Farnesol, a quorum-sensing molecule, inhibits Candida albicans hyphal formation, affects its biofilm formation and dispersal, and impacts its stress response. Several aspects of farnesol's mechanism of action remain incompletely uncharacterized.
KEYWORDS: Candida albicans, farnesol, zinc cluster transcription factor
ABSTRACT
Farnesol, a quorum-sensing molecule, inhibits Candida albicans hyphal formation, affects its biofilm formation and dispersal, and impacts its stress response. Several aspects of farnesol's mechanism of action remain incompletely uncharacterized. Among these are a thorough accounting of the cellular receptors and transporters for farnesol. This work suggests these processes are linked through the Zn cluster transcription factors Tac1 and Znc1 and their induction of the multidrug efflux pump Cdr1. Specifically, we have demonstrated that Tac1 and Znc1 are functionally activated by farnesol through a mechanism that mimics other means of hyperactivation of Zn cluster transcription factors. This is consistent with our observation that many genes acutely induced by farnesol are dependent on TAC1, ZNC1, or both. A related molecule, 1-dodecanol, invokes a similar TAC1-ZNC1 response, while several other proposed C. albicans quorum-sensing molecules do not. Tac1 and Znc1 both bind to and upregulate the CDR1 promoter in response to farnesol. Differences in inducer and DNA binding specificity lead to Tac1 and Znc1 having overlapping, but nonidentical, regulons. Induction of genes by farnesol via Tac1 and Znc1 was inversely related to the level of CDR1 present in the cell, suggesting a model in which induction of CDR1 by Tac1 and Znc1 leads to an increase in farnesol efflux. Consistent with this premise, our results show that CDR1 expression, and its regulation by TAC1 and ZNC1, facilitates growth in the presence of high farnesol concentrations in C. albicans and in certain strains of its close relative, C. dubliniensis.
INTRODUCTION
Candida albicans is a major opportunistic human fungal pathogen that can cause life-threatening systemic infections in immunocompromised individuals (1–3). Multiple important C. albicans virulence-related traits, including the morphological switch between yeast and hyphal growth (4, 5), biofilm formation and dispersal (6), interspecies communication with bacteria (7), and response to oxidative stress (8), can be modulated by its quorum-sensing molecule (QSM), farnesol, the first identified QSM for eukaryotes (9–14).
Among multiple Candida species, C. albicans has been found to produce the most significant amounts of farnesol, followed by its close relative, C. dubliniensis (15, 16). Dense cultures of C. albicans, in certain media, can accumulate farnesol to concentrations as high as 50 μM (15, 16). The known mechanisms underlying the biological activity of farnesol in C. albicans include modulation of signaling pathways, such as the Ras1-Cyr1/cAMP-PKA cascade, in part via direct inhibition of Cyr1 (17–19). Farnesol exposure also results in a transcriptional response in C. albicans in both sessile and planktonic cells (12, 20–23).
One of the outstanding questions regarding farnesol activity in C. albicans is the existence of specific farnesol receptors and transporters (13). Adenylyl cyclase Cyr1 is a cytoplasmic target of farnesol, as it binds and is inhibited by farnesol (18). Transcription factors that directly respond to farnesol as a nuclear receptor/effector to regulate gene expression, however, have not been identified. Growth of C. albicans, in the white cell form, is remarkably resistant to growth inhibition by high concentrations of farnesol compared to other fungal species (14, 24). This property might result from an efficient farnesol efflux by a certain transporter(s). The ABC (ATP-binding cassette) transporter Cdr1 was found upregulated upon 2 to 24 h of farnesol treatment (21, 22) and has been proposed to play a role in farnesol efflux (22). Expression of CDR1 and another ABC transporter, CDR2, in C. albicans is regulated by the Zn(II)Cys6 transcription factor Tac1 (25). Gain-of-function (GOF) mutations in TAC1 are often found in clinical isolates of C. albicans that are resistant to treatment with azole drugs due to high levels of CDR1 expression (25–27). Tac1 binds to a 13-bp drug-responsive-element (DRE) at the CDR1 and CDR2 promoters and activates transcription upon acquisition of gain-of-function mutations or treatment with certain xenobiotics, such as fluphenazine (25, 26, 28). The C. albicans TAC1 gene is located in a zinc cluster region on chromosome 5 (25), where it neighbors two other transcription factors from its family, Hal9 and Znc1. Interestingly, Znc1, when activated artificially, also increases CDR1 expression (29).
In this work, we investigated whether Tac1 functions as a farnesol nuclear receptor/effector to activate CDR1 expression and searched for other transcription factors with a similar function. Our work showed that Tac1 and Znc1 contributed individually and in tandem to the transcriptional activation response to farnesol. We also found that CDR1 expression, and its regulation by TAC1 and ZNC1, facilitates growth in the presence of farnesol in both C. albicans and C. dubliniensis.
RESULTS
Farnesol and 1-dodecanol rapidly induce CDR1 expression.
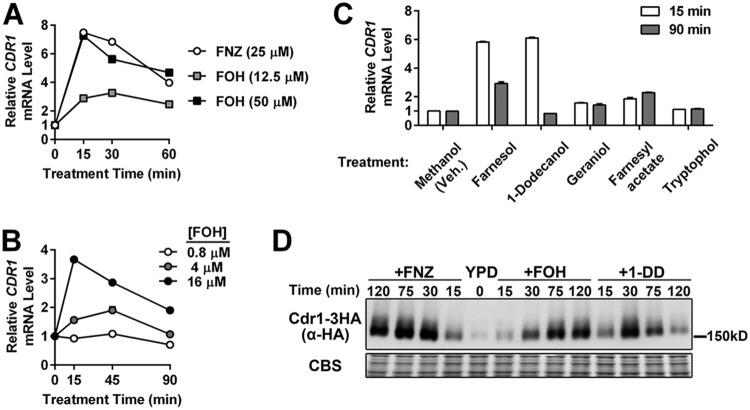
Since specific xenobiotic inducers evoke an acute activation of the C. albicans CDR1 promoter (28, 30), we tested whether the known physiologic inducer of CDR1, farnesol (FOH), also led to a rapid transcriptional induction of the efflux pump gene. FOH addition to exponentially growing cells led to an increase in CDR1 mRNA expression with an amplitude and temporal pattern comparable to that of fluphenazine (FNZ), a well-studied inducer of CDR1 (Fig. 1A). FOH induces CDR1 expression in a dose-dependent manner, starting at concentrations as low as 4 μM (Fig. 1B). The 12-carbon backbone and hydroxyl group of FOH are required for its full inhibition of C. albicans hyphal growth (9, 31). Several different terpene alcohols and FOH derivatives were tested for their ability to rapidly induce CDR1. Geraniol and farnesyl acetate are unable to induce levels of CDR1 expression comparable to those of FOH (Fig. 1C). 1-Dodecanol (1-DD), however, another 12-carbon molecule that inhibits hyphal growth (32), induces CDR1 expression at concentrations similar to those of FOH (Fig. 1C). Tryptophol (Fig. 1C), an aromatic amino acid-derived alcohol with fungal quorum-sensing activity (33, 34), and tyrosol (see Fig. S1 in the supplemental material), another C. albicans quorum-sensing molecule (33), do not induce CDR1. Tracking the expression of a 3× hemagglutinin (HA)-tagged CDR1 allele by immunoblot analysis confirmed that FOH and 1-DD also induced Cdr1 at the protein level (Fig. 1D). Cdr1 protein levels, especially in response to FNZ and FOH, appear to stay at the peak induced level longer than the mRNA, indicating that Cdr1 is fairly stable.
FIG 1.
CDR1 induction by farnesol and 1-dodecanol treatment. (A) RT-qPCR analysis of CDR1 mRNA expression in a C. albicans wild-type strain (yLM167) grown in YPD and treated with farnesol (FOH) or fluphenazine (FNZ). CDR1 basal expression (mRNA level prior to treatment; 0 min) was set to 1 to calculate the relative CDR1 level across conditions. ACT1 level was used as an internal reference. Results from one representative experiment were presented by the means and standard deviations (values may not be large enough to give a visible error bar) of two qPCR measurements on the same set of cDNA samples. (B) RT-qPCR analysis of CDR1 mRNA expression induced by increasing concentrations of FOH. CDR1 expression, in the absence of treatment, in the tested strain (yLM167) was set to 1. (C) RT-qPCR analysis of changes in CDR1 expression upon exposure to molecules structurally or functionally related to FOH. Each compound tested (or an equal volume of methanol [vehicle]) was added into log-phase cultures of a wild-type strain (yLM167) at a final concentration of 50 μM. The CDR1 mRNA level in the methanol-treated samples (15 min) was set to 1. (D) Immunoblot analysis of whole-cell extracts made from a strain expressing C-terminally 3×HA-tagged Cdr1 (yLM505) treated with FNZ (25 μM), FOH (50 μM), or 1-DD (50 μM) for the indicated amount of time. Extracts were resolved on a 6% SDS-PAGE gel and probed by an α-HA antibody or stained by Coomassie blue (CBS) as a loading control.
Tac1 is required for the induction of some, but not all, FOH and 1-DD target genes.
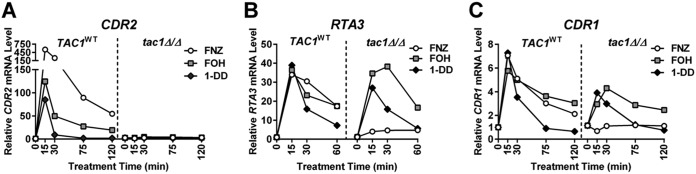
Induction of CDR1, CDR2, and RTA3 by xenobiotics, such as FNZ and estradiol, is dependent on the zinc cluster transcription factor Tac1 (25, 28, 29, 35). The observation that CDR1, CDR2, and RTA3 expression was induced by FOH and 1-DD treatment (Fig. 1A and 2A and B) suggested Tac1 hyperactivation as a mechanism for FOH- and 1-DD-induced transcription. Unlike FNZ induction of CDR1, CDR2, and RTA3, which was entirely Tac1 dependent, only FOH and 1-DD induction of CDR2 was entirely dependent on Tac1 (Fig. 2A to C). The residual CDR1 induction, and virtually unaffected RTA3 induction, suggest that additional transcription factors respond to FOH and 1-DD at these promoters.
FIG 2.
TAC1 dependence of CDR2, RTA3, and CDR1 induction by FOH and 1-DD. RT-qPCR analysis of CDR2 (A), RTA3 (B), and CDR1 (C) mRNA expression after treatment with FNZ (25 μM), FOH (50 μM), and 1-DD (50 μM) in a wild-type (TAC1WT; yLM167 or yLM660) and tac1 deletion strain (tac1Δ/Δ; yLM166 or yLM663). The expression level of each gene, in the absence of treatment (0 min) in the wild-type background, was set to 1.
Znc1 contributes to the induction of multiple FOH and 1-DD target genes.
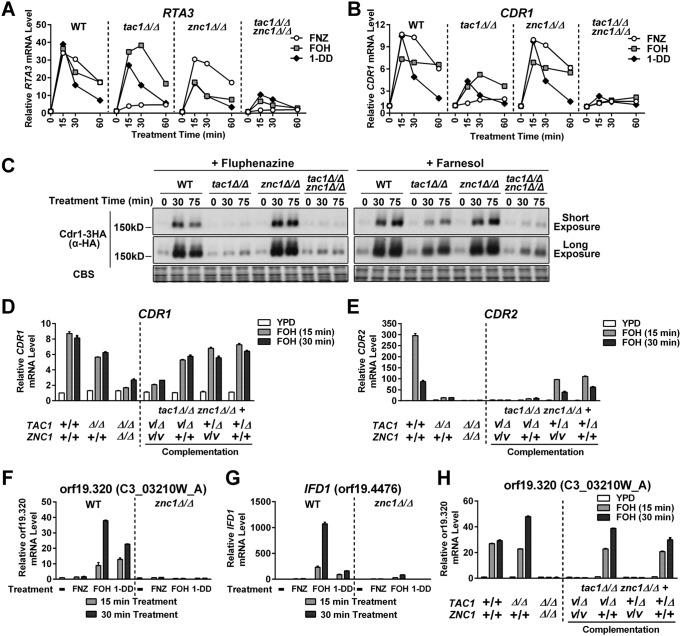
The first candidate transcription factor that we tested for Tac1-independent induction of CDR1 and RTA3 by FOH (and 1-DD) was Znc1. Znc1 is a zinc cluster transcription factor that is encoded adjacent to TAC1 and whose sequence bares the greatest similarity to Tac1 of all other members of the C. albicans zinc cluster transcription factor family (Candida Genome Database [25]). Znc1 was previously identified as a potential regulator of CDR1 and RTA3 in an experiment in which a potent activation domain was fused to the full-length wild-type Znc1 (29). RTA3 induction by FOH and 1-DD was decreased in a znc1Δ/Δ strain, while RTA3 induction by FNZ was largely unaffected (Fig. 3A). FNZ, FOH, and 1-DD induction of CDR1 was largely unaffected in the znc1Δ/Δ strain; however, FOH and 1-DD induction of CDR1 and RTA3 was decreased in a tac1Δ/Δ znc1Δ/Δ strain compared to that of either single mutant (Fig. 3A and B). The pattern of Cdr1 protein expression was consistent with the epistasis analysis of CDR1 mRNA expression in the tac1 and znc1 strains (Fig. 3C). FOH induction of CDR1 in the tac1Δ/Δ znc1Δ/Δ strain was restored by complementation with either TAC1 or ZNC1 (Fig. 3D), while CDR2 induction was only restored upon TAC1 complementation (Fig. 3E).
FIG 3.
Znc1 and Tac1 contribute individually and in tandem to the regulation of specific FOH- and 1-DD-induced promoters. (A and B) RT-qPCR analysis of RTA3 (A) and CDR1 (B) mRNA expression in tac1Δ/Δ and znc1Δ/Δ strains treated with FNZ, FOH, or 1-DD. A wild-type strain (yLM660) and mutants carrying a tac1 deletion (yLM663), znc1 deletion (yLM661), or double deletion (yLM664) were treated with FNZ (25 μM), FOH (50 μM), or 1-DD (50 μM). CDR1 and RTA3 basal expression in the untreated wild-type strain (yLM660) was individually set to 1. (C) Immunoblot analysis of Cdr1 protein levels in tac1Δ/Δ, znc1Δ/Δ, and double deletion strains treated with FNZ or FOH. Wild-type (yLM665), tac1Δ/Δ (yLM666), znc1Δ/Δ (yLM667), and tac1Δ/Δ znc1Δ/Δ (yLM668) strains expressing C-terminally 3×HA-tagged Cdr1 were treated with FNZ (25 μM) or FOH (50 μM). Cell lysates were resolved on 6% SDS-PAGE gels and probed with an anti-HA antibody. Blot images, acquired at two exposure times, are presented, and Coomassie blue staining (CBS) images are presented as a loading control. (D to E) RT-qPCR analysis of CDR1 (D) and CDR2 (E) mRNA expression in tac1Δ/Δ and znc1Δ/Δ strains, and complementation controls, treated with FOH. A tac1Δ/Δ znc1Δ/Δ strain was complemented by ZNC1 (yLM676), TAC1 (yLM677), or both (yLM675) and treated with FOH (50 μM). Parallel experiments were also conducted in wild-type (yLM660), tac1 deletion (yLM663), parental tac1 znc1 double deletion (yLM664), and mock complementation (yLM678) strains for comparison. A plus sign labels a native or restored gene locus, while Δ and V (vector) mark an unrestored gene disruption and a mock complementation by introducing an empty vector. (F and G) RT-qPCR analysis of orf19.320 (F) and IFD1 (G) mRNA expression in the wild-type ZNC1 or znc1 deletion background (yLM660 and yLM661, respectively) treated with FNZ (25 μM), FOH (50 μM), and 1-DD (50 μM) for the indicated period of time. orf19.320 and IFD1 basal expression in the wild-type strain was individually set to 1. (H) qPCR analysis of orf19.320 expression in the complementation strains using cDNA samples tested in panels D and E. Noninduced expression levels in the wild-type strain (yLM660) were set to 1.
In addition to CDR1 and RTA3, the previous Znc1 activation domain fusion analysis (29) suggested that several other genes, including orf19.320 and IFD1 (orf19.4476), were direct Znc1 targets. Both orf19.320 and IFD1 are induced by FOH and 1-DD but not by FNZ (Fig. 3F and G). Induction of orf19.320 and IFD1 by FOH and 1-DD was ZNC1 dependent but not affected by TAC1 (Fig. 3H). Consistent with these results, FOH induction of orf19.320 in a tac1Δ/Δ znc1Δ/Δ strain was restored by reintroduction of ZNC1 but not TAC1 (Fig. 3H).
Hyperactivation of Tac1 and Znc1 by farnesol and 1-dodecanol.
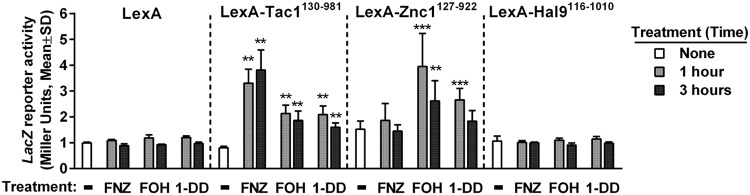
The mechanism by which small molecules regulate the ability of Tac1, as well as other members of the PDR1 family, to activate transcription has not been clearly defined. Direct binding to these small molecules/xenobiotics has been shown, in the case of C. glabrata PDR1 (36), to play an important role in this process. Among the strongest pieces of evidence for direct xenobiotic hyperactivation in C. albicans is the finding that a heterologous DNA binding domain (DBD) fused to Tac1 (minus the DBD) activated reporter genes in response to xenobiotic (30, 37). We used a C. albicans one-hybrid assay to test whether Tac1 or Znc1, with its native DBD replaced by the LexA DBD, could activate a LacZ reporter in response to FOH and 1-DD. The LexA-Tac1 fusion protein activated its reporter gene in response to both FOH and 1-DD at levels comparable to, although somewhat lower than, the levels previously observed (30) for FNZ (Fig. 4A). The LexA-Znc1 construct induces the LacZ reporter in response to FOH and 1-DD but does not respond to treatment with FNZ. The fold activation by FOH and 1-DD are similar for LexA-Znc1 and LexA-Tac1, while LexA-Znc1 has a basal activation potential that is slightly higher than that of the Tac1 construct. Hal9, the C. albicans transcription factor with next highest similarity to Tac1 and Znc1, fused to LexA does not activate the reporter in response to FNZ, FOH, or 1-DD (Fig. 4).
FIG 4.
Direct hyperactivation of Tac1 and Znc1 by FOH and 1-DD. C. albicans one-hybrid assay assessing Tac1- and Znc1-dependent reporter activation under multiple inducing conditions. A C. albicans one-hybrid strain expressing the LexA DNA binding domain (yLM567) or the fusion of the LexA DBD with Tac1, Znc1, or Hal9 fragments (yLM568, yLM680 and yLM681, respectively) were treated with FNZ (25 μM), FOH (50 μM), and 1-DD (50 μM) for 1 h and 3 h before measurement of LacZ activity. Untreated (none) and methanol-treated (not shown) cultures showed the same level of basal activity. Statistical significance for reporter activation was determined by comparing the basal and induced LacZ activity in t tests (**, P < 0.01; ***, P < 0.001).
Hyperactivation of Tac1 tightly correlates with its phosphorylation by the Mediator complex and can be detected by a decrease in gel mobility (30). FOH and 1-DD treatment both result in an N-terminal His Flag-tagged Tac1 mobility shift that is slightly lower than the shift caused by FNZ (Fig. S2A). The Tac1 band shift by FOH is unaffected in the znc1 deletion mutant (Fig. S2B), ruling out a potential competitive effect by hyperactivated Znc1. The variability of Tac1 phosphorylation pattern suggests inducer-specific conformations of hyperactive Tac1 that lead to differential phosphorylation by Mediator. To test if hyperactivated Znc1 is also subject to phosphorylation, we generated strains expressing C-terminally 3×HA-tagged Znc1. The tagging does not compromise Znc1 activation competence at the CDR1 and orf19.320 promoters (Fig. S2C and S2D). As opposed to Tac1, FOH or 1-DD treatment does not induce detectable changes in Znc1-3HA mobility in either the wild-type or tac1 deletion background (Fig. S2A and E).
Tac1 and Znc1 promoter occupancy correlates with their impact on target gene induction by FOH.
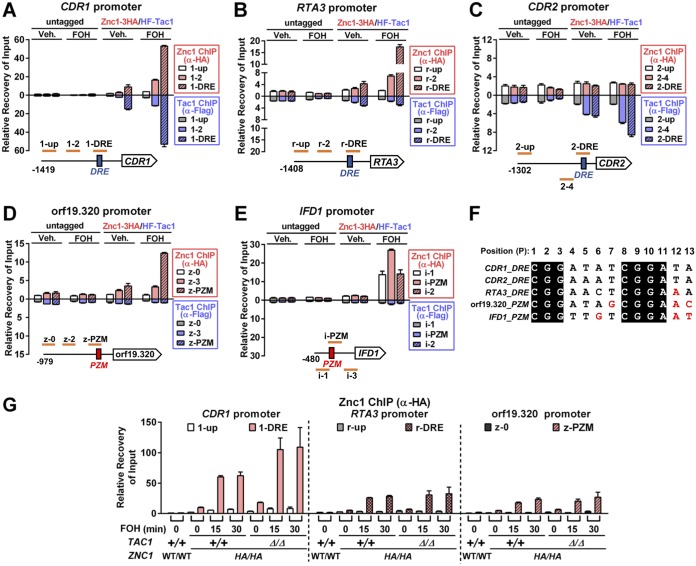
Chromatin immunoprecipitation (ChIP) analysis was performed to test whether Tac1 and Znc1 promoter occupancy determined their FOH-induced target gene specificity. Tac1 and Znc1 occupancy are enriched at the CDR1 DRE in the absence of inducer, and this occupancy is enhanced by treatment with FOH (Fig. 5A). There is also a weak but reproducible enrichment of Tac1 and Znc1 occupancy at the RTA3 DRE under noninducing conditions. Similar to our observation that Znc1 was the primary regulator of RTA3 expression in response to FOH, only Znc1 occupancy at the RTA3 DRE was increased by treatment with FOH (Fig. 3B). Tac1 and Znc1 occupancy was specific to the CDR2 and orf19.320 promoters, respectively, under noninducing conditions and was enhanced by induction with FOH (Fig. 5C and D). Another Znc1-dependent promoter, IFD1, is exclusively bound by Znc1, but only after FOH treatment (Fig. 5E). The ChIP assay results allowed identification of potential cis elements for Znc1 at the tested promoters. High Znc1 occupancy at the CDR1 and RTA3 DRE suggests DNA binding preference of Znc1 similar to that of Tac1. Thirteen-bp DRE-like CGG triplet sequences were found in the orf19.320 and IFD1 promoter regions, whose locations correlated to the region of highest local enrichment for Znc1 ChIP signal. Thus, we refer to these 13-bp elements as potential Znc1 binding motifs (PZMs). DREs and PZMs in the tested genes share a core consensus of CGGNNNNCGGAN (Fig. 5F). Multiple bases found in PZMs (labeled red in Fig. 5F) have been reported to impair CDR2 DRE function (26) and may specifically reduce Tac1 binding. The ChIP (Fig. 5B) and expression analysis (Fig. 3A) suggest that one such nucleotide in the RTA3 DRE, P12 A, is better tolerated by the Znc1 DBD than the Tac1 DBD. One model for the partially redundant function of Tac1 and Znc1 at the CDR1 promoter is that both transcription factors competently bind to the CDR1 promoter in the absence of the other. To test this hypothesis, we performed Znc1 ChIP in a tac1Δ/Δ strain. Znc1 occupancy is observed at the CDR1, RTA3, or orf19.320 promoters in a tac1Δ/Δ strain and even increases at the CDR1 DRE (Fig. 5G) compared to that of a wild-type TAC1 strain. This last finding indicates Tac1 and Znc1 compete for DRE binding at promoters where we detected cooccupancy. Additionally, we have found in ChIP assays that FNZ treatment has only a minor effect on Znc1 occupancy compared to its effect on Tac1 at the CDR1 promoter (Fig. S3A) or the effect of FOH/1-DD on Znc1 occupancy at the RTA3 promoter (Fig. S3B). Previous studies have shown that Tac1 GOF mutants can confer fluconazole resistance through a mechanism that relies on the ability of Tac1 to bind and activate the CDR1 promoter (25, 26, 28, 30). We have found that the fluconazole MIC in TAC1GOF mutant strains does not decrease in the znc1Δ/Δ strain (Table S1). Collectively, this evidence supports the hypothesis that Tac1 and Znc1 bind promoters independently of each other.
FIG 5.
Tac1 and Znc1 occupancy at FOH target promoters. (A to E) ChIP analysis of Tac1 and Znc1 occupancy at CDR1 (A), RTA3 (B), CDR2 (C), orf19.320 (D), and IFD1 (E) upon treatment with FOH. A strain carrying two copies of N-terminally 6His3Flag-tagged TAC1 and two copies of C-terminally 3×HA-tagged ZNC1 (Znc1-3HA/HF-Tac1; yLM686) and a strain with native TAC1 and ZNC1 (untagged; yLM660) were treated with FOH (50 μM) or vehicle (Veh.; methanol) for 15 min before fixation. Each sample was immunoprecipitated by an anti-Flag antibody and an anti-HA antibody in separate reactions. Promoter regions tested for Tac1 and Znc1 binding and their relative positions to the known Tac1 cis elements at the CDR1, RTA3, and CDR2 promoters (DRE; blue boxes) or the CGG triplet motifs found at the orf19.320 and IFD1 promoters (PZMs; red boxes) are schematically shown in each panel. Percent recovery of input (Input%) at the CDR1 promoter 1-up region in the anti-Flag/anti-HA ChIP products obtained from the methanol-treated untagged strain was set to 1 to normalize Tac1/Znc1 binding across conditions and promoter regions. Hence, the strength of ChIP signals (y axis value) can be compared across panels. (F) Alignment of the CDR1, CDR2, and RTA3 DREs and the CGG triplet motifs (PZMs) at the orf19.320 and IFD1 promoters. The 13 nucleotide positions are numbered in order. Individual nucleotide substitutions that have been found to be deleterious in the CDR2 DRE (26) are highlighted in red. (G) ChIP analysis of Znc1 occupancy at target promoters in a tac1 deletion background. Wild-type (yLM684) and tac1 deletion (yLM685) strains expressing two copies of C-terminally 3×HA-tagged ZNC1 (HA/HA) were treated with 50 μM FOH for the indicated period of time before fixation for an anti-HA ChIP assay. Percent recovery of input (Input%) at the 1-up region in a wild-type strain with native Tac1 (+/+) and Znc1 (WT/WT) was set to 1 to normalize recoveries across conditions and promoters.
FOH-induced Znc1 works through a Mediator-dependent coactivator mechanism.
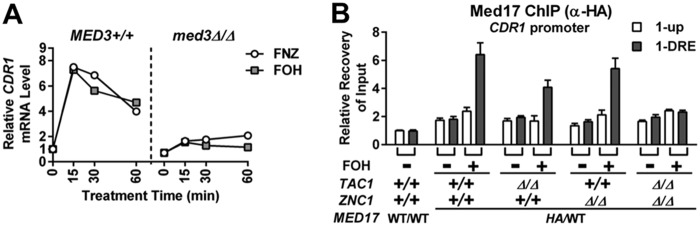
Our previous work showed recruitment of Mediator complex is critical to FNZ-induced Tac1-dependent CDR1 activation (30). Here, we found the Mediator tail module is also important for FOH-induced CDR1 expression (Fig. 6A) and that either Tac1 or Znc1 is competent for Mediator recruitment at the CDR1 DRE under these conditions (Fig. 6B). Therefore, Tac1 and Znc1 both show DRE binding and Mediator recruitment at the CDR1 promoter in the presence of FOH that is independent of the other.
FIG 6.
Mediator requirement for, and recruitment by, Tac1- and Znc1-dependent induction of CDR1 by farnesol. (A) RT-qPCR analysis of CDR1 mRNA expression in a wild-type strain (yLM167) and a med3 null strain (yLM232) after treatment with 50 μM FOH. FNZ (25 μM) induction (30) was performed as a reference. (B) Anti-HA ChIP analysis of Mediator occupancy at the CDR1 promoter in wild-type (yLM695), tac1Δ/Δ (yLM696), znc1Δ/Δ (yLM697), and tac1Δ/Δ znc1Δ/Δ (yLM698) strains expressing C-terminally 3×HA-tagged Med17 treated with 50 μM FOH. Percent recovery of input (Input%) at the 1-up region in a ChIP product obtained from a strain with native MED17 (WT/WT; yLM660) was set to 1.
Additional transcription factors regulate FOH- and 1-DD-induced transcription in a promoter-specific manner.
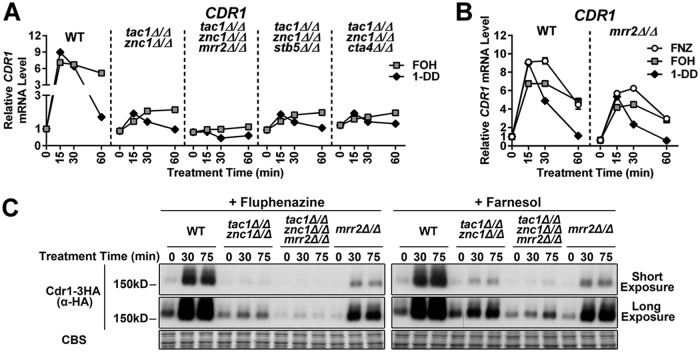
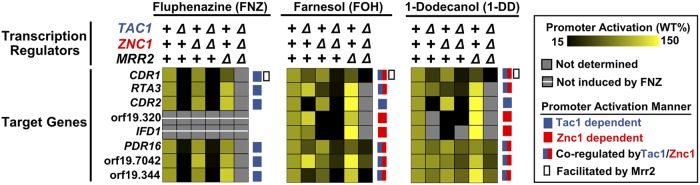
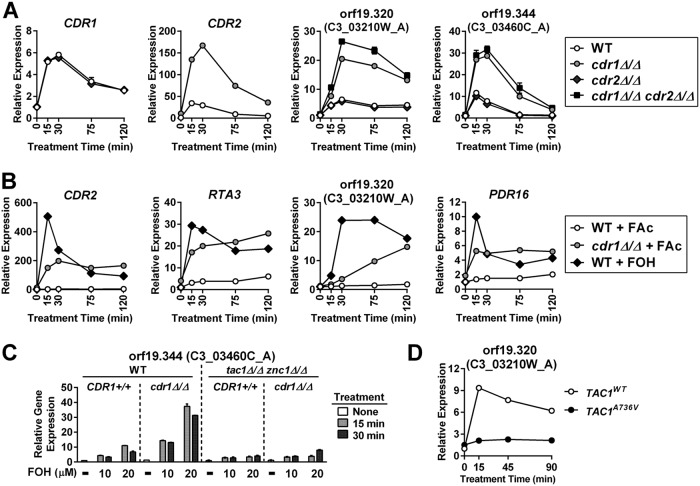
Despite the FOH induction of CDR1 being severely compromised in the tac1Δ/Δ znc1Δ/Δ strain, a small residual induction was observed in this background (Fig. 7A). This finding suggested that another transcription factor was involved in CDR1 activation by certain inducers. A genetic screen of zinc cluster transcription factors identified Mrr2, Stb5, and Cta4 as potential regulators of CDR1 (29). The tac1 znc1 cta4 and tac1 znc1 stb5 triple deletion mutants showed unaffected FOH and 1-DD induction of all genes tested compared to that of a tac1 znc1 double null strain (Fig. 7A). Deletion of mrr2, however, largely eliminates the residual induction of CDR1 mRNA by FOH (and 1-DD) in the tac1Δ/Δ znc1Δ/Δ strain (Fig. 7A), while deletion of mrr2 also compromises CDR1 activation by FNZ, FOH, and 1-DD when Tac1 and Znc1 are both present (Fig. 7B). Deletion of mrr2 in the tac1Δ/Δ znc1Δ/Δ background also decreases induced Cdr1 protein levels (Fig. 7C). Interestingly, mrr2 deletion appears to cause a greater decrease in the induced Cdr1 protein levels than in mRNA levels (Fig. 7B and C), suggesting Mrr2 also regulates CDR1 posttranscriptionally. In addition to CDR1, the genes CDR2 (Fig. 2A), PDR16 (Fig. S4A), orf19.7042 (Fig. S4B), and orf19.344 (Fig. S4C) are dependent on TAC1, ZNC1, or a combination of the two under different induction conditions (29). Deletion of mrr2, stb5, or cta4 did not impact the expression of these additional Tac1/Znc1 target genes when we assayed their induction in a tac1Δ/Δ znc1Δ/Δ background (Fig. S4D to F). Likewise, deletion of mrr2 in an otherwise wild-type background did not compromise induction of the tested Tac1/Znc1 target genes, other than CDR1, by FNZ, FOH, or 1-DD (Fig. S4G to K). Among the genes tested, Mrr2 functions as a specific modulator of CDR1 expression rather than a broad regulator of FOH and 1-DD induction. The transcription factor(s) responsible for the residual FOH or 1-DD induction of PDR16, orf19.7042, and orf19.344 in the tac1Δ/Δ znc1Δ/Δ strains remain unidentified. Figure 8 provides a heat map summary of the impact of Tac1, Znc1, and Mrr2 on expression of the fluphenazine-, farnesol-, and 1-dodecanol-induced genes analyzed in this work.
FIG 7.
Influence of Mrr2 on FOH and 1-DD target gene expression. (A) RT-qPCR analysis of CDR1 mRNA expression in a wild-type strain (yLM660) and tac1Δ/Δ znc1Δ/Δ strains (yLM701 to yLM704) generated from an otherwise wild-type, mrr2Δ/Δ, stb5Δ/Δ, or cta4Δ/Δ background and treated with FOH (50 μM) and 1-DD (50 μM). CDR1 expression, in the absence of treatment, in yLM660 was set to 1. (B) RT-qPCR analysis of CDR1 mRNA expression in wild-type (yLM660) and mrr2 null (yLM662) strains treated with FNZ (25 μM), FOH (50 μM), or 1-DD (50 μM). CDR1 expression level, in the absence of treatment, in the wild-type strain was set to 1. (C) Anti-HA immunoblot analysis of C-terminally 3×HA-tagged CDR1 protein expression in MRR2 wild-type strains (yLM665 and yLM705) and deletion mutants (yLM707 and yLM706) in the presence and absence of TAC1 and ZNC1 and treatment with FNZ (25 μM) or FOH (50 μM). Blot images acquired at different exposures are presented with a Coomassie blue stain (CBS) as a loading control.
FIG 8.
Combinatorial control of gene expression by Tac1, Znc1, and Mrr2. A summary of the effect of TAC1, ZNC1, and MRR2 on gene expression induced by FNZ (25 μM), FOH (50 μM), and 1-DD (50 μM) is shown. The means of the relative gene expression after 15 and 30 min of exposure to the inducer were used to compare promoter activation in different strain backgrounds after being normalized to the induced expression level in the wild-type strain as percentages. Effects of transcription factor deletion on gene activation are visualized by a black-yellow chromatic scale. Transcription factor(s) dependence for each promoter was illustrated by the colors and symbols as coded in the legend box.
Cdr1-mediated feedback regulation of the transcriptional FOH response.
It has been proposed that C. albicans Cdr1 can decrease intracellular FOH concentration via its efflux pump activity (22). This led us to hypothesize that FOH-induced CDR1 expression is part of a negative feedback mechanism to downregulate the cellular response to FOH. To test whether increased CDR1 expression downregulated the transcriptional response to FOH, we compared FOH-induced gene expression in a wild-type and cdr1 deletion background. In a cdr1Δ/Δ strain, CDR2, orf19.320, and orf19.344 are all expressed at higher levels by the same concentration of exogenous FOH (Fig. 9A). cdr2 deletion does not affect FOH induction and had a minimal effect when combined with cdr1 deletion, suggesting CDR1 plays a specific role in modulating this response. Based on this finding, we sought to determine whether the efficiency of Cdr1-mediated xenobiotic transport governed the transcriptional response to other small molecules. Farnesyl acetate, an FOH-like molecule, causes little to no induction of FOH-inducible promoters in a wild-type strain (Fig. 9B). In the absence of CDR1, however, farnesyl acetate and FOH result in comparable induction of several FOH target genes (Fig. 9B). This finding suggests that the Cdr1-dependent transport of farnesyl acetate, rather than an inability to activate the relevant transcription factors, is the limiting factor in response to farnesyl acetate. Compared to an FOH induction curve that peaks and then decreases, the induction curve for farnesyl acetate in the cdr1 null shows a plateau or gradual increase (Fig. 9A and B). Similar to FOH, 1-DD treatment induces higher levels of target gene expression in the absence of cdr1 (Fig. S5A and B), while geraniol and tryptophol do not (Fig. S5C). Deletion of cdr1 only enhances the FOH/1-DD response to specific inducers rather than a pool of broadly related molecules. The CDR1-facilitated negative feedback model predicts that the increase in FOH target gene expression in the cdr1 null strain will also be dependent on Tac1 and Znc1. Indeed, FOH induction in wild-type and cdr1Δ/Δ strains exhibits a similar dependence on TAC1 and ZNC1 (Fig. 9C and Fig. S4D to F). The negative feedback model also predicts that overexpression of Cdr1 would increase farnesol efflux and desensitize C. albicans to FOH induction. A strain carrying a TAC1 GOF allele, which overexpresses CDR1, showed a dramatically lower degree of Znc1 target gene induction than a wild-type strain at identical concentrations of FOH (Fig. 9D). These results further support a model where FOH-induced Cdr1 expression facilitates FOH clearance.
FIG 9.
Feedback modulation of FOH induction by Cdr1. (A) RT-qPCR analysis of CDR1, CDR2, orf19.320, and orf19.344 mRNA expression in a wild-type strain (yLM660) and mutant strains carrying a deletion in cdr1 (yLM708), cdr2 (yLM709), or both (yLM710) after treatment with FOH (20 μM). Expression of each gene in the wild-type strain, in the absence of treatment, was set to 1. Symbol legends are in the box on the right. (B) RT-qPCR analysis of CDR2, RTA3, orf19.320, and PDR16 mRNA expression in wild-type (yLM660) and cdr1 null (yLM708) strains treated with 50 μM farnesyl acetate (FAc) or 50 μM FOH. Expression of each gene in the wild-type strain, in the absence of treatment, was set to 1. Symbol legends are in the box on the right. (C) RT-qPCR analysis of orf19.344 mRNA expression in TAC1/ZNC1 wild-type (WT; yLM660 and yLM708) and tac1Δ/Δ znc1Δ/Δ (yLM664 and yLM711) strains, derived from a wild-type or cdr1Δ/Δ background, after treatment with increasing concentrations of FOH. Expression of orf19.344 in the wild-type strain (yLM660), in the absence of treatment, was set to 1. (D) RT-qPCR analysis of orf19.320 mRNA expression in strains carrying a wild-type (TAC1WT; yLM167) or a GOF mutant TAC1 allele (TAC1A736V; yLM169) treated with 16 μM FOH. Expression of orf19.320 in the TAC1WT strain, in the absence of treatment, was set to 1.
Cdr1 facilitates C. albicans and C. dubliniensis resistance to FOH exposure.
C. albicans is able to tolerate significantly higher levels of exogenous FOH than other ascomycetes through a mechanism that is not entirely understood (13, 14, 24). To determine whether the CDR1 transcriptional response pathway described above could detoxify FOH, we investigated the role of Cdr1 in C. albicans FOH survival. Under the conditions tested, our C. albicans wild-type strain did not exhibit a major decrease in colony size when grown on YPD agar containing 200 μM FOH (Table 1). The growth of a cdr1 deletion strain, however, was compromised or completely inhibited at 50 or 100 μM FOH (Table 1). Colony growth, within the FOH concentration range tested, was not affected in a cdr2 null strain. FOH tolerance, however, was further decreased in a cdr1Δ/Δ cdr2Δ/Δ strain versus a cdr1Δ/Δ strain. Consistent with this finding, deletion of tac1 further sensitizes a cdr1 null mutant to FOH, while TAC1 GOF mutants, which overexpress Cdr2, increase FOH tolerance in a cdr1Δ/Δ strain in a manner that is dependent on CDR2 (Table 1). A killing assay was performed to determine if FOH inhibits growth of cdr1 deletion mutants through a fungistatic or fungicidal effect. The cdr1Δ/Δ cdr2Δ/Δ strain showed decreased viability upon treatment with 200 μM FOH, as only ∼25% of mutant cells survived after 6 h of treatment (Fig. 10A). Identical treatment with vehicle had no effect on viability (Fig. S6A) The viability of the cdr1Δ/Δ cdr2Δ/Δ and cdr1Δ/Δ strains decreases at a similar rate during the first 6 h of FOH exposure. Deletion of the transcription factors that drive FOH induction of CDR1 and CDR2 had a lesser impact on viability after FOH exposure than complete deletion of CDR1 and CDR2 (Fig. 10A). Deletion of tac1, znc1, and mrr2 individually, or tac1 znc1 simultaneously, does not affect FOH tolerance (Table 1). The tac1Δ/Δ znc1Δ/Δ mrr2Δ/Δ strain, however, showed mildly compromised colony formation and decreased cell growth upon FOH exposure (Table 1 and Fig. 10B). There was no difference in growth sensitivity of the wild-type and the cdr1Δ/Δ cdr2Δ/Δ strains to SDS, indicating that the mutants did not impact the membrane integrity of the cells (Fig. S6B).
TABLE 1.
Relative colony radius of C. albicans strains grown on farnesol-containing YPD agara
| Strainb | Vehicle | [FOH] (μM) |
|||
|---|---|---|---|---|---|
| 25 | 50 | 100 | 200 | ||
| Wild type | ++++ | +++ | +++ | +++ | +++ |
| cdr1Δ/Δ | ++++ | +++ | + | NG | NG |
| cdr2Δ/Δ | ++++ | +++ | +++ | +++ | +++ |
| cdr1Δ/Δ cdr2Δ/Δ | ++++ | ++ | NG | NG | NG |
| tac1Δ/Δ | ++++ | +++ | +++ | +++ | +++ |
| tac1Δ/Δ cdr1Δ/Δ | ++++ | +++ | NG | NG | NG |
| znc1Δ/Δ | ++++ | +++ | +++ | +++ | +++ |
| znc1Δ/Δ cdr1Δ/Δ | ++++ | +++ | + | NG | NG |
| tac1Δ/Δ znc1Δ/Δ | ++++ | +++ | +++ | +++ | +++ |
| tac1Δ/Δ znc1Δ/Δ cdr1Δ/Δ | ++++ | +++ | NG | NG | NG |
| mrr2Δ/Δ | ++++ | +++ | +++ | +++ | +++ |
| tac1Δ/Δ znc1Δ/Δ mrr2Δ/Δ | ++++ | +++ | +++ | ++ | ++ |
| TAC1WT | ++++ | +++ | +++ | +++ | +++ |
| TAC1WT cdr1Δ/Δ | ++++ | +++ | ++ | NG | NG |
| TAC1WT cdr1Δ/Δ cdr2Δ/Δ | ++++ | +++ | NG | NG | NG |
| TAC1A736V | ++++ | +++ | +++ | +++ | +++ |
| TAC1A736V cdr1Δ/Δ | ++++ | +++ | +++ | +++ | +++ |
| TAC1A736V cdr1Δ/Δ cdr2Δ/Δ | ++++ | +++ | NG | NG | NG |
| TAC1N977D | ++++ | +++ | +++ | +++ | +++ |
| TAC1N977D cdr1Δ/Δ | ++++ | +++ | +++ | +++ | +++ |
| TAC1N977D cdr1Δ/Δ cdr2Δ/Δ | ++++ | +++ | NG | NG | NG |
Each entry in the table represents the colony size observed for a particular combination of strain and treatment. The average radius of colonies formed by a wild-type reference strain (wild type or TAC1WT) on non-FOH-containing plates (vehicle) was set to 100% to normalize colony size of its derivative mutants under each growth condition. Data were symbolized by the following transformation: 90 to 110%, ++++; 70 to 90%, +++; 50 to 70%, ++; 30 to 50%, +. NG, no visible colony formation was seen after 40 h of incubation at 30°C.
Strains tested in this table are marked by their genotypic feature in Table S2. Strains (and their growth data) are divided (horizontal line) with respect to their parental wild-type background (designated wild type or TAC1WT).
FIG 10.
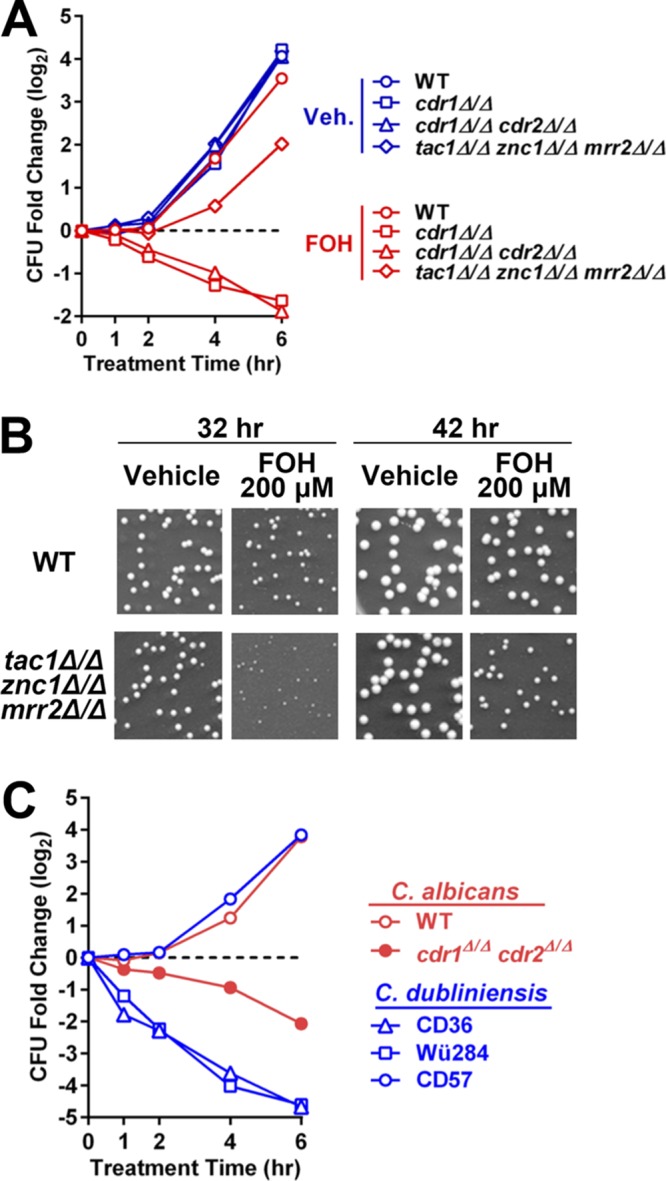
Dependence of C. albicans and C. dubliniensis farnesol sensitivity on CDR1 expression. (A) Colony formation analysis comparing cell viability between wild-type and cdr1 null strains after 1 to 6 h of FOH or vehicle (methanol) exposure. Wild-type (yLM660), cdr1 null (yLM708), cdr1 cdr2 double deletion (yLM710), and tac1 znc1 mrr2 triple deletion (yLM702) strains were each diluted from overnight YPD cultures and treated with 200 μM FOH in YPD medium. Aliquots, after an appropriate dilution (if needed), were spread on YPD plates at the indicated time points. Fold change in CFU was calculated by setting the CFU before treatment (not shown) to 1 and is presented in a logarithmic form (base 2). (B) Representative plate scans showing a high FOH concentration in agar medium reduces colony growth of a tac1Δ/Δ znc1Δ/Δ mrr2Δ/Δ strain. Dilutions of a wild-type strain (yLM660) and a tac1 znc1 mrr2 triple deletion mutant (yLM702) from overnight cultures were plated on YPD agar supplemented with 200 μM FOH or the same volume of vehicle (methanol). Plates were imaged after incubation for the indicated amounts of time at 30°C. (C) Colony formation analysis comparing cell viability between three C. dubliniensis strains (CD36, Wü284, and CD57) after 1 to 6 h of FOH or vehicle (methanol) exposure. Wild-type (yLM660) and cdr1 cdr2 double deletion (yLM710) C. albicans strains were tested in parallel as a control. The strains were each diluted from overnight YPD cultures and treated with 200 μM FOH in YPD medium. Aliquots, after an appropriate dilution (if needed), were spread on YPD plates at the indicated time points. Fold change in CFU was calculated by setting the CFU before treatment (not shown) to 1 and is presented in a logarithmic form (base 2).
The C. dubliniensis genome-sequencing strain CD36, a close relative of C. albicans, has been reported to have much higher sensitivity to FOH-induced cell death (24). It is also known that multiple C. dubliniensis strains within genotype group I, including CD36, do not express functional full-length Cdr1 protein due to a homozygous variant that creates a stop codon at CDR1 amino acid 756 (38). All cdr1756stop/756stop C. dubliniensis strains (CD36, CD38, and Wü284) we tested showed complete or partial growth inhibition by 50 μM FOH (Table 2 and Fig. 10C). There are multiple CDR1+/+ C. dubliniensis strains (such as CD57), and we have observed that they exhibit resistance comparable to that of C. albicans at FOH levels as high as 200 μM (Table 2 and Fig. 10C). Additionally, we have found that deletion of cdr1 sensitizes CD57 to farnesol, while deletion of cdr1 in the cdr1756stop/756stop strain Wü284 does not further sensitize it to farnesol (Table 2).
TABLE 2.
Relative colony radius of C. dubliniensis strains grown on farnesol-containing YPD agara
| Strainb | Vehicle | [FOH] (μM) |
|||
|---|---|---|---|---|---|
| 25 | 50 | 100 | 200 | ||
| CD36 (I; cdr1756stop/cdr1756stop) | ++++ | +++ | ++ | NG | NG |
| CD38 (I; cdr1756stop/cdr1756stop) | ++++ | ++ | + | NG | NG |
| Wü284 (I; cdr1756stop/cdr1756stop) | ++++ | ++ | NG | NG | NG |
| Wü284 cdr1Δ/Δ | ++++ | ++ | NG | NG | NG |
| CD57 (I; CDR1/CDR1) | ++++ | +++ | +++ | +++ | +++ |
| CD57 cdr1Δ/Δ | ++++ | ++ | ++ | ++ | ++ |
| CD57 tac1Δ/Δ | ++++ | +++ | +++ | +++ | +++ |
| CD57 tac1Δ/Δ znc1Δ/Δ | ++++ | +++ | +++ | +++ | +++ |
| CM1 (I; CDR1/CDR1) | ++++ | +++ | +++ | +++ | +++ |
| CBS8500 (I; CDR1/CDR1) | ++++ | +++ | +++ | +++ | +++ |
| CD506 (II; CDR1/CDR1) | ++++ | ++ | ++ | ++ | +++ |
| CAN6 (II; CDR1/CDR1) | ++++ | +++ | +++ | +++ | +++ |
| p7718 (IV; CDR1/CDR1) | ++++ | +++ | +++ | +++ | +++ |
The average radius of colonies formed by each wild-type C. dubliniensis isolate on non-FOH-containing plates (vehicle) was set to 100% to normalize its colony size or its derivative mutants colony size under each growth condition. Data were symbolized by the following transformation: 90 to 110%, ++++; 70 to 90%, +++; 50 to 70%, ++; 30 to 50%, +. NG, no visible colony formation was seen after 40 h of incubation at 30°C.
The genotype group (I, II, or IV) of each C. dubliniensis isolate and the presence or absence of the cdr1756stop allele are denoted in parentheses. The genotype of each mutant tested in this table is described in detail in Table S2.
Tac1 and Znc1 mediate the CDR1 transcriptional FOH response in C. dubliniensis.
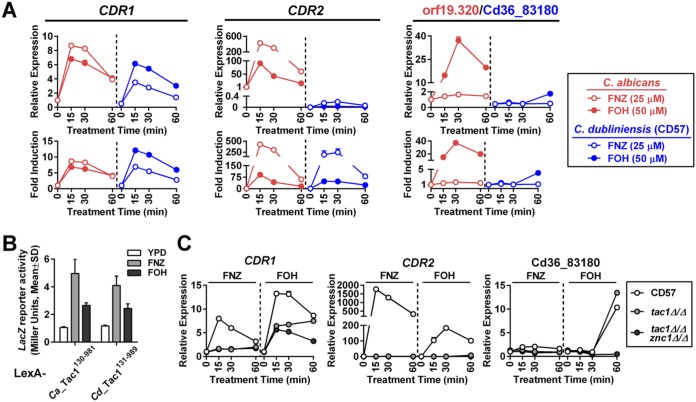
Our standard protocol showed that FOH induces CDR1 expression in CD57 with kinetics and amplitude similar to those of C. albicans (Fig. 11A). Although CD57 showed an extremely low expression level of C. dubliniensis CDR2 compared to that of its C. albicans ortholog, FOH treatment results in a comparable fold induction of CDR2 in the two species (Fig. 11A). The ortholog of orf19.320, a C. albicans Znc1-dependent promoter, in C. dubliniensis (Cd36.83180) had an expression pattern in which induction was only observed after 1 h of FOH treatment (Fig. 11A). A LexA-CdTac1 fusion protein, constructed in a fashion similar to that for the earlier LexA-CaTac1 fusion (Fig. 4), activated LacZ expression upon FOH and FNZ treatment when tested in a one-hybrid reporter assay in C. albicans (Fig. 11B). Representatives of the three different classes of FNZ/FOH-induced promoters defined in C. albicans, Tac1/Znc1 dependent (CDR1), Tac1 dependent (CDR2), and Znc1 dependent (Ca orf19.320/Cd36_83180), have a very similar dependence on these same transcription factors in C. dubliniensis (Fig. 11C). An exception was the observation that deletion of znc1 in the tac1Δ/Δ CD57 strain did not decrease FOH CDR1 induction as strongly as it does in C. albicans, suggesting the existence of another regulator(s) governing FOH induction of the C. dubliniensis CDR1 (CdCDR1) promoter.
FIG 11.
Tac1 and Znc1 regulate gene induction by FOH in C. dubliniensis. (A) RT-qPCR analysis comparing FOH induction of CDR1, CDR2, and orf19.320 orthologs in C. albicans and C. dubliniensis. A wild-type C. albicans strain (yLM660) and a C. dubliniensis isolate (CD57) were treated with 25 μM FNZ and 50 μM FOH in YPD culture for the indicated amount of time before collection for RNA extraction. Expression of each pair of orthologs was measured by pan-primers and compared across species by setting the basal expression in the C. albicans strain to 1 (relative expression; upper). Gene fold induction in each species is shown in the lower panels by setting gene basal expression in yLM660 and CD57 individually to 1. (B) LacZ reporter activation by CdTac1 under FNZ and FOH treatment conditions. C. albicans one-hybrid strains expressing LexA-CdTac1131–989 (yLM766) or LexA-CaTac1130–980 (yLM568) fusion proteins were treated with 25 μM FNZ or 50 μM FOH for 2 h and measured for β-galactosidase activity. (C) RT-qPCR analysis showing the effect of tac1 deletion and tac1 znc1 double deletion on FNZ and FOH induction of CDR1, CDR2, and Cd36.83180 in C. dubliniensis. CD57 and its tac1Δ/Δ (yLM764) and tac1Δ/Δ znc1Δ/Δ (yLM765) derivatives were treated with 25 μM FNZ and 50 μM FOH. Basal expression of each gene in CD57 was individually set to 1.
DISCUSSION
Our characterization of Tac1, Znc1, and Mrr2 as essential signal targets (direct or indirect) for farnesol provides a new framework for thinking about how the C. albicans cell coordinates its transcriptional response to the quorum-sensing molecule. The demonstration that multiple Zn cluster transcription factors can be activated by overlapping yet nonredundant small molecules reveals a previously underappreciated combinatorial complexity that allows these factors to regulate complex patterns of gene regulation. Additionally, the dependence of CDR1 expression on this transcriptional response, combined with the regulation of this response by the action of this important efflux pump, supports the mounting evidence for CDR1 serving as a farnesol transporter and suggests the presence of a negative feedback loop modulating its action.
Tac1 and Znc1 act as targets in Candida for farnesol.
Earlier work had indicated that CDR1 expression was upregulated upon treatment with farnesol (21, 22) and that Tac1 was an important regulator of CDR1 (25, 27). This work demonstrates that farnesol can activate an acute transcriptional response via the hyperactivation of Tac1 and Znc1. The kinetics and amplitude of this response are very similar to the well-characterized response of Tac1 to xenobiotics, such as fluphenazine and estradiol (28), and expands the range of C. albicans Tac1 hyperactivators into the realm of physiological small molecules. Our discovery that farnesol can lead to the upregulation of CDR1 through the hyperactivation of Znc1 reveals that the control of efflux pump expression is more complex than previously appreciated. The finding that Tac1 and Znc1 share overlapping but nonidentical targeting to sequences in the promoter elements in farnesol-induced genes extends our knowledge of how a diverse set of genes can be upregulated via farnesol. It is uncertain, at this point, whether farnesol activates Tac1 and Znc1 through a direct binding mechanism, similar to the activation of Pdr1 in C. glabrata by azoles (36), or through an indirect mechanism, such as posttranslational modification. The observed subcellular localization of exogenously added farnesol includes nuclear enrichment (39), which would allow farnesol to hyperactivate Tac1 and Znc1 through direct binding. Parallels have previously be drawn between the mechanism of action of the C. glabrata Pdr1 zinc cluster transcription factor and mammalian nuclear receptors (36, 40), and it is interesting that the mammalian bile acid receptor FXR (farnesoid X-activated receptor) is also activated by farnesol (41). However, strong physiological FXR agonists, such as chenodeoxycholic acid (CDCA) and deoxycholic acid (DA) (42, 43), do not activate CDR1 expression in C. albicans (see Fig. S1 in the supplemental material), suggesting the lack of a broader overlap between Tac1/Znc1 and mammalian FXR inducers. If direct binding of inducers to the Zn cluster transcription factors is the mechanism of hyperactivation, the structural differences between Tac1 inducers (farnesol, dodecanol, fluphenazine, and estradiol) suggest a low-affinity/specificity interaction similar to that observed for the FXR receptor. The observation that Znc1 can respond to farnesol (and dodecanol) but not fluphenazine indicates that it is possible for the Zn cluster transcription factors to make structural distinctions between these molecules. Although we cannot rule out posttranslational modification as a mechanism, the absence of a mobility shift in Znc1 upon farnesol treatment compared to the hyperactive Tac1 phosphorylation mobility shift (30) suggests that phosphorylation is not a requirement for farnesol activation of Tac1 and Znc1.
Mode of Tac1- and Znc1-DNA interaction.
In fungi, paralogous zinc cluster transcription factors have been reported to form both homo- and heterodimers, for example, Pdr1/Pdr3 (44) and Oaf1/Pip2 (45, 46) in Saccharomyces cerevisiae. Our observation of distinct binding of Tac1 and Znc1 at promoters where they show nonredundant activation, as well as the binding of Znc1 to promoters in a tac1 null strain, does not support the idea that Tac1 and Znc1 bind promoters as a stable heterodimer. The previous observation that Tac1 requires the presence of both CGG triplet elements in a DRE for gene activation (26) largely rules out the possibility that monomeric Tac1 and Znc1 each bind to one CGG triplet in a single DRE, a mode of DNA binding infrequently observed for zinc cluster transcription factors (47). The evidence presented here supports a model where Tac1 homodimers and Znc1 homodimers competitively bind to coregulated promoters at a single paired CGG triplet element. Given the variation in DRE and PZM sequences at Tac1-dependent promoters (i.e., CDR2) and Znc1-dependent promoters (i.e., orf19.320), it is likely that Tac1 and Znc1 have overlapping but nonidentical sequence specificity. The DNA binding specificity of zinc cluster transcription factors, namely, the sequence between and surrounding the CGG triplet(s), is thought to be determined by the amino acid sequence in the linker region between the zinc clusters and the dimerization domain (48–50). Divergence in Tac1 and Znc1 in their linker regions may contribute to their different occupancies at the DREs and PZMs. Since it has previously been noted that Tac1 binds to DREs regardless of promoter status (35), it was reasonable to think that hyperactivation of Tac1 did not work by a mechanism that increased DNA binding of the transcription factor. The ChIP analysis shows, however, that DRE/PZM occupancy is clearly increased in an inducer-specific manner. It is unclear whether this reflects a direct increase in binding affinity of the transcription factor or the formation of a more stable transcription factor complex involving interactions with coactivators and enhanced chromatin remodeling.
The complex regulation of the CDR1 promoter.
The new regulatory mechanisms revealed by our study further demonstrate the complexity of the CDR1 promoter. Previous studies of activation of the CDR1 promoter focused on gain-of-function mutants in Tac1 or xenobiotic hyperactivation of Tac1 (25, 26, 28, 51). Our study now adds farnesol and 1-dodecanol treatment to the limited number of conditions where CDR1 expression can be induced chemically in the absence of Tac1 (37). Tac1-independent CDR1 induction by farnesol can be largely attributed to Znc1 (and Mrr2) function. Interestingly, unlike Tac1 (27) and Mrr2 (52), no gain-of-function mutants of Znc1 have been reported to drive azole resistance in C. albicans. Of the well-characterized CDR1 inducers, farnesol is the only one considered to be a C. albicans metabolite.
Identification of Tac1 and Znc1 as farnesol-induced transcription activators of the CDR1 promoter allowed us to test whether the proposed CDR1-mediated farnesol efflux (22) provided feedback to the transcriptional response. The amplification of the Tac1- and Znc1-driven transcriptional response to farnesol in cells lacking Cdr1 function, as well as the dampening of the Znc1-driven transcriptional response to farnesol in TAC1 gain-of-function cells that overexpress CDR1 (Fig. 9), support the idea that Cdr1 serves to regulate intracellular levels of farnesol via an efflux mechanism. However, the observation that the transcriptional response to farnesol still exhibits attenuation after rapid induction in a cdr1 null strain (Fig. 9) indicates that there are additional farnesol transporters in C. albicans. In contrast to this response, the plateau observed for the transcriptional response to farnesyl acetate in cdr1 null strain indicates that Cdr1 is the sole efflux pump for farnesyl acetate (Fig. 9B).
The role of CDR1 in modulating farnesol sensitivity.
Although the phenotypic regulation of C. albicans by farnesol is not the central focus of the work presented here, the suggested circuit involving farnesol, Zn cluster transcription factors, and Cdr1 prompted us to begin to investigate how these factors might interact to influence Candida biology. Under our experimental conditions (cells grown in YPD), as well as synthetic medium conditions tested in several other studies (53–55), farnesol concentrations as high as 300 μM showed minor effects on the growth or viability of wild-type C. albicans. These concentrations of farnesol are typically toxic to most other fungi (14, 24). Our analysis of farnesol toxicity to C. albicans and C. dubliniensis strains with and without functional Cdr1 consistently suggests Cdr1-mediated farnesol efflux plays a protective role under these growth conditions (Fig. 10 and Tables 1 and 2). It is somewhat surprising that transcription factor mutants in C. albicans and C. dubliniensis do not show a more dramatic change in farnesol sensitivity given the decrease in CDR1 induction. It is possible that expression of other genes that influence farnesol sensitivity was affected by tac1, znc1, and/or mrr2 deletion in a way that compensated for the compromised CDR1 induction in these mutants. Elsewhere it has been reported that farnesol treatment under a non-medium condition (phosphate-buffered saline) induced apoptosis in C. albicans through a Cdr1-dependent mechanism (22), suggesting Cdr1 regulates C. albicans farnesol sensitivity in either direction depending on the treatment condition. Since activity of Cdr1, an ATP-dependent transporter, is strongly affected by cellular energy status (56, 57), the availability of nutrients may affect the role of Cdr1 during farnesol exposure.
The results of this study lead to the ability to ask new questions about both the general role and mechanism of zinc cluster transcription factors in the response to physiological fungal metabolites, as well as the action of farnesol as a quorum-sensing molecule. For instance, how do Tac1 and Znc1 achieve specificity in their response to farnesol? Are there other metabolite ligands? Do the other genes in the Tac1/Znc1 farnesol regulon play an important role in quorum sensing? It has been observed that an increase in CDR1 and CDR2 expression in sessile C. albicans cells compared to that in planktonic cells (58, 59) contributes to drug resistance in early C. albicans biofilm, in cooperation with the upregulation of the major facilitator transporter, MDR1 (60). The mechanism underlying the induction of these pumps in biofilm has not been clarified. Farnesol induction of CDR1 and CDR2 suggests accumulation of a quorum-sensing molecule favored by static growth as a possible answer.
MATERIALS AND METHODS
Strains and plasmids.
Strains and plasmids used in this study are listed in Tables S2 and S3, respectively, in the supplemental material. Construction of the strains and plasmids are described in detail in the supplemental material. Primers used for generating the strains and plasmids are listed in Table S4. C. albicans transformation was performed by electroporation and selected by Clonat resistance (1% yeast extract, 2% peptone, 2% glucose, 2% agar, 0.1 mM uridine, 100 μg/ml Clonat) or complementation of auxotrophy (6.7 g/liter Difco YNB without amino acids [BD], appropriately supplemented with 1.5 g/liter drop-out mix synthetic medium lacking uracil, histidine, arginine, andleucine [US Biological] and with 2% glucose and 2% agar). Expression of flippase was induced by growth in YPMal (1% yeast extract, 2% peptone, 2% maltose, 0.1 mM uridine) liquid medium for 24 h. Successful eviction of the SAT1 marker was selected by sensitivity to 100 μg/ml Clonat.
Cell growth and drug treatment.
Cells were grown in liquid YPD medium (1% yeast extract, 2% peptone, 2% glucose, 0.1 mM uridine) at 30°C if not specified otherwise. Drug treatment was performed by adding fluphenazine (6 mg/ml aqueous stock; Alfa Aesar), farnesol (mixed isoforms; biweekly 50 mM or fresh 200 mM methanolic dilution; MP Bio), 1-dodecanol (50 mM methanolic dilution; Sigma), farnesyl acetate (50 mM methanolic dilution; Fluka [mixed isoforms]), geraniol (50 mM methanolic dilution; Sigma), tryptophol (50 mM methanolic stock; Sigma), tyrosol (50 mM aqueous stock; Alfa Aesar), chenodeoxycholic acid (50 mM dimethyl sulfoxide [DMSO] stock; Cayman), or deoxycholic acid (50 mM DMSO stock; Fisher) into mid-log-phase C. albicans or C. dubliniensis culture to the final concentration specified for each experiment in the figure legends. To test cell growth in the presence of farnesol, an overnight culture, after appropriate dilution, was spread on YPD plates supplemented with each concentration of FOH or the same volume of methanol. Colony number and size were analyzed by OpenCFU (61) after 40 h of incubation at 30°C. In farnesol killing assays, an overnight culture of each strain to be tested was diluted to an optical density (OD) of 0.05 in fresh YPD medium for treatment with 200 μM farnesol or the same volume of methanol. Aliquots taken at each of the indicated time points, after an appropriate dilution (if needed), were plated on YPD agar (no farnesol). Colony number was counted after 40 h of incubation at 30°C.
Reverse transcription-quantitative PCR (RT-qPCR).
RNA samples were prepared from collected frozen cell pellets and reverse transcribed as described previously (62). qPCR was performed using the relative standard curve method (StepOne; Life Technologies). ACT1 abundance, measured by ZL712/ZL713, was used as an internal reference to compare CDR1 (ZL540/ZL541), CDR2 (ZL542/ZL543), RTA3 (ZL544/ZL545), orf19.320 (ZL951/ZL958), IFD1 (ZL823/ZL824), PDR16 (M2PT-1/M2PT-2), orf19.7042 (M2PT-23/M2PT-24), orf19.344 (M2PT-15/M2PT-16), or expression across strains and conditions. ZL540/ZL541, ZL1008/ZL1009, and ZL951/ZL958 were used as respective pan-primers to compare expression and induction of CDR1, CDR2, and orf19.320 homologs in C. albicans and C. dubliniensis.
Immunoblotting.
Immunoblot analysis was used to compare Cdr1-3HA expression or 6His3Flag-Tac1 or Znc1-3HA gel mobility. Cell lysates were prepared, resolved by SDS-PAGE, and probed by an α-HA (3F10; Roche) or α-Flag (F7425; Sigma) antibody as described previously (30). A lower-molecular-weight region of a gel, which typically did not contain the immunoblotting signals of interest in this study, was stained by Coomassie blue as the loading reference.
ChIP.
ChIP experiments were performed as described previously (30) to analyze 6His3Flag-Tac1, Znc1-3HA, and Mediator (Med17-3HA) occupancy at target promoters. Results of ChIP experiments are presented in the form of relative recovery of input. The absolute recovery at the CDR1 promoter 1-up region in a non-farnesol-treated untagged strain (as specified in the figure legends) was set to 1 to normalize recoveries at other promoter regions across conditions. Primers used in the ChIP assay are listed in Table S4, with their probing region denoted.
Liquid β-galactosidase activity assays.
C. albicans one-hybrid strains were diluted from overnight culture, grown for 3 to 4 h in fresh YPD medium, and treated with fluphenazine (∼25 μM), farnesol (50 μM), 1-dodecanol (50 μM), or methanol for 1 h or 3 h before collection for β-galactosidase activity measurement by an SDS-chloroform method (30, 63). β-Galactosidase activity, in Miller units, was calculated by the following simplified formula: 1,000 × A420/(T × C), where A420 is the absorbance of the reaction product at 420 nm, T is the reaction time in minutes, and C is the total amounts of cells in total OD at 600 nm (OD600) used in the reaction. A420 and OD600 values were measured with a Beckman Coulter DU-7300 spectrophotometer. The activity of each LexA fusion protein was tested in at least three confirmed transformants.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by NIH grants 5R21AI113390 and 5R21AI115253 to L.C.M.
We thank Deborah Hogan and the Hogan laboratory for sharing their expertise and reagents relating to farnesol biology. We also thank Gary Moran for providing strains.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00968-18.
REFERENCES
- 1.Pfaller MA, Diekema DJ. 2010. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 2.Martins N, Ferreira IC, Barros L, Silva S, Henriques M. 2014. Candidiasis: predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia 177:223–240. doi: 10.1007/s11046-014-9749-1. [DOI] [PubMed] [Google Scholar]
- 3.Antinori S, Milazzo L, Sollima S, Galli M, Corbellino M. 2016. Candidemia and invasive candidiasis in adults: a narrative review. Eur J Intern Med 34:21–28. doi: 10.1016/j.ejim.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Martin R, Albrecht-Eckardt D, Brunke S, Hube B, Hunniger K, Kurzai O. 2013. A core filamentation response network in Candida albicans is restricted to eight genes. PLoS One 8:e58613. doi: 10.1371/journal.pone.0058613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudbery PE. 2011. Growth of Candida albicans hyphae. Nat Rev Microbiol 9:737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- 6.Nobile CJ, Johnson AD. 2015. Candida albicans biofilms and human disease. Annu Rev Microbiol 69:71–92. doi: 10.1146/annurev-micro-091014-104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindsay AK, Hogan DA. 2014. Candida albicans: molecular interactions with Pseudomonas aeruginosa and Staphylococcus aureus. Fungal Biol Rev 28:85–96. doi: 10.1016/j.fbr.2014.10.002. [DOI] [Google Scholar]
- 8.Dantas Ada S, Day A, Ikeh M, Kos I, Achan B, Quinn J. 2015. Oxidative stress responses in the human fungal pathogen, Candida albicans. Biomolecules 5:142–165. doi: 10.3390/biom5010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, Dussault P, Nickerson KW. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol 67:2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL. 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol 68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Kohler JR, Kadosh D, Lopez-Ribot JL. 2010. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog 6:e1000828. doi: 10.1371/journal.ppat.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deveau A, Piispanen AE, Jackson AA, Hogan DA. 2010. Farnesol induces hydrogen peroxide resistance in Candida albicans yeast by inhibiting the Ras-cyclic AMP signaling pathway. Eukaryot Cell 9:569–577. doi: 10.1128/EC.00321-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nickerson KW, Atkin AL. 2017. Deciphering fungal dimorphism: farnesol's unanswered questions. Mol Microbiol 103:567–575. doi: 10.1111/mmi.13601. [DOI] [PubMed] [Google Scholar]
- 14.Albuquerque P, Casadevall A. 2012. Quorum sensing in fungi–a review. Med Mycol 50:337–345. doi: 10.3109/13693786.2011.652201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber K, Schulz B, Ruhnke M. 2010. The quorum-sensing molecule E,E-farnesol–its variable secretion and its impact on the growth and metabolism of Candida species. Yeast 27:727–739. doi: 10.1002/yea.1769. [DOI] [PubMed] [Google Scholar]
- 16.Weber K, Sohr R, Schulz B, Fleischhacker M, Ruhnke M. 2008. Secretion of E,E-farnesol and biofilm formation in eight different Candida species. Antimicrob Agents Chemother 52:1859–1861. doi: 10.1128/AAC.01646-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis-Hanna A, Piispanen AE, Stateva LI, Hogan DA. 2008. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol 67:47–62. doi: 10.1111/j.1365-2958.2007.06013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall RA, Turner KJ, Chaloupka J, Cottier F, De Sordi L, Sanglard D, Levin LR, Buck J, Muhlschlegel FA. 2011. The quorum-sensing molecules farnesol/homoserine lactone and dodecanol operate via distinct modes of action in Candida albicans. Eukaryot Cell 10:1034–1042. doi: 10.1128/EC.05060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindsay AK, Deveau A, Piispanen AE, Hogan DA. 2012. Farnesol and cyclic AMP signaling effects on the hypha-to-yeast transition in Candida albicans. Eukaryot Cell 11:1219–1225. doi: 10.1128/EC.00144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao YY, Cao YB, Xu Z, Ying K, Li Y, Xie Y, Zhu ZY, Chen WS, Jiang YY. 2005. cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Antimicrob Agents Chemother 49:584–589. doi: 10.1128/AAC.49.2.584-589.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt M, Barker S, Essmann M, Larsen B. 2008. Effect of commonly used herbicides on the virulence factor CDR1 in Candida albicans. Environ Toxicol Chem 27:2346–2351. doi: 10.1897/08-060.1. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Krom BP, Sanglard D, Intapa C, Dawson CC, Peters BM, Shirtliff ME, Jabra-Rizk MA. 2011. Farnesol-induced apoptosis in Candida albicans is mediated by Cdr1-p extrusion and depletion of intracellular glutathione. PLoS One 6:e28830. doi: 10.1371/journal.pone.0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uppuluri P, Mekala S, Chaffin WL. 2007. Farnesol-mediated inhibition of Candida albicans yeast growth and rescue by a diacylglycerol analogue. Yeast 24:681–693. doi: 10.1002/yea.1501. [DOI] [PubMed] [Google Scholar]
- 24.Jabra-Rizk MA, Shirtliff M, James C, Meiller T. 2006. Effect of farnesol on Candida dubliniensis biofilm formation and fluconazole resistance. FEMS Yeast Res 6:1063–1073. doi: 10.1111/j.1567-1364.2006.00121.x. [DOI] [PubMed] [Google Scholar]
- 25.Coste AT, Karababa M, Ischer F, Bille J, Sanglard D. 2004. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot Cell 3:1639–1652. doi: 10.1128/EC.3.6.1639-1652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coste AT, Crittin J, Bauser C, Rohde B, Sanglard D. 2009. Functional analysis of cis- and trans-acting elements of the Candida albicans CDR2 promoter with a novel promoter reporter system. Eukaryot Cell 8:1250–1267. doi: 10.1128/EC.00069-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanglard D, Coste A, Ferrari S. 2009. Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Res 9:1029–1050. doi: 10.1111/j.1567-1364.2009.00578.x. [DOI] [PubMed] [Google Scholar]
- 28.de Micheli M, Bille J, Schueller C, Sanglard D. 2002. A common drug-responsive element mediates the upregulation of the Candida albicans ABC transporters CDR1 and CDR2, two genes involved in antifungal drug resistance. Mol Microbiol 43:1197–1214. doi: 10.1046/j.1365-2958.2002.02814.x. [DOI] [PubMed] [Google Scholar]
- 29.Schillig R, Morschhauser J. 2013. Analysis of a fungus-specific transcription factor family, the Candida albicans zinc cluster proteins, by artificial activation. Mol Microbiol 89:1003–1017. doi: 10.1111/mmi.12327. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Myers LC. 2017. Mediator tail module is required for Tac1-activated CDR1 expression and azole resistance in Candida albicans. Antimicrob Agents Chemother 61:e01342-17. doi: 10.1128/AAC.01342-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shchepin R, Hornby JM, Burger E, Niessen T, Dussault P, Nickerson KW. 2003. Quorum sensing in Candida albicans: probing farnesol's mode of action with 40 natural and synthetic farnesol analogs. Chem Biol 10:743–750. doi: 10.1016/S1074-5521(03)00158-3. [DOI] [PubMed] [Google Scholar]
- 32.Hogan DA, Vik A, Kolter R. 2004. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol 54:1212–1223. doi: 10.1111/j.1365-2958.2004.04349.x. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Fujita M, Feng Q, Clardy J, Fink GR. 2004. Tyrosol is a quorum-sensing molecule in Candida albicans. Proc Natl Acad Sci U S A 101:5048–5052. doi: 10.1073/pnas.0401416101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Fink GR. 2006. Feedback control of morphogenesis in fungi by aromatic alcohols. Genes Dev 20:1150–1161. doi: 10.1101/gad.1411806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu TT, Znaidi S, Barker KS, Xu L, Homayouni R, Saidane S, Morschhauser J, Nantel A, Raymond M, Rogers PD. 2007. Genome-wide expression and location analyses of the Candida albicans Tac1p regulon. Eukaryot Cell 6:2122–2138. doi: 10.1128/EC.00327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thakur JK, Arthanari H, Yang F, Pan SJ, Fan X, Breger J, Frueh DP, Gulshan K, Li DK, Mylonakis E, Struhl K, Moye-Rowley WS, Cormack BP, Wagner G, Naar AM. 2008. A nuclear receptor-like pathway regulating multidrug resistance in fungi. Nature 452:604–609. doi: 10.1038/nature06836. [DOI] [PubMed] [Google Scholar]
- 37.Schneider S, Morschhauser J. 2015. Induction of Candida albicans drug resistance genes by hybrid zinc cluster transcription factors. Antimicrob Agents Chemother 59:558–569. doi: 10.1128/AAC.04448-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moran G, Sullivan D, Morschhauser J, Coleman D. 2002. The Candida dubliniensis CdCDR1 gene is not essential for fluconazole resistance. Antimicrob Agents Chemother 46:2829–2841. doi: 10.1128/AAC.46.9.2829-2841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shchepin R, Dumitru R, Nickerson KW, Lund M, Dussault PH. 2005. Biologically active fluorescent farnesol analogs. Chem Biol 12:639–641. doi: 10.1016/j.chembiol.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Thakur JK, Arthanari H, Yang F, Chau KH, Wagner G, Naar AM. 2009. Mediator subunit Gal11p/MED15 is required for fatty acid-dependent gene activation by yeast transcription factor Oaf1p. J Biol Chem 284:4422–4428. doi: 10.1074/jbc.M808263200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. 1995. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 42.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. 1999. Identification of a nuclear receptor for bile acids. Science 284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 43.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. 1999. Bile acids: natural ligands for an orphan nuclear receptor. Science 284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 44.Mamnun YM, Pandjaitan R, Mahe Y, Delahodde A, Kuchler K. 2002. The yeast zinc finger regulators Pdr1p and Pdr3p control pleiotropic drug resistance (PDR) as homo- and heterodimers in vivo. Mol Microbiol 46:1429–1440. doi: 10.1046/j.1365-2958.2002.03262.x. [DOI] [PubMed] [Google Scholar]
- 45.Trzcinska-Danielewicz J, Ishikawa T, Micialkiewicz A, Fronk J. 2008. Yeast transcription factor Oaf1 forms homodimer and induces some oleate-responsive genes in absence of Pip2. Biochem Biophys Res Commun 374:763–766. doi: 10.1016/j.bbrc.2008.07.105. [DOI] [PubMed] [Google Scholar]
- 46.Rottensteiner H, Kal AJ, Hamilton B, Ruis H, Tabak HF. 1997. A heterodimer of the Zn2Cys6 transcription factors Pip2p and Oaf1p controls induction of genes encoding peroxisomal proteins in Saccharomyces cerevisiae. Eur J Biochem 247:776–783. doi: 10.1111/j.1432-1033.1997.00776.x. [DOI] [PubMed] [Google Scholar]
- 47.Cahuzac B, Cerdan R, Felenbok B, Guittet E. 2001. The solution structure of an AlcR-DNA complex sheds light onto the unique tight and monomeric DNA binding of a Zn(2)Cys(6) protein. Structure 9:827–836. doi: 10.1016/S0969-2126(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 48.Reece RJ, Ptashne M. 1993. Determinants of binding-site specificity among yeast C6 zinc cluster proteins. Science 261:909–911. doi: 10.1126/science.8346441. [DOI] [PubMed] [Google Scholar]
- 49.Mamane Y, Hellauer K, Rochon MH, Turcotte B. 1998. A linker region of the yeast zinc cluster protein leu3p specifies binding to everted repeat DNA. J Biol Chem 273:18556–18561. doi: 10.1074/jbc.273.29.18556. [DOI] [PubMed] [Google Scholar]
- 50.Johnston M, Dover J. 1987. Mutations that inactivate a yeast transcriptional regulatory protein cluster in an evolutionarily conserved DNA binding domain. Proc Natl Acad Sci U S A 84:2401–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coste A, Turner V, Ischer F, Morschhauser J, Forche A, Selmecki A, Berman J, Bille J, Sanglard D. 2006. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics 172:2139–2156. doi: 10.1534/genetics.105.054767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Liu JY, Shi C, Li WJ, Zhao Y, Yan L, Xiang MJ. 2015. Mutations in transcription factor Mrr2p contribute to fluconazole resistance in clinical isolates of Candida albicans. Int J Antimicrob Agents 46:552–559. doi: 10.1016/j.ijantimicag.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Hala G, Rachel G, Arthur B, Oceane V, Anne-Laure B, Jean-Michel C, Francoise A. 2014. Changes in glutathione-dependent redox status and mitochondrial energetic strategies are part of the adaptive response during the filamentation process in Candida albicans. Free Radic Biol Med 75(Suppl 1):S22. doi: 10.1016/j.freeradbiomed.2014.10.736. [DOI] [PubMed] [Google Scholar]
- 54.Langford ML, Hasim S, Nickerson KW, Atkin AL. 2010. Activity and toxicity of farnesol towards Candida albicans are dependent on growth conditions. Antimicrob Agents Chemother 54:940–942. doi: 10.1128/AAC.01214-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polke M, Sprenger M, Scherlach K, Alban-Proano MC, Martin R, Hertweck C, Hube B, Jacobsen ID. 2017. A functional link between hyphal maintenance and quorum sensing in Candida albicans. Mol Microbiol 103:595–617. doi: 10.1111/mmi.13526. [DOI] [PubMed] [Google Scholar]
- 56.Szczepaniak J, Lukaszewicz M, Krasowska A. 2015. Detection of inhibitors of Candida albicans Cdr transporters using a diS-C3(3) fluorescence. Front Microbiol 6:176. doi: 10.3389/fmicb.2015.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wada S, Niimi M, Niimi K, Holmes AR, Monk BC, Cannon RD, Uehara Y. 2002. Candida glabrata ATP-binding cassette transporters Cdr1p and Pdh1p expressed in a Saccharomyces cerevisiae strain deficient in membrane transporters show phosphorylation-dependent pumping properties. J Biol Chem 277:46809–46821. doi: 10.1074/jbc.M207817200. [DOI] [PubMed] [Google Scholar]
- 58.Ramage G, Bachmann S, Patterson TF, Wickes BL, Lopez-Ribot JL. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother 49:973–980. doi: 10.1093/jac/dkf049. [DOI] [PubMed] [Google Scholar]
- 59.Nett JE, Lepak AJ, Marchillo K, Andes DR. 2009. Time course global gene expression analysis of an in vivo Candida biofilm. J Infect Dis 200:307–313. doi: 10.1086/599838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukherjee PK, Chandra J, Kuhn DM, Ghannoum MA. 2003. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun 71:4333–4340. doi: 10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geissmann Q. 2013. OpenCFU, a new free and open-source software to count cell colonies and other circular objects. PLoS One 8:e54072. doi: 10.1371/journal.pone.0054072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang A, Liu Z, Myers LC. 2013. Differential regulation of white-opaque switching by individual subunits of Candida albicans mediator. Eukaryot Cell 12:1293–1304. doi: 10.1128/EC.00137-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guarente L. 1983. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol 101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.