-TB drugs.
PK parameter values (maximum concentration [Cmax] and partial area under the concentration-time curve [AUC] over 6 h [AUC0–6]) and concentration-time profiles, by DM status, are shown in Table 2 and Fig. S1 in the supplemental material. Of note, PK-PD analyses used the Cmax to avoid bias in the values of AUC0–6 attributed to the delayed absorption in diabetic patients. The Cmax for rifampin was similar among patients with and without DM. Similarly, there were no differences in the rifampin Cmax by HbA1c category (<5.6%, 5.7 to 6.5%, and >6.5%) (Table S1). The isoniazid Cmax was lower in patients with DM than in patients without DM during the intensive phase of TB treatment, but this difference did not remain statistically significant in models adjusting for age, sex, and weight. The odds of the time to the maximum concentration (Tmax) of isoniazid being observed at 6 h versus 2 h was significantly higher in patients with DM than in patients without DM (odds ratio [OR] = 2.10, 95% CI =1.30 to 3.40, P = 0.002). Pyrazinamide was given exclusively in the intensive phase. The pyrazinamide Cmax was significantly lower in diabetic patients than in nondiabetic participants (21.6 versus 32.8 μg/ml), even after adjusting for age, weight, and sex (adjusted geometric mean ratio [aGMR] = 0.74; 95% 95% CI = 0.56 to 0.97, P = 0.03).
TABLE 2.
Geometric means and univariate and adjusted geometric mean ratios with 95% CI comparing the Cmaxs and the AUC0–6 of anti-TB drugs in patients with DM versus patients without DMa
| Drug, phase, and parameter | Geometric mean value for patients with: |
GMR (95% CI) | P value for GMR | Adjusted GMR (95% CI) | P value for adjusted GMR | |
|---|---|---|---|---|---|---|
| No diabetes | Diabetes | |||||
| Rifampin | ||||||
| Intensive phase | ||||||
| Cmax (μg/ml) | 4.32 | 3.63 | 0.84 (0.62, 1.14) | 0.26 | 0.75 (0.48, 1.19) | 0.22 |
| AUC0–6 (μg · h/ml) | 15.34 | 12.19 | 0.79 (0.60, 1.05) | 0.11 | 0.79 (0.55, 1.15) | 0.22 |
| Continuation phase | ||||||
| Cmax (μg/ml) | 2.98 | 3.81 | 1.28 (0.90, 1.82) | 0.17 | 1.00 (0.67, 1.49) | 0.99 |
| AUC0–6 (μg · h/ml) | 11.13 | 14.46 | 1.30 (0.92, 1.84) | 0.14 | 1.08 (0.73, 1.61) | 0.69 |
| Isoniazid | ||||||
| Intensive phase | ||||||
| Cmax (μg/ml) | 7.91 | 5.97 | 0.75 (0.58, 0.98) | 0.03 | 0.77 (0.56, 1.03) | 0.08 |
| AUC0–6 (μg · h/ml) | 26.84 | 20.0 | 0.75 (0.57, 0.97) | 0.03 | 0.74 (0.54, 1.01) | 0.06 |
| Continuation phase | ||||||
| Cmax (μg/ml) | 7.63 | 7.42 | 0.97 (0.72, 1.32) | 0.86 | 0.82 (0.53, 1.27) | 0.38 |
| AUC0–6 (μg · h/ml) | 26.79 | 23.79 | 0.89 (0.65, 1.21) | 0.45 | 0.75 (0.50, 1.14) | 0.18 |
| Pyrazinamide, intensive phase | ||||||
| Cmax (μg/ml) | 32.75 | 21.59 | 0.66 (0.51, 0.85) | 0.002 | 0.74 (0.56, 0.97) | 0.03 |
| AUC0–6 μg · h/ml) | 128.43 | 80.89 | 0.63 (0.49, 0.80) | <0.001 | 0.65 (0.49, 0.86) | 0.003 |
The models are adjusted for age, sex, and weight. Visit 1 was during the intensive phase of TB treatment, while visit 2 took place during the continuation phase of TB treatment. GMR, geometric mean ratio.
Reaching the therapeutic targets of anti-TB drugs.
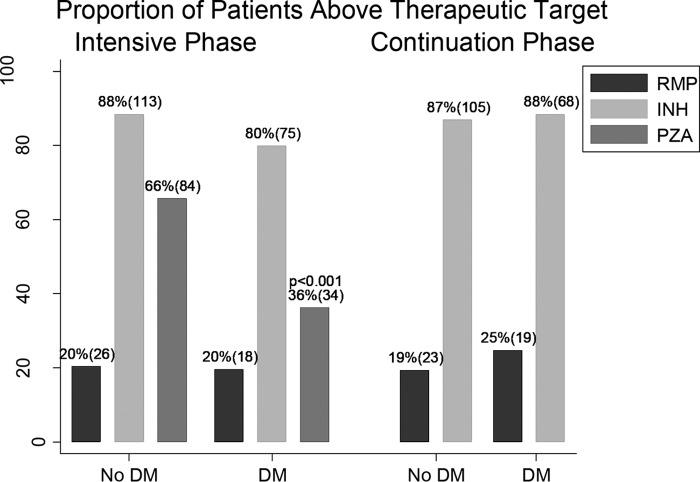
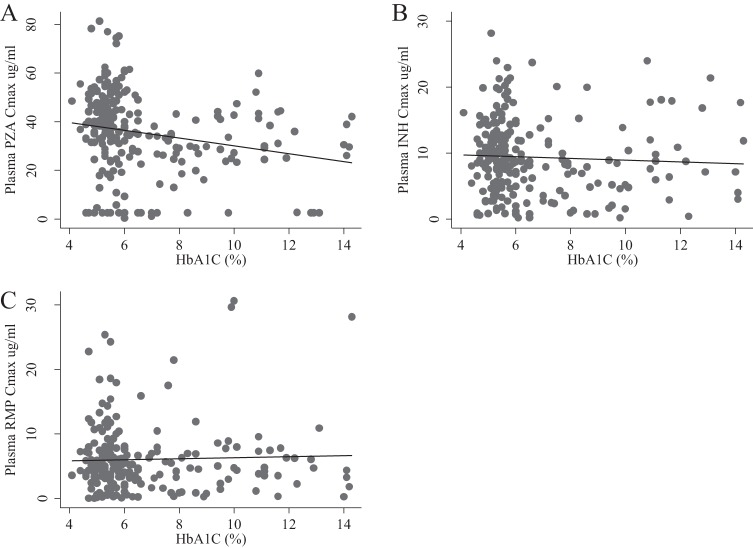
Commonly cited therapeutic targets for rifampin, isoniazid, and pyrazinamide are 8 μg/ml, 3 μg/ml, and 35 μg/ml, respectively. Overall, 79.3% of all study participants had rifampin Cmax values below the therapeutic target, and there was no difference between groups (Fig. 1). For isoniazid, a smaller proportion of patients (about 15%) had Cmax values below the target. During the intensive phase, 20.2% of TB patients with DM and 11.7% of those without DM had isoniazid Cmax below the target (P = 0.082), while 63.8% of the patients with DM and 34.4% of the patients without DM had pyrazinamide concentrations below the target (P < 0.001). Further, pyrazinamide concentrations decreased as the HbA1c value increased (Fig. 2).
FIG 1.
Proportion of patients with and without diabetes mellitus who achieved the therapeutic target concentrations of rifampin (RMP), isoniazid (INH), and pyrazinamide (PZA).
FIG 2.
Maximum concentration (Cmax; indicated on the x axis [in micrograms per milliliter]) of pyrazinamide (PZA) (A), isoniazid (INH) (B), and rifampin (RMP) (C) by HbA1c value in the intensive phase.
PK-PD analyses. (i) Microbiologic results.
PK-PD analyses were performed to assess the relationship between drug concentrations and microbiologic outcomes (culture results over time), taking into account factors that may influence treatment outcomes in patients with and without DM. In univariate models, female sex and a lower baseline bacterial load were significantly associated with a shorter time to culture conversion, whereas a higher pyrazinamide Cmax was associated with a longer time to culture conversion (Table 3). In multivariate models that included diabetes status, age, sex, weight, baseline bacterial burden, and the rifampin, isoniazid, or pyrazinamide maximum concentration, female sex (adjusted hazard ratio [aHR] = 1.76, 95% CI = 1.26 to 2.45, P = 0.001), a lower baseline bacterial load (aHR = 1.37, 95% CI = 1.16 to 1.63, P < 0.001), and a higher pyrazinamide Cmax (aHR = 0.99, 95% CI = 0.98 to 1.0, P = 0.006) were independently associated with a shorter time to culture conversion. In addition, having a pyrazinamide Cmax above the therapeutic target (35 μg/ml) was associated with an increased time to culture conversion in the multivariate model (aHR = 0.72, 95% CI = 0.54 to 0.98, P = 0.04). As this was a surprising finding, we performed subanalyses in patients with and without DM. In patients without DM, while sex and baseline bacterial load remained statistically significant in multivariate models, the concentrations of isoniazid, rifampin, and pyrazinamide were not statistically significantly associated with the time to culture conversion (all P values were >0.5) (Table 4). In patients with DM, in the multivariate model, higher rifampin (aHR = 1.05, 95% CI = 1.03 to 1.09, P < 0.001) and higher isoniazid (aHR = 1.08, 95% CI = 1.04 to 1.12, P < 0.001) values were positively associated with a hazard of culture conversion, while pyrazinamide concentrations were negatively associated (aHR = 0.96, 0.95 to 0.98, P < 0.001).
TABLE 3.
Time to culture negativity and percentage of patients with conversion of sputum culture to negative by 8 weeks of therapy, by characteristica
| Characteristic | Median TTN (days [95% CI]) | HR (95% CI) | P value for HR | No. (%) of patients with culture conversion at 8 wk | P value for culture conversion |
|---|---|---|---|---|---|
| DM | |||||
| Yes | 39 (30, 43) | 1.08 (0.82, 1.41) | 0.59 | 73 (83.91) | 0.79 |
| No | 39 (28, 42) | 99 (82.50) | |||
| HbA1c value (%) | |||||
| <5.6 | 39 (28, 42) | 1.07 (0.92, 1.26) | 0.37 | 78 (83.87) | 0.59 |
| 5.6–6.5 | 39 (28, 42) | 0.92 (0.66, 1.28) | 0.61 | 45 (78.95) | |
| >6.5 | 37 (28, 43) | 1.18 (0.86, 1.62) | 0.31 | 49 (85.96) | |
| Age (yr) | |||||
| <50 | 39 (30, 42) | 0.85 (0.62, 1.17) | 0.32 | 138 (83.13) | 0.92 |
| >50 | 40 (28, 56) | 33 (82.50) | |||
| Sex | |||||
| Male | 41 (37, 44) | 1.54 (1.15, 2.07) | 0.004 | 106 (79.10) | 0.04 |
| Female | 28 (27, 39) | 65 (90.28) | |||
| Bacterial burden | |||||
| High | 42 (39, 85) | 1.40 (1.18, 1.66) | <0.001 | 17 (73.9) | 0.01 |
| Medium | 42 (39, 53) | 67 (75.28) | |||
| Low | 31 (28, 42) | 58 (92.06) | |||
| Very low | 26 (16, 34) | 26 (92.86) | |||
| Cavity | |||||
| No | 42 (34, 52) | 0.88 (0.64 1.19) | 0.42 | 82 (81.19) | |
| Yes | 44 (39, 54) | 81 (84.38) | |||
| Rifampin Cmax | |||||
| Below target | 39 (31, 42) | 1.10 (0.74 1.64) | 0.65 | 133 (82.6) | 0.77 |
| Above target | 40 (27, 43) | 38 (84.44) | |||
| Isoniazid Cmax | |||||
| Below target | 43 (28, 57) | 1.10 (0.79 1.52) | 0.58 | 21 (80.77) | 0.74 |
| Above target | 39 (30, 42) | 151 (83.43) | |||
| Pyrazinamide Cmax | |||||
| Below target | 37 (28, 40) | 0.72 (0.54 0.95) | 0.02 | 80 (89.89) | 0.02 |
| Above target | 41 (30, 42) | 81 (77.14) |
TTN, time to culture negativity; HR, hazard ratio.
TABLE 4.
Exposure-response relationships between anti-TB drugs and time to culture conversion, in adjusted models, by diabetes status
| Characteristic | No diabetes |
Diabetes |
||||
|---|---|---|---|---|---|---|
| aHR | 95% CI | P value | aHR | 95% CI | P value | |
| Age | 0.99 | 0.97, 1.00 | 0.07 | 0.98 | 0.96, 1.0 | 0.04 |
| Sex | 1.78 | 1.20, 2.65 | 0.004 | 1.69 | 0.81, 3.54 | 0.17 |
| Wt | 1.02 | 1.0, 1.04 | 0.1 | 1.0 | 0.98, 1.01 | 0.73 |
| Baseline bacterial burden | 1.41 | 1.09, 1.84 | 0.01 | 1.38 | 1.06, 1.78 | 0.02 |
| Rifampin Cmax | 1.00 | 0.95, 1.05 | 0.99 | 1.05 | 1.03, 1.09 | <0.001 |
| Isoniazid Cmax | 1.00 | 0.96, 1.05 | 0.96 | 1.08 | 1.04, 1.12 | <0.001 |
| Pyrazinamide Cmax | 1.0 | 0.98, 1.00 | 0.55 | 0.96 | 0.95, 0.98 | <0.001 |
Regarding 8-week culture conversion, a commonly used early biomarker of the treatment response in trials, among those with and without DM, 83.91% and 82.50% had converted their sputum to negative by 8 weeks of treatment, respectively (Table 3). In multivariate models, female sex (adjusted odds ratio [aOR] = 3.44, 95% CI = 1.25 to 9.45, P = 0.02) and a lower baseline bacterial burden (aOR = 1.74 95% CI = 1.14 to 2.65, P = 0.01) were associated with a higher likelihood of 8-week culture conversion. Pyrazinamide exposure was negatively associated with 8-week culture conversion, but only in those with DM (aOR = 0.94, 95% CI = 0.96 to 1.03, P = 0.02) (Table S3B).
(ii) Clinical outcomes.
The purpose of these analyses was to explore the effect of drug exposures on clinical outcomes (failure, relapse, and death), taking into account potential confounders, in patients with and without DM. Overall, there were 61 participants with an unfavorable outcome; 26 had DM and 35 did not. There were 14 deaths (6 in DM patients, 8 in non-DM patients), 23 failures (8 in DM patients, 15 in non-DM patients), and 28 relapses (12 in DM patients, 16 in non-DM patients). In a multivariate model that included DM status, age, sex, weight, baseline bacterial load, and Cmax values for all drugs, only the baseline bacterial load was statistically significantly associated with the odds of a composite unfavorable outcome (aOR = 0.66, CI = 0.47 to 0.93, P = 0.02). Even in analyses stratified by DM status, drug concentrations were not significant predictors of a composite unfavorable outcome. When we performed exploratory multivariate analyses separately for death, relapse, and failure, we found no clear predictors for death or relapse. However, there was a trend toward an association between pyrazinamide exposure and a risk of failure (adjusted incidence rate ratio [aIRR] = 1.02, 95% CI = 1.0 to 1.05, P = 0.05). Among those with DM with poorly controlled DM (defined as an HbA1c value of >8%), in the univariate analysis, the baseline bacterial load (IRR = 0.36, 95% CI = 0.18 to 0.72, P = 0.004) and a higher pyrazinamide exposure (IRR = 1.08, 95% CI = 1.4 to 1.12, P < 0.001) were associated with an increased risk of failure, but in the multivariate analysis, the effect of pyrazinamide did not reach statistical significance (aIRR = 1.12, 95% CI = 0.99 to 1.27, P = 0.08) (Table S4). Univariate analysis of the proportion of patients who had drug concentrations above or below the therapeutic target showed that a pyrazinamide concentration above the therapeutic target was associated with a higher odds of an overall unfavorable outcome (OR = 1.92, 95% CI = 1.03 to 3.59, P = 0.04) (Table S5); this trend, though not significant, was also observed among patients with HbA1c values of ≥8 (OR = 3.8, 95% CI = 0.93 to 15.57, P = 0.06).
DISCUSSION
In this study, conducted among Indian patients with pulmonary TB, we found that rifampin concentrations were universally low and that the concentrations of isoniazid and pyrazinamide were reduced in patients with DM compared to those in patients without DM. Surprisingly, however, high pyrazinamide concentrations were associated with a longer time to sputum culture conversion. In subgroup analyses, in patients without DM, drug concentrations did not have an impact on microbiologic outcomes, but in patients with DM, the time to culture conversion was positively associated with isoniazid and rifampin concentrations and negatively associated with pyrazinamide levels. To our knowledge, this is the first study to show differences in PK-PD relationships in patients DM with versus patients without DM.
What is the mechanism by which DM reduces anti-TB drug exposures, and why does the effect vary by drug? In our study, rifampin levels were low in the majority of patients, and the levels of this drug were not different by DM status. In the first study to look at the relationship between DM and rifampin PK, investigators in Indonesia found that rifampin exposures were significantly reduced in patients with DM compared to patients without DM who were age and sex matched (17). A subsequent study by the same group, however, showed that these differences disappeared when weight was a matching factor (22). Data from other settings are mixed, with some studies showing a reduced and slower absorption of rifampin (with a lower Cmax) with DM (18, 19) and others showing no meaningful effect of DM on rifampin PK (21). It is likely that discrepancies in the results can be attributed to differences in demographics, DM severity, drug dosing, and study design. While it is reassuring that DM did not impact rifampin concentrations meaningfully in our study (and, thus, that patients with DM do not necessarily need different dosing than patients without DM to achieve similar exposures), it is alarming that such a large portion of patients had low levels overall. The patients in our study were given a dose of 450 mg of rifampin thrice weekly, a dose that is too low for intermittent dosing and has been associated with worsened clinical outcomes (24). Higher, daily doses are needed; daily dosing is now the norm in India. Patients with DM did have lower isoniazid exposures than patients without DM, but this was true only during the intensive phase, and in multivariate models with adjustment for weight, the effect of DM on isoniazid concentrations was less pronounced. The PK of pyrazinamide were impacted most dramatically by DM, with 67% of patients without DM and 36% of patients with DM achieving target pyrazinamide Cmax values of 35 μg/ml or higher. This effect was not mitigated after adjusting for other factors. These results are in keeping with the results from other studies that reported lower pyrazinamide and isoniazid Cmax concentrations in DM patients in the intensive phase, even after adjusting for possible confounders (23, 25).
For isoniazid, reductions in drug concentrations in patients with DM were in part attributable to using fixed doses rather than adjusting for weight, with diabetics being heavier than patients without DM, though strict milligram-per-kilogram dosing (rather than the use of data-informed weight banding) also results in poor uniformity across individuals of different weights (26). We observed that the time to the maximum concentration (Tmax) of isoniazid was more commonly delayed in patients with DM, suggesting that lower concentrations may also be related to DM-induced changes in intestinal motility (27, 28). Diabetes may also affect P-glycoprotein transporter expression and activity, which may, in turn, affect anti-TB drug absorption (29, 30). However, isoniazid and pyrazinamide are not known to be substrates of P-glycoprotein. Isoniazid is a drug that gets metabolized mainly through N-acetyltransferase 2 (NAT2), the expression of which has been associated with obesity, at least in children (31, 32). Whether or not NAT2 activity or cytochrome P450 2E1 is meaningfully impacted by DM is unknown (33–36). In our study, most patients had relatively high isoniazid levels, likely because the members of the local population are mostly slow NAT2 acetylators and the isoniazid dose was 600 mg.
Pyrazinamide is a prodrug that gets metabolized by deamidase to form the active metabolite pyrazinoic acid (POA), which, in turn, gets metabolized to 5-hydroxypyrazinoic acid (5-OH-POA) by xanthine oxidase (37). Xanthine oxidase also metabolizes pyrazinamide to 5-hydroxypyrazinamide (5-OH-PZA) (38, 39). Interestingly, DM has been shown to increase the plasma and hepatic levels of xanthine oxidase significantly in animals with type 1 DM (40, 41). One study was conducted in Malaysia among 650 patients with type 2 DM and 280 healthy participants to evaluate the effect of glycemic control on xanthine oxidase and antioxidant indices. The investigators found that patients with type 2 DM had higher levels of xanthine oxidase with a strong association between HbA1c values and oxidative indices (42). Similar results were found in subsequent studies (43–45). Interestingly, a study involving six healthy participants to examine the effect of the coadministration of pyrazinamide with allopurinol, a xanthine oxidase inhibitor, showed that there was a significant increase in POA and 5-OH-PZA exposure (46). One might speculate, based on these data, that the DM-induced xanthine oxidase elevation contributes to reductions in pyrazinamide concentrations, but that has yet to be proven.
Should patients with DM get higher anti-TB drug doses? One might argue that DM patients should receive higher doses of isoniazid (particularly if they are heavier) and pyrazinamide based simply on PK differences between people with and without DM. In our study, higher isoniazid and rifampin exposures were associated with a shorter time to culture conversion in patients with DM but not in patients without DM. The reasons for this are unknown but may be related to the dysregulation of immune responses in DM and, in that setting, an increased role for antimicrobial agents in eradicating the pathogen (47–52). Therefore, ensuring adequate doses of isoniazid (which brings down the bacterial burden quickly) and rifampin (which is the key sterilizing agent) is especially important in DM. Further, and unexpectedly, in our study, there was an inverse relationship between pyrazinamide concentrations and the time to culture conversion, and this effect seemed to be restricted to patients with DM. While we did not have the power to explore PK-PD relationships for relevant clinical outcomes fully, there was a trend toward a higher risk of failure with higher pyrazinamide exposure over the observed concentration range.
What might explain this negative relationship between pyrazinamide concentrations and TB treatment outcomes in DM? One possibility is that DM potentiates pyrazinamide-associated side effects (hypothetically, through increasing xanthine oxidase expression and increasing the production of more toxic metabolites) and that this toxicity occurs more commonly in patients with higher drug levels, thus leading to reduced adherence. Since pyrazinamide is part of a fixed-dose combination, not taking pyrazinamide means also not taking companion drugs. However, we are not aware of reduced adherence in patients with DM in our study. Another potential explanation is that there is a concentration range for pyrazinamide that is more commonly experienced by diabetics and for which there is antagonism with isoniazid and/or rifampin (53, 54), two drugs that are needed more critically in patients with DM than in those without. A third possibility is that the positive effects of metformin in DM are somehow antagonized by pyrazinamide (16, 55). Another possibility may be attributed to the effect of pyrazinamide in inhibiting PARP1, a protein that is involved in activation of different monocytes and T cells involved in inflammation and immunity (56). TB starts by inhalation of M. tuberculosis bacilli that then invade alveolar macrophages, replicate, and spread to thoracic lymph nodes, where the antigen-specific T cells initiate a cascade of cytokine activity to target infected macrophages, providing effective antimicrobial activity. In chronic hyperglycemic animals, the presence of inflammatory cells and mediators is comparable to and sometimes higher than that in euglycemic animals; however, there is a delay in the cellular immune response. This was also manifested in inadequate binding and ingesting of the peripheral blood monocytes with M. tuberculosis in patients with type 2 diabetes (57). A speculated mechanism for the defective ability of macrophages to respond to M. tuberculosis in patients with diabetes may be due to the reduction in nitric oxide production and H2O2 in the macrophages (58, 59), processes in which PARP1 is actively involved (60, 61). Therefore, one may speculate that the effect of pyrazinamide inhibition of PARP1 on the immune modulation may be more important in diabetic patients, which may, in turn, explain why higher concentrations of pyrazinamide in diabetic patients and patients with HbA1c values above 8% were associated with a longer time to culture conversion and a higher incidence of failure.
This is not the only situation in which pyrazinamide has been observed to have adverse effects: in one study of patients with TB meningitis and HIV infection, pyrazinamide concentrations in the cerebrospinal fluid were strongly correlated with neurologic toxicity and mortality (62). Whether or not differences in physiology, including in the microenvironments where TB must be killed to cure the patient, exist between patients with DM and other TB patients is unknown. Further, one-size-fits-all PK targets, which are based more on population norms than on careful PK-PD analysis, may not fit all.
Untangling the contributors to unfavorable treatment outcomes in diabetic patients will be important so that the right measures can be taken to give these patients the best possibility for cure without relapse. Some studies, including ours, found that patients with DM have a higher baseline bacterial load than patients without DM (14), and in that case, intensification of treatment (by dose or duration) may perhaps help. Others have shown that controlling DM may result in better TB outcomes (6, 63, 64), in which case enhanced care for DM may be beneficial. Assuming that the time to culture conversion represents an early biomarker of drug activity with regard to the prevention of failure or relapse (65), our study suggests that ensuring adequate concentrations of isoniazid and rifampin, which we found were associated with a reduced time to culture conversion, is important for DM-associated TB treatment success. Whether or not the PK-PD relationships seen with higher, daily dosing (whereby unfavorable outcomes are expected to be rarer) are similar in nature to those seen with thrice-weekly dosing remains to be studied (66–68). Further, the reasons for the apparent negative relationship between pyrazinamide concentrations (in the range experienced by our patients) need to be uncovered, after first checking to see if these results are replicated in other large cohort studies in different populations. Further studies may focus on the effects of DM on pyrazinamide metabolic pathways and immune modulators as well as pyrazinamide PK-PD relationships across a broader range of pyrazinamide doses in diabetic patients.
Our study has several limitations. First, our PK sampling scheme was imperfect, especially in this patient cohort, which included many patients with delayed and prolonged absorption (the concentration in the sample collected at 0.5 h was often below the limit of quantitation and that in the sample collected at 6 h was higher than that in the sample collected at 2 h in most patients, so we could not characterize absorption or clearance well to estimate the daily AUC; subsequent patients in our cohort had sampling at 1 h and 8 h). For most anti-TB drugs, though, these are the time points at which samples for therapeutic drug monitoring are typically collected (to capture Cmax), allowing us to compare data across studies. Second, our analyses of the relationship between drug exposures and clinical outcomes could only be exploratory, as there was not power to explore these associations fully. Further, we did not adjust for multiple comparisons in our analyses, so it is possible that some associations were present by chance. However, we hope that discoveries made in these exploratory analyses will inspire investigations in other cohorts that will help inform TB-DM management best practices. We did not account for antidiabetic drug adherence or diabetic complications in this set of analyses. Also, weight was collected only at the baseline visit, so we cannot provide information about weight gain during the study (though drugs were not dosed by weight). Of the 61 patients who did have baseline and follow-up values, weight gain was the norm (54 to 59 kg in patients with DM, 44 to 51 kg in patients without DM). Despite these limitations, our study demonstrates the low anti-TB drug concentrations achieved for patients with DM and the associations between drug concentrations and unfavorable outcomes that appear to be restricted to diabetic patients.
Conclusion.
Patients with DM had lower concentrations of isoniazid, mostly related to higher weight, and slower isoniazid absorption. Rifampin concentrations were not affected by DM or HbA1c values. Pyrazinamide concentrations were lower in patients with DM than in those without DM, even after adjusting for weight. Despite low pyrazinamide concentrations, over the range of pyrazinamide concentrations observed, there was a negative PK-PD relationship with microbiologic outcomes, leading us to wonder about the mechanism by which pyrazinamide is potentially harmful in patients with DM. In diabetics, isoniazid and rifampin concentrations were important predictors of treatment outcome, and so it is especially important that these drugs achieve therapeutic concentrations in diabetics. It will be important to explore whether the PK-PD relationships that we observed to be different in patients with DM versus patients without DM are seen in other cohorts and/or in other disease states (e.g., TB meningitis).
MATERIALS AND METHODS
Study population.
Adult patients (age, ≥18 years) with newly diagnosed smear-positive or smear-negative pulmonary TB were recruited between 23 December 2013 and 4 January 2017 from the Byramjee-Jeejeebhoy Government Medical College-Sassoon General Hospitals (BJGMC-SGH) clinical research site (CRS) in Pune, India. BJGMC-SGH is a Maharashtra State Government-run public-sector teaching hospital and receives referrals of eligible participants from Revised National TB Control Program (RNTCP) centers in Pune and Pimpri-Chinchwad municipal corporations. BJGMC-SGH serves a population of approximately 7 million in the surrounding urban, semiurban, and rural low-income populations. The study also enrolled eligible participants from the Dr. D. Y. Patil Medical College, Pune, India, a private medical college affiliated with a large hospital, beginning in July 2016. The following were exclusion criteria: >1 week of TB treatment at the time of screening, infection with an M. tuberculosis strain resistant to rifampin or isoniazid, HIV coinfection, and pregnancy. Patients were considered to have DM if they had an HbA1c value of ≥6.5%, a fasting blood glucose concentration of ≥126 mg/dl, or a random blood glucose level of ≥200 mg/dl or if they self-reported as having DM or were taking medications for DM. The study was approved by the ethics committees of the participating sites. All participants provided written informed consent.
Study procedures.
Patients with newly diagnosed pulmonary TB were screened for DM as part of a prospective cohort study in which patients with and without DM initiating treatment for TB were followed for treatment outcomes (69). Study participants received standard TB treatment through the RNTCP. At the time of the start of the study, standard treatment was given thrice weekly, as follows: rifampin at 450 mg, isoniazid at 600 mg, pyrazinamide at 1,500 mg, and ethambutol at 1,200 mg during the intensive phase of TB treatment, followed by rifampin and isoniazid (at the same doses) during the continuation phase. This PK-PD study involves those study participants who received thrice-weekly treatment, prior to a nationwide change in dosing frequency to daily. Participants were followed for 18 months to assess TB treatment outcomes (cure, treatment completion, death, treatment failure, or loss to follow-up) and relapse. Failure was defined as having a sputum culture that remained positive ≥4 months after the start of treatment. Relapse was defined as TB recurrence after completion of TB treatment up to the 18-month follow-up visit. All-cause mortality was assessed through the 18-month follow-up visit by study staff.
During the first 2 months of TB treatment, participants underwent study visits every 2 weeks. During the continuation phase of TB treatment, participants had monthly visits. Follow-up visits were also scheduled at 12 and 18 months. Anthropomorphic measurements were taken at baseline and then 3 and 6 months after the initiation of TB treatment. HbA1c values and fasting glucose levels were measured at baseline and 3, 6, and 12 months. Sputum specimens for detection of acid-fast bacilli (AFB) and culture on liquid medium (MGIT) were collected at baseline, and sputum specimen collection was repeated at each study visit up until the 6-month visit. A sputum specimen for culture was also collected during follow-up if there were clinical signs or symptoms of TB. The baseline bacterial burden was quantified using the Xpert MTB/RIF assay, which gives ordered categorical quantification of the bacterial DNA concentration based on the cycle threshold (CT) value of the device (high, medium, low, and very low) required to detect the organism: higher values mean a lower bacterial burden. The MGIT culture results were displayed as “detected” if there was mycobacterial growth and “not detected” if there was no growth. Sparse PK sampling for rifampin, pyrazinamide, and isoniazid was performed once during the intensive phase (weeks 4 to 6) and once during the continuation phase (weeks 10 to 12). PK samples were collected predose and then at 0.5, 2, and 6 h postdose. The therapeutic target concentrations considered in this analysis were 8 μg/ml, 3 μg/ml, and 35 μg/ml for rifampin, isoniazid, and pyrazinamide, respectively (70–72).
Drug quantification in plasma.
Blood was collected into a heparinized Vacutainer tube, and the plasma was separated by centrifugation. Ascorbic acid was added to the plasma to prevent the oxidation of rifampin. Aliquots of plasma were stored at −80°C prior to analysis. High-performance liquid chromatography (HPLC) was used for the drug concentration analysis (Shimadzu Corporation, Kyoto, Japan). Acetonitrile was used for rifampin extraction using a C18 column at 254 nm for the analysis. para-Hydrobenzaldehyde and trifluoroacetic acid were used for isoniazid and pyrazinamide extraction, respectively, using a C8 column at 267 nm for the analysis. The retention times for rifampin, pyrazinamide, and isoniazid were 1.7, 3, and 5.5 min, respectively, and the lower limits of quantification were 0.25, 1.25, and 0.25 μg/ml, respectively. All the drugs had less than 10% within- and between-run variation.
Data analysis. (i) PK analyses.
Noncompartmental analysis using the Phoenix WinNonLin program was performed for PK analyses to estimate the maximum concentration (Cmax), the time to the maximum concentration (Tmax), and the area under the concentration-time curve (AUC) for the study drugs. Concentrations below the limit of quantitation (BLQ) were assigned a value of BLQ divided by 2 in the analyses. Statistical analysis was performed using the STATA program (version 14; StataCorp, College Station, TX, USA). Results are presented as medians with interquartile ranges (IQR). The test of comparison for PK parameters was the Wilcoxon rank sum test, and Pearson's chi-square test was used for categorical variables. To compare the differences in the Cmax between patients with DM and patients without DM, generalized equation models were used to account for the correlation between the two PK visits. The Cmax was expressed as the geometric mean and its 95% CI. Missing values were rare (less than 10%) and thus were omitted from the analysis. The test of normality for the variables and for the residuals versus fitted values was performed, with data transformations being attempted for data that were not normally distributed. The generalized estimating equation (GEE) was used to assess the effect of DM or HbA1c values on the change in the Cmax and AUC of the anti-TB drugs. Geometric mean ratio was used to present those PK parameters. Adjustment for the possible confounder and the selection of the final model were done based on the QIC (quasi-likelihood under independence model criterion) value and the scatter plots of the residuals versus the fitted values. The known clinical confounders were also considered in the final selection of the model.
(ii) PK-PD analyses.
Survival analysis and Cox regression were performed to assess the relationship between drug concentrations and microbiologic outcomes, adjusting for key variables known to affect TB treatment outcomes (e.g., cavitary disease status) and those factors that, in univariate analysis, were associated with treatment outcome at a P value of <0.1. Variance inflation tests were performed to assess for multicollinearity among variables. The main microbiologic outcome was the time to culture negativity, defined as the time from treatment initiation to sustained sputum culture negativity. Participants who had negative cultures throughout treatment (including at the baseline visit) were excluded from the analysis. The proportional hazard assumption was assessed using the Schoenfeld test, and model selection was done on the basis of the Akaike information criterion (AIC) value and the clinical information for the possible confounders. Poisson regression was used to assess the relationship between drug concentrations and clinical outcomes, adjusting for key factors, as described above. An unfavorable treatment composite outcome was defined as treatment failure, relapse, or death. Postmodeling diagnostics were based on the AIC (for Poisson, linear regression, and Cox regressions) and the QIC for the generalized estimating equation model. In addition, scatter plots of the residuals versus the fitted values were considered. Additionally, the known clinical confounders were considered in the model.
Supplementary Material
ACKNOWLEDGMENTS
We thank the clinic and research staff of BJGMC-SGH and the Dr. D. Y. Patil Medical College for their immense contributions. We are grateful to the patients and their families for their participation in this study.
This work was primarily supported by the National Institutes of Health (NIH/NIAID R01AI097494 to J.E.G.). This work was also supported by the NIH-funded Johns Hopkins Baltimore-Washington-India Clinical Trials Unit for NIAID Networks (UM1AI069465 to V.M., N.G., and A.G.). Data in this report were also collected as part of the Regional Prospective Observational Research for Tuberculosis (RePORT) India Consortium, Indo (DBT)-US (NIH) (USB1-31147-XX-13 CRDF/NIH), which is funded in part with federal funds from the Government of India's (GOI) Department of Biotechnology (DBT), the Indian Council of Medical Research (ICMR), the United States NIH, the National Institute of Allergy and Infectious Diseases (NIAID), and the Office of AIDS Research (OAR) and distributed in part by CRDF Global. R.L. was supported by the BJGMC JHU HIV TB program, funded by the Fogarty International Center, National Institutes of Health (NIH D43TW009574).
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the NIH, DBT, the ICMR, or CRDF Global. Any mention of trade names, commercial projects, or organizations does not imply endorsement by any of the sponsoring organizations.
We all declare that we have no competing interests to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01383-18.
REFERENCES
- 1.World Health Organization. 2017. Global tuberculosis report 2017. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/global_report/en/ Accessed 3 May 2018.
- 2.World Health Organization. 2017. Diabetes. World Health Organization, Geneva, Switzerland. http://www.who.int/en/news-room/fact-sheets/detail/diabetes Accessed 3 May 2018.
- 3.Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. 2017. Association between diabetes mellitus and active tuberculosis: a systematic review and meta-analysis. PLoS One 12:e0187967. doi: 10.1371/journal.pone.0187967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Workneh MH, Bjune GA, Yimer SA. 2017. Prevalence and associated factors of tuberculosis and diabetes mellitus comorbidity: a systematic review. PLoS One 12:e0175925. doi: 10.1371/journal.pone.0175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung CC, Lam TH, Chan WM, Yew WW, Ho KS, Leung GM, Law WS, Tam CM, Chan CK, Chang KC. 2008. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol 167:1486–1494. doi: 10.1093/aje/kwn075. [DOI] [PubMed] [Google Scholar]
- 6.Mahishale V, Avuthu S, Patil B, Lolly M, Eti A, Khan S. 2017. Effect of poor glycemic control in newly diagnosed patients with smear-positive pulmonary tuberculosis and type-2 diabetes mellitus. Iran J Med Sci 42:144–151. [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar Nathella P, Babu S. 2017. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology 152:13–24. doi: 10.1111/imm.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alisjahbana B, Sahiratmadja E, Nelwan EJ, Purwa AM, Ahmad Y, Ottenhoff TH, Nelwan RH, Parwati I, van der Meer JW, van Crevel R. 2007. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis 45:428–435. doi: 10.1086/519841. [DOI] [PubMed] [Google Scholar]
- 9.Salindri AD, Kipiani M, Kempker RR, Gandhi NR, Darchia L, Tukvadze N, Blumberg HM, Magee MJ. 2016. Diabetes reduces the rate of sputum culture conversion in patients with newly diagnosed multidrug-resistant tuberculosis. Open Forum Infect Dis 3:ofw126. doi: 10.1093/ofid/ofw126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Workneh MH, Bjune GA, Yimer SA. 2016. Diabetes mellitus is associated with increased mortality during tuberculosis treatment: a prospective cohort study among tuberculosis patients in South-Eastern Amahra Region, Ethiopia. Infect Dis Poverty 5:22. doi: 10.1186/s40249-016-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viswanathan V, Vigneswari A, Selvan K, Satyavani K, Rajeswari R, Kapur A. 2014. Effect of diabetes on treatment outcome of smear-positive pulmonary tuberculosis—a report from South India. J Diabetes Complications 28:162–165. doi: 10.1016/j.jdiacomp.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura A, Hagiwara E, Hamai J, Taguri M, Terauchi Y. 2014. Impact of underlying diabetes and presence of lung cavities on treatment outcomes in patients with pulmonary tuberculosis. Diabet Med 31:707–713. doi: 10.1111/dme.12414. [DOI] [PubMed] [Google Scholar]
- 13.Banu Rekha VV, Balasubramanian R, Swaminathan S, Ramachandran R, Rahman F, Sundaram V, Thyagarajan K, Selvakumar N, Adhilakshmi AR, Iliayas S, Narayanan PR. 2007. Sputum conversion at the end of intensive phase of category-1 regimen in the treatment of pulmonary tuberculosis patients with diabetes mellitus or HIV infection: an analysis of risk factors. Indian J Med Res 126:452–458. [PubMed] [Google Scholar]
- 14.Singla R, Khan N, Al-Sharif N, Ai-Sayegh MO, Shaikh MA, Osman MM. 2006. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. Int J Tuberc Lung Dis 10:74–79. [PubMed] [Google Scholar]
- 15.Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lonnroth K, Ottmani SE, Goonesekera SD, Murray MB. 2011. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degner NR, Wang JY, Golub JE, Karakousis PC. 2018. Metformin use reverses the increased mortality associated with diabetes mellitus during tuberculosis treatment. Clin Infect Dis 66:198–205. doi: 10.1093/cid/cix819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nijland HM, Ruslami R, Stalenhoef JE, Nelwan EJ, Alisjahbana B, Nelwan RH, van der Ven AJ, Danusantoso H, Aarnoutse RE, van Crevel R. 2006. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin Infect Dis 43:848–854. doi: 10.1086/507543. [DOI] [PubMed] [Google Scholar]
- 18.Medellin-Garibay SE, Cortez-Espinosa N, Milan-Segovia RC, Magana-Aquino M, Vargas-Morales JM, Gonzalez-Amaro R, Portales-Perez DP, Romano-Moreno S. 2015. Clinical pharmacokinetics of rifampin in patients with tuberculosis and type 2 diabetes mellitus: association with biochemical and immunological parameters. Antimicrob Agents Chemother 59:7707–7714. doi: 10.1128/AAC.01067-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babalik A, Ulus IH, Bakirci N, Kuyucu T, Arpag H, Dagyildizi L, Capaner E. 2013. Plasma concentrations of isoniazid and rifampin are decreased in adult pulmonary tuberculosis patients with diabetes mellitus. Antimicrob Agents Chemother 57:5740–5742. doi: 10.1128/AAC.01345-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang MJ, Chae JW, Yun HY, Lee JI, Choi HD, Kim J, Park JS, Cho YJ, Yoon HI, Lee CT, Shin WG, Lee JH. 2015. Effects of type 2 diabetes mellitus on the population pharmacokinetics of rifampin in tuberculosis patients. Tuberculosis (Edinb) 95:54–59. doi: 10.1016/j.tube.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Requena-Mendez A, Davies G, Ardrey A, Jave O, Lopez-Romero SL, Ward SA, Moore DA. 2012. Pharmacokinetics of rifampin in Peruvian tuberculosis patients with and without comorbid diabetes or HIV. Antimicrob Agents Chemother 56:2357–2363. doi: 10.1128/AAC.06059-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruslami R, Nijland HM, Adhiarta IG, Kariadi SH, Alisjahbana B, Aarnoutse RE, van Crevel R. 2010. Pharmacokinetics of antituberculosis drugs in pulmonary tuberculosis patients with type 2 diabetes. Antimicrob Agents Chemother 54:1068–1074. doi: 10.1128/AAC.00447-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemanth Kumar AK, Kannan T, Chandrasekaran V, Sudha V, Vijayakumar A, Ramesh K, Lavanya J, Swaminathan S, Ramachandran G. 2016. Pharmacokinetics of thrice-weekly rifampicin, isoniazid and pyrazinamide in adult tuberculosis patients in India. Int J Tuberc Lung Dis 20:1236–1241. doi: 10.5588/ijtld.16.0048. [DOI] [PubMed] [Google Scholar]
- 24.Long MW, Snider DE Jr, Farer LS. 1979. U.S. Public Health Service cooperative trial of three rifampin-isoniazid regimens in treatment of pulmonary tuberculosis. Am Rev Respir Dis 119:879–894. [DOI] [PubMed] [Google Scholar]
- 25.Kumar AK, Chandrasekaran V, Kannan T, Murali AL, Lavanya J, Sudha V, Swaminathan S, Ramachandran G. 2017. Anti-tuberculosis drug concentrations in tuberculosis patients with and without diabetes mellitus. Eur J Clin Pharmacol 73:65–70. doi: 10.1007/s00228-016-2132-z. [DOI] [PubMed] [Google Scholar]
- 26.Court R, Chirehwa MT, Wiesner L, Wright B, Smythe W, Kramer N, McIlleron H. 2018. Quality assurance of rifampicin-containing fixed-drug combinations in South Africa: dosing implications. Int J Tuberc Lung Dis 22:537–543. doi: 10.5588/ijtld.17.0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rayner CK, Schwartz MP, van Dam PS, Renooij W, de Smet M, Horowitz M, Smout AJ, Samsom M. 2002. Small intestinal glucose absorption and duodenal motility in type 1 diabetes mellitus. Am J Gastroenterol 97:3123–3130. doi: 10.1111/j.1572-0241.2002.07109.x. [DOI] [PubMed] [Google Scholar]
- 28.Yarandi SS, Srinivasan S. 2014. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterol Motil 26:611–624. doi: 10.1111/nmo.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dash RP, Ellendula B, Agarwal M, Nivsarkar M. 2015. Increased intestinal P-glycoprotein expression and activity with progression of diabetes and its modulation by epigallocatechin-3-gallate: evidence from pharmacokinetic studies. Eur J Pharmacol 767:67–76. doi: 10.1016/j.ejphar.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Nawa A, Fujita-Hamabe W, Tokuyama S. 2011. Altered intestinal P-glycoprotein expression levels in a monosodium glutamate-induced obese mouse model. Life Sci 89:834–838. doi: 10.1016/j.lfs.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Pradhan K, Zhong XB, Ma X. 2016. Isoniazid metabolism and hepatotoxicity. Acta Pharm Sin B 6:384–392. doi: 10.1016/j.apsb.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. 2012. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet 51:277–304. doi: 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Song BJ, Akbar M, Jo I, Hardwick JP, Abdelmegeed MA. 2015. Translational implications of the alcohol-metabolizing enzymes, including cytochrome P450-2E1, in alcoholic and nonalcoholic liver disease. Adv Pharmacol 74:303–372. doi: 10.1016/bs.apha.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Tomankova V, Liskova B, Skalova L, Bartikova H, Bousova I, Jourova L, Anzenbacher P, Ulrichova J, Anzenbacherova E. 2015. Altered cytochrome P450 activities and expression levels in the liver and intestines of the monosodium glutamate-induced mouse model of human obesity. Life Sci 133:15–20. doi: 10.1016/j.lfs.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 35.van Rongen A, Valitalo PA, Peeters MY, Boerma D, Huisman FW, van Ramshorst B, van Dongen EP, van den Anker JN, Knibbe CA. 2016. Morbidly obese patients exhibit increased CYP2E1-mediated oxidation of acetaminophen. Clin Pharmacokinet 55:833–847. doi: 10.1007/s40262-015-0357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiney MS, Schwarzenberg SJ, Johnson LA. 2011. Altered xanthine oxidase and N-acetyltransferase activity in obese children. Br J Clin Pharmacol 72:109–115. doi: 10.1111/j.1365-2125.2011.03959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shih TY, Pai CY, Yang P, Chang WL, Wang NC, Hu OY. 2013. A novel mechanism underlies the hepatotoxicity of pyrazinamide. Antimicrob Agents Chemother 57:1685–1690. doi: 10.1128/AAC.01866-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lacroix C, Hoang TP, Nouveau J, Guyonnaud C, Laine G, Duwoos H, Lafont O. 1989. Pharmacokinetics of pyrazinamide and its metabolites in healthy subjects. Eur J Clin Pharmacol 36:395–400. doi: 10.1007/BF00558302. [DOI] [PubMed] [Google Scholar]
- 39.Whitehouse LW, Lodge BA, By AW, Thomas BH. 1987. Metabolic disposition of pyrazinamide in the rat: identification of a novel in vivo metabolite common to both rat and human. Biopharm Drug Dispos 8:307–318. doi: 10.1002/bdd.2510080402. [DOI] [PubMed] [Google Scholar]
- 40.Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, Sastre J, Vina J. 2002. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes 51:1118–1124. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto S, Koshiishi I, Inoguchi T, Nawata H, Utsumi H. 2003. Confirmation of superoxide generation via xanthine oxidase in streptozotocin-induced diabetic mice. Free Radic Res 37:767–772. doi: 10.1080/1071576031000107344. [DOI] [PubMed] [Google Scholar]
- 42.Kuppusamy UR, Indran M, Rokiah P. 2005. Glycaemic control in relation to xanthine oxidase and antioxidant indices in Malaysian type 2 diabetes patients. Diabet Med 22:1343–1346. doi: 10.1111/j.1464-5491.2005.01630.x. [DOI] [PubMed] [Google Scholar]
- 43.Miric DJ, Kisic BM, Filipovic-Danic S, Grbic R, Dragojevic I, Miric MB, Puhalo-Sladoje D. 2016. Xanthine oxidase activity in type 2 diabetes mellitus patients with and without diabetic peripheral neuropathy. J Diabetes Res 2016:4370490. doi: 10.1155/2016/4370490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klisic A, Kocic G, Kavaric N, Jovanovic M, Stanisic V, Ninic A. 2018. Xanthine oxidase and uric acid as independent predictors of albuminuria in patients with diabetes mellitus type 2. Clin Exp Med 18:283–290. doi: 10.1007/s10238-017-0483-0. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Meng X, Gao X, Pang X, Wang Y, Wu X, Deng X, Zhang Q, Sun C, Li Y. 2018. Elevated serum xanthine oxidase activity is associated with the development of type 2 diabetes: a prospective cohort study. Diabetes Care 41:884–890. doi: 10.2337/dc17-1434. [DOI] [PubMed] [Google Scholar]
- 46.Lacroix C, Guyonnaud C, Chaou M, Duwoos H, Lafont O. 1988. Interaction between allopurinol and pyrazinamide. Eur Respir J 1:807–811. [PubMed] [Google Scholar]
- 47.Martinez N, Kornfeld H. 2014. Diabetes and immunity to tuberculosis. Eur J Immunol 44:617–626. doi: 10.1002/eji.201344301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamashiro S, Kawakami K, Uezu K, Kinjo T, Miyagi K, Nakamura K, Saito A. 2005. Lower expression of Th1-related cytokines and inducible nitric oxide synthase in mice with streptozotocin-induced diabetes mellitus infected with Mycobacterium tuberculosis. Clin Exp Immunol 139:57–64. doi: 10.1111/j.1365-2249.2005.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. 2007. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol 37:518–524. doi: 10.1165/rcmb.2006-0478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lecube A, Pachon G, Petriz J, Hernandez C, Simo R. 2011. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One 6:e23366. doi: 10.1371/journal.pone.0023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tater D, Tepaut B, Bercovici JP, Youinou P. 1987. Polymorphonuclear cell derangements in type I diabetes. Horm Metab Res 19:642–647. doi: 10.1055/s-2007-1011899. [DOI] [PubMed] [Google Scholar]
- 52.Vallerskog T, Martens GW, Kornfeld H. 2010. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol 184:6275–6282. doi: 10.4049/jimmunol.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swaminathan S, Pasipanodya JG, Ramachandran G, Hemanth Kumar AK, Srivastava S, Deshpande D, Nuermberger E, Gumbo T. 2016. Drug concentration thresholds predictive of therapy failure and death in children with tuberculosis: bread crumb trails in random forests. Clin Infect Dis 63:S63–S74. doi: 10.1093/cid/ciw471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rockwood N, Pasipanodya JG, Denti P, Sirgel F, Lesosky M, Gumbo T, Meintjes G, McIlleron H, Wilkinson RJ. 2017. Concentration-dependent antagonism and culture conversion in pulmonary tuberculosis. Clin Infect Dis 64:1350–1359. doi: 10.1093/cid/cix158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee YJ, Han SK, Park JH, Lee JK, Kim DK, Chung HS, Heo EY. 16 March 2018. The effect of metformin on culture conversion in tuberculosis patients with diabetes mellitus. Korean J Intern Med. doi: 10.3904/kjim.2017.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krug SKB, Dawson VL, Dawson TM, Bishai WR. 2018. Host DAMP activator PARP-1 is induced in TB-infected mice. Abstr Tuberculosis: Translating Scientific Findings for Clinical and Public Health Impact Keystone Symposia Global Health Series, Whistler, BC, Canada. [Google Scholar]
- 57.Gomez DI, Twahirwa M, Schlesinger LS, Restrepo BI. 2013. Reduced Mycobacterium tuberculosis association with monocytes from diabetes patients that have poor glucose control. Tuberculosis (Edinb) 93:192–197. doi: 10.1016/j.tube.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugawara I, Mizuno S. 2008. Higher susceptibility of type 1 diabetic rats to Mycobacterium tuberculosis infection. Tohoku J Exp Med 216:363–370. doi: 10.1620/tjem.216.363. [DOI] [PubMed] [Google Scholar]
- 59.Wang CH, Yu CT, Lin HC, Liu CY, Kuo HP. 1999. Hypodense alveolar macrophages in patients with diabetes mellitus and active pulmonary tuberculosis. Tuber Lung Dis 79:235–242. doi: 10.1054/tuld.1998.0167. [DOI] [PubMed] [Google Scholar]
- 60.Buzzo CL, Medina T, Branco LM, Lage SL, Ferreira LC, Amarante-Mendes GP, Hottiger MO, De Carvalho DD, Bortoluci KR. 2017. Epigenetic regulation of nitric oxide synthase 2, inducible (Nos2) by NLRC4 inflammasomes involves PARP1 cleavage. Sci Rep 7:41686. doi: 10.1038/srep41686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mathews MT, Berk BC. 2008. PARP-1 inhibition prevents oxidative and nitrosative stress-induced endothelial cell death via transactivation of the VEGF receptor 2. Arterioscler Thromb Vasc Biol 28:711–717. doi: 10.1161/ATVBAHA.107.156406. [DOI] [PubMed] [Google Scholar]
- 62.Torok ME, Aljayyoussi G, Waterhouse D, Chau T, Mai N, Phu NH, Hien TT, Hope W, Farrar JJ, Ward SA. 2018. Suboptimal exposure to anti-TB drugs in a TBM/HIV+ population is not related to antiretroviral therapy. Clin Pharmacol Ther 103:449–457. doi: 10.1002/cpt.646. [DOI] [PubMed] [Google Scholar]
- 63.Yoon YS, Jung JW, Jeon EJ, Seo H, Ryu YJ, Yim JJ, Kim YH, Lee BH, Park YB, Lee BJ, Kang H, Choi JC. 2017. The effect of diabetes control status on treatment response in pulmonary tuberculosis: a prospective study. Thorax 72:263–270. doi: 10.1136/thoraxjnl-2015-207686. [DOI] [PubMed] [Google Scholar]
- 64.Magee MJ, Bloss E, Shin SS, Contreras C, Huaman HA, Ticona JC, Bayona J, Bonilla C, Yagui M, Jave O, Cegielski JP. 2013. Clinical characteristics, drug resistance, and treatment outcomes among tuberculosis patients with diabetes in Peru. Int J Infect Dis 17:e404–e412. doi: 10.1016/j.ijid.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallis RS, Peppard T, Hermann D. 2015. Month 2 culture status and treatment duration as predictors of recurrence in pulmonary tuberculosis: model validation and update. PLoS One 10:e0125403. doi: 10.1371/journal.pone.0125403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bose A, Kalita S, Rose W, Tharyan P. 2014. Intermittent versus daily therapy for treating tuberculosis in children. Cochrane Database Syst Rev 2014:CD007953. doi: 10.1002/14651858.CD007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kasozi S, Clark J, Doi SA. 2015. Intermittent versus daily pulmonary tuberculosis treatment regimens: a meta-analysis. Clin Med Res 13:117–138. doi: 10.3121/cmr.2015.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Menon PR, Lodha R, Sivanandan S, Kabra SK. 2010. Intermittent or daily short course chemotherapy for tuberculosis in children: meta-analysis of randomized controlled trials. Indian Pediatr 47:67–73. doi: 10.1007/s13312-010-0009-2. [DOI] [PubMed] [Google Scholar]
- 69.Mave V, Meshram S, Lokhande R, Kadam D, Dharmshale S, Bharadwaj R, Kagal A, Pradhan N, Deshmukh S, Atre S, Sahasrabudhe T, Barthwal M, Meshram S, Kakrani A, Kulkarni V, Raskar S, Suryavanshi N, Shivakoti R, Chon S, Selvin E, Gupte A, Gupta A, Gupte N, Golub JE. 2017. Prevalence of dysglycemia and clinical presentation of pulmonary tuberculosis in western India. Int J Tuberc Lung Dis 21:1280–1287. doi: 10.5588/ijtld.17.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chideya S, Winston CA, Peloquin CA, Bradford WZ, Hopewell PC, Wells CD, Reingold AL, Kenyon TA, Moeti TL, Tappero JW. 2009. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis 48:1685–1694. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peloquin CA. 2002. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 62:2169–2183. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 72.Donald PR, Maritz JS, Diacon AH. 2011. The pharmacokinetics and pharmacodynamics of rifampicin in adults and children in relation to the dosage recommended for children. Tuberculosis (Edinb) 91:196–207. doi: 10.1016/j.tube.2011.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.